Chronic Venous Disease during Pregnancy Causes a Systematic Increase in Maternal and Fetal Proinflammatory Markers
Abstract
:1. Introduction
2. Results
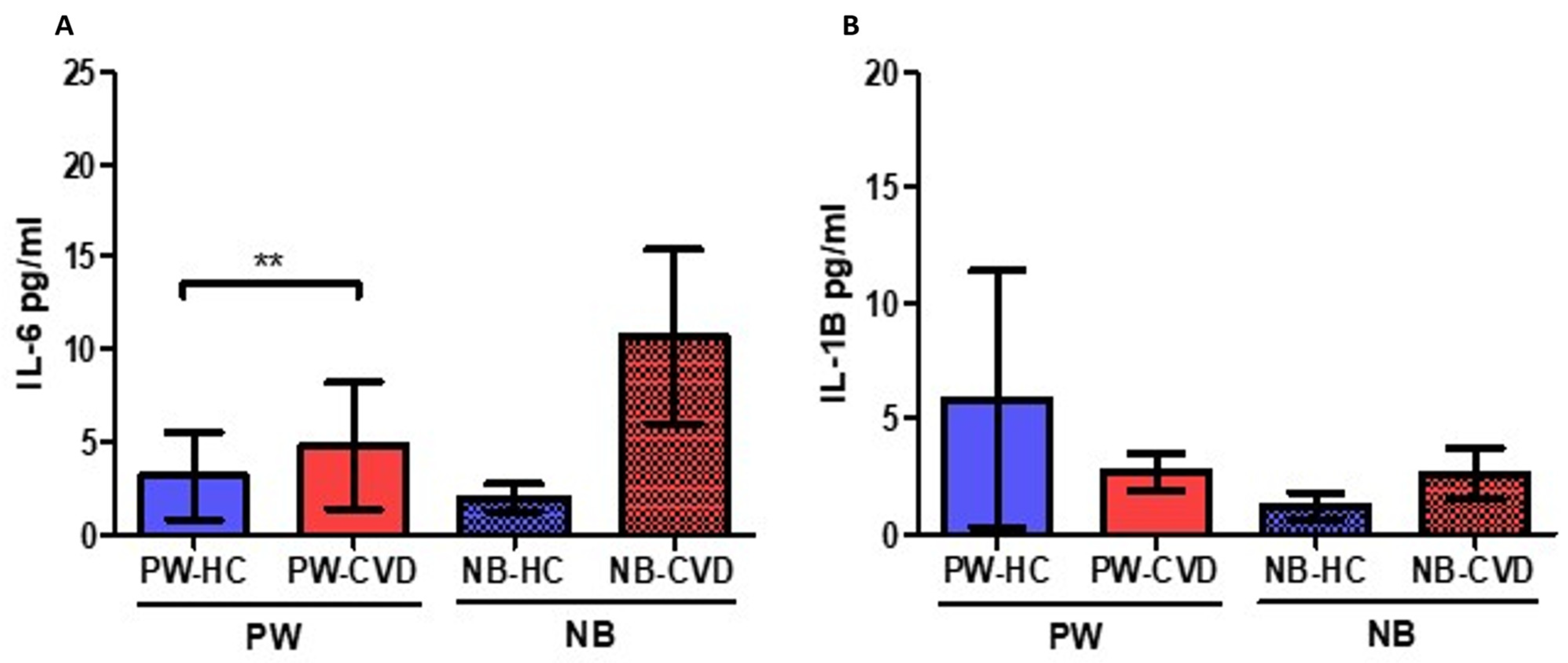
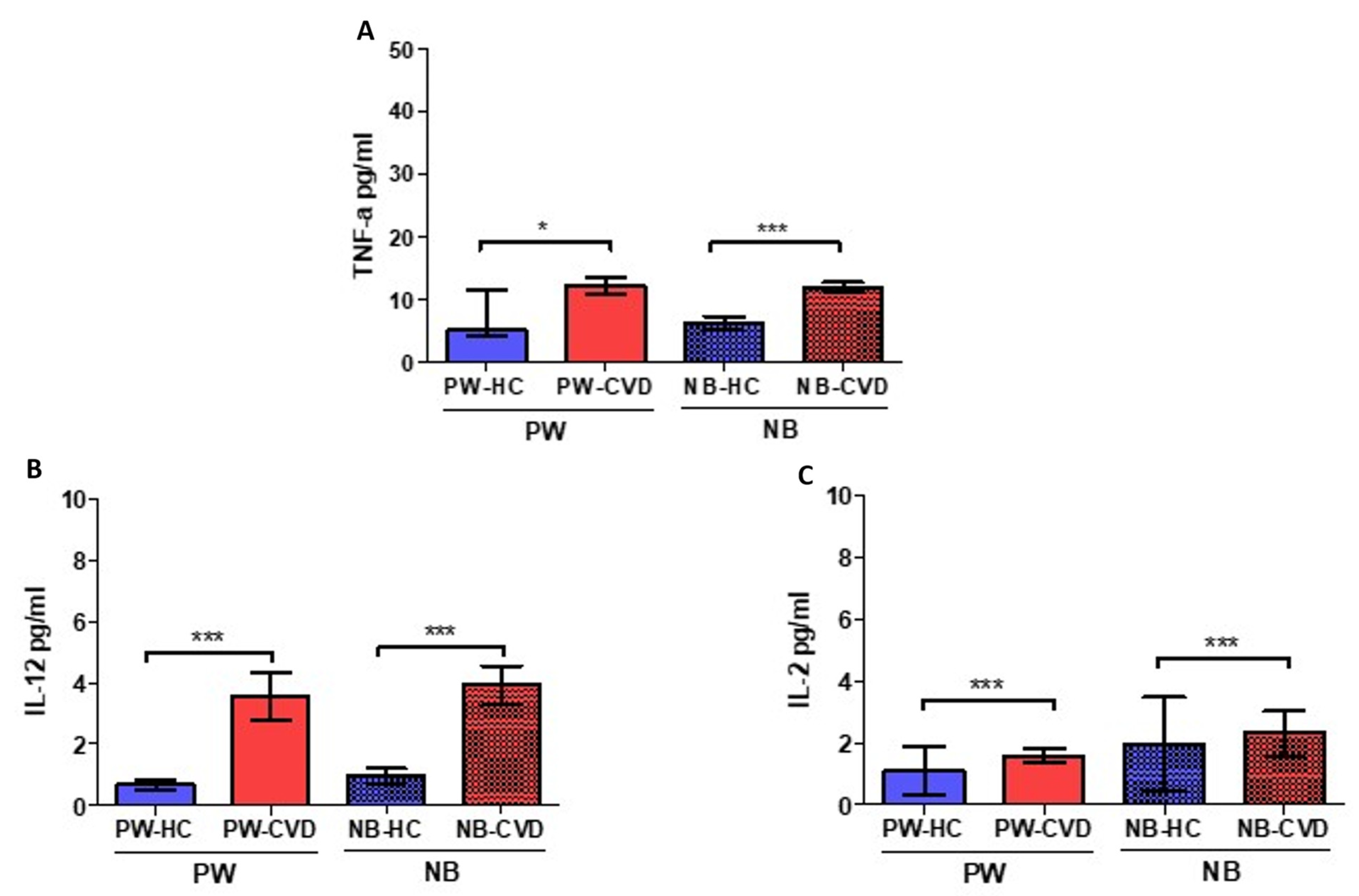
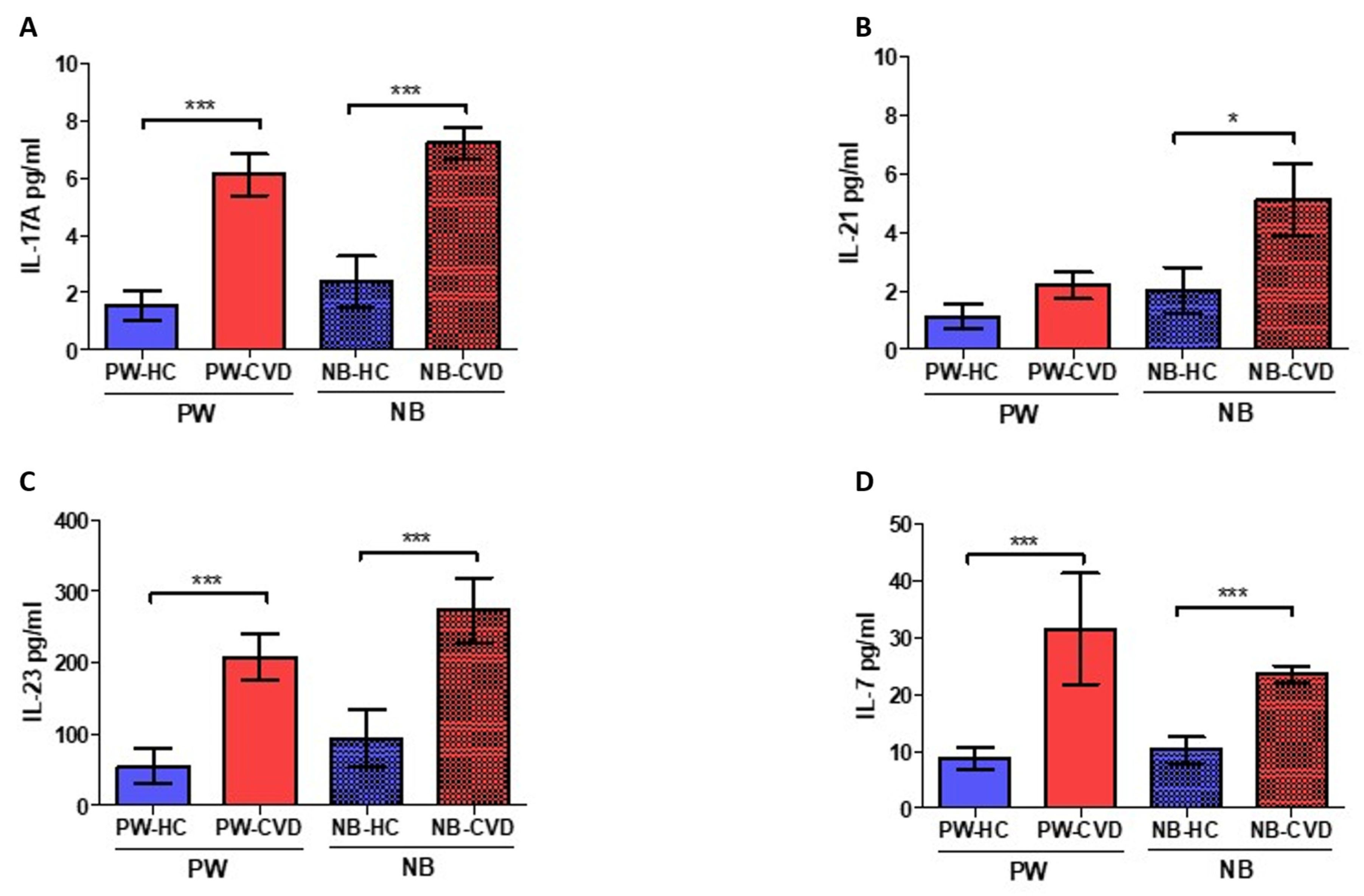
