Sensory-Motor Perturbations in Larval Zebrafish (Danio rerio) Induced by Exposure to Low Levels of Neuroactive Micropollutants during Development
Abstract
:1. Introduction
2. Results
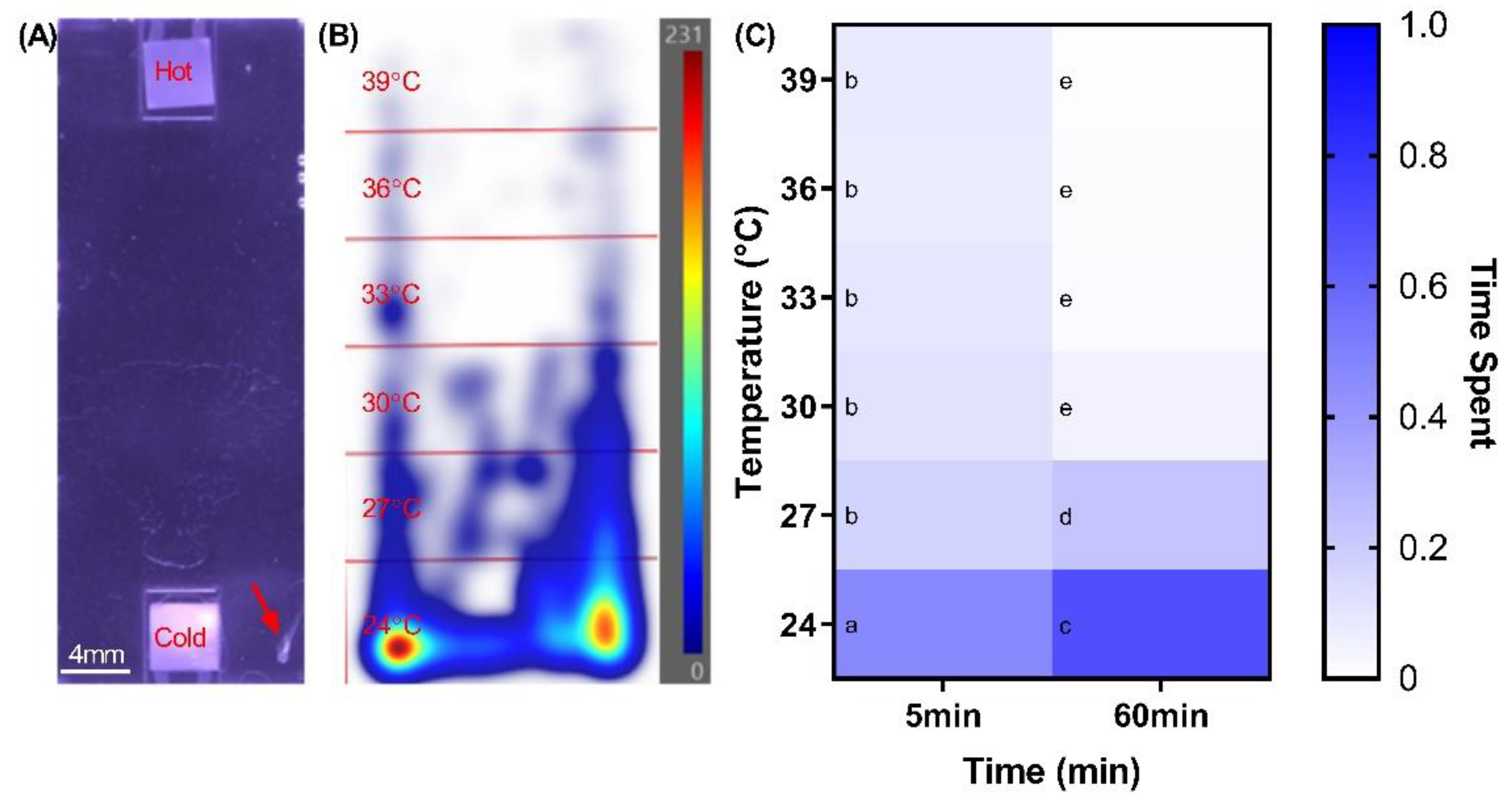
2.1. Defining Zebrafish Thermotactic Responses
2.2. Chemo-Behavioral Alterations upon Exposure to Environmentally Relevant Concentrations of Pharmaceutical Micropollutants
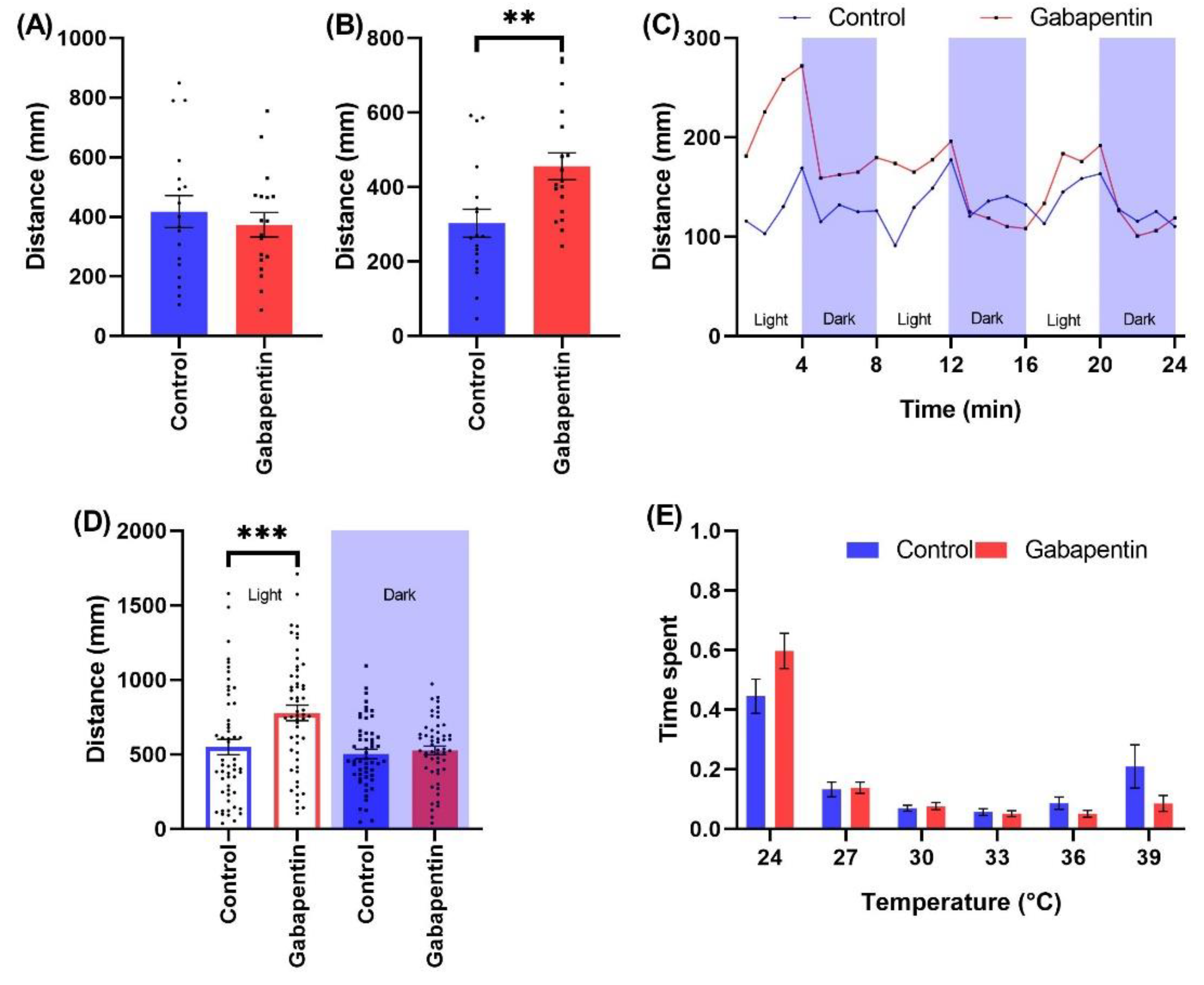
2.2.1. Anticonvulsants
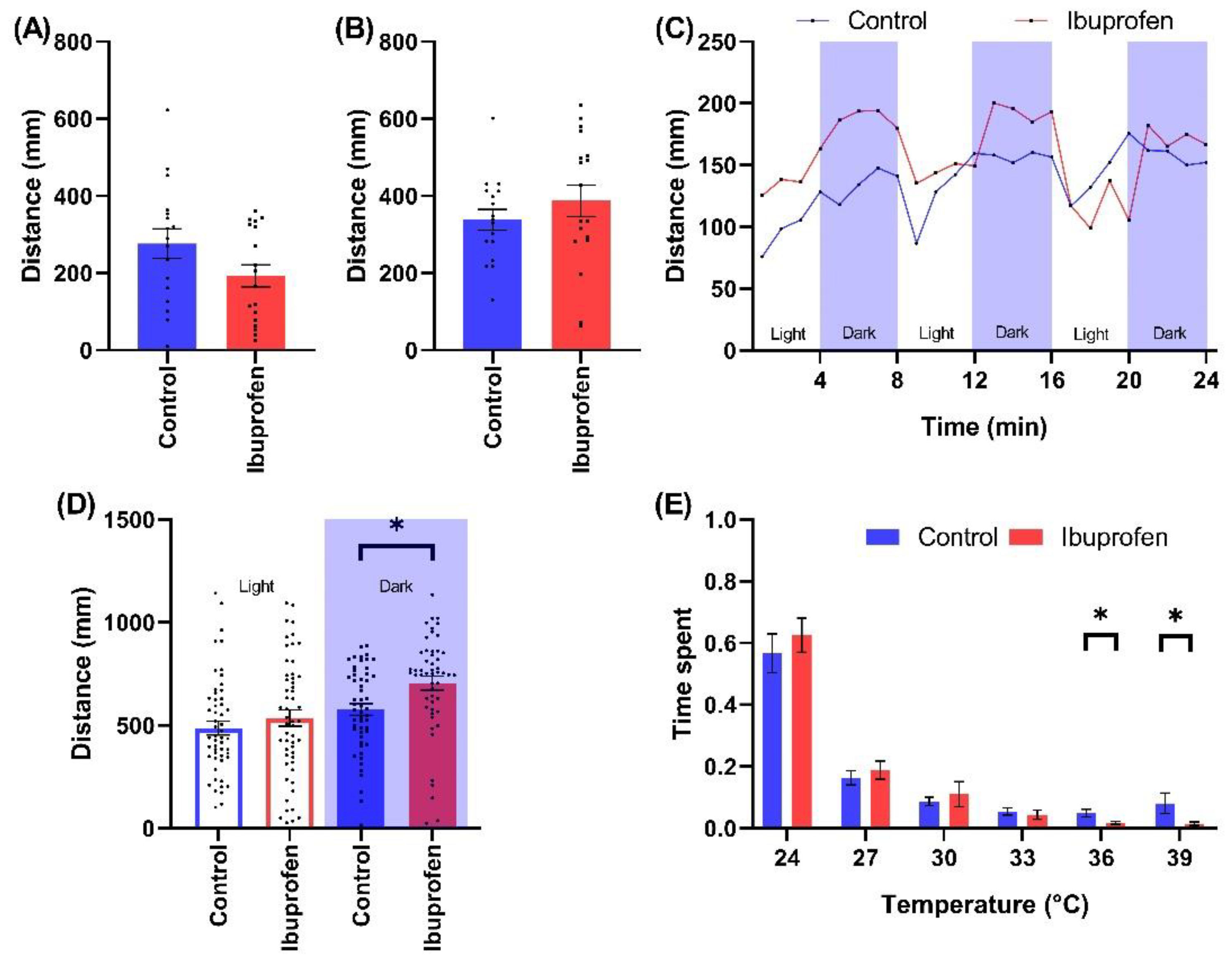
2.2.2. NSAID
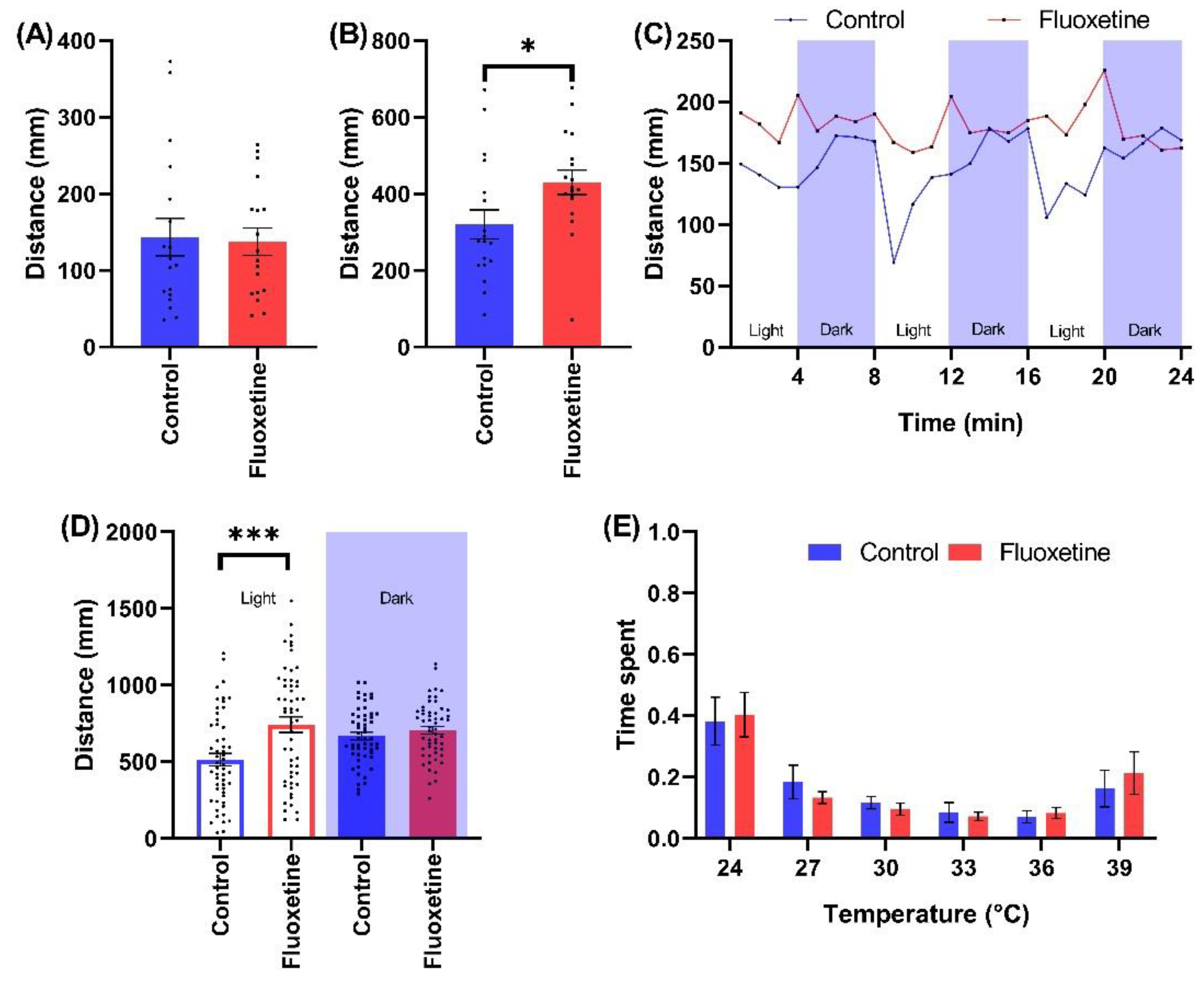
2.2.3. SSRI Antidepressants
3. Discussion
3.1. Larval Zebrafish Thermotactic Behavior Enables a New Sensory-Motor Biotest
3.2. Behavioral Effects upon Exposure to Pharmaceutical Micropollutants during Fish Neuro-Development
3.2.1. Impact of Anticonvulsants
3.2.2. Impact of NSAID
3.2.3. Impact of SSRI Antidepressants
4. Materials and Methods
4.1. Biological Specimens
4.2. Chemicals and Materials
4.3. Chemical Exposure Conditions
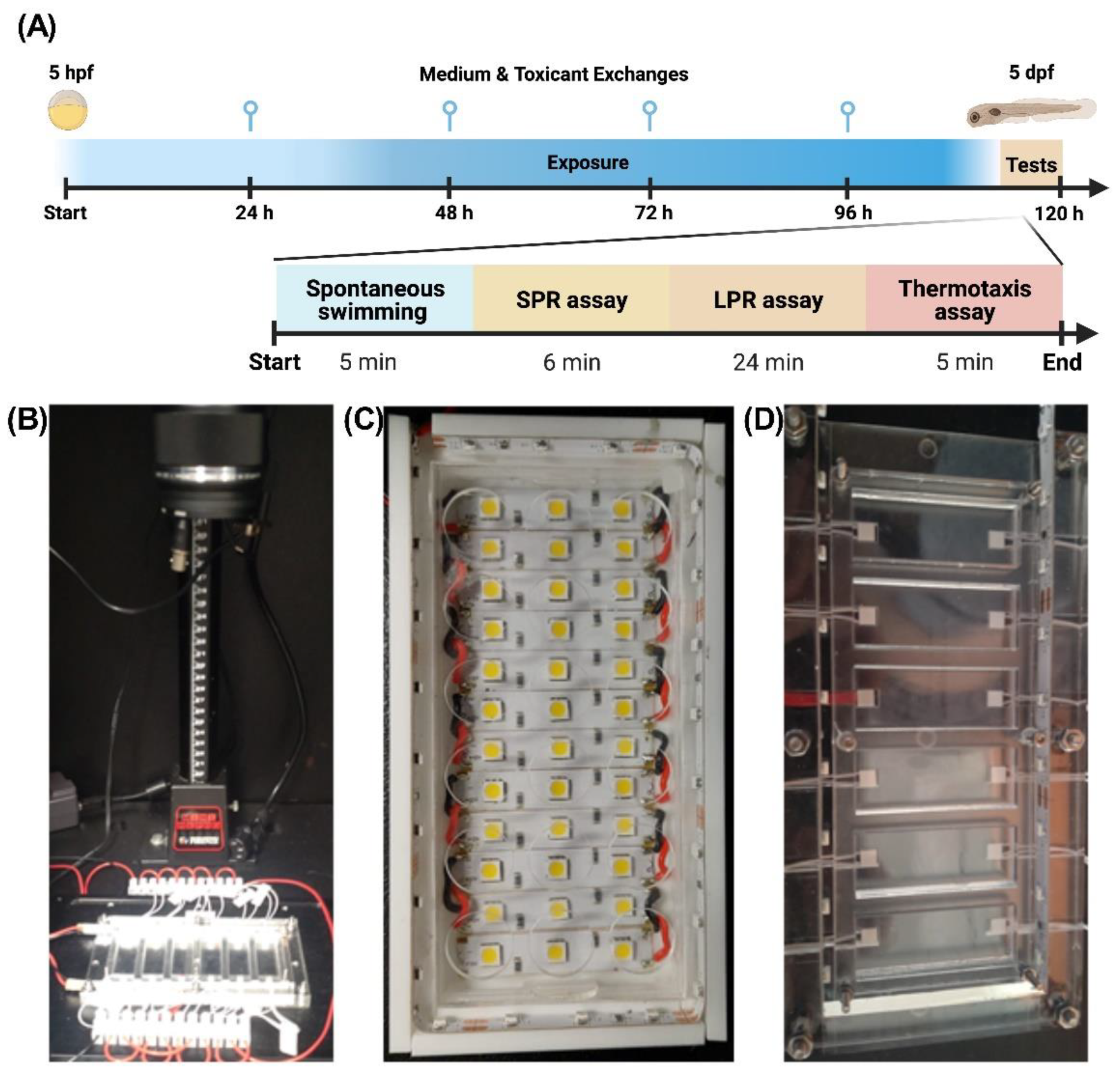
4.4. Behavioral Biotests
4.4.1. Spontaneous Swimming (SS) Assay
4.4.2. Simulated Predator Response (SPR) Assay
4.4.3. Larval Photomotor Response (LPR) Assay
4.4.4. Thermal Preference Assay
4.5. Behavioral Data Acquisition
4.6. Data Analysis and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ford, A.T.; Ågerstrand, M.; Brooks, B.W.; Allen, J.; Bertram, M.G.; Brodin, T.; Dang, Z.; Duquesne, S.; Sahm, R.; Hoffmann, F.; et al. The Role of Behavioral Ecotoxicology in Environmental Protection. Environ. Sci. Technol. 2021, 55, 5620–5628. [Google Scholar] [CrossRef]
- Ågerstrand, M.; Arnold, K.; Balshine, S.; Brodin, T.; Brooks, B.W.; Maack, G.; McCallum, E.S.; Pyle, G.; Saaristo, M.; Ford, A.T. Emerging investigator series: Use of behavioural endpoints in the regulation of chemicals. Environ. Sci. Process. Impacts 2020, 22, 49–65. [Google Scholar] [CrossRef]
