Cellular Mechanisms Underlying the Cardioprotective Role of Allicin on Cardiovascular Diseases
Abstract
1. Introduction
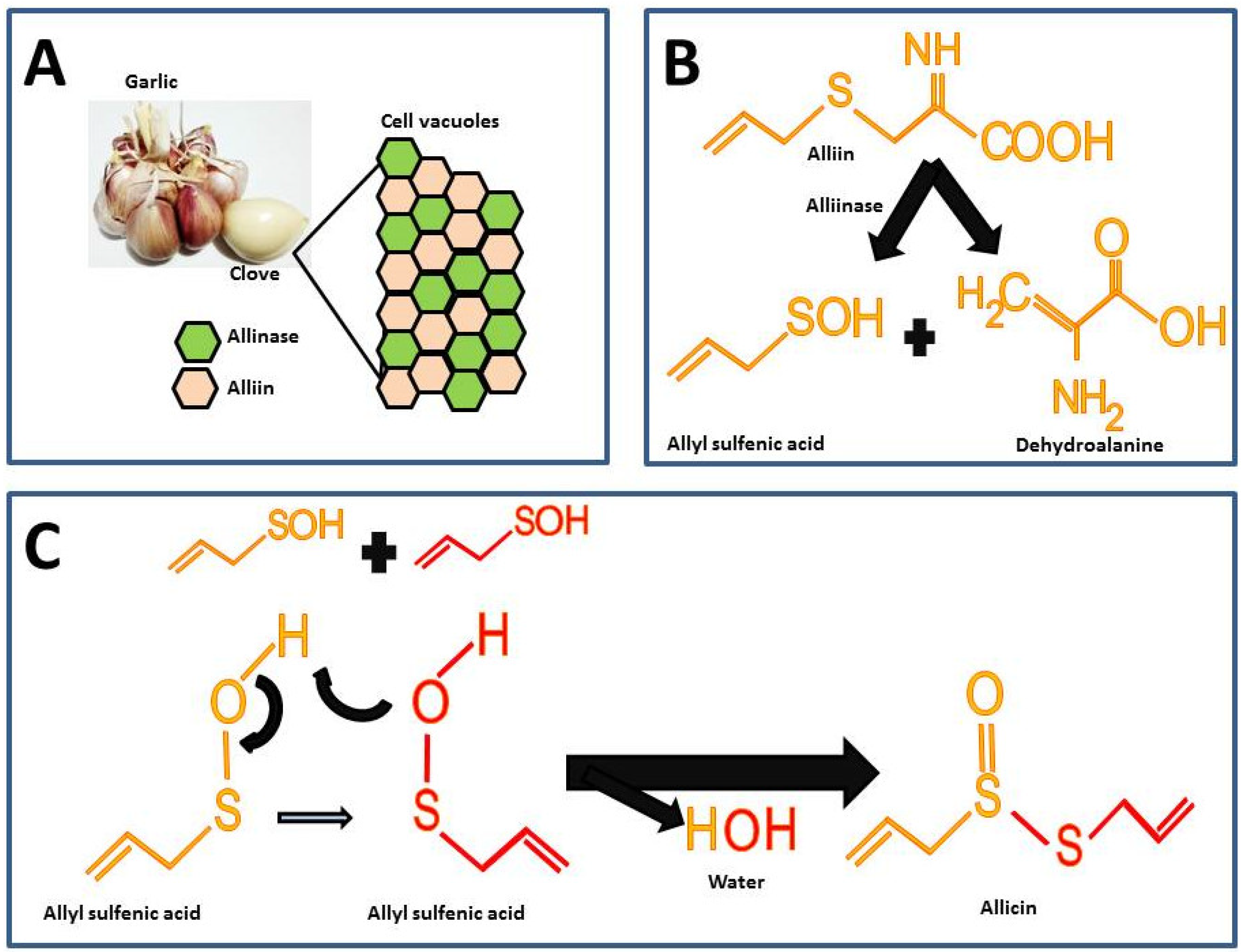
2. Allicin
2.1. Garlic as a Natural Source of Allicin
| Substance or Compound | Percent in 100 g Dry Weight | |
|---|---|---|
| Water | 50% | |
| Carbohydrates | 30% | |
| Proteins | 10% | |
| Alliinase | 10 mg/gr | |
| Free amino acid | 1.0% | |
| Lipids | 3.5% | |
| Fiber | 1.0% | |
| Kilocalories | 149 Kcal | |
| Vitamins | ||
| B1 | 0.16 mg | |
| B2 | 0.02 mg | |
| B6 | 0.32 mg | |
| Nicotinic Acid | 0.12 mg | |
| Ascorbic Acid | 14 mg | |
| Minerals | ||
| Potassium | 446 mg | |
| Phosphorous | 134 mg | |
| Sodium | 19 mg | |
| Calcium | 17 mg | |
| Iron | 1.2 mg | |
| Magnesium | 24.1 mg | |
| Zinc | 1.1 mg | |
| Iodine | 4.7 µg | |
| Selenium | 2 µg | |
| Sulfur compounds | 3.5% | |
| γ-glutamyl peptides: 80–85% | ||
| γ-L-glutamyl-S-(2-propenyl)-L-cysteine (GSAC) | 40–60% | |
| γ-L-glutamyl-S-(trans-1-propenil)-L-cysteine (GSPC) | 10–18% | |
| γ-L-glutamyl-S-methyl-L-cysteine (GSMC) | 10–18% | |
| Sulfoxides produced by the allinase action: | ||
