Influence of Microbial Metabolites and Itaconic Acid Involved in Bacterial Inflammation on the Activity of Mitochondrial Enzymes and the Protective Role of Alkalization
Abstract
:1. Introduction
2. Results
2.1. Influence of Indolic Acids on the Oxidation of Mitochondrial Substrates Measured by the MTT Assay
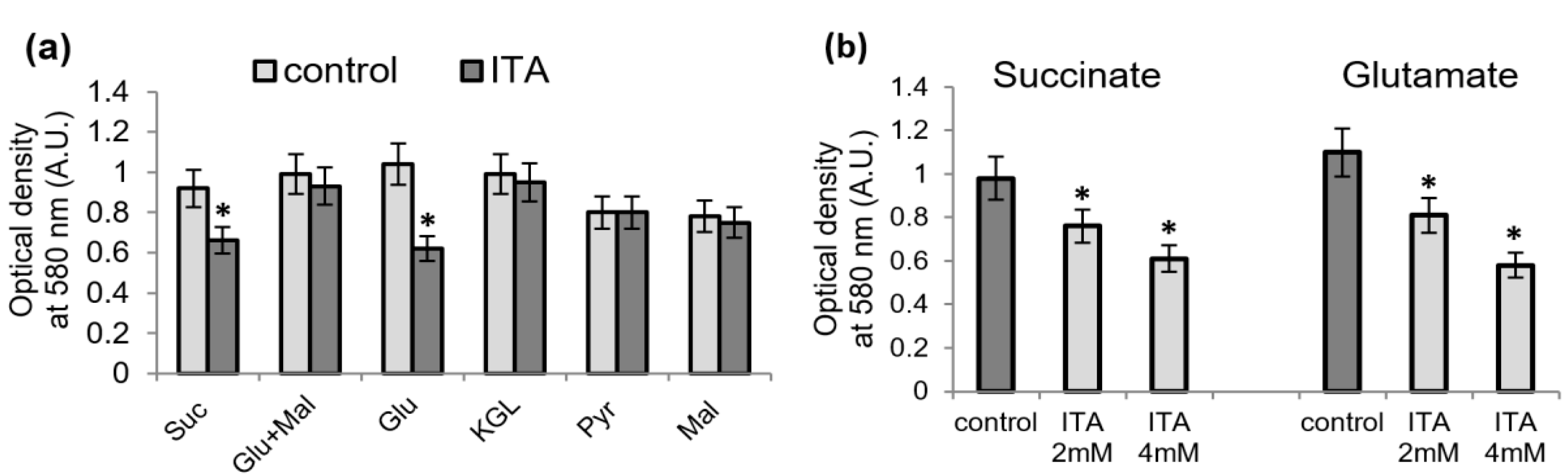
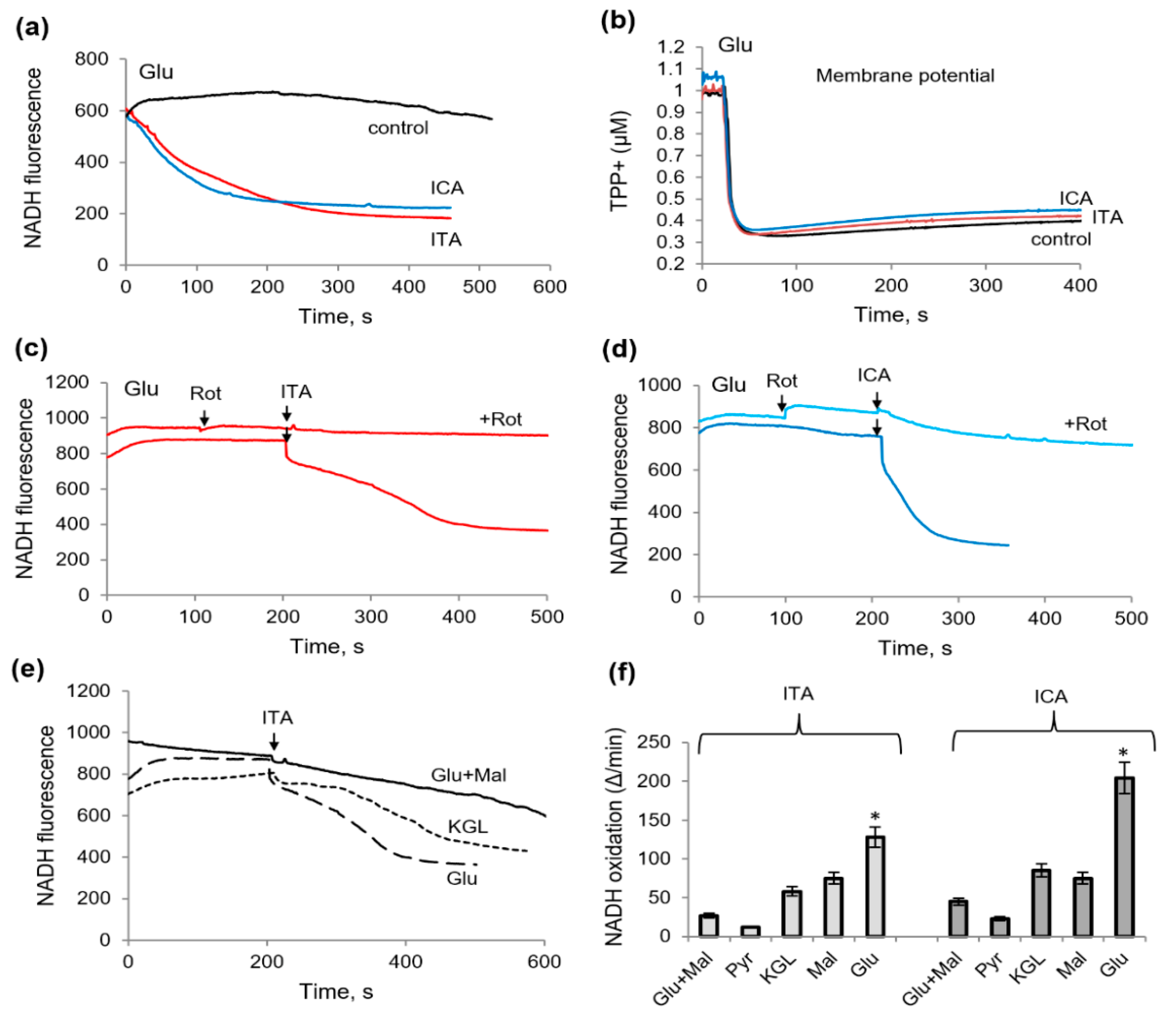
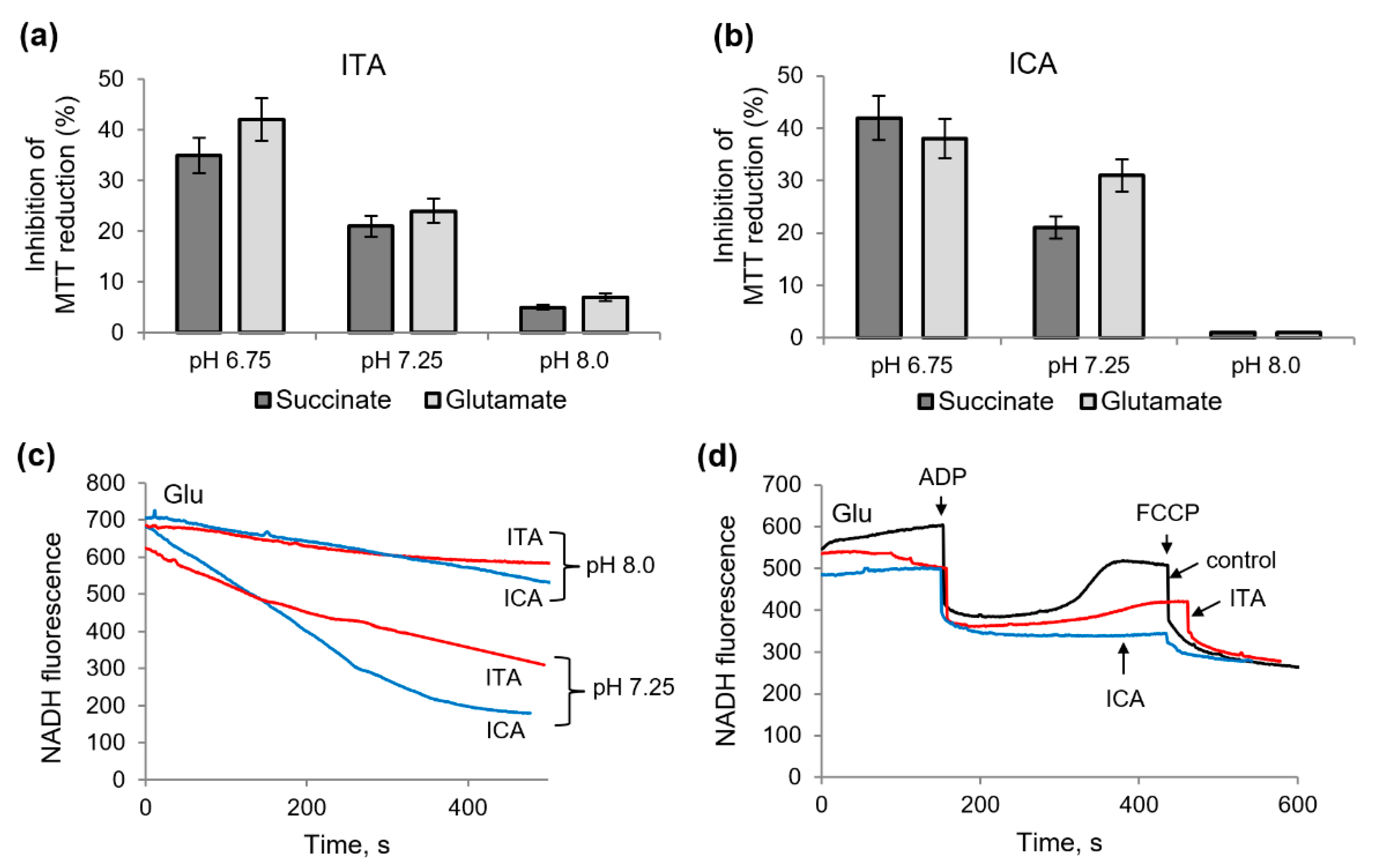
2.2. Influence of Itaconic Acid on the Oxidation of Mitochondrial Substrates, as Measured by the MTT Reduction
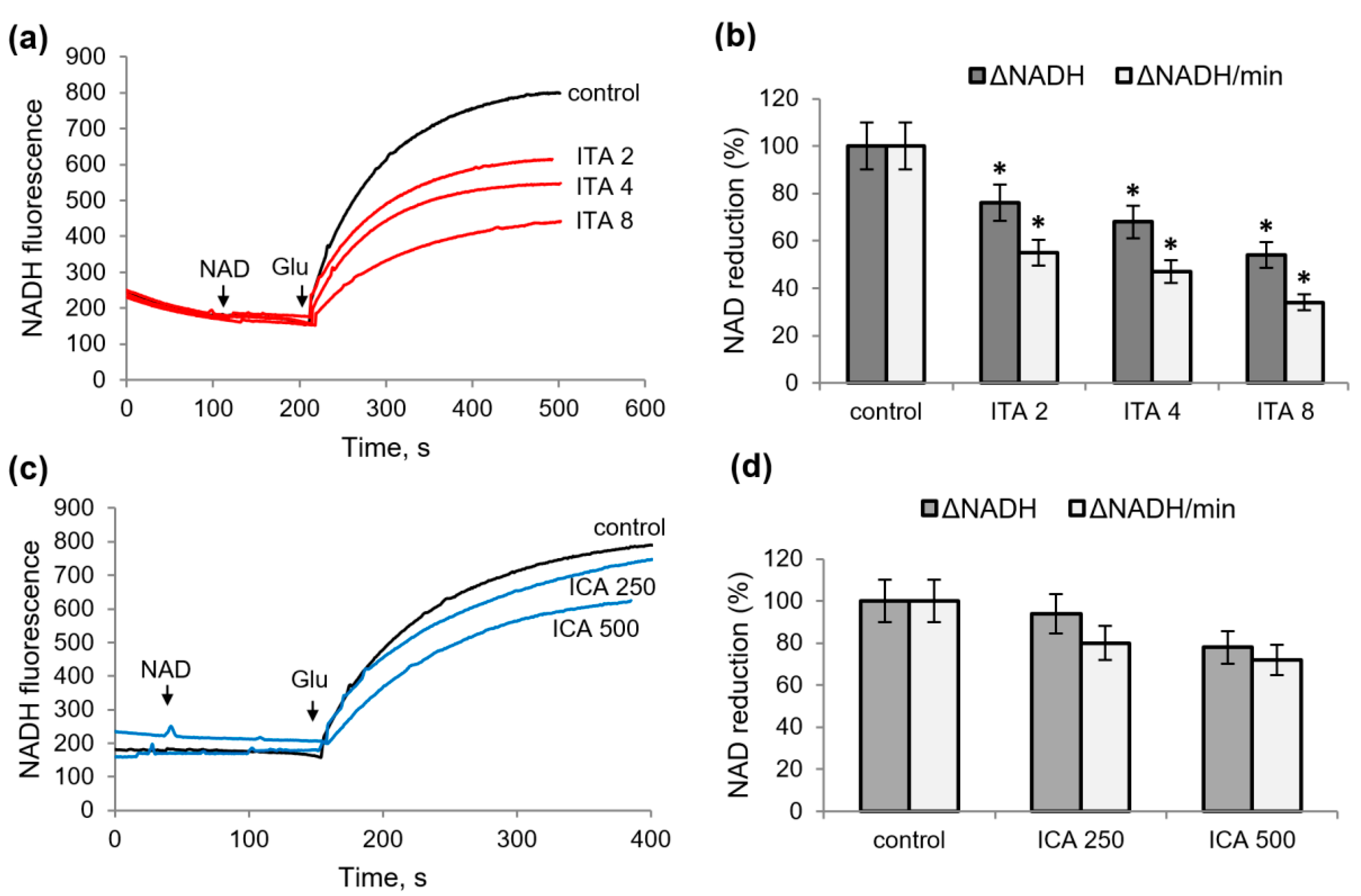
2.3. Influence of Itaconic and Indolcarboxylic Acids on the Oxidation of NAD-Dependent Substrates
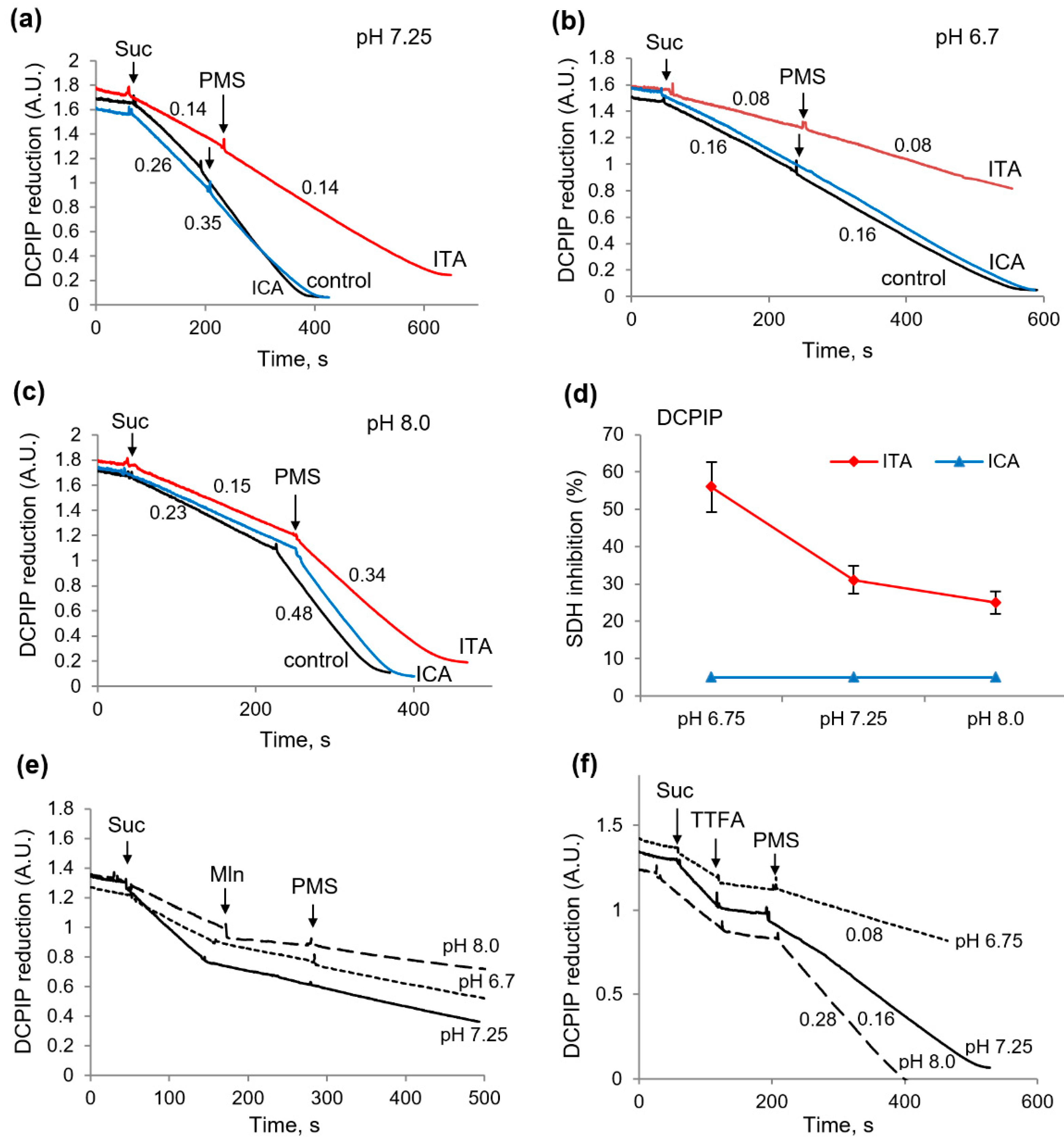
2.4. Influence of Itaconic and Indolcaboxylic Acids on the Activity of SDH, as Measured by DCPIP Reduction
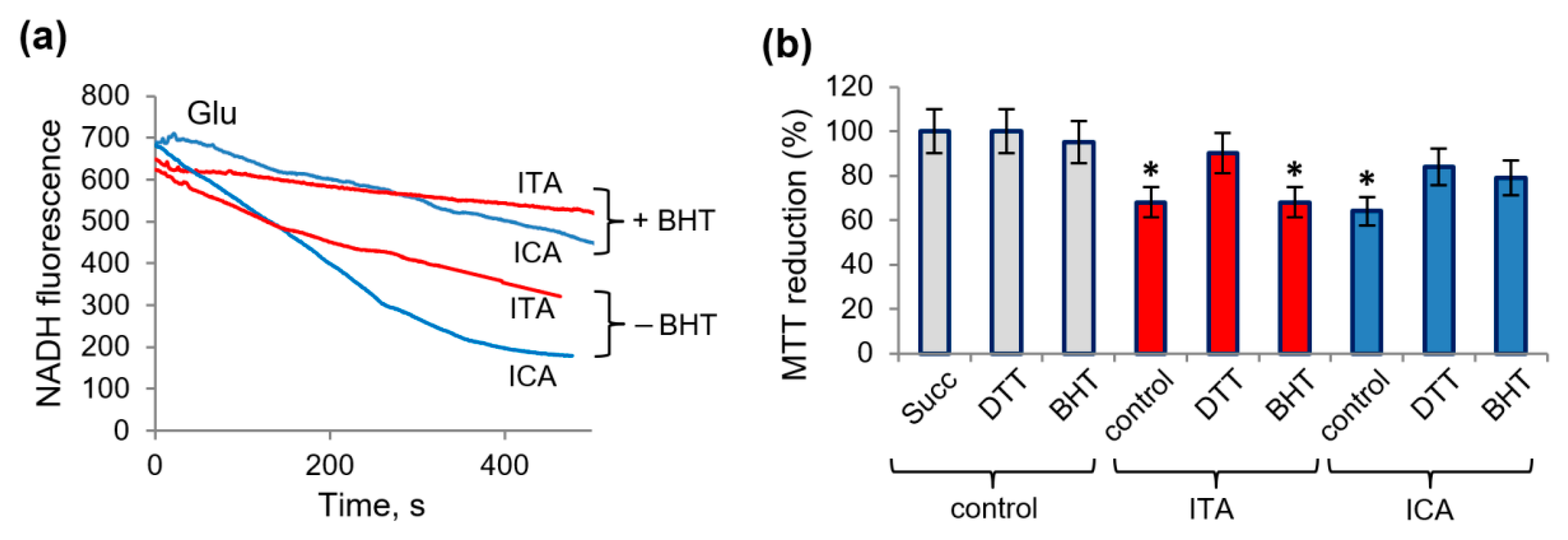
2.5. Protective Role of Alkalization against the Influence of Itaconic and Indolcaboxylic Acids on the Activity of Mitochondrial Enzymes
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Preparation of Rat Liver Mitochondria
4.3. Determination of Succinate Dehydrogenase Activity from Reduction of Methyl Thiazolyl Tetrazolium (MTT) and Dichlorophenolindophenol (DCPIP)
4.4. Determination of Mitochondrial Membrane Potential
4.5. Determination of the Redox State of Pyridine Nucleotides and Oxidative Phosphorylation in Mitochondria