2.1. Women with CVD during Pregnancy Show an Increase in Different Proinflammatory Cytokines
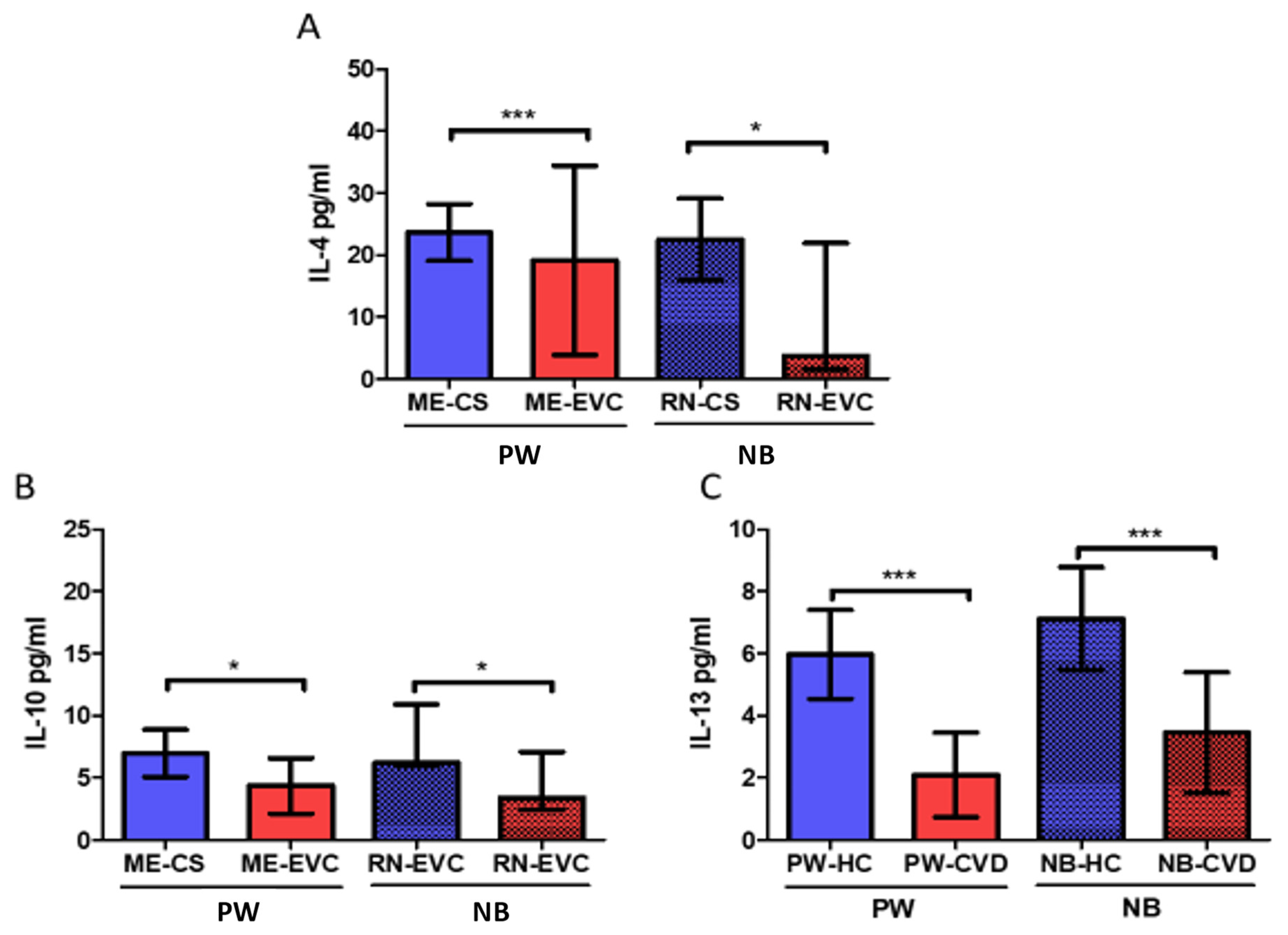