| (+)-S-(2-propenyl)-L-cysteine sulfoxide (alliin) | 60–80% | |
| (+)-S-(trans-1-propenil)-L-cysteine sulfoxide (isoalline) | ||
| (+)-S-methyl-L-cysteine sulfoxide (methiine) | ||
| (1S, 3R, 5S) -5-methyl-1, 4-thiazan-3-carboxylic acid 1-oxide (cycloaliine). |
2.2. Synthetic Allicin
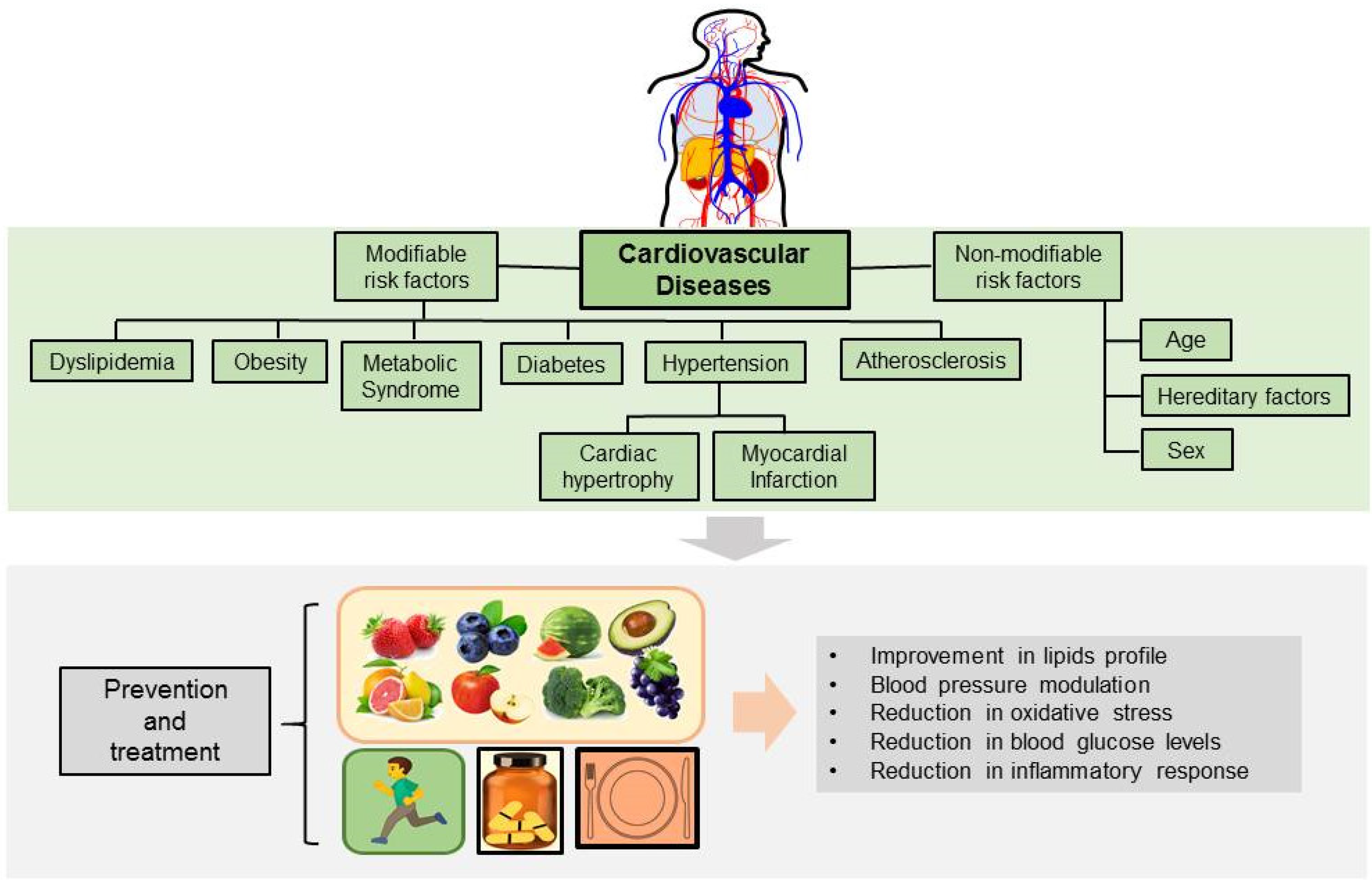
3. Effects of Allicin on Cardiovascular Risk Factors
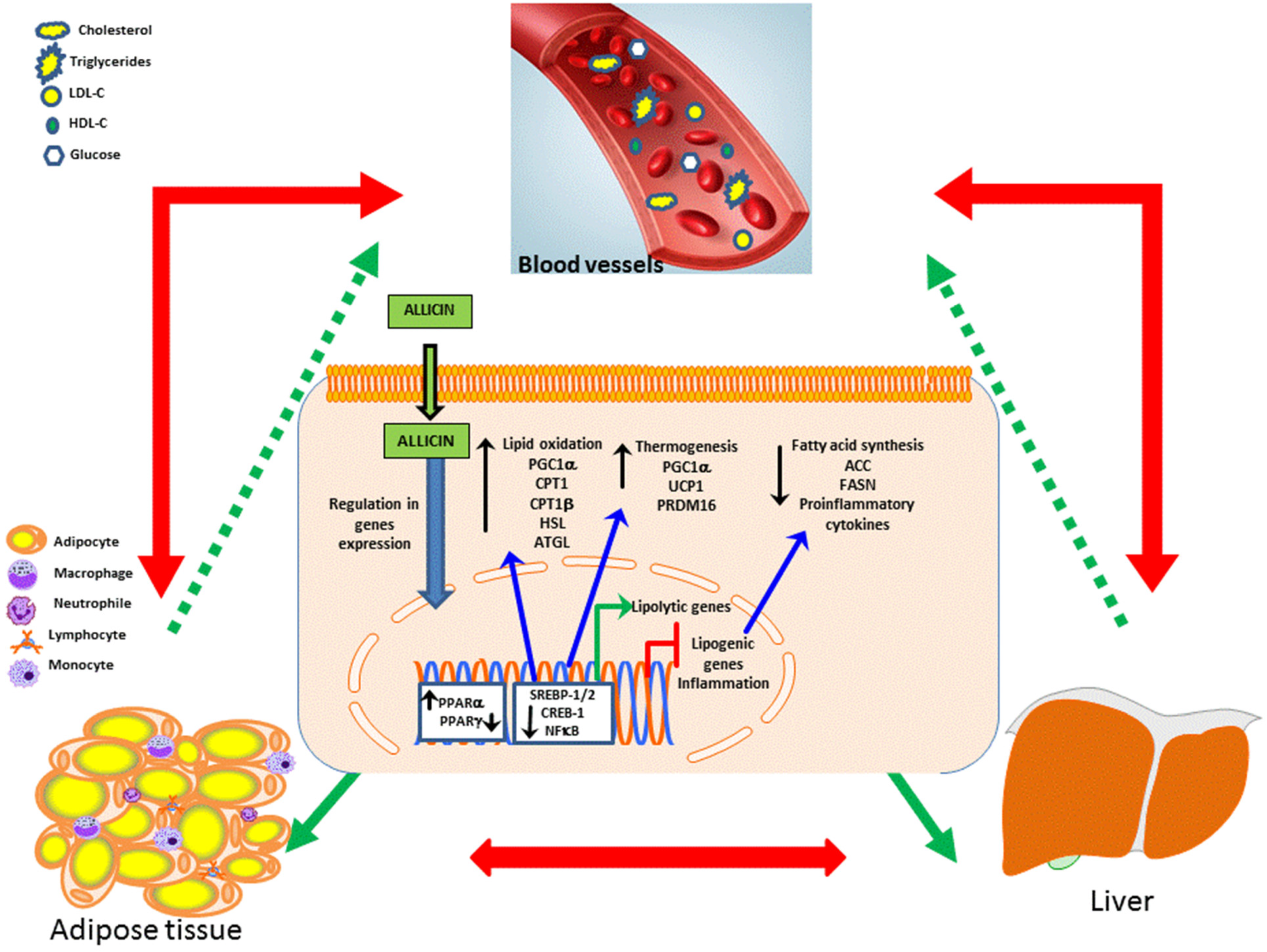
3.1. Dyslipidemia and Obesity
3.2. Atherosclerosis
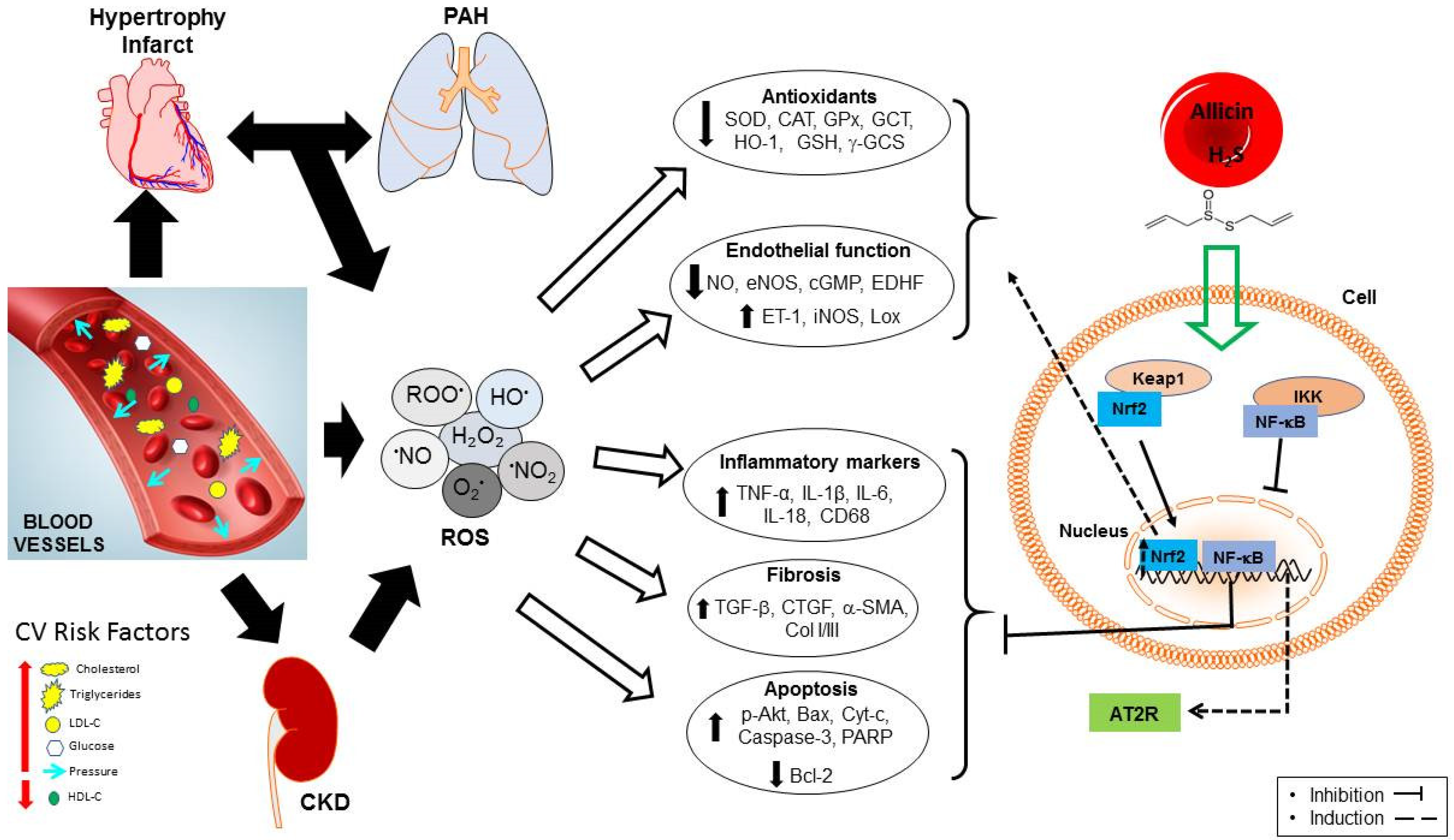
3.3. Endothelial Dysfunction and Oxidative Stress
3.4. Myocardial Infarction
3.5. Hypertension and Cardiac Hypertrophy
3.6. Diabetic Cardiomyopathy and Arrhythmias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarre-Álvarez, D.; Cabrera-Jardines, R.; Rodríguez-Weber, F.; Díaz-Greene, E. Enfermedad cardiovascular aterosclerótica. Revisión de las escalas de riesgo y edad cardiovascular. Med. Int. Méx 2018, 34, 910–923. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.; Zayeri, F.; Salehi, M. Trend analysis of cardiovascular disease mortality, incidence, and mortality-to-incidence ratio: Results from global burden of disease study 2017. BMC Public Health 2021, 21, 401. