Abstract
Fatty acid synthase (FAS) is a key enzyme in the lipid synthesis pathway, however, its roles in insects remain largely unknown. Here, we firstly identified two FAS genes from the transcriptome dataset of the general cutworm Spodoptera litura, which is a destructive insect pest of many crops. Both SlFAS1 and SlFAS2 were highly expressed in third instar larvae and in their fat bodies. Then, we successfully silenced SlFAS1 in third instar larvae and the content of α-linolenic acid and triglyceride was significantly decreased. Besides that, the effect of FAS on the metamorphic development in S. litura was evaluated. The results indicate that after silencing SlFAS1, the survival rates of S. litura larvae decreased significantly compared to the control groups. Silencing SlFAS1 in fifth instar larvae resulted in more malformed pupae and adults, and the emergence rates were significantly reduced. Furthermore, the ecdysone content in the haemolymph of fifth instar larvae was significantly decreased after silencing SlFAS1. In addition, knocking down SlFAS1 significantly alters the expression of other key genes in the lipogenesis pathway, implying that FAS has an impact on the lipogenesis pathway. The present study deepens the understanding of FAS in insects and provides novel potential targets for managing insect pests.
1. Introduction
Lipids are essential in organisms and play multiple roles such as energy storage, signal transduction, hormone synthesis, and cell membrane component formation, and they are vital for maintaining normal life activities. Lipid synthesis is a highly complex process catalysed by a battery of enzymes, among which fatty acid synthases (FASs) are key and multifunctional enzymes in fatty acid synthesis and lipid metabolism. There are two types of FASs existing in organisms. Type I FASs are predominantly found in animals and fungi. The type I FASs of fungi are encoded by two genes that assemble an α6β6-heterododecamer [1], but mammalian type I FASs are expressed as a single protein and assemble as a homodimer [2]. In contrast, type Ⅱ FASs are common in bacteria and eukaryotic organelles, found especially in chloroplasts and mitochondria, and are expressed as discrete proteins in the cytosol [3]. The general FAS monomer contains seven functional domains including β-ketoacyl synthase (KS), malonyl/acetyl transferase (MAT), β-hydroxyacyl dehydratase (DH), enoyl reductase (ER), β-ketoacyl reductase (KR), acyl carrier protein (ACP), and thioesterase (TE) [4].
Until now, the identification, as well as the function, of FAS genes has only been studied in a narrow range of insect species [5,6]. The first reported insect FAS was purified from the fat body of Drosophila melanogaster and there are three FAS genes (FASCG3523, FASCG3524, FASCG17374) identified from D. melanogaster [7]. FASCG3523 is expressed in the fat body while FASCG3524 and FASCG17374 are expressed in oenocytes. Silencing FASCG3524 and FASCG17374 can induce the lethal phenotype [8]. Generally, FAS genes are mainly expressed in the fat body and oenocytes, and some others are also expressed in different tissues with different biological functions [9]. In the fat body, FASs are responsible for the biosynthesis and storage of lipids, mainly deposited in the form of triglyceride, and transport it to different tissues in the form of diacylglycerols through the haemolymph [10]. In Aedes aegypti, the expression level of FAS1 was highest in the fat body after 48 h, but highest in the ovary 72 h after blood feeding. Besides that, triglyceride and phospholipid levels of A. aegypti adults were significantly reduced after silencing FAS1 [11].
Triglyceride is the main component of lipids and FAS regulates de novo lipogenesis by converting acetyl-CoA to palmitate, further leading to the production and storage of triglyceride [12]. The fatty acid synthesis pathway also involves other enzymes, such as acetyl-CoA carboxylase (ACC), desaturases (Desat), and lipase. Furthermore, considering the importance of triglyceride in insect development, the exact roles of FASs still receive less attention. Previous research revealed that silencing GpylFAS1 in a mulberry pest, Glyphodes pyloalis, significantly reduced the content of α-linolenic acid, resulting in more malformed pupa and lower emergence rates [13]. Studies have shown that two hormones in insects, juvenile hormone and ecdysone, also participate in the regulation of triglyceride metabolism [14]. However, it remains unknown whether FAS regulates the dynamics of juvenile hormone and ecdysone during metamorphic development.
The general cutworm, Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) is a serious worldwide polyphagous pest. It causes heavy damage to dozens of crops, such as potato, tomato, corn, and mulberry trees every year [15]. At present, studies on lipid metabolism in S. litura have been reported [16], but there is no reference to the role of FASs in regulating lipid metabolism in S. litura.
In the present study, we obtained two FAS genes from the transcriptome of S. litura and focused on the role of SlFASs in the synthesis of lipids and in the development and metamorphosis of S. litura larvae. The present study reveals the molecular mechanism of FAS during the development of S. litura, providing a novel potential target for the integrated biological control of pests.
2. Results
2.1. Gene Identification and Sequence Analysis of FAS Genes in S. litura
Two FAS genes were identified from our previously constructed S. litura larvae transcriptome database and named SlFAS1 (LOC111359194) and SlFAS2 (LOC111359981). The sequences of SlFAS1 and SlFAS2 contained complete ORFs of 6240 and 7269 bp which encoded 2079 and 2422 amino acid residues, respectively. The protein sequences of SlFAS1 have eight functional domains, including Ketoacyl-synt (ks, pfam00109), Ketoacyl-synt_C (KsC, pfam02801), KAsynt_C_assoc (KCa, pfam16197), Acyl_transf_1 (At1, pfam00698), PS-DH (PF14765), PKS_ER (smart00829), PKS_KR (smart00822), and PP-binding (Pb, pfam00550). However, SlFAS2 contains nine functional domains, with an additional Thioesterase domain (Th, pfam00975), compared to SlFAS1 (Figure 1). Sequence alignment showed 35.63% amino acid identity between SlFAS1 and SlFAS2 (Figure S1). Phylogenetic analysis of FASs revealed that SlFAS1 and FASs in Agrotis ipsilon and Trichoplusia ni were clustered into a subcluster, and SlFAS2 was clustered with FASs in Spodoptera frugiperda into a subbranch (Figure 2).
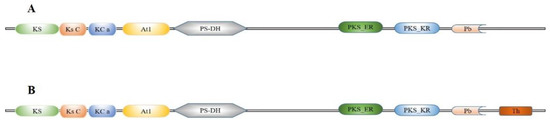
Figure 1.
Domain schematic of SlFAS1 (A) and SlFAS2 (B) in S. litura. KS, Ketoacyl-synt (pfam00109); Ks C, Ketoacyl-synt_C (pfam02801); KC a, KAsynt_C_assoc (pfam16197); At1, Acyl_transf_1 (pfam00698); PS-DH (PF14765); PKS_ER (smart00829); PKS_KR (smart00822); Pb, PP-binding (pfam00550); Th, Thioesterase (pfam00975).