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, C.H. Immune regulation by microbiome metabolites. Immunology 2018, 154, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Caspani, G.; Swann, J. Small talk: Microbial metabolites involved in the signaling from microbiota to brain. Curr. Opin. Pharmacol. 2019, 48, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Visconti, A.; Le Roy, C.I.; Rosa, F.; Rossi, N.; Martin, T.C.; Mohney, R.P.; Li, W.; de Rinaldis, E.; Bell, J.T.; Venter, J.C.; et al. Interplay between the human gut microbiome and host metabolism. Nat. Commun. 2019, 10, 4505. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.I.; Kapila, R. Dietary metabolites derived from gut microbiota: Critical modulators of epigenetic changes in mammals. Nutr. Rev. 2017, 75, 374–389. [Google Scholar] [CrossRef] [PubMed]
- Beloborodova, N.; Pautova, A.; Sergeev, A.; Fedotcheva, N. Serum Levels of Mitochondrial and Microbial Metabolites Reflect Mitochondrial Dysfunction in Different Stages of Sepsis. Metabolites 2019, 9, 196. [Google Scholar] [CrossRef]
- Bajpai, P.; Darra, A.; Agrawal, A. Microbe-mitochondrion crosstalk and health: An emerging paradigm. Mitochondrion 2018, 39, 20–25. [Google Scholar] [CrossRef]
- Cordes, T.; Wallace, M.; Michelucci, A.; Divakaruni, A.S.; Sapcariu, S.C.; Sousa, C.; Koseki, H.; Cabrales, P.; Murphy, A.N.; Hiller, K.; et al. Immunoresponsive Gene 1 and Itaconate Inhibit Succinate Dehydrogenase to Modulate Intracellular Succinate Levels. J. Biol. Chem. 2016, 291, 14274–14284. [Google Scholar] [CrossRef]
- Peace, C.G.; O’Neill, L.A. The role of itaconate in host defense and inflammation. J. Clin. Invest. 2022, 132, e148548. [Google Scholar] [CrossRef]