2.2. Women with CVD during Pregnancy Show a Decrease in Anti-Inflammatory Cytokines
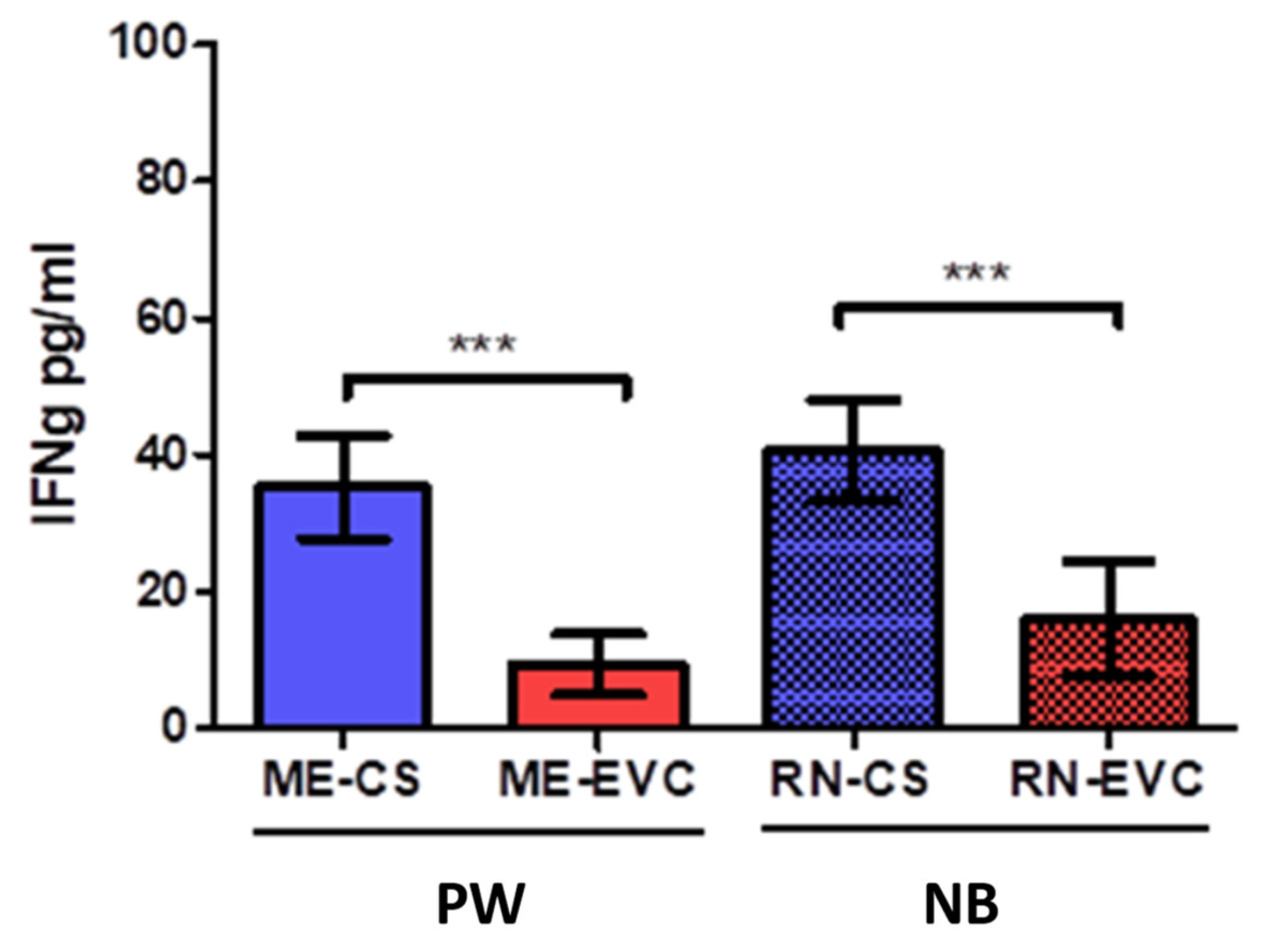
2.3. Women with CVD during Pregnancy