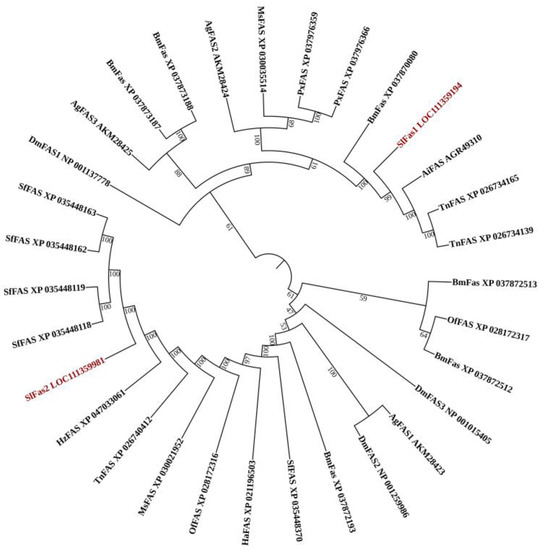
Figure 2.
Phylogenetic tree of FAS amino acid sequences in different species. Spodoptera litura (Sl), Bombyx mori (Bm), Drosophila melanogaster (Dm), Spodoptera frugiperda (Sf), Helicoverpa zea (Hz), Trichoplusia ni (Tn), Ostrinia furnacalis (Of), Helicoverpa armigera (Ha), Plutella xylostella (Px), Agrotis ipsilon (Ai).
2.2. Expression Patterns of SlFAS1 and SlFAS2
SlFAS1 had the highest expression level in adults, and the expression level in males was significantly higher than that in females. In the immature stages, the expression level of SlFAS1 was higher in third instar larvae (Figure 3A). In contrast, SlFAS2 had the highest expression level in third instar larvae compared to other stages followed by first and fourth instar larvae (Figure 3B). Besides that, both SlFAS1 and SlFAS2 were expressed at the highest levels in the fat body and the lowest in the midgut (Figure 3C,D).
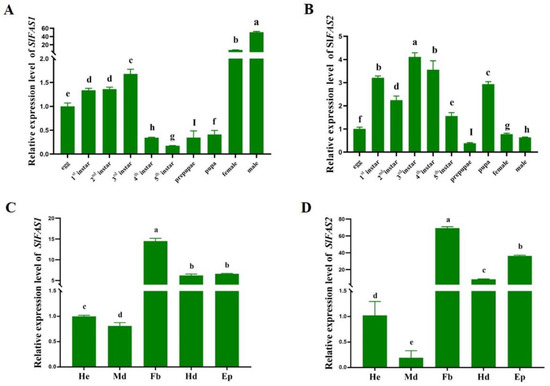
Figure 3.
Expression of SlFAS1 and SlFAS2 in different development stages and tissues. (A) Expression patterns of SlFAS1 in different development stages. (B) Expression patterns of SlFAS2 in different development stages. (C) Expression patterns of SlFAS1 in different tissues. (D) Expression patterns of SlFAS2 in different tissues. He, haemolymph; Md, midgut; Fb, fat body; Hd, head; Ep, epidermis. Significant differences in expression levels among developmental stages and different tissues were compared using a one-way analysis of variance (ANOVA) with Tukey’s post-hoc test. Different lowercase letters above the bars indicate significant differences (p < 0.05).
2.3. Analysis of the Function of SlFASs Using RNAi
To further evaluate the function of SlFASs, both SlFAS1 and SlFAS2 were successfully silenced in third instar S. litura larvae, with expression levels decreased significantly 24 h after dsRNA injection (Figure 4A,B). However, silencing SlFAS2 failed 48 h after dsRNA injection (Figure 4B); therefore, SlFAS1 was chosen for subsequent functional verification.
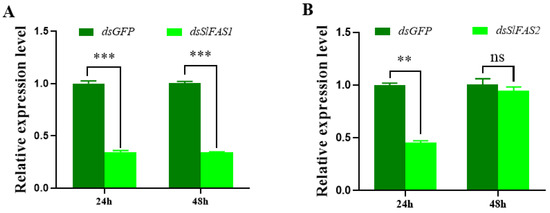
Figure 4.
Expression levels of SlFAS1 and SlFAS2 after RNAi. (A) SlFAS1 expression levels after dsSlFAS1 injection. (B) SlFAS2 expression levels after dsSlFAS2 injection. Significant differences in the expression levels of each target gene were compared using a one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. Significant differences are indicated by asterisks (** p < 0.01; *** p < 0.001; ns: no significant differences).
Gas chromatography determination showed that the content of methyl α-linolenate was significantly reduced 48 h after silencing SlFAS1, but no obvious change was observed at 24 h compared to the dsGFP-injection group (Figure 5A). Moreover, after silencing SlFAS1, the content of methyl palmitate, methyl oleate, and methyl linoleate did not change significantly compared to the control cohorts at both 24 and 48 h (Figure 5B–D).
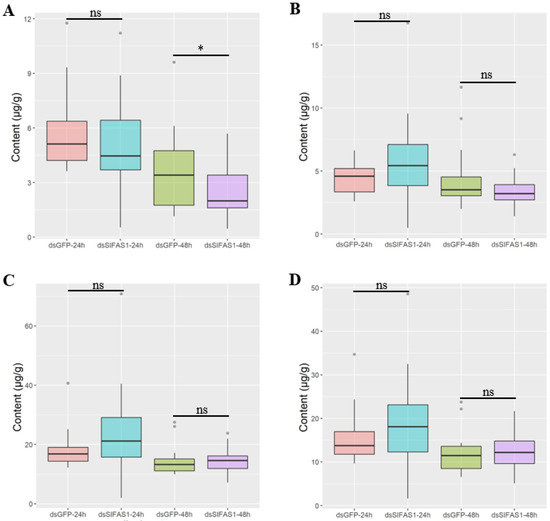
Figure 5.
Effect of silencing SlFAS1 on the fatty acid content in S. litura larvae. (A) Effect of silencing SlFAS1 on methyl α-linolenate content. (B) Effect of silencing SlFAS1 on methyl palmitate content. (C) Effect of silencing SlFAS1 on methyl oleate content. (D) Effect of silencing SlFAS1 on methyl linoleate content. Significant differences are indicated by asterisks (* p < 0.01; ns: no significant differences; non parametric Mann–Whitney test).
2.4. Effect of Silencing SlFAS1 on Lipid Accumulation in S. litura Larvae
To understand the effect of silencing SlFAS1 on lipid accumulation in third instar S. litura larvae, we detected the triglyceride content after dsSlFAS1 injection. Nile red staining revealed that the density of lipid droplets was significantly reduced in the fat body at 24 and 48 h compared to the control group after dsRNA injection, respectively (Figure 6A). Besides that, the triglyceride content of the fat body decreased significantly at 24 and 48 h after dsSlFAS1 injection compared to the dsGFP group, respectively (Figure 6B).