- Bownik, A.; Wlodkowic, D. Applications of advanced neuro-behavioral analysis strategies in aquatic ecotoxicology. Sci. Total Environ. 2021, 772, 145577. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.; Wlodkowic, D. High-throughput animal tracking in chemobehavioral phenotyping: Current limitations and future perspectives. Behav. Processes 2020, 180, 104226. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.; Rodriguez, A.; Wlodkowic, D. Impact of digital video analytics on accuracy of chemobehavioural phenotyping in aquatic toxicology. PeerJ 2019, 7, e7367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hagstrom, D.; Hayes, P.; Graham, A.; Collins, E.S. Multi-Behavioral Endpoint Testing of an 87-Chemical Compound Library in Freshwater Planarians. Toxicol. Sci. 2019, 167, 26–44. [Google Scholar] [CrossRef]
- Hagstrom, D.; Cochet-Escartin, O.; Zhang, S.; Khuu, C.; Collins, E.-M.S. Freshwater Planarians as an Alternative Animal Model for Neurotoxicology. Toxicol. Sci. 2015, 147, 270–285. [Google Scholar] [CrossRef]
- Bai, Y.; Henry, J.; Campana, O.; Wlodkowic, D. Emerging prospects of integrated bioanalytical systems in neuro-behavioral toxicology. Sci. Total Environ. 2021, 756, 143922. [Google Scholar] [CrossRef]
- Gau, P.; Poon, J.; Ufret-Vincenty, C.; Snelson, C.D.; Gordon, S.E.; Raible, D.W.; Dhaka, A. The zebrafish ortholog of TRPV1 is required for heat-induced locomotion. J. Neurosci. 2013, 33, 5249–5260. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Yamashita, T.; Agata, K. Thermosensory Signaling by TRPM Is Processed by Brain Serotonergic Neurons to Produce Planarian Thermotaxis. J. Neurosci. 2014, 34, 15701–15714. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, E.; Hernández, F.; Ibáñez, M.; Rico, A.; Pitarch, E.; Bijlsma, L. Occurrence and ecological risks of pharmaceuticals in a Mediterranean river in Eastern Spain. Environ. Int. 2020, 144, 106004. [Google Scholar] [CrossRef]