- Zasłona, Z.; O’Neill, L.A.J. Cytokine-like Roles for Metabolites in Immunity. Mol. Cell. 2020, 78, 814–823. [Google Scholar] [CrossRef]
- Erlich, J.R.; To, E.E.; Liong, S.; Brooks, R.; Vlahos, R.; O’Leary, J.J.; Brooks, D.A.; Selemidis, S. Targeting Evolutionary Conserved Oxidative Stress and Immunometabolic Pathways for the Treatment of Respiratory Infectious Diseases. Antioxid. Redox. Signal. 2020, 32, 993–1013. [Google Scholar] [CrossRef]
- Sánchez-García, F.J.; Pérez-Hernández, C.A.; Rodríguez-Murillo, M.; Moreno-Altamirano, M.M.B. The Role of Tricarboxylic Acid Cycle Metabolites in Viral Infections. Front. Cell Infect. Microbiol. 2021, 11, 725043. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host. Microbe. 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.R.; Yu, J.B.; Fu, J.; Pan, L.B.; Yu, H.; Han, P.; Zhang, Z.W.; Peng, R.; Xu, H.; Wang, Y. Determination and Application of Nineteen Monoamines in the Gut Microbiota Targeting Phenylalanine, Tryptophan, and Glutamic Acid Metabolic Pathways. Molecules 2021, 26, 1377. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Perdew, G.H. The aryl hydrocarbon receptor as a mediator of host-microbiota interplay. Gut. Microbes. 2020, 12, 1859812. [Google Scholar] [CrossRef]
- Roager, H.M.; Lich, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef]
- Thome, T.; Salyers, Z.R.; Kumar, R.A.; Hahn, D.; Berru, F.N.; Ferreira, L.F.; Scali, S.T.; Ryan, T.E. Uremic metabolites impair skeletal muscle mitochondrial energetics through disruption of the electron transport system and matrix dehydrogenase activity. Am. J. Physiol. Cell. Physiol. 2019, 317, C701–C713. [Google Scholar] [CrossRef]