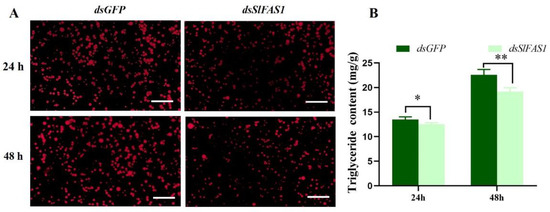
Figure 6.
Lipid accumulation in the fat bodies of S. litura larvae after dsSlFAS1. (A) The fat body of S. litura was stained with Nile red 24 and 48 h after dsSlFAS1 injection and examined by laser scanning microscope. Scale bar, 20 µm. (B) Triglyceride content in the fat body of S. litura after dsSlFAS1 injection. Differences in the Triglyceride content was compared using a one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. Significant differences were indicated by asterisks (* p < 0.05; ** p < 0.01).
2.5. Effect of Silencing SlFAS1 on the Development of S. litura Larvae
To study the effect of SlFAS1 on the development of S. litura larvae, we analysed survival rates and the growth trajectory of body weight after dsSlFAS1 injection in third instar larvae. The results showed that the survival rates of dsSlFAS1-injection larvae were significantly reduced by approximately 45% compared to the dsGFP control (Figure 7A). In addition, compared to the dsGFP-injection group, the growth trajectory in terms of body weight at each time point between the dsSlFAS1-injection and dsGFP-injection groups was not significantly different (Figure 7B).
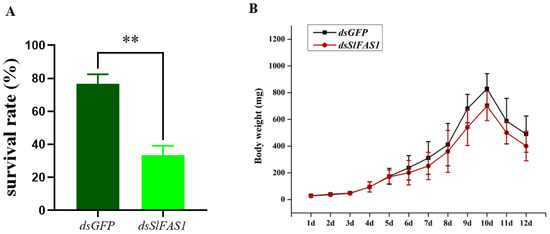
Figure 7.
Effect of SlFAS1 on the development of S. litura larvae. (A) Survival rate of S. litura larvae after injection of dsSlFAS1 into third instar larvae. (B) Growth trajectory of S. litura larvae after injection of dsSlFAS1 into the third instar larvae. Significant differences were compared using a one-way analysis of variance (ANOVA). Significant differences were indicated by asterisks (** p < 0.01).
After the dsSlFAS1 injection in the fifth instar larvae, the proportion of abnormal pupae was significantly higher compared to the dsGFP-injection groups (Figure 8A,B,E). Subsequently, the emergence rate of pupae derived from the dsSlFAS1-injected fifth instar larvae was significantly lower than that of the dsGFP control group (Figure 8F). Meanwhile, more morphologically abnormal adult moths were observed in the dsSlFAS1-injection group than in the dsGFP-injection group (Figure 8C,D,G). At the same time, we also determined the content of juvenile hormone and ecdysone in the haemolymph of fifth instar larvae after silencing SlFAS1; the results showed that the content of ecdysone decreased significantly 24 h after silencing SlFAS1, but no significant change was observed 48 h post knocking down SlFAS1 (Figure 9A). By contrast, the content of juvenile hormone was changed neither 24 h nor 48 h after silencing SlFAS1 (Figure 9B).
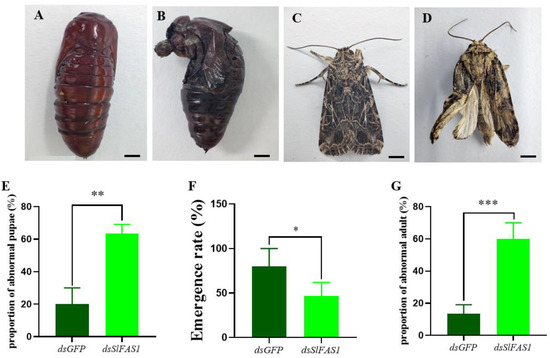
Figure 8.
Morphology of pupae and adults after knocking down SlFAS1 in fifth instar S. litura. (A) Normal pupae after dsGFP injection. (B) Abnormal pupae after dsSlFAS1 injection. (C) Normal adults after dsGFP injection. (D) Abnormal adults after dsSlFAS1 injection. (E) Effect of silencing SlFAS1 on the proportion of abnormal pupae in S. litura. (F) Effect of silencing SlFAS1 on emergence rate in S. litura. (G) Effect of silencing SlFAS1 on the proportion of abnormal adults in S. litura. Significant differences were compared using a one-way analysis of variance (ANOVA). Significant differences were indicated by asterisks (* p < 0.05; ** p < 0.01; *** p < 0.001); scale bars in A and B: 2 mm; scale bars in C and D: 2 mm.
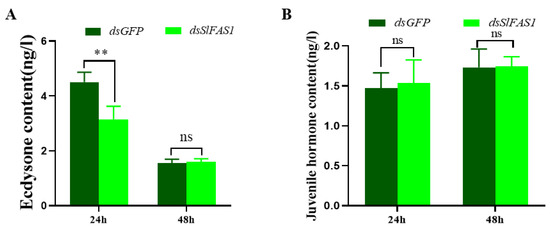
Figure 9.
Effect of knocking down SlFAS1 on the content of juvenile hormone and ecdysone in the haemolymph of fifth instar larvae. (A) Effect of silencing SlFAS1 on ecdysone content in the haemolymph of fifth S. litura larvae. (B) Effect of silencing SlFAS1 on juvenile hormone content in the haemolymph of fifth instar S. litura larvae. Significant differences were compared using a one-way analysis of variance (ANOVA). Significant differences were indicated by asterisks (** p < 0.01; ns, no significant differences).
2.6. Effect of Silencing SlFAS1 on the Expression of other Key Genes in the Lipogenesis Pathway
To explore the effect of silencing SlFAS1 on the lipogenesis pathway, the expression levels of other key genes in the fatty acid synthesis pathway after dsSlFAS1 injection were validated by qRT-PCR. The expression levels of SlFAS2 and SlDesat were significantly upregulated24 h after dsSlFAS1 injection, and the expression of SlDesat was also increased 48 h after dsSlFAS1 injection. The expression levels of SlACC and SlLIPase were significantly upregulated 48 h after dsSlFAS1 injection; in addition, SlLIPase was significantly downregulated 24 h after silencing SlFAS1 (Figure 10).