- Lopez, F.; Pitarch, E.; Botero-Coy, A.; Fabregat-Safont, D.; Ibáñez, M.; Marin, J.; Peruga, A.; Ontañón, N.; Martínez-Morcillo, S.; Olalla, A.; et al. Removal efficiency for emerging contaminants in a WWTP from Madrid (Spain) after secondary and tertiary treatment and environmental impact on the Manzanares River. Sci. Total Environ. 2022, 812, 152567. [Google Scholar] [CrossRef] [PubMed]
- Cunha, D.L.; Mendes, M.P.; Marques, M. Environmental risk assessment of psychoactive drugs in the aquatic environment. Environ. Sci. Pollut. Res. Int. 2019, 26, 78–90. [Google Scholar] [CrossRef]
- Baali, H.; Cosio, C. Effects of carbamazepine in aquatic biota. Environ. Sci. Process. Impacts 2022, 24, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Oldenkamp, R.; Hoeks, S.; Čengić, M.; Barbarossa, V.; Burns, E.E.; Boxall, A.B.; Ragas, A.M.J. A High-Resolution Spatial Model to Predict Exposure to Pharmaceuticals in European Surface Waters: ePiE. Environ. Sci. Technol. 2018, 52, 12494–12503. [Google Scholar] [CrossRef] [PubMed]
- Moreno Rios, A.L.; Gutierrez-Suarez, K.; Carmona, Z.; Ramos, C.G.; Silva Oliveira, L.F. Pharmaceuticals as emerging pollutants: Case naproxen an overview. Chemosphere 2022, 291 Pt 1, 132822. [Google Scholar] [CrossRef] [PubMed]
- Pyle, G.; Ford, A.T. Behaviour revised: Contaminant effects on aquatic animal behaviour. Aquat. Toxicol. 2017, 182, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.H.; Bury, N.R.; Owen, S.F.; MacRae, J.I.; Barron, L.P. A review of the pharmaceutical exposome in aquatic fauna. Environ. Pollut. 2018, 239, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Fursdon, J.B.; Martin, J.M.; Bertram, M.G.; Lehtonen, T.K.; Wong, B.B.M. The pharmaceutical pollutant fluoxetine alters reproductive behaviour in a fish independent of predation risk. Sci. Total Environ. 2019, 650 Pt 1, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Polverino, G.; Martin, J.M.; Bertram, M.G.; Soman, V.R.; Tan, H.; Brand, J.A.; Mason, R.T.; Wong, B.B.M. Psychoactive pollution suppresses individual differences in fish behaviour. Proc. Biol. Sci. 2021, 288, 20202294. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.; Brand, J.A.; Bai, Y.; Martin, J.M.; Wong, B.B.M.; Wlodkowic, D. Multi-generational impacts of exposure to antidepressant fluoxetine on behaviour, reproduction, and morphology of freshwater snail Physa acuta. Sci. Total Environ. 2022, 814, 152731. [Google Scholar] [CrossRef] [PubMed]