- Fedotcheva, T.A.; Fedotcheva, N.I. Protectors of the Mitochondrial Permeability Transition Pore Activated by Iron and Doxorubicin. Curr. Cancer Drug Targets. 2021, 21, 514–525. [Google Scholar] [CrossRef]
- Fedotcheva, N.I.; Mokhova, E.N. Mitochondrial models of pathologies with oxidative stress. Efficiency of alkalization to reduce mitochondrial damage. Biochem 2013, 78, 1293–1297. [Google Scholar] [CrossRef]
- Umbrasas, D.; Čižas, P.; Arandarčikaitė, O.; Vanagas, T.; Borutaitė, V. Effects of itaconic acid on neuronal viability and brain mitochondrial functions. J. Bioenerg Biomembr. 2021, 53, 499–511. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Meredith, D. The SLC16 gene family—From monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflug. Arch. 2004, 447, 619–628. [Google Scholar] [CrossRef]
- Andrienko, T.N.; Pasdois, P.; Pereira, G.C.; Ovens, M.J.; Halestrap, A.P. The role of succinate and ROS in reperfusion injury—A critical appraisal. J. Mol. Cell. Cardiol. 2017, 110, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.P.; Singer, P.; Wierzchowska-McNew, R.A.; Viana, M.V.; Ben-David, I.A.; Pantet, O.; Thaden, J.J.; Ten Have, G.A.M.; Engelen, M.P.K.J.; Berger, M.M. Comprehensive metabolic amino acid flux analysis in critically ill patients. Clin. Nutr. 2021, 40, 2876–2897. [Google Scholar] [CrossRef] [PubMed]