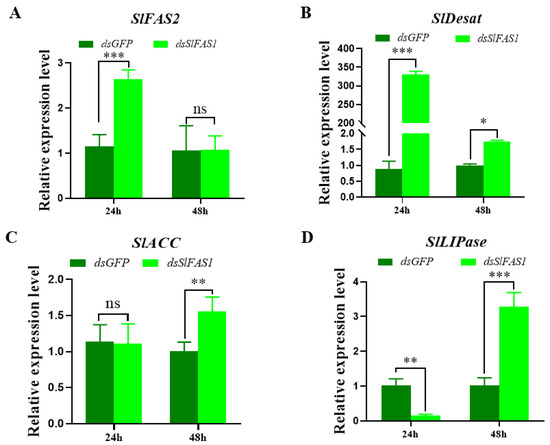
Figure 10.
Effect of silencing SlFAS1 on the expression levels of other key genes in the lipid synthesis pathway in S. litura larvae. (A) SlFAS2 expression levels after dsSlFAS1 injection. (B) SlDesat expression levels after dsSlFAS1 injection. (C) SlACC expression levels after dsSlFAS1 injection. (D) SlLIPase expression levels after dsSlFAS1 injection. One-way analysis of variance (ANOVA) with Tukey’s post hoc test was used to compare significant differences in the expression levels of each target gene. Significant differences were indicated by asterisks (* p < 0.05; ** p < 0.01; *** p < 0.001; ns, no significant differences).
3. Discussion
Lipid is important for energy homeostasis in insect development, reproduction and hormone synthesis [17]. The synthesis of lipids is an intricate process regulated by multiple enzymes. Among these, FAS is a vital participant mainly contributing to fatty acid synthesis and its functional characterization has been reported in bacteria [18] and fungi [19]. However, the identification and characterization of FAS in insects have still received less attention.
In the present study, we firstly identified two FAS genes from our previously constructed transcriptome database. The number of FAS genes identified here was similar to that present in other insects, such as Colaphellus bowringi (two FAS genes) [20]. The relatively small number suggests the conservative function of FAS. On the other hand, sequence analysis showed that SlFAS1 and SlFAS2 shared only 35.63% amino acid identity with each other, and conserved domain prediction revealed that unlike SlFAS2, SlFAS1 lacks a Thioesterase domain (pfam00975), implying the potential functional divergence of these two genes.
To explore the function of the SlFASs, we analysed their expression patterns in S. litura larvae by qRT-PCR. Although SlFAS1 was expressed highest in adults, it was also expressed higher in third instar larvae, suggesting that SlFAS1 still plays a role in S. litura larvae. Meanwhile, SlFAS2 was highly expressed in the third instar larvae of S. litura. Previous studies have reported this similar development stage-biased expression pattern. The expression levels of MpulFAS1 and MpulFAS2 reach a peak at the adult stage but the MpulFAS3 and MpulFAS4 expressions peaks at the larval stage in Meteorus pulchricornis [21]. The divergence of the FAS expression at various different developmental stages indicates that FAS plays distinct functions at different time periods and its role is more complicated. For tissue expression, both SlFAS1 and SlFAS2 were highest in fat bodies. This fat body-biased expression is also reported in other insect species and attributed to the main role of the fat body in lipid metabolism [22]. In Rhodnius prolixus, FAS1 is predominantly expressed in the fat body [23]. A high transcript abundance of FAS2 has been observed in the fat body of diapaused female Colaphellus bowringi [20]. Meanwhile, a higher expression of SlFASs in other tissues such as the epidermis needs to be noted in future studies for revealing the potential role of FASs in insects.
FAS is a central enzyme in de novo lipogenesis, which catalyses the production of C14, C16, and C18 fatty acyl-CoAs, and produces multiple fatty acids [24,25]. Although this catalysis is well summarized in model organisms, there is still a lack of sufficient evidence to support whether FAS undertakes a consistent mission in insects. In the present study, changes in the content of four major fatty acids were examined after silencing SlFAS1, and the GC results showed that the content of methyl α-linolenate was decreased significantly only at 48 h after silencing SlFAS1. However, no significant changes in the other three fatty acids were observed. This result strongly suggests that SlFAS1 is involved in α-linolenic acid synthesis in third instar S. litura larvae. Wang et al. [13] reported that the content of methyl α-linolenate, as well as methyl palmitate, methyl oleate, and methyl linoleate, is decreased significantly after silencing GpylFAS1 in the pupae of the lepidopteran pest Glyphodes pyloalis. Similarly, knocking down Bgfas1 significantly reduces the cuticle free fatty acid content in Blattella germanica [26,27]. Our results not only show that SlFAS1 solely affects α-linolenic acid, but also indicate that the synthesis of insect fatty acids may be regulated by diverse FAS genes or even other enzymes, and further research is needed to confirm this hypothesis.
The fat body is a crucial biosynthetic and metabolic factory in insects [28], and lipids are mainly stored in the fat body in the form of triglyceride. In the present study, Nile red staining indicated that lipid droplets in the fat body were significantly reduced at 24 and 48 h after dsSlFAS1 injection compared to the dsGFP group. Meanwhile, the triglyceride content was significantly decreased compared to the dsGFP control group at 24 and 48 h. These results reveal a close relationship between SlFAS1 and lipid synthesis in S. litura larvae, and this correlation has been reported in a limited number of species. For example, the level of triglyceride was severely decreased after knocking down FAS1 in the mosquito Aedes aegypti [11]. In Serratia-infected Acyrthosiphon pisum, the triglyceride content was reduced by feeding with dsFASN1 compared with the control [29]. Therefore, the present study demonstrates that FAS is not only responsible for fatty acid synthesis, but also affects the lipid dynamic in insects.
It is well accepted that lipid accumulation affects insect growth and development [30]. In the present study, our results show that body weight in each developmental time point was not significantly different between the dsSlFAS1-injection larvae and dsGFP-injection cohorts. However, the survival rates of the dsSlFAS1-injection third S. litura larvae were reduced sharply compared to the dsGFP-injection group. In the model organism C. elegans, silencing FASN-1 at the larval stage ceases normal development and juvenile nematodes are not able to reach adulthood successfully [31]. In a recent study, Yang et al. [32] reported that the injection of dsLmFAS1 and dsLmFAS3 caused about 80% mortality in Locusta migratoria. Therefore, FAS may be essential for insect development.