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Rodriguez, J.; Peñaranda-Flores, D.; Reyes-Saenz, A.; Caceres-Arevalo, K.; Cañizares-Perez, Y. Prevalence of cardiovascular risk factors in Latin America: A review of the published evidence 2010–2015. Rev. Mex. De Cardiol. 2015, 26, 125–139. [Google Scholar]
- Inegi. Comunicado de Prensa Inegi, NÚM. 61/21 27 de Enero de 2021 PÁGINA 1/4. 2021, 61, 45. Available online: https://www.inegi.org.mx/contenidos/saladeprensa/boletines/2021/EstSociodemo/DefuncionesRegistradas2020_Pnles.pdf (accessed on 21 January 2022).
- Available online: https://www.who.int/es/news/item/09-12-2020-who-reveals-leading-causes-of-death-and-disability-worldwide-2000-2019 (accessed on 21 January 2022).
- Cui, Z.; Dewey, S.; Gomes, A.V. Cardioproteomics: Advancing the discovery of signaling mechanisms involved in cardiovascular diseases. Am. J. Cardiovasc. Dis. 2011, 1, 274–292. [Google Scholar] [PubMed]
- International Diabetes Federation. Diabetes and Cardiovascular Disease: Brussels, Belgium. Int. Diabetes Fed. 2016.