- Lado-Abeal, J.; Martinez-Sánchez, N.; Cocho, J.A.; Martín-Pastor, M.; Castro-Piedras, I.; Couce-Pico, M.L.; Saha, A.K.; López, M. Lipopolysaccharide (LPS)-induced septic shock causes profound changes in myocardial energy metabolites in pigs. Metabolomics 2018, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Belosludtsev, K.N.; Belosludtseva, N.V.; Kosareva, E.A.; Talanov, E.Y.; Gudkov, S.V.; Dubinin, M.V. Itaconic acid impairs the mitochondrial function by the inhibition of complexes II and IV and induction of the permeability transition pore opening in rat liver mitochondria. Biochimie 2020, 176, 150–157. [Google Scholar] [CrossRef]
- Qin, W.; Qin, K.; Zhang, Y.; Jia, W.; Chen, Y.; Cheng, B.; Peng, L.; Chen, N.; Liu, Y.; Zhou, W.; et al. S-glycosylation-based cysteine profiling reveals regulation of glycolysis by itaconate. Nat. Chem. Biol. 2019, 15, 983–991. [Google Scholar] [CrossRef]
- Liao, S.T.; Han, C.; Xu, D.Q.; Fu, X.W.; Wang, J.S.; Kong, L.Y. 4-Octyl itaconate inhibits aerobic glycolysis by targeting GAPDH to exert anti-inflammatory effects. Nat. Commun. 2019, 10, 5091. [Google Scholar] [CrossRef]
- Liu, X.; Shi, B.; Suo, R.; Xiong, S.; Wang, X.; Liang, X.; Li, X.; Li, G. Itaconate regulates macrophage function through stressful iron-sulfur cluster disrupting and iron metabolism rebalancing. FASEB J. 2021, 35, e21936. [Google Scholar] [CrossRef]
- Lin, J.; Ren, J.; Gao, D.S.; Dai, Y.; Yu, L. The Emerging Application of Itaconate: Promising Molecular Targets and Therapeutic Opportunities. Front. Chem. 2021, 9, 669308. [Google Scholar] [CrossRef]