For insects, pupation and emergence are key scenarios of metamorphosis [33,34]. Knocking down GpylFAS1 or GpylDesat5, which are essential for metamorphosis in G. pyloalis, leads to more abnormal pupae and adults, and lower emergence rates [13]. Analogously, our results showed that silencing SlFAS1 can induce many more abnormal pupae and adults, and the emergence rates were decreased significantly. Similarly, knocking down LdJHAMT, which is an essential metabolism-related enzyme, causes pupation failure and lower emergence rates in the potato beetle Leptinotarsa decemlineata [35]. Furthermore, it is well demonstrated that the hormones in insect larvae significantly shape the process of metamorphosis, and this regulation can be easily measured by monitoring the content of hormones or the expression of hormone genes. Silencing juvenile hormone acid methyltransferase (JHAMT) in the beetle Tribolium castaneum reduces juvenile hormone levels, resulting in the deceleration of lipid catabolism [36]. In the mosquito A. aegypti, 20-hydroxyecdysone (20E) can induce the expression of the transcription factor hepatocyte nuclear factor 4 (HNF4), which accelerates β-oxidation and produces the substances and energy required for oocyte development [37]. Interestingly, our results showed that the content of juvenile hormone in the haemolymph of fifth instar S. litura was not significantly changed after SlFAS1 injection, while the content of ecdysone was significantly decreased at 24 h, suggesting a positive linkage between SlFAS and the ecdysone. Tan et al. [20] reported that the transcription of FAS2 is suppressed by the juvenile hormone and the juvenile hormone receptor methoprene-tolerant. It can be speculated that FAS can regulate the ecdysone metabolism and eventually affect insect metamorphosis.
To further explore the effect of silencing SlFAS1 on other genes in the lipid synthesis pathway, we detected alterations in the expression of SlFAS2, SlDesat, SlACC, and SlLIPase by qRT-PCR. Our results suggest that the expression of these genes changes to varying degrees after knocking down SlFAS1, indicating that these genes may be connected with SlFAS1 to jointly regulate the synthesis of lipids in the body. Yang et al. [38] revealed that the glucose-6-phosphate (G6P) metabolism of the host D. melanogaster parasitized by Pachycrepoideus vindemmiae is affected by PvG6PDH; the expression levels of the host G6P-metabolism-related genes changed at different time points after the host was injected with PvG6PDH, suggesting the expression of nutrition–related genes can be affected by other genes. In a recent study, Wang et al. [13] also reported that silencing GpylFAS1 affected the expressions of the other fatty acid synthesis-related genes in G. pyloalis. Taken together, it can be concluded that lipid metabolism-related genes can be regulated by FAS and future studies are required to reveal the detailed molecular mechanism of this regulation.
4. Materials and Methods
4.1. Insects
The S. litura larvae were collected from the mulberry fields at the campus of Jiangsu University of Science and Technology, Zhenjiang City, Jiangsu Province, China, and reared in the insectary [26 ± 2 °C, 60–80% relative humidity, and photoperiod of 14:10 (L:D) h]. The adult moths were fed with 10% (w/w) honey solution and provided with strips of paper as the substrate for egg deposition in organza-covered cages (23 cm × 23 cm × 21 cm). After hatching, the newly hatched S. litura larvae were fed with artificial diets daily under the same conditions.
4.2. Bioinformatics Analysis of FAS Genes in S. litura
The sequences of FASs were identified from the previously constructed S. litura transcriptome database (BioProject Acc. in NCBI: PRJNA810583). An open reading frame (ORF) finder (https://www.ncbi.nlm.nih.gov/orffinder/) (accessed on 21 February 2021) was used to predict the ORFs of putative SlFAS1 and SlFAS2 genes. The NCBI Conserved Domain Search website (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) (accessed on 21 February 2021) was used to predict the protein functional domains. The Simple Modular Architecture Research Tool (SMART) online software (Version: 3.3.2, EMBL, Heidelberg, Germany) (http://smart.embl-heidelberg.de/) (accessed on 21 February 2021) was used to predict the conserved motifs. DNAMAN 8.0 (Lynnon Corporation, Quebec City, QC, Canada) was used for multiple alignments of various protein sequences. Phylogenetic analysis was conducted using the neighbour-joining method by Molecular Evolutionary Genetic Analysis 6.0 (MEGA 6.0, Mega Limited, Auckland, New Zealand) software with 1000 bootstrap replications. The Interactive Tree Of Life (iTOL) online tool (https://itol.embl.de) (accessed on 21 February 2021) was used to generate the circular phylogenetic tree. SlFASs homologous amino acid sequences of Homo sapiens, Spodoptera litura, Bombyx mori, Drosophila melanogaster, Spodoptera frugiperda, Helicoverpa zea, Trichoplusia ni, Ostrinia furnacalis, Helicoverpa armigera, Plutella xylostella, Agrotis ipsilon were downloaded from GenBank (http://www.ncbi.nlm.nih.gov/) (accessed on 21 February 2021).
4.3. RNA Isolation and Quantitative RT-PCR Validation
Total RNA from the whole body or different tissues (head, midgut, haemolymph, fat body, epidermis) was extracted using a TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s recommendations. The concentration of RNA samples was evaluated according to the absorbance at 260 nm and the purity of RNA was confirmed by the OD260/280 ratio using a 2100 Bioanalyzer (Agilent Technologies, California, CA, USA). Then, the RNA integrity was verified by using 1% agarose gel electrophoresis. Subsequently, 1 μg of total RNA was used to synthesize the first-strand cDNA with a PrimeScript® RT reagent kit (Takara, Dalian, China) following the manufacturer’s instructions. The total qRT-PCR reaction volume was 20 μL, containing 10 μL of 2 × iQTM SYBR® Green I, 1 μL of 10 μM primer of each of the forward and reverse primers, 2 μL of cDNA template, and 6 μL of RNA-free enzyme water. The qRT-PCR validation was conducted using a Roche LightCycler 96 (Roche, Switzerland) and the programme was as follows: 95 °C for 5 min and 40 cycles of 95 °C for 15 s and 60 °C for 31 s. Each sample was repeated three technical times. Meanwhile, the no-template controls (NTCs) of each primer were negative with non-detection of Cq value. The elongation factor-1 alpha (EF1) (GenBank accession: DQ192234.1) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) genes (GenBank accession: MZ393966.1) were used as the reference genes to normalize the mRNA expression levels. LightCycler® 96 software (Roche, Switzerland) was used to analyse the qRT-PCR results. The relative expression levels were calculated using the 2−ΔΔCt method [39]. The primers were designed using the Primer-BLAST online programmer (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) (accessed on 21 February 2021) (Table S1). Each sample was run in triplicate for technical repeats, and three biological replicates were performed simultaneously.
4.4. RNA Interference in S. litura Larvae
The oligonucleotide sequences were designed using BLOCK-iTTM RNAi Designer (https://rnaidesigner.thermofisher.com/) (accessed on 21 February 2021) (Table S2). dsSlFAS1 and dsSlFAS2 were synthesized in vitro using a Transcription T7 kit (Taktableara Biotechnology Co. Ltd., Dalian, China) following the manufacturer’s protocol, and the dsRNA of the green fluorescent protein (GFP) gene was synthesized as the negative control. A NanoDrop 2000 spectrophotometer was used to detect the concentration and purity of synthesized dsRNA, and 1% agarose gel electrophoresis was used to examine the RNA integrity.
dsSlFAS1 and dsSlFAS2 (500 ng) were injected into the third abdominal segment of 1-day-old third instar S. litura larvae using a microsyringe (Drummond Scientific, Broomall, PA, USA), respectively. The samples were collected 24 and 48 h after dsGFP- and dsSlFAS- were injected, and then RNA samples were extracted and the cDNA was synthesized to verify interference efficiency using qRT-PCR. These procedures were the same as described above.