- Guler, Y.; Ford, A.T. Anti-depressants make amphipods see the light. Aquat. Toxicol. 2010, 99, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Wlodkowic, D.; Bownik, A.; Leitner, C.; Stengel, D.; Braunbeck, T. Beyond the behavioural phenotype: Uncovering mechanistic foundations in aquatic eco-neurotoxicology. Sci. Total Environ. 2022, 829, 154584. [Google Scholar] [CrossRef] [PubMed]
- Qiang, L.; Cheng, J.; Yi, J.; Rotchell, J.M.; Zhu, X.; Zhou, J. Environmental concentration of carbamazepine accelerates fish embryonic development and disturbs larvae behavior. Ecotoxicology 2016, 25, 1426–1437. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, Q.; Di Paolo, C.; Shao, Y.; Hollert, H.; Seiler, T.-B. Behavioral profile alterations in zebrafish larvae exposed to environmentally relevant concentrations of eight priority pharmaceuticals. Sci. Total Environ. 2019, 664, 89–98. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jia, D.; Du, S.; Zhu, R.; Zhou, W.; Pan, S.; Zhang, Y. Toxicity of gabapentin-lactam on the early developmental stage of zebrafish (Danio rerio). Environ. Pollut. 2021, 287, 117649. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, X.; Jia, D.; Zhang, W.; Zhang, T.; Yu, Y.; Xu, Y.; Zhang, Y. A transcriptomics-based analysis of the toxicity mechanisms of gabapentin to zebrafish embryos at realistic environmental concentrations. Environ. Pollut. 2019, 251, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Láng, J.; Kőhidai, L. Effects of the aquatic contaminant human pharmaceuticals and their mixtures on the proliferation and migratory responses of the bioindicator freshwater ciliate Tetrahymena. Chemosphere 2012, 89, 592–601. [Google Scholar] [CrossRef] [PubMed]