Showed a Decrease in IFN-ɣ
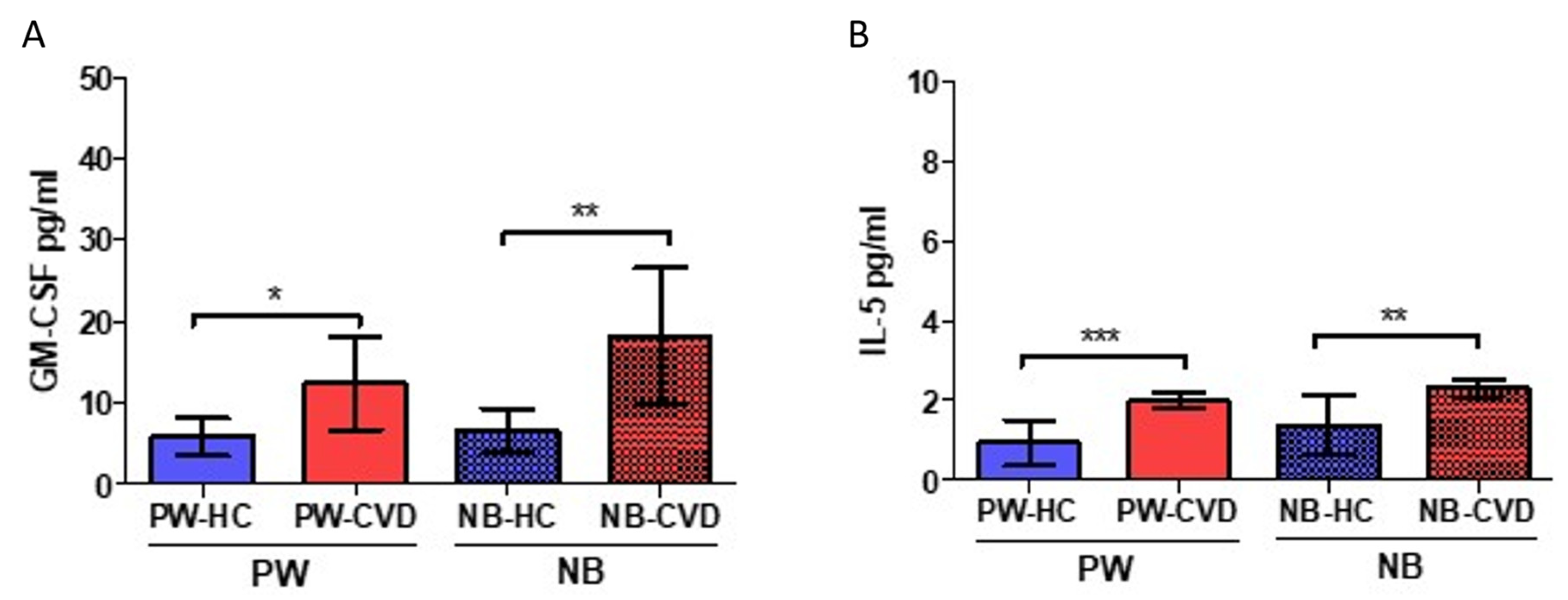
2.4. Women with CVD during Pregnancy Show an Increase in the Eosinopoietins GM-CSF and IL-5