4.5. Determination of Fatty Acid Content
The fatty acid content was determined according to the method described by Wang et al. [13] with some minor modifications. In brief, third instar S. litura larvae were collected 24 and 48 h after dsRNA injection, and dried at 65 °C for fatty acid determination. Each individual was weighed, and transferred to a 10 mL centrifuge tube, and then mashed with a plastic pestle to which 2 mL of n-hexane was added. The homogenate was centrifuged at 10,000 rpm for 10 min, and then 500 μL of supernatant was transferred to a new 10 mL centrifuge tube, adding 1.5 mL of n-hexane and 2 mL of 0.5 mol L−1 KOH-CH3OH solution. The mixture was reacted at 60 °C for 1 h. After the mixture was cooled to room temperature, 4 mL of ultrapure water was added, and the resulting mixture was centrifuged at 10,000 rpm for 10 min. The supernatant was removed to 2 mL Eppendorf (EP) tubes for gas chromatography (GC) analysis [40]. The content of methyl α-linolenate, methyl palmitate, methyl oleate, and methyl linoleate was detected using an Agilent 6820 gas chromatograph (Agilent, Santa Clara, CA, USA) equipped with a Supelco capillary column (HP-INNOWax, 30 m × 0.25 mm, i.d. = 0.20 μm; Agilent), a split injection port, and a flame ionization detector. The initial oven temperature was 200 °C, held for 1 min, then increased to 240 °C at 1.5 °C min−1 and held for 1 min. The detector was set at 280 °C and the injector was set at 250 °C. Nitrogen was used as the carrier gas at a flow rate of 1 mL min−1. The split ratio was 50:1 and the sample size was 1 μL [40].
The content of four fatty acids was calculated according to the standard curves for methyl palmitate, methyl oleate, methyl linoleic, and methyl linoleate, respectively. The content of fatty acids was measured based on content per capita (total content divided by individual weight). For each treatment, 15–20 individuals were determined.
4.6. Measurements of Triglyceride Content
The triglyceride content of the fat body was measured using a triglyceride ELISA kit (BYabscience, China) 24 and 48 h after dsRNA injection, following the manufacturer’s instructions. Briefly, the fat body of the larvae was collected and then homogenized in 1 × phosphate-buffered saline (PBS, PH = 7.4). The samples were centrifuged for 10 min at 12,000 rpm at 4 °C, and the supernatants were removed to new 1.5 mL EP tubes for detection. Subsequently, the supernatant was transferred into a 96-well plate, incubated with the reaction mix at room temperature in the dark for 30 min, and measured using a spectrophotometer (Thermo 1500, Waltham, MA, USA). The experiments were repeated three times.
4.7. Nile Red Staining
The lipid of fat bodies in third instar larvae was observed by staining with Nile red [30]. The fat bodies of S. litura larvae were dissected and washed with 1 × PBS (pH = 7.4) three times, each washing procedure lasting 5 min; subsequently, the fat bodies were fixed with 4% paraformaldehyde on a glass slide for 30 min at 4 °C. After fixation, the samples were washed with 1 × PBST (1 × PBS containing 0.1% Triton and 0.05% Tween20) three times for 5 min each. For lipid staining, the samples were incubated for 30 min in a 1:1000 dilution of 1 mg ml−1 Nile red solution (Aladdin, Shanghai, China) in 1 × PBST, and then rinsed three times with 1 × PBS (pH = 7.4). The samples were visualized using a fluorescent microscope (LSM 710, Carl Zeiss, Germany) at Ex 543/Em 626 nm.
4.8. Development of S. litura
To investigate the effect of SlFASs on the growth and development of S. litura larvae, we measured the weight and survival rate of third instar S. litura larvae and the emergence rate of fifth instar S. litura larvae after silencing the SlFAS, respectively. One-day-old third instar S. litura larvae were injected with dsSlFAS or dsGFP and weighed daily until pupation. A total of 30 third instar S. litura larvae were tested. To test the effects of silencing SlFAS on the metamorphosis of S. litura, the morphology and the emergence of the pupae were observed after injecting dsSlFAS1 into fifth instar S. litura larvae. Each treatment was tested on 30 individuals and the dsGFP-injection groups were taken as the control.
4.9. Content of Juvenile Hormone and Ecdysone in Haemolymph of Fifth Instar S. litura after Silencing SlFAS
To determine the effects of silencing SlFAS on the dynamics of the juvenile hormone and ecdysone in the haemolymph of fifth instar S. litura larvae, the content of the juvenile hormone and ecdysone in larvae haemolymph was measured using an insect juvenile hormone and ecdysone ELISA kit (Rui Fanbioon, Shanghai, China) according to the manufacturer’s protocols. In brief, the haemolymph was collected from the fifth instar S. litura at 24 and 48 h after dsSlFAS1 injection and centrifuged at 1000× g for 5 min at 4 °C. The supernatant was transferred into a new centrifuge tube. Subsequently, 10 μL of supernatant was added to a 96-well plate and incubated with the reaction mixture for 15 min at 37 °C. The optical density value was read at 450 nm using a spectrophotometer (Thermo1500, Waltham, MA, USA). Each sample contained three biological replicates.
4.10. Effect of Silencing SlFAS1 on the Expression of Other Key Genes in the Lipid Synthesis Pathway
To explore the effects of SlFAS1 on other genes in the lipid synthesis pathway in the third instar S. litura larvae, the expression levels of acetyl-CoA carboxylase (ACC), Desaturase (Desat), and Lipase, which were key genes in the lipid synthesis pathway, were validated 24 and 48 h after dsSlFAS1 or dsGFP injection by qRT-PCR; the procedures were the same as mentioned above. The primers of each target gene are listed in Table S1.
4.11. Statistical Analysis
Mann-Whitney tests were used to analyse the significant differences in fatty acid content; data analysis was performed using R software 3.4.0 (R Development Core Team, Vienna, Austria) [41]. One-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test was used to compare the differences in the relative expression levels and the triglyceride content, as well as the content of juvenile hormone and ecdysone.