- Cruz Robles, D.; de la Peña Díaz, A.; Arce Fonseca, M.; García Trejo, J.J.; Pérez Méndez, Ó.A.; Vargas Alarcón, G. Genética y biología molecular de las cardiopatías congénitas y adquiridas. Arch. Cardiol. Méx. 2005, 75, 467–482. [Google Scholar]
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. Lancet 2013, 383, 999–1008. [Google Scholar] [CrossRef]
- Oktaviono, Y.H.; Amadis, M.R.; Al-Farabi, M.J. High Dose Allicin with Vitamin C Improves EPCs Migration from the Patient with Coronary Artery Disease. Pharmacogn. J. 2020, 12, 232–235. [Google Scholar] [CrossRef]
- Corrigendum to: European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur. Heart J. 2020, 41, 4507. [CrossRef] [PubMed]
- Jorge-Galarza, E.; Martínez-Sánchez, F.D.; Javier-Montiel, C.I.; Msc, A.X.M.; Posadas-Romero, C.; Rd, M.C.G.; Osorio-Alonso, H.; Msc, A.S.A.; Juárez-Rojas, J.G. Control of blood pressure levels in patients with premature coronary artery disease: Results from the Genetics of Atherosclerotic Disease study. J. Clin. Hypertens. 2020, 22, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Gooding, H.C.; Gidding, S.S.; Moran, A.E.; Redmond, N.; Allen, N.B.; Bacha, F.; Burns, T.L.; Catov, J.M.; Grandner, M.A.; Harris, K.M.; et al. Challenges and Opportunities for the Prevention and Treatment of Cardiovascular Disease Among Young Adults: Report From a National Heart, Lung, and Blood Institute Working Group. J. Am. Heart Assoc. 2020, 9, e016115. [Google Scholar] [CrossRef] [PubMed]
- Medina-Urrutia, A.X.; Martínez-Sánchez, F.D.; Posadas-Romero, C.; Jorge-Galarza, E.; Martínez-Alvarado, M.D.R.; González-Salazar, M.D.C.; Osorio-Alonso, H.; Juárez-Rojas, J.G. Metabolic control achievement in a population with premature coronary artery disease: Results of the genetics of atherosclerotic disease study. Ther. Adv. Endocrinol. Metab. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Tenore, G.C.; Novellino, E. Nutraceuticals: A paradigm of proactive medicine. Eur. J. Pharm. Sci. 2017, 96, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.A. Catechins as Emerging and Promising Antiparasitic Agents. Biomed. J. Sci. Tech. Res. 2020, 30, 23084–23088. [Google Scholar] [CrossRef]
- Sánchez-Gloria, J.L.; Osorio-Alonso, H.; Arellano-Buendía, A.S.; Carbó, R.; Hernández-Díazcouder, A.; Guzmán-Martín, C.A.; Rubio-Gayosso, I.; Sánchez-Muñoz, F. Nutraceuticals in the Treatment of Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2020, 21, 4827. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, T.; Zhang, W.; Zhao, Z.; Sun, J. Natural Drugs as a Treatment Strategy for Cardiovascular Disease through the Regulation of Oxidative Stress. Oxidative Med. Cell. Longev. 2020, 2020, 5430407. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rodrigues, C.F.; Sharopov, F.; Docea, A.O.; Can Karaca, A.; Sharifi-Rad, M.; Kahveci Karıncaoglu, D.; Gülseren, G.; Özçelik, B.; Demircan, E.; et al. Diet, Lifestyle and Cardiovascular Diseases: Linking Pathophysiology to Cardioprotective Effects of Natural Bioactive Compounds. Int. J. Environ. Res. Public Health 2020, 17, 2326. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Suleria, H.A.R.; Butt, M.S.; Khalid, N.; Sultan, S.; Raza, A.; Aleem, M.; Abbas, M. Garlic (Allium sativum): Diet based therapy of 21st century–a review. Asian Pac. J. Trop. Dis. 2015, 5, 271–278. [Google Scholar] [CrossRef]
- Bose, S.; Laha, B.; Banerjee, S. Anti-inflammatory activity of isolated allicin from garlic with post-acoustic waves and microwave radiation. J. Adv. Pharm. Educ. Res. 2013, 3, 512–515. [Google Scholar]
- Villamiel, M.; Corzo-Martinez, M.; Soria, A.C. A comprehensive survey of Garlic functionality. Garlic Consum. Health 2010, 5, 642–649. [Google Scholar]
- Ashraf, R.; Aamir, K.; Shaikh, A.R.; Ahmed, T. Effects of garlic on dyslipidemia in patients with type 2 diabetes mellitus. J. Ayub Med Coll. Abbottabad JAMC 2005, 17, 60–64. [Google Scholar] [PubMed]
- Lawson, L.D.; Hunsaker, S.M. Allicin Bioavailability and Bioequivalence from Garlic Supplements and Garlic Foods. Nutrients 2018, 10, 812. [Google Scholar] [CrossRef]
- Ilic, D.; Nikolic, V.; Nikolic, L.; Stankovic, M.; Stanojevic, L.; Cakic, M. Allicin and related compounds: Biosynthesis, synthesis and pharmacological activity. Facta Univ.-Ser. Phys. Chem. Technol. 2011, 9, 9–20. [Google Scholar] [CrossRef]
- Zhai, B.; Zhang, C.; Sheng, Y.; Zhao, C.; He, X.; Xu, W.; Huang, K.; Luo, Y. Hypoglycemic and hypolipidemic effect of S-allyl-cysteine sulfoxide (alliin) in DIO mice. Sci. Rep. 2018, 8, 3527. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, M.A.; Zepeda-Morales, A.S.M.; Carrera-Quintanar, L.; Viveros-Paredes, J.M.; Franco-Arroyo, N.N.; Godínez-Rubí, M.; Ortuño-Sahagun, D.; López-Roa, R.I. Alliin, an Allium sativum Nutraceutical, ReducesMetaflammation Markers in DIO Mice. Nutrients 2020, 12, 624. [Google Scholar] [CrossRef]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar] [CrossRef]
- Redondo, G.L.M.; Gutiérrez, A.L.; Barrantes, J.B.; Navarro, M.P.; Monge, M.C.O.; Vargas, M.J.C. Aspectos generales del Allium sativumuna revisión. Ars Pharm. 2021, 62, 471–481. [Google Scholar] [CrossRef]
- Cardelle, A.; Soria, A.; Corzo, N.; Villamiel, M. A Comprehensive Survey of Garlic Functionality; Nova Science Publishers, Inc.: New York, NY, USA, 2010; pp. 1–60. [Google Scholar]
- Argüello-García, R.; Medina-Campos, O.N.; Pérez-Hernández, N.; Pedraza-Chaverrí, J.; Ortega-Pierres, G. Hypochlorous Acid Scavenging Activities of Thioallyl Compounds from Garlic. J. Agric. Food Chem. 2010, 58, 11226–11233. [Google Scholar] [CrossRef]
- Fujisawa, H.; Suma, K.; Origuchi, K.; Seki, T.; Ariga, T. Thermostability of Allicin Determined by Chemical and Biological Assays. Biosci. Biotechnol. Biochem. 2008, 72, 2877–2883. [Google Scholar] [CrossRef]
- Miron, T.; Rabinkov, A.; Mirelman, D.; Wilchek, M.; Weiner, L. The mode of action of allicin: Its ready permeability through phospholipid membranes may contribute to its biological activity. Biochim. Biophys. Acta-Biomembr. 2000, 1463, 20–30. [Google Scholar] [CrossRef]