- Fedotcheva, N.; Olenin, A.; Beloborodova, N. Influence of Microbial Metabolites on the Nonspecific Permeability of Mitochondrial Membranes under Conditions of Acidosis and Loading with Calcium and Iron Ions. Biomedicines 2021, 9, 558. [Google Scholar] [CrossRef]
- Jansen, T.C.; van Bommel, J.; Bakker, J. Blood lactate monitoring in critically ill patients: A systematic health technology assessment. Crit. Care Med. 2009, 37, 2827–2839. [Google Scholar]
- Cheng, H.H.; Chen, F.C.; Change, M.W.; Kung, C.T.; Cheng, C.Y.; Tsai, T.C.; Hsiao, S.Y.; Su, C.M. Difference between elderly and non-elderly patients in using serum lactate level to predict mortality caused by sepsis in the emergency department. Medicine 2018, 97, e0209. [Google Scholar] [CrossRef] [PubMed]
- Yagi, K.; Fujii, T. Management of acute metabolic acidosis in the ICU: Sodium bicarbonate and renal replacement therapy. Crit. Care. 2021, 25, 314. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, P.; Wang, C.; Han, C.; Meng, J.; Liu, X.; Xu, S.; Li, N.; Wang, Q.; Shi, X.; et al. Immune responsive gene 1 (IRG1) promotes endotoxin tolerance by ncreasing A20 expression in macrophages through reactive oxygen species. Biol. Chem. 2013, 288, 16225–16234. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Lee, S.H.; Chung, K.S.; Ku, N.S.; Hyun, Y.M.; Chun, S.; Park, M.S.; Lee, S.G. Development and validation of a novel sepsis biomarker based on amino acid profiling. Clin. Nutr. 2021, 40, 3668–3676. [Google Scholar] [CrossRef]