5. Conclusions
In the present study, we studied the function of FAS genes in a major pest, S. litura, and found that silencing SlFAS1 of the third instar S. litura larvae can depress the synthesis of fatty acids and the content of triglycerides in the fat body and, furthermore, affect development. Meanwhile, we also demonstrated that SlFAS1 is necessary for the metamorphosis of S. litura, and ecdysone alteration may be involved in this process. This study provides a new insight into lipid synthesis in insects, especially in insect pests, and would provide novel targets in pest management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23169064/s1.
Author Contributions
Y.S. conducted experiments, analyzed data, and wrote the manuscript; F.G., Z.L. (Zhixiang Liu) and Z.L. (Zongnan Li) reared insects and conducted experiments; F.W. and S.S. conceived research; S.S. guided and supervised the research. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Qing Lan Project of Jiangsu Province (Year of 2021), the Key Project of University Science Research of Jiangsu Province (20KJA210004), the Jiangsu Agricultural Science and Technology Innovation Fund (CX(21)3179), the Postgraduate Research & Scientific Research Innovation Program of Jiangsu Province (KYCX22_3760).
Institutional Review Board Statement
The work does not include studies involving humans or other animals.
Informed Consent Statement
The study does not involve humans.
Data Availability Statement
All data used in the work have been included in the manuscript.
Acknowledgments
We are grateful for the assistance of Jiao Wang and Chenghai Yan at Jiangsu University of Science and Technology, Zhenjiang, China.
Conflicts of Interest
The authors declare no competing or financial interest.
References
- Jenni, S.; Leibundgut, M.; Boehringer, D.; Frick, C.; Mikolásek, B.; Ban, N. Structure of fungal fatty acid synthase and impli-cations for iterative substrate shuttling. Science 2007, 316, 254–261. [Google Scholar] [CrossRef]
- Maier, T.; Leibundgut, M.; Ban, N. The Crystal Structure of a Mammalian Fatty Acid Synthase. Science 2008, 321, 1315–1322. [Google Scholar] [CrossRef]
- Finzel, K.; Lee, D.J.; Burkart, M.D. Using Modern Tools To Probe the Structure-Function Relationship of Fatty Acid Synthases. ChemBioChem 2015, 16, 528–547. [Google Scholar] [CrossRef] [PubMed]
- Finck, J.; Berdan, E.L.; Mayer, F.; Ronacher, B.; Geiselhardt, S. Divergence of cuticular hydrocarbons in two sympatric grass-hopper species and the evolution of fatty acid synthases and elongases across insects. Sci. Rep. 2016, 6, 33695. [Google Scholar] [CrossRef] [PubMed]
- Parvy, J.-P.; Napal, L.; Rubin, T.; Poidevin, M.; Perrin, L.; Wicker-Thomas, C.; Montagne, J. Drosophila melanogaster Ace-tyl-CoA-carboxylase sustains a fatty acid-dependent remote signal to waterproof the respiratory system. PLoS Genet. 2012, 8, e1002925. [Google Scholar] [CrossRef]
- Wicker-Thomas, C.; Garrido, D.; Bontonou, G.; Napal, L.; Mazuras, N.; Denis, B.; Rubin, T.; Parvy, J.-P.; Montagne, J. Flexible origin of hydrocarbon/pheromone precursors in Drosophila melanogaster. J. Lipid Res. 2015, 56, 2094–2101. [Google Scholar] [CrossRef]
- Renobales, M.; Woodin, T.S.; Blomquist, G.J. Drosophila melanogaster fatty acid synthetase: Characteristics and effect of protease inhibitors. Insect Biochem. 1986, 16, 887–894. [Google Scholar] [CrossRef]
- Garrido, D.; Rubin, T.; Poidevin, M.; Maroni, B.; Le Rouzic, A.; Parvy, J.-P.; Montagne, J. Fatty Acid Synthase Cooperates with Glyoxalase 1 to Protect against Sugar Toxicity. PLoS Genet. 2015, 11, e1004995. [Google Scholar] [CrossRef]
- Chung, H.; Loehlin, D.W.; Dufour, H.D.; Vaccarro, K.; Millar, J.G.; Carroll, S.B. A single gene affects both ecological di-vergence and mate choice in Drosophila. Science 2014, 343, 1148–1151. [Google Scholar] [CrossRef] [PubMed]
- Arrese, E.L.; Canavoso, L.E.; Jouni, Z.E.; Pennington, J.E.; Tsuchida, K.; Wells, M.A. Lipid storage and mobilization in insects: Current status and future directions. Insect Biochem. Mol. Biol. 2001, 31, 7–17. [Google Scholar] [CrossRef]
- Alabaster, A.; Isoe, J.; Zhou, G.; Lee, A.; Murphy, A.; Day, W.A.; Miesfeld, R.L. Deficiencies in acetyl-CoA carboxylase and fatty acid synthase 1 differentially affect eggshell formation and blood meal digestion in Aedes aegypti. Insect Biochem. Mol. Biol. 2011, 41, 946–955. [Google Scholar] [CrossRef]
- Menendez, J.A.; Lupu, R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer 2007, 7, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, Y.; Hwarari, D.T.; Liang, X.-H.; Ding, J.-H.; Yan, M.W.; Wu, F.A.; Wang, J.; Sheng, S. Fatty acid synthases and desaturases are essential for the biosynthesis of α-linolenic acid and metamorphosis in a major mulberry pest, Glyphodes pyloalis walker (Lepidoptera: Pyralidae). Pest Manag. Sci. 2022, 78, 2629–2642. [Google Scholar] [CrossRef] [PubMed]
- Gondim, K.C.; Atella, G.C.; Pontes, E.G.; Majerowicz, D. Lipid metabolism in insect disease vectors. Insect Biochem. Mol. Biol. 2018, 101, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Hussain, D.; Ghouse, G.; Abbas, M.; Fisher, S.W. Monitoring of insecticide resistance in Spodoptera litura (Lepi-doptera: Noctuidae) from four districts of Punjab, Pakistan to conventional and new chemistry insecticides. Crop Prot. 2016, 79, 177–184. [Google Scholar] [CrossRef]
- Wen, L.; Gao, G.P.; Huang, Z.Q.; Zheng, S.C.; Feng, Q.L.; Liu, L. Expression, regulation and binding affinity of fatty ac-id-binding protein 2 in Spodoptera litura. J. Integr. Agr. 2020, 19, 2095–3119. [Google Scholar] [CrossRef]