- Elkayam, A.; Mirelman, D.; Peleg, E.; Wilchek, M.; Miron, T.; Rabinkov, A.; Sadetzki, S.; Rosenthal, T. Allicin Enalapril on Blood Pressure, Insulin and Triglycerides Levels in Fructose-Induced Hyperinsulinemic-Hyperlipidemic Hypertensive Rats. Am. J. Hyperten. 2000, 14, 377–381. [Google Scholar]
- Elkayam, A.; Mirelman, D.; Peleg, E.; Wilchek, M.; Miron, T.; Rabinkov, A.; Sadetzki, S.; Rosenthal, T. The effects of allicin and enalapril in fructose-induced hyperinsulinemic hyperlipidemic hypertensive rats. Am. J. Hypertens. 2001, 14, 377–381. [Google Scholar] [CrossRef]
- Lu, Y.; He, Z.; Shen, X.; Xu, X.; Fan, J.; Wu, S.; Zhang, D. Cholesterol-Lowering Effect of Allicin on Hypercholesterolemic ICR Mice. Oxidative Med. Cell. Longev. 2012, 2012, 489690. [Google Scholar] [CrossRef]
- Eilat, S.; Oestraicher, Y.; Rabinkov, A.; Ohad, D.; Mirelman, D.; Battler, A.; Eldar, M.; Vered, Z. Alteration of lipid profile in hyperlipidemic rabbits by allicin, an active constituent of garlic. Coron. Artery Dis. 1995, 6, 985–990. [Google Scholar]
- Faisal, A.N.; Almoussawi, Z.A. The Role of Allicin in Regulating Insulin and Glycemic Level in White Mice with Induced Insulin Resistance. Ann. Rom. Soc. Cell Biol. 2021, 25, 10921–10928. [Google Scholar]
- Elkayam, A.; Mirelman, D.; Peleg, E.; Wilchek, M.; Miron, T.; Rabinkov, A.; Oron-Herman, M.; Rosenthal, T. The effects of allicin on weight in fructose-induced hyperinsulinemic, hyperlipidemic, hypertensive rats. Am. J. Hypertens. 2003, 16, 1053–1056. [Google Scholar] [CrossRef]
- Ali, M.; Al-Qattan, K.; Al-Enezi, F.; Khanafer, R.; Mustafa, T. Effect of allicin from garlic powder on serum lipids and blood pressure in rats fed with a high cholesterol diet. Prostaglandins Leukot. Essent. Fat. Acids 2000, 62, 253–259. [Google Scholar] [CrossRef]
- Saradeth, T.; Seidl, S.; Resch, K.; Ernst, E. Does garlic alter the lipid pattern in normal volunteers? Phytomedicine 1994, 1, 183–185. [Google Scholar] [CrossRef]
- Liu, D.; Wang, S.; Li, J.; Liang, E.; Yan, M.; Gao, W. Allicin improves carotid artery intima-media thickness in coronary artery disease patients with hyperhomocysteinemia. Exp. Ther. Med. 2017, 14, 1722–1726. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Song, G.; Zuo, C.; Qiao, X.; Qin, S. The effect of combination therapy of allicin and fenofibrate on high fat diet-induced vascular endothelium dysfunction and liver damage in rats. Lipids Health Dis. 2010, 9, 131. [Google Scholar] [CrossRef]
- Lu, J.; Cheng, B.; Fang, B.; Meng, Z.; Zheng, Y.; Tian, X.; Guan, S. Protective effects of allicin on 1,3-DCP-induced lipid metabolism disorder in HepG2 cells. Biomed. Pharmacother. 2017, 96, 1411–1417. [Google Scholar] [CrossRef]
- Cheng, B.; Li, T.; Li, F. Use of Network Pharmacology to Investigate the Mechanism by Which Allicin Ameliorates Lipid Metabolism Disorder in HepG2 Cells. Evid.-Based Complement. Altern. Med. 2021, 2021, 3956504. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, X.; Chu, X.; Wang, J.; Xie, B.; Ge, J.; Guo, Y.; Li, X.; Yang, G. Allicin Improves Metabolism in High-Fat Diet-Induced Obese Mice by Modulating the Gut Microbiota. Nutrients 2019, 11, 2909. [Google Scholar] [CrossRef]
- Peirce, V.; Carobbio, S.; Vidal-Puig, A. The different shades of fat. Nature 2014, 510, 76–83. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, N.-N.; Yin, R.-L.; Ma, W.; Yan, C.; Feng, Y.-M.; Zhang, C.-H.; Zhao, D. Activation of brown adipocytes by placental growth factor. Biochem. Biophys. Res. Commun. 2018, 504, 470–477. [Google Scholar] [CrossRef]
- Kajimura, S.; Spiegelman, B.M.; Seale, P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 2015, 22, 546–559. [Google Scholar] [CrossRef]
- Wu, J.; Cohen, P.; Spiegelman, B.M. Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes Dev. 2013, 27, 234–250. [Google Scholar] [CrossRef]
- Lee, P.; Bova, R.; Schofield, L.; Bryant, W.; Dieckmann, W.; Slattery, A.; Govendir, M.A.; Emmett, L.; Greenfield, J.R. Brown Adipose Tissue Exhibits a Glucose-Responsive Thermogenic Biorhythm in Humans. Cell Metab. 2016, 23, 602–609. [Google Scholar] [CrossRef]
- Lee, C.G.; Rhee, D.K.; Kim, B.O.; Um, S.H.; Pyo, S. Allicin induces beige-like adipocytes via KLF15 signal cascade. J. Nutr. Biochem. 2018, 64, 13–24. [Google Scholar] [CrossRef]
- Zhang, C.; He, X.; Sheng, Y.; Xu, J.; Yang, C.; Zheng, S.; Liu, J.; Li, H.; Ge, J.; Yang, M.; et al. Allicin Regulates Energy Homeostasis through Brown Adipose Tissue. Science 2020, 23, 101113. [Google Scholar] [CrossRef]
- Soleimani, D.; Moosavian, S.P.; Zolfaghari, H.; Paknahad, Z. Effect of garlic powder supplementation on blood pressure and hs-C-reactive protein among nonalcoholic fatty liver disease patients: A randomized, double-blind, placebo-controlled trial. Food Sci. Nutr. 2021, 9, 3556–3562. [Google Scholar] [CrossRef]
- Abramovitz, D.; Gavri, S.; Harats, D.; Levkovitz, H.; Mirelman, D.; Miron, T.; Eilat-Adar, S.; Rabinkov, A.; Wilchek, M.; Eldar, M.; et al. Allicin-induced decrease in formation of fatty streaks (atherosclerosis) in mice fed a cholesterol-rich diet. Coron. Artery Dis. 1999, 10, 515–520. [Google Scholar] [CrossRef]
- Gonen, A.; Harats, D.; Rabinkov, A.; Miron, T.; Mirelman, D.; Wilchek, M.; Weiner, L.; Ulman, E.; Levkovitz, H.; Ben-Shushan, D.; et al. The Antiatherogenic Effect of Allicin: Possible Mode of Action. Pathobiology 2005, 72, 325–334. [Google Scholar] [CrossRef]
- Dirsch, V.M.; Kiemer, A.K.; Wagner, H.; Vollmar, A.M. Effect of allicin and ajoene, two compounds of garlic, on inducible nitric oxide synthase. Atherosclerosis 1998, 139, 333–339. [Google Scholar] [CrossRef]
- Liu, D.-S.; Gao, W.; Liang, E.-S.; Wang, S.-L.; Lin, W.-W.; Zhang, W.-D.; Jia, Q.; Guo, R.-C.; Zhang, J.-D. Effects of allicin on hyperhomocysteinemia-induced experimental vascular endothelial dysfunction. Eur. J. Pharmacol. 2013, 714, 163–169. [Google Scholar] [CrossRef]