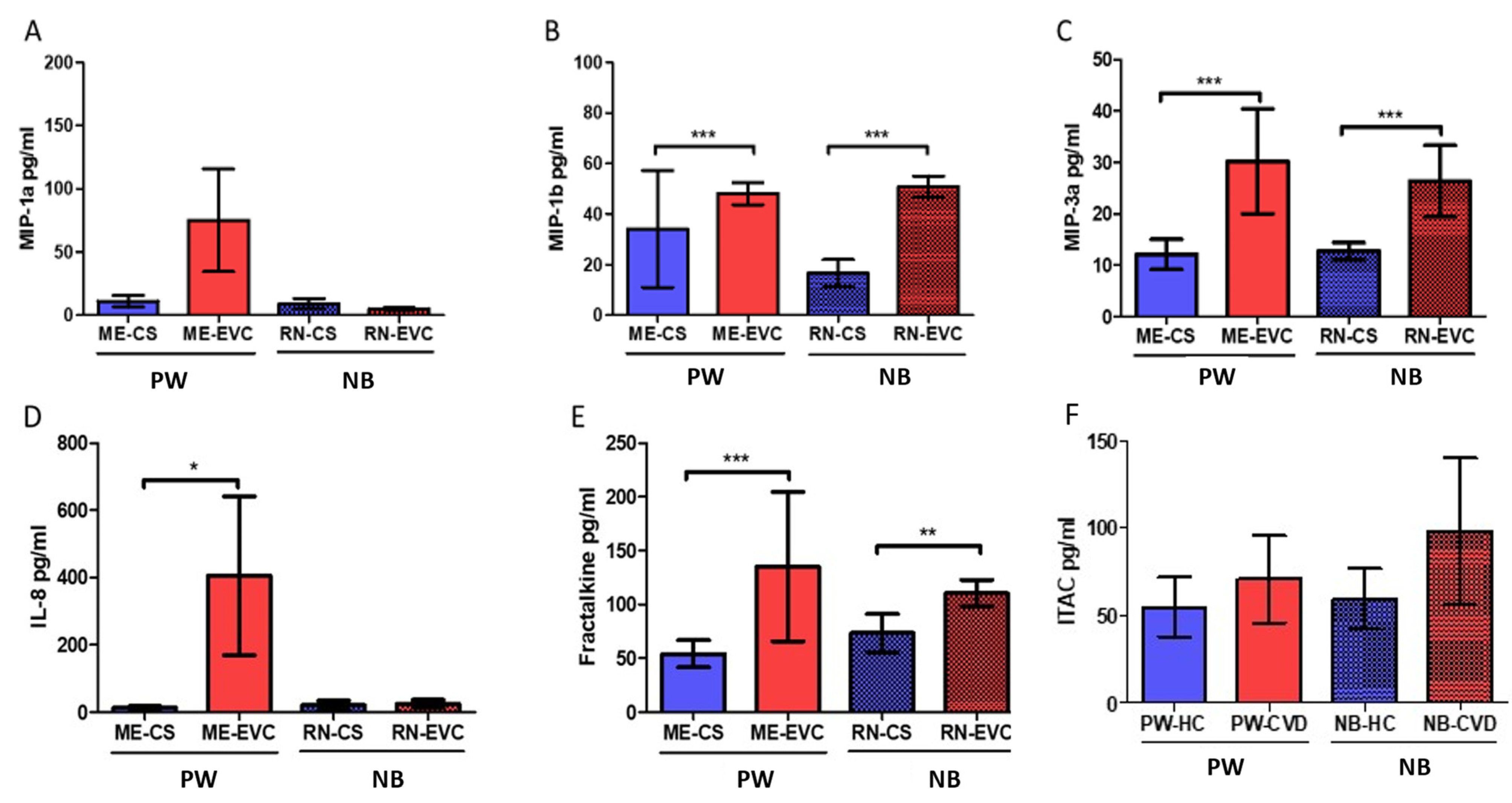
2.5. Women with CVD during Pregnancy Show a Significant Increase in Plasmatic Chemokines