- Poeze, M.; Luiking, Y.C.; Breedveld, P.; Manders, S.; Deutz, N.E. Decreased plasma glutamate in early phases of septic shock with acute liver dysfunction is an independent predictor of survival. Clin. Nutr 2008, 27, 523–530. [Google Scholar] [CrossRef]
- Kurtz, P.; d’Avila, J.C.; Prado, D.; Madeira, C.; Vargas-Lopes, C.; Panizzutti, R.; Azevedo, L.C.; Bozza, F.A. Cerebral Multimodal Monitoring in Sepsis: An Experimental Study. Shock. 2019, 51, 228–234. [Google Scholar] [CrossRef]
- Pastor Arroyo, E.M.; Yassini, N.; Sakiri, E.; Russo, G.; Bourgeois, S.; Mohebbi, N.; Amann, K.; Joller, N.; Wagner, C.A.; Imenez Silva, P.H. Alkali therapy protects renal function, suppresses inflammation, and improves cellular metabolism in kidney disease. Clin. Sci. 2022, 136, 557–577. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, S.D.; Shao, J.; Buysse, J.; Bushinsky, D.A. Effects of Treatment of Metabolic Acidosis in CKD: A Systematic Review and Meta-Analysis. Clin. J. Am. Soc. Nephrol. 2019, 14, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Teplova, V.V.; Kruglov, A.G.; Kovalyov, L.I.; Nikiforova, A.B.; Fedotcheva, N.I.; Lemasters, J.J. Glutamate contributes to alcohol hepatotoxicity by enhancing oxidative stress in mitochondria. J. Bioenerg Biomembr. 2017, 49, 253–264. [Google Scholar] [CrossRef]
- Fedotcheva, N.I.; Litvinova, E.G.; Zakharchenko, M.V.; Khunderyakova, N.V.; Fadeev, R.S.; Teplova, V.V.; Kondrashova, M.N.; Fedotcheva, T.A.; Beloborodova, N.V. Substrate-specific reduction of tetrazolium salts by isolated mitochondria, tissues, and leukocytes. Biochemistry 2017, 82, 192–204. [Google Scholar] [CrossRef]