- Panyod, S.; Wu, W.-K.; Chen, P.-C.; Chong, K.-V.; Yang, Y.-T.; Chuang, H.-L.; Chen, C.-C.; Chen, R.-A.; Liu, P.-Y.; Chung, C.-H.; et al. Atherosclerosis amelioration by allicin in raw garlic through gut microbiota and trimethylamine-N-oxide modulation. NPJ Biofilms Microbiomes 2022, 8, 4. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef]
- Schiattarella, G.; Sannino, A.; Toscano, E.; Giugliano, G.; Gargiulo, G.; Franzone, A.; Trimarco, B.; Esposito, G.; Perrino, C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: A systematic review and dose-response meta-analysis. Eur. Heart J. 2017, 38, 2948–2956. [Google Scholar] [CrossRef]
- Ren, S.-C.; Mao, N.; Yi, S.; Ma, X.; Zou, J.-Q.; Tang, X.; Fan, J.-M. Vascular Calcification in Chronic Kidney Disease: An Update and Perspective. Aging Dis. 2022, 13, 673. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lin, P.; Feng, L.; Ren, Q.; Xie, X.; Zhang, B. Ameliorative effect of allicin on vascular calcification via inhibiting endoplasmic reticulum stress. Vascular 2021. [Google Scholar] [CrossRef]
- Scioli, M.G.; Storti, G.; D’Amico, F.; Guzmán, R.R.; Centofanti, F.; Doldo, E.; Miranda, E.M.C.; Orlandi, A. Oxidative Stress and New Pathogenetic Mechanisms in Endothelial Dysfunction: Potential Diagnostic Biomarkers and Therapeutic Targets. J. Clin. Med. 2020, 9, 1995. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.-Y.; Tsui, H.-T.; Chung, I.Y.-M.; Chan, R.Y.-K.; Kwan, Y.-W.; Chan, S.-W. Allicin protects rat cardiomyoblasts (H9c2 cells) from hydrogen peroxide-induced oxidative injury through inhibiting the generation of intracellular reactive oxygen species. Int. J. Food Sci. Nutr. 2014, 65, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Yang, P.; Gao, T.; Liu, M.; Li, X. Allicin attenuates myocardial apoptosis, inflammation and mitochondrial injury during hypoxia-reoxygenation: An in vitro study. BMC Cardiovasc. Disord. 2021, 21, 200. [Google Scholar] [CrossRef]
- Chen, X.; Pang, S.; Lin, J.; Xia, J.; Wang, Y. Allicin prevents oxidized low-density lipoprotein-induced endothelial cell injury by inhibiting apoptosis and oxidative stress pathway. BMC Complement. Altern. Med. 2016, 16, 133. [Google Scholar] [CrossRef]
- Lu, Q.; Lu, P.-M.; Piao, J.-H.; Xu, X.-L.; Chen, J.; Zhu, L.; Jiang, J.-G. Preparation and physicochemical characteristics of an allicin nanoliposome and its release behavior. LWT Food Sci. Technol. 2014, 57, 686–695. [Google Scholar] [CrossRef]
- Grune, T.; Scherat, T.; Behrend, H.; Conradi, E.; Brenke, R.; Siems, W. Influence of Allium sativum on oxidative stress status—A clinical investigation. Phytomedicine 1996, 2, 205–207. [Google Scholar] [CrossRef]
- Trejo, E.M.G.; Buendía, A.S.A.; Reyes, O.S.; Arroyo, F.E.G.; Garcia, F.; Mendoza, M.L.L.; Tapia, E.; Lozada, L.G.S.; Alonso, H.O. The Beneficial Effects of Allicin in Chronic Kidney Disease Are Comparable to Losartan. Int. J. Mol. Sci. 2017, 18, 1980. [Google Scholar] [CrossRef]
- Oktaviono, Y.H.; Pikir, B.S.; Alzahra, F.; Al-Farabi, M.J.; Putri, A.Y. Garlic Extract (Allicin) Improves the Proliferation of Endothelial Progenitor Cell (EPC) from Patients with Stable Coronary Artery Disease. Open Access Maced. J. Med. Sci. 2020, 8, 65–69. [Google Scholar] [CrossRef]
- Liu, S.; He, Y.; Shi, J.; Liu, L.; Ma, H.; He, L.; Guo, Y. Allicin Attenuates Myocardial Ischemia Reperfusion Injury in Rats by Inhibition of Inflammation and Oxidative Stress. Transplant. Proc. 2019, 51, 2060–2065. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.-N.; Li, L.-D.; Li, S.-C.; Hao, X.-M.; Zhang, J.-Y.; He, P.; Li, Y.-K. Allicin improves cardiac function by protecting against apoptosis in rat model of myocardial infarction. Chin. J. Integr. Med. 2016, 23, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, S.; Li, S.; Deng, L.; Li, Y.; Li, H. Effect of Allicin against Ischemia/Hypoxia-Induced H9c2 Myoblast Apoptosis via eNOS/NO Pathway-Mediated Antioxidant Activity. Evid.-Based Complement. Altern. Med. 2018, 2018, 3207973. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Yang, P.; Fu, D.; Liu, M.; Deng, X.; Shao, M.; Liao, J.; Jiang, H.; Li, X. The protective effect of allicin on myocardial ischemia-reperfusion by inhibition of Ca2+ overload-induced cardiomyocyte apoptosis via the PI3K/GRK2/PLC-γ/IP3R signaling pathway. Aging 2021, 13, 19643–19656. [Google Scholar] [CrossRef]
- Wang, S.-L.; Liu, D.-S.; Liang, E.-S.; Gao, Y.-H.; Cui, Y.; Liu, Y.-Z.; Gao, W. Protective effect of allicin on high glucose/hypoxia-induced aortic endothelial cells via reduction of oxidative stress. Exp. Ther. Med. 2015, 10, 1394–1400. [Google Scholar] [CrossRef]
- Xu, W.; Li, X.-P.; Li, E.-Z.; Liu, Y.-F.; Zhao, J.; Wei, L.-N.; Ma, L. Protective Effects of Allicin on ISO-Induced Rat Model of Myocardial Infarction via JNK Signaling Pathway. Pharmacology 2020, 105, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Liu, W.; Yu, C.; Ren, J.; Li, Y.; Shi, X.; Li, Q.; Zhang, J. Protective Effects of Allicin on Acute Myocardial Infarction in Rats via Hydrogen Sulfide-mediated Regulation of Coronary Arterial Vasomotor Function and Myocardial Calcium Transport. Front. Pharmacol. 2022, 12, 752244. [Google Scholar] [CrossRef]
- Liu, M.; Yang, P.; Fu, D.; Gao, T.; Deng, X.; Shao, M.; Liao, J.; Jiang, H.; Li, X. Allicin protects against myocardial I/R by accelerating angiogenesis via the miR-19a-3p/PI3K/AKT axis. Aging 2021, 13, 22843–22855. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.M.; Soliman, K.E.; Alhumaydhi, F.A.; Almatroudi, A.; Allemailem, K.S.; Alsahli, M.A.; Alrumaihi, F.; Aljasir, M.; Alwashmi, A.S.; Ahmed, A.A.; et al. Could allicin alleviate trastuzumab-induced cardiotoxicity in a rat model through antioxidant, anti-inflammatory, and antihyperlipidemic properties? Life Sci. 2022, 302, 120656. [Google Scholar] [CrossRef] [PubMed]