- Zhou, J.-M.; Yan, J.; You, K.-K.; Chen, X.; Yuan, Z.-N.; Zhou, Q.; Lu, K. Characterization of a Nilaparvata lugens (Stal) brummer gene and analysis of its role in lipid metabolism. Arch. Insect Biochem. Physiol. 2018, 97, e21442. [Google Scholar] [CrossRef]
- Lambert, C.; Poyart, C.; Gruss, A.; Fouet, A. FabT, a Bacterial Transcriptional Repressor That Limits Futile Fatty Acid Biosynthesis. Microbiol. Mol. Biol. Rev. 2022, 21, e0002922. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Li, B.; Huang, B.-C.; Wang, F.-B.; Zhang, Y.-Q.; Zhao, S.-G.; Li, M.; Wang, H.-Y.; Yu, X.-J.; Liu, X.-Y.; et al. Production, Biosynthesis, and Commercial Applications of Fatty Acids From Oleaginous Fungi. Front. Nutr. 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.-Q.; Liu, W.; Zhu, F.; Lei, C.-L.; Wang, X.-P. Fatty acid synthase 2 contributes to diapause preparation in a beetle by regulating lipid accumulation and stress tolerance genes expression. Sci. Rep. 2017, 7, 40509. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shen, L.-W.; Xing, X.-R.; Xie, Y.-Q.; Li, Y.-J.; Liu, Z.-X.; Wang, J.; Wu, F.-A.; Sheng, S. Lipid Dynamics, Identification, and Expression Patterns of Fatty Acid Synthase Genes in an Endoparasitoid, Meteorus pulchricornis (Hymenoptera: Braconidae). Int. J. Mol. Sci. 2020, 21, 6228. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect Fat Body: Energy, Metabolism, and Regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Moriconi, D.E.; Dulbecco, A.B.; Juárez, M.P.; Calderón-Fernández, G.M. A fatty acid synthase gene (FASN3) from the in-tegument tissue of Rhodnius prolixus contributes to cuticle water loss regulation. Insect Mol. Biol. 2019, 28, 850–861. [Google Scholar] [CrossRef]
- Stanley-Samuelson, D.W.; Jurenka, R.A.; Cripps, C.; Blomquist, G.J.; de Renobales, M. Fatty acids in insects: Composition, metabolism, and biological significance. Arch. Insect Biochem. Physiol. 1988, 9, 1–33. [Google Scholar] [CrossRef]
- Dembeck, L.M.; Böröczky, K.; Huang, W.; Schal, C.; Anholt, R.R.H.; Mackay, T.F.C. Genetic architecture of natural variation in cuticular hydrocarbon composition in Drosophila melanogaster. eLife 2015, 4, e09861. [Google Scholar] [CrossRef]
- Pei, X.-J.; Chen, N.; Bai, Y.; Qiao, J.-W.; Li, S.; Fan, Y.-L.; Liu, T.-X. BgFas1: A fatty acid synthase gene required for both hydro-carbon and cuticular fatty acid biosynthesis in the German cockroach, Blattella germanica (L.). Insect Biochem. Mol. Biol. 2019, 112, 103203. [Google Scholar] [CrossRef]
- Paszkiewicz, M.; Sikora, A.; Boguś, M.I.; Włóka, E.; Stepnowski, P.; Gołębiowski, M. Effect of exposure to chlorpyrifos on the cuticular and internal lipid composition of Blattella germanica males. Insect Sci. 2016, 23, 94–104. [Google Scholar] [CrossRef]
- Law, J.H.; Wells, M.A. Insects as biochemical models. J. Biol. Chem. 1989, 264, 16335–16338. [Google Scholar] [CrossRef]
- Zhou, X.; Ling, X.; Guo, H.; Zhu-Salzman, K.; Ge, F.; Sun, Y. Serratia symbiotica Enhances Fatty Acid Metabolism of Pea Aphid to Promote Host Development. Int. J. Mol. Sci. 2021, 22, 5951. [Google Scholar] [CrossRef]
- Cheng, Y.; Lu, T.; Guo, J.; Lin, Z.; Jin, Q.; Zhang, X.; Zou, Z. Helicoverpa armigera miR-2055 regulates lipid metabolism via fatty acid synthase expression. Open Biol. 2022, 12. [Google Scholar] [CrossRef]
- Li, Y.; Paik, Y.-K. A potential role for fatty acid biosynthesis genes during molting and cuticle formation in Caenorhabditis elegans. BMB Rep. 2011, 44, 285–290. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, X.; Niu, N.; Zhao, Y.; Liu, W.; Moussian, B.; Zhang, J. Two fatty acid synthase genes from the integument contribute to cuticular hydrocarbon biosynthesis and cuticle permeability in Locusta migratoria. Insect Mol. Biol. 2020, 29, 555–568. [Google Scholar] [CrossRef]
- Fang, H.; Wang, X.; Liu, X.; Michaud, J.P.; Wu, Y.; Zhang, H.; Li, Y.; Li, Z. Molecular characterization of insulin receptor (IR) in oriental fruit moth, Grapholita molesta (Lepidoptera: Tortricidae), and elucidation of its regulatory roles in glucolipid homeostasis and metamorphosis through interaction with miR-982490. Insect Mol. Biol. 2022. [Google Scholar] [CrossRef]
- Sun, R.; Xu, Y.; Liu, J.; Yang, L.; Cui, G.; Zhong, G.; Yi, X. Proteomic profiling for ovarian development and azadirachtin ex-posure in Spodoptera litura during metamorphosis from pupae to adults. Ecotoxicol. Environ. Saf. 2022, 237, 113548. [Google Scholar] [CrossRef]
- Fu, K.-Y.; Li, Q.; Zhou, L.-T.; Meng, Q.-W.; Lü, F.-G.; Guo, W.-C.; Li, G.-Q. Knockdown of juvenile hormone acid methyl transferase severely affects the performance of Leptinotarsa decemlineata (Say) larvae and adults. Pest Manag. Sci. 2016, 72, 1231–1241. [Google Scholar] [CrossRef]
- Xu, J.-J.; Sheng, Z.-T.; Palli, S.R. Juvenile Hormone and Insulin Regulate Trehalose Homeostasis in the Red Flour Beetle, Tribolium castaneum. PLoS Genet. 2013, 9, e1003535. [Google Scholar] [CrossRef]
- Wang, X.; Hou, Y.; Saha, T.T.; Pei, G.; Raikhel, A.S.; Zou, Z. Hormone and receptor interplay in the regulation of mosquito lipid metabolism. Proc. Natl. Acad. Sci. USA 2017, 114, E2709–E2718. [Google Scholar] [CrossRef]
- Yang, L.; Qiu, L.; Fang, Q.; Wu, S.; Ye, G. A venom protein of ectoparasitoid Pachycrepoideus vindemiae, PvG6PDH, contributes to parasitism by inhibiting host glucose-6-phosphate metabolism. Insect Sci. 2022, 29, 399–410. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, J.; Wu, W.; Wang, X.; Wang, M.; Wu, F. An effective GC method for the determination of the fatty acid composition in silkworm pupae oil using a two-step methylation process. J. Serbian Chem. Soc. 2015, 80, 9–20. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; Volume 14, pp. 12–21. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).