- Fedotcheva, N.I.; Kondrashova, M.N.; Litvinova, E.G.; Zakharchenko, M.V.; Khunderyakova, N.V.; Beloborodova, N.V. Modulation of the activity of succinate dehydrogenase by acetylation with chemicals, drugs, and microbial metabolites. Biophysics 2018, 63, 743–750. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedotcheva, N.; Beloborodova, N. Influence of Microbial Metabolites and Itaconic Acid Involved in Bacterial Inflammation on the Activity of Mitochondrial Enzymes and the Protective Role of Alkalization. Int. J. Mol. Sci. 2022, 23, 9069. https://doi.org/10.3390/ijms23169069
Fedotcheva N, Beloborodova N. Influence of Microbial Metabolites and Itaconic Acid Involved in Bacterial Inflammation on the Activity of Mitochondrial Enzymes and the Protective Role of Alkalization. International Journal of Molecular Sciences. 2022; 23(16):9069. https://doi.org/10.3390/ijms23169069
Chicago/Turabian StyleFedotcheva, Nadezhda, and Natalia Beloborodova. 2022. "Influence of Microbial Metabolites and Itaconic Acid Involved in Bacterial Inflammation on the Activity of Mitochondrial Enzymes and the Protective Role of Alkalization" International Journal of Molecular Sciences 23, no. 16: 9069. https://doi.org/10.3390/ijms23169069
APA StyleFedotcheva, N., & Beloborodova, N. (2022). Influence of Microbial Metabolites and Itaconic Acid Involved in Bacterial Inflammation on the Activity of Mitochondrial Enzymes and the Protective Role of Alkalization. International Journal of Molecular Sciences, 23(16), 9069. https://doi.org/10.3390/ijms23169069