- De Lange, H.J.; Noordoven, W.; Murk, A.J.; Lürling, M.; Peeters, E.T.H.M. Behavioural responses of Gammarus pulex (Crustacea, Amphipoda) to low concentrations of pharmaceuticals. Aquat. Toxicol. 2006, 78, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Villa, S.; Di Nica, V.; Pescatore, T.; Bellamoli, F.; Miari, F.; Finizio, A.; Lencioni, V. Comparison of the behavioural effects of pharmaceuticals and pesticides on Diamesa zernyi larvae (Chironomidae). Environ. Pollut. 2018, 238, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.M.; Furlong, E.T.; Kolpin, D.W.; Werner, S.L.; Schoenfuss, H.L.; Barber, L.B.; Blazer, V.S.; Norris, D.O.; Vajda, A.M. Antidepressant pharmaceuticals in two U.S. effluent-impacted streams: Occurrence and fate in water and sediment, and selective uptake in fish neural tissue. Environ. Sci. Technol. 2010, 44, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Batucan, N.S.T.; Tremblay, L.A.; Northcott, G.L.; Matthaei, C.D. Medicating the environment? A critical review on the risks of carbamazepine, diclofenac and ibuprofen to aquatic organisms. Environ. Adv. 2022, 7, 100164. [Google Scholar] [CrossRef]
- Tolou-Ghamari, Z.; Zare, M.; Habibabadi, J.M.; Najafi, M.R. A quick review of carbamazepine pharmacokinetics in epilepsy from 1953 to 2012. J. Res. Med. Sci. 2013, 18 (Suppl. 1), S81–S85. [Google Scholar] [PubMed]
- Cheng, J.K.; Chiou, L.C. Mechanisms of the antinociceptive action of gabapentin. J. Pharmacol. Sci. 2006, 100, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.P.; Gee, N.S.; Su, T.Z.; Kocsis, J.D.; Welty, D.F.; Brown, J.P.; Dooley, D.J.; Boden, P.; Singh, L. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res. 1998, 29, 233–249. [Google Scholar] [CrossRef]
- Bertrand, S.; Ng, G.Y.; Purisai, M.G.; Wolfe, S.E.; Severidt, M.W.; Nouel, D.; Robitaille, R.; Low, M.J.; O’Neill, G.P.; Metters, K.; et al. The anticonvulsant, antihyperalgesic agent gabapentin is an agonist at brain gamma-aminobutyric acid type B receptors negatively coupled to voltage-dependent calcium channels. J. Pharmacol. Exp. Ther. 2001, 298, 15–24. [Google Scholar]