- Al-Thubiani, W.S.; Abuzinadah, O.A.H.; El-Aziz, G.S.A. Betanin and Allicin Ameliorate Adriamycin-Induced Cardiotoxicity in Rats by Ameliorating Cardiac Ischemia and Improving Antioxidant Efficiency. J. Pharm. Res. Int. 2021, 33, 39–56. [Google Scholar] [CrossRef]
- Shan, Y.; Chen, D.; Hu, B.; Xu, G.; Li, W.; Jin, Y.; Jin, X.; Jin, X.; Jin, L. Allicin ameliorates renal ischemia/reperfusion injury via inhibition of oxidative stress and inflammation in rats. Biomed. Pharmacother. 2021, 142, 112077. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ning, J.; Huang, H.; Jiang, S.; Zhuo, D. Allicin protects against renal ischemia–reperfusion injury by attenuating oxidative stress and apoptosis. Int. Urol. Nephrol. 2021, 54, 1761–1768. [Google Scholar] [CrossRef]
- Saheera, S.; Krishnamurthy, P. Cardiovascular Changes Associated with Hypertensive Heart Disease and Aging. Cell Transplant. 2020, 29, 0963689720920830. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Liu, W.; Chen, S.; Yu, C.; Li, Y.; Zhang, J.-Y. Antihypertensive effects of allicin on spontaneously hypertensive rats via vasorelaxation and hydrogen sulfide mechanisms. Biomed. Pharmacother. 2020, 128, 110240. [Google Scholar] [CrossRef]
- Dubey, H.; Singh, A.; Patole, A.M.; Tenpe, C.R.; Ghule, B.V. Allicin, a SUR2 opener: Possible mechanism for the treatment of diabetic hypertension in rats. Rev. Bras. de Farm. 2012, 22, 1053–1059. [Google Scholar] [CrossRef]
- Bhardwaj, K.; Verma, M.; Verma, N.; Bhardwaj, S.; Mishra, S. Effect of long term supplementation of active garlic allicin in reducing blood pressure in hypertensive subjects. Int. J. Adv. Med. 2015, 2, 231–234. [Google Scholar] [CrossRef]
- McMahon, F.G.; Vargas, R. Can garlic lower blood pressure? A pilot study. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1993, 13, 406–407. [Google Scholar]
- Ba, L.; Gao, J.; Chen, Y.; Qi, H.; Dong, C.; Pan, H.; Zhang, Q.; Shi, P.; Song, C.; Guan, X.; et al. Allicin attenuates pathological cardiac hypertrophy by inhibiting autophagy via activation of PI3K/Akt/mTOR and MAPK/ERK/mTOR signaling pathways. Phytomedicine 2018, 58, 152765. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cao, F.; Tang, Q.-Z.; Yan, L.; Dong, Y.-G.; Zhu, L.-H.; Wang, L.; Bian, Z.-Y.; Li, H. Allicin protects against cardiac hypertrophy and fibrosis via attenuating reactive oxygen species-dependent signaling pathways. J. Nutr. Biochem. 2010, 21, 1238–1250. [Google Scholar] [CrossRef]
- Li, X.-H.; Li, C.-Y.; Xiang, Z.-G.; Hu, J.-J.; Lu, J.-M.; Tian, R.-B.; Jia, W. Allicin Ameliorates Cardiac Hypertrophy and Fibrosis through Enhancing of Nrf2 Antioxidant Signaling Pathways. Cardiovasc. Drugs Ther. 2012, 26, 457–465. [Google Scholar] [CrossRef] [PubMed]
- García-Trejo, E.M.A.; Arellano-Buendía, A.S.; Argüello-García, R.; Loredo-Mendoza, M.L.; García-Arroyo, F.E.; Arellano-Mendoza, M.G.; Castillo-Hernández, M.C.; Guevara-Balcázar, G.; Tapia, E.; Sánchez-Lozada, L.G.; et al. Effects of Allicin on Hypertension and Cardiac Function in Chronic Kidney Disease. Oxidative Med. Cell. Longev. 2016, 2016, 3850402. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Cao, Y.; Gao, J.; Fu, B.; Ren, J.; Ba, L.; Song, C.; Qi, H.; Huang, W.; Guan, X.; et al. Allicin improves the function of cardiac microvascular endothelial cells by increasing PECAM-1 in rats with cardiac hypertrophy. Phytomedicine 2018, 51, 241–254. [Google Scholar] [CrossRef]
- Liu, Q.; Fu, Q.; DU, J.; Liu, X. Experimental study on the role and mechanism of Allicin in ventricular remodeling through PPARα and PPARγ signaling pathways. Food Sci. Technol. 2022, 42, e31121. [Google Scholar] [CrossRef]
- Rosenkranz, S.; Howard, L.S.; Gomberg-Maitland, M.; Hoeper, M.M. Systemic Consequences of Pulmonary Hypertension and Right-Sided Heart Failure. Circulation 2020, 141, 678–693. [Google Scholar] [CrossRef]
- Sun, X.; Ku, D.D. Allicin in garlic protects against coronary endothelial dysfunction and right heart hypertrophy in pulmonary hypertensive rats. Am. J. Physiol. Circ. Physiol. 2006, 291, H2431–H2438. [Google Scholar] [CrossRef]
- Sánchez-Gloria, J.L.; Martínez-Olivares, C.E.; Rojas-Morales, P.; Hernández-Pando, R.; Carbó, R.; Rubio-Gayosso, I.; Arellano-Buendía, A.S.; Rada, K.M.; Sánchez-Muñoz, F.; Osorio-Alonso, H. Anti-Inflammatory Effect of Allicin Associated with Fibrosis in Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2021, 22, 8600. [Google Scholar] [CrossRef] [PubMed]
- Borghetti, G.; von Lewinski, D.; Eaton, D.M.; Sourij, H.; Houser, S.R.; Wallner, M. Diabetic Cardiomyopathy: Current and Future Therapies. Beyond Glycemic Control. Front. Physiol. 2018, 9, 1514. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, H.; Wang, Y.; Wu, M.; Cao, Y.; Huang, W.; Li, L.; Ji, Z.; Sun, H. Allicin protects against myocardial apoptosis and fibrosis in streptozotocin-induced diabetic rats. Phytomedicine 2012, 19, 693–698. [Google Scholar] [CrossRef]
- Horuzsko, D.; LaCavera, M.; Ma, H.; Wu, Y.; Zhu, S.; White, R. Allicin Reverses Diabetes-Induced Dysfunction of Human Coronary Artery Endothelial Cells. FASEB J. 2019, 33, lb500. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Cao, Y.-G.; Qi, H.-P.; Li, L.; Bai, B.; Liu, Y.; Sun, H.-L. Antiarrhythmic effects and ionic mechanisms of allicin on myocardial injury of diabetic rats induced by streptozotocin. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2013, 386, 697–704. [Google Scholar] [CrossRef]
- Cao, H.; Huang, C.; Wang, X. Allicin inhibits transient outward potassium currents in mouse ventricular myocytes. Exp. Ther. Med. 2016, 11, 1896–1900. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Xu, L.; Liu, P.; Liu, Y.; Sun, C.; Yin, Y. Allicin disrupts cardiac Cav1.2 channels via trafficking. Pharm. Biol. 2019, 57, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, Y.; Bai, J.; Liu, C.; Ma, S.; Li, J.; Lu, X.; Fu, Z.; Fang, L.; Li, Y.; et al. Effects of Allicin on Late Sodium Current Caused by ΔKPQ-SCN5A Mutation in HEK293 Cells. Front. Physiol. 2021, 12, 636485. [Google Scholar] [CrossRef]