- Van Haaften, K.A.; Forsythe, L.R.E.; Stelow, E.A.; Bain, M.J. Effects of a single preappointment dose of gabapentin on signs of stress in cats during transportation and veterinary examination. J. Am. Vet. Med. Assoc. 2017, 251, 1175–1181. [Google Scholar] [CrossRef]
- Boleda, M.R.; Galceran, M.T.; Ventura, F. Validation and uncertainty estimation of a multiresidue method for pharmaceuticals in surface and treated waters by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2013, 1286, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Gumbi, B.P.; Moodley, B.; Birungi, G.; Ndungu, P.G. Detection and quantification of acidic drug residues in South African surface water using gas chromatography-mass spectrometry. Chemosphere 2017, 168, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Fekadu, S.; Alemayehu, E.; Dewil, R.; Van der Bruggen, B. Pharmaceuticals in freshwater aquatic environments: A comparison of the African and European challenge. Sci. Total Environ. 2019, 654, 324–337. [Google Scholar] [CrossRef]
- Xia, L.; Zheng, L.; Zhou, J.L. Effects of ibuprofen, diclofenac and paracetamol on hatch and motor behavior in developing zebrafish (Danio rerio). Chemosphere 2017, 182, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.J.; Myers, B.L.; Badia, P. Nonsteroidal anti-inflammatory drugs alter body temperature and suppress melatonin in humans. Physiol. Behav. 1996, 59, 133–139. [Google Scholar] [CrossRef]
- Hong, X.; Zhao, G.; Zhou, Y.; Chen, R.; Li, J.; Zha, J. Risks to aquatic environments posed by 14 pharmaceuticals as illustrated by their effects on zebrafish behaviour. Sci. Total Environ. 2021, 771, 145450. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.W.; Foran, C.M.; Richards, S.M.; Weston, J.; Turner, P.K.; Stanley, J.K.; Solomon, K.R.; Slattery, M.; La Point, T.W. Aquatic ecotoxicology of fluoxetine. Toxicol. Lett. 2003, 142, 169–183. [Google Scholar] [CrossRef]
- Fong, P.P.; Bury, T.B.; Dworkin-Brodsky, A.D.; Jasion, C.M.; Kell, R.C. The antidepressants venlafaxine (“Effexor”) and fluoxetine (“Prozac”) produce different effects on locomotion in two species of marine snail, the oyster drill (Urosalpinx cinerea) and the starsnail (Lithopoma americanum). Mar. Environ. Res. 2015, 103, 89–94. [Google Scholar] [CrossRef]
- Zindler, F.; Stoll, S.; Baumann, L.; Knoll, S.; Huhn, C.; Braunbeck, T. Do environmentally relevant concentrations of fluoxetine and citalopram impair stress-related behavior in zebrafish (Danio rerio) embryos? Chemosphere 2020, 261, 127753. [Google Scholar] [CrossRef] [PubMed]
- Atzei, A.; Jense, I.; Zwart, E.P.; Legradi, J.; Venhuis, B.J.; van der Ven, L.T.M.; Heusinkveld, H.J.; Hessel, E.V.S. Developmental Neurotoxicity of Environmentally Relevant Pharmaceuticals and Mixtures Thereof in a Zebrafish Embryo Behavioural Test. Int. J. Environ. Res. Public Health 2021, 18, 6717. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.A.; Arnold, V.I.; Vijayan, M.M. Venlafaxine in Embryos Stimulates Neurogenesis and Disrupts Larval Behavior in Zebrafish. Environ. Sci. Technol. 2017, 51, 12889–12897. [Google Scholar] [CrossRef]
- Thompson, W.A.; Vijayan, M.M. Zygotic Venlafaxine Exposure Impacts Behavioral Programming by Disrupting Brain Serotonin in Zebrafish. Environ. Sci. Technol. 2020, 54, 14578–14588. [Google Scholar] [CrossRef]
- Melnyk-Lamont, N.; Best, C.; Gesto, M.; Vijayan, M.M. The antidepressant venlafaxine disrupts brain monoamine levels and neuroendocrine responses to stress in rainbow trout. Environ. Sci. Technol. 2014, 48, 13434–13442. [Google Scholar] [CrossRef]
- Henry, J.; Bai, Y.; Kreuder, F.; Mawdsley, D.; Kaslin, J.; Wlodkowic, D. Accelerating Chemobehavioral Phenotypic Screening in Neurotoxicology Using a Living Embryo Array System. Zebrafish 2022, 19, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Walpitagama, M.; Carve, M.; Douek, A.M.; Trestrail, C.; Bai, Y.; Kaslin, J.; Wlodkowic, D. Additives migrating from 3D-printed plastic induce developmental toxicity and neuro-behavioural alterations in early life zebrafish (Danio rerio). Aquat. Toxicol. 2019, 213, 105227. [Google Scholar] [CrossRef] [PubMed]