- Chan, J.Y.-Y.; Yuen, A.C.-Y.; Chan, R.Y.-K.; Chan, S.-W. A Review of the Cardiovascular Benefits and Antioxidant Properties of Allicin. Phytother. Res. 2013, 27, 637–646. [Google Scholar] [CrossRef]
- Trio, P.Z.; You, S.; He, X.; He, J.; Sakao, K.; Hou, D.-X. Chemopreventive functions and molecular mechanisms of garlic organosulfur compounds. Food Funct. 2014, 5, 833–844. [Google Scholar] [CrossRef]
- Pedraza-Chaverrí, J.; Barrera, D.; Maldonado, P.D.; Chirino, Y.I.; Macías-Ruvalcaba, N.A.; Medina-Campos, O.N.; Castro, L.; Salcedo, M.I.; Hernández-Pando, R. S-allylmercaptocysteine scavenges hydroxyl radical and singlet oxygen in vitro and attenuates gentamicin-induced oxidative and nitrosative stress and renal damage in vivo. BMC Clin. Pharmacol. 2004, 4, 5. [Google Scholar] [CrossRef]
- Buendía, A.S.A.; González, M.T.; Reyes, O.S.; Arroyo, F.E.G.; García, R.A.; Tapia, E.; Lozada, L.G.S.; Alonso, H.O. Immunomodulatory Effects of the Nutraceutical Garlic Derivative Allicin in the Progression of Diabetic Nephropathy. Int. J. Mol. Sci. 2018, 19, 3107. [Google Scholar] [CrossRef] [PubMed]
- Horev-Azaria, L.; Eliav, S.; Izigov, N.; Pri-Chen, S.; Mirelman, D.; Miron, T.; Rabinkov, A.; Wilchek, M.; Jacob-Hirsch, J.; Amariglio, N.; et al. Allicin up-regulates cellular glutathione level in vascular endothelial cells. Eur. J. Nutr. 2008, 48, 67–74. [Google Scholar] [CrossRef]
- Miron, T.; Rabinkov, A.; Peleg, E.; Rosenthal, T.; Mirelman, D.; Wilchek, M. Allylmercaptocaptopril: A new antihypertensive drug. Am. J. Hypertens. 2004, 17, 71–73. [Google Scholar] [CrossRef][Green Version]
- Oron-Herman, M.; Rosenthal, T.; Mirelman, D.; Miron, T.; Rabinkov, A.; Wilchek, M.; Sela, B.-A. The effects of S-allylmercaptocaptopril, the synthetic product of allicin and captopril, on cardiovascular risk factors associated with the metabolic syndrome. Atherosclerosis 2005, 183, 238–243. [Google Scholar] [CrossRef]
- Ma, C.; Li, S.; Yin, Y.; Xu, W.; Xue, T.; Wang, Y.; Liu, X.; Liu, F. Preparation, characterization, formation mechanism and stability of allicin-loaded emulsion gel. LWT 2022, 161, 113389. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Shao, J.-J.; Wang, Z.-L.; Lu, Z.-X. Study of allicin microcapsules in β-cyclodextrin and porous starch mixture. Food Res. Int. 2012, 49, 641–647. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Helden, E.; Conner, W.T. Garlic Extract Therapy in Children with Hypercholesterolemia. Arch. Pediatr. Adolesc. Med. 1998, 152, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Ansary, J.; Forbes-Hernández, T.Y.; Gil, E.; Cianciosi, D.; Zhang, J.; Elexpuru-Zabaleta, M.; Simal-Gandara, J.; Giampieri, F.; Battino, M. Potential Health Benefit of Garlic Based on Human Intervention Studies: A Brief Overview. Antioxidants 2020, 9, 619. [Google Scholar] [CrossRef]
- Asdaq, S.M.B.; Yasmin, F.; Alsalman, A.J.; Al Mohaini, M.; Kamal, M.; Al Hawaj, M.A.; Alsalman, K.J.; Imran, M.; Sreeharsha, N. Obviation of dyslipidemia by garlic oil and its organosulfur compound, diallyl disulphide, in experimental animals. Saudi J. Biol. Sci. 2021, 29, 2520–2525. [Google Scholar] [CrossRef]
| Type | Affection | Clinical Manifestation |
|---|---|---|
| Cerebrovascular Disease | Alterations in blood vessels and circulation that supply blood to the brain [1,2,7] | Embolism |
| Thrombosis | ||
| Ischemic Stroke | ||
| Intracerebral hemorrhage | ||
| Transient ischemic attack | ||
| Coronary Heart Disease | Impaired flow in the blood vessels that supply blood to the heart [1,2,7] | Hypertensive diseases |
| Myocardial infarction | ||
| Heart failure | ||
| Sudden death | ||
| Atherosclerotic heart disease | ||
| Pulmonary arterial hypertension | ||
| Peripheral Arterial Disease | The narrowing of the blood vessels reduces blood flow to the arms and legs [1,2,7] | Atherosclerosis |
| Aneurysm | ||
| Arterial thrombosis | ||
| Deep vein thrombosis | ||
| Acute limb ischemia | ||
| Arrhythmias | Alteration in rate or rhythm of the heartbeat [2,7] | Tachycardia |
| Bradycardia | ||
| Premature contractions | ||
| Atrial fibrillation | ||
| Rheumatic Heart Disease | Damage to the muscle and valves in the heart [1,2,7] | Rheumatic fever |
| Congenital Heart Defects | Malformations of the heart or great vessels present at birth [2,9] | Abnormal heart valves |
| Septal defects | ||
| Patent ductus arteriosus | ||
| Atresia | ||
| Pulmonary arterial hypertension | ||
| Coarctation of aorta |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Gloria, J.L.; Arellano-Buendía, A.S.; Juárez-Rojas, J.G.; García-Arroyo, F.E.; Argüello-García, R.; Sánchez-Muñoz, F.; Sánchez-Lozada, L.G.; Osorio-Alonso, H. Cellular Mechanisms Underlying the Cardioprotective Role of Allicin on Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 9082. https://doi.org/10.3390/ijms23169082
Sánchez-Gloria JL, Arellano-Buendía AS, Juárez-Rojas JG, García-Arroyo FE, Argüello-García R, Sánchez-Muñoz F, Sánchez-Lozada LG, Osorio-Alonso H. Cellular Mechanisms Underlying the Cardioprotective Role of Allicin on Cardiovascular Diseases. International Journal of Molecular Sciences. 2022; 23(16):9082. https://doi.org/10.3390/ijms23169082
Chicago/Turabian StyleSánchez-Gloria, José L., Abraham S. Arellano-Buendía, Juan G. Juárez-Rojas, Fernando E. García-Arroyo, Raúl Argüello-García, Fausto Sánchez-Muñoz, Laura G. Sánchez-Lozada, and Horacio Osorio-Alonso. 2022. "Cellular Mechanisms Underlying the Cardioprotective Role of Allicin on Cardiovascular Diseases" International Journal of Molecular Sciences 23, no. 16: 9082. https://doi.org/10.3390/ijms23169082
APA StyleSánchez-Gloria, J. L., Arellano-Buendía, A. S., Juárez-Rojas, J. G., García-Arroyo, F. E., Argüello-García, R., Sánchez-Muñoz, F., Sánchez-Lozada, L. G., & Osorio-Alonso, H. (2022). Cellular Mechanisms Underlying the Cardioprotective Role of Allicin on Cardiovascular Diseases. International Journal of Molecular Sciences, 23(16), 9082. https://doi.org/10.3390/ijms23169082