- Bruni, G.; Rennekamp, A.J.; Velenich, A.; McCarroll, M.; Gendelev, L.; Fertsch, E.; Taylor, J.; Lakhani, P.; Lensen, D.; Evron, T.; et al. Zebrafish behavioral profiling identifies multitarget antipsychotic-like compounds. Nat. Chem. Biol. 2016, 12, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Bruni, G.; Lakhani, P.; Kokel, D. Discovering novel neuroactive drugs through high-throughput behavior-based chemical screening in the zebrafish. Front. Pharmacol. 2014, 5, 153. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henry, J.; Bai, Y.; Kreuder, F.; Saaristo, M.; Kaslin, J.; Wlodkowic, D. Sensory-Motor Perturbations in Larval Zebrafish (Danio rerio) Induced by Exposure to Low Levels of Neuroactive Micropollutants during Development. Int. J. Mol. Sci. 2022, 23, 8990. https://doi.org/10.3390/ijms23168990
Henry J, Bai Y, Kreuder F, Saaristo M, Kaslin J, Wlodkowic D. Sensory-Motor Perturbations in Larval Zebrafish (Danio rerio) Induced by Exposure to Low Levels of Neuroactive Micropollutants during Development. International Journal of Molecular Sciences. 2022; 23(16):8990. https://doi.org/10.3390/ijms23168990
Chicago/Turabian StyleHenry, Jason, Yutao Bai, Florian Kreuder, Minna Saaristo, Jan Kaslin, and Donald Wlodkowic. 2022. "Sensory-Motor Perturbations in Larval Zebrafish (Danio rerio) Induced by Exposure to Low Levels of Neuroactive Micropollutants during Development" International Journal of Molecular Sciences 23, no. 16: 8990. https://doi.org/10.3390/ijms23168990
APA StyleHenry, J., Bai, Y., Kreuder, F., Saaristo, M., Kaslin, J., & Wlodkowic, D. (2022). Sensory-Motor Perturbations in Larval Zebrafish (Danio rerio) Induced by Exposure to Low Levels of Neuroactive Micropollutants during Development. International Journal of Molecular Sciences, 23(16), 8990. https://doi.org/10.3390/ijms23168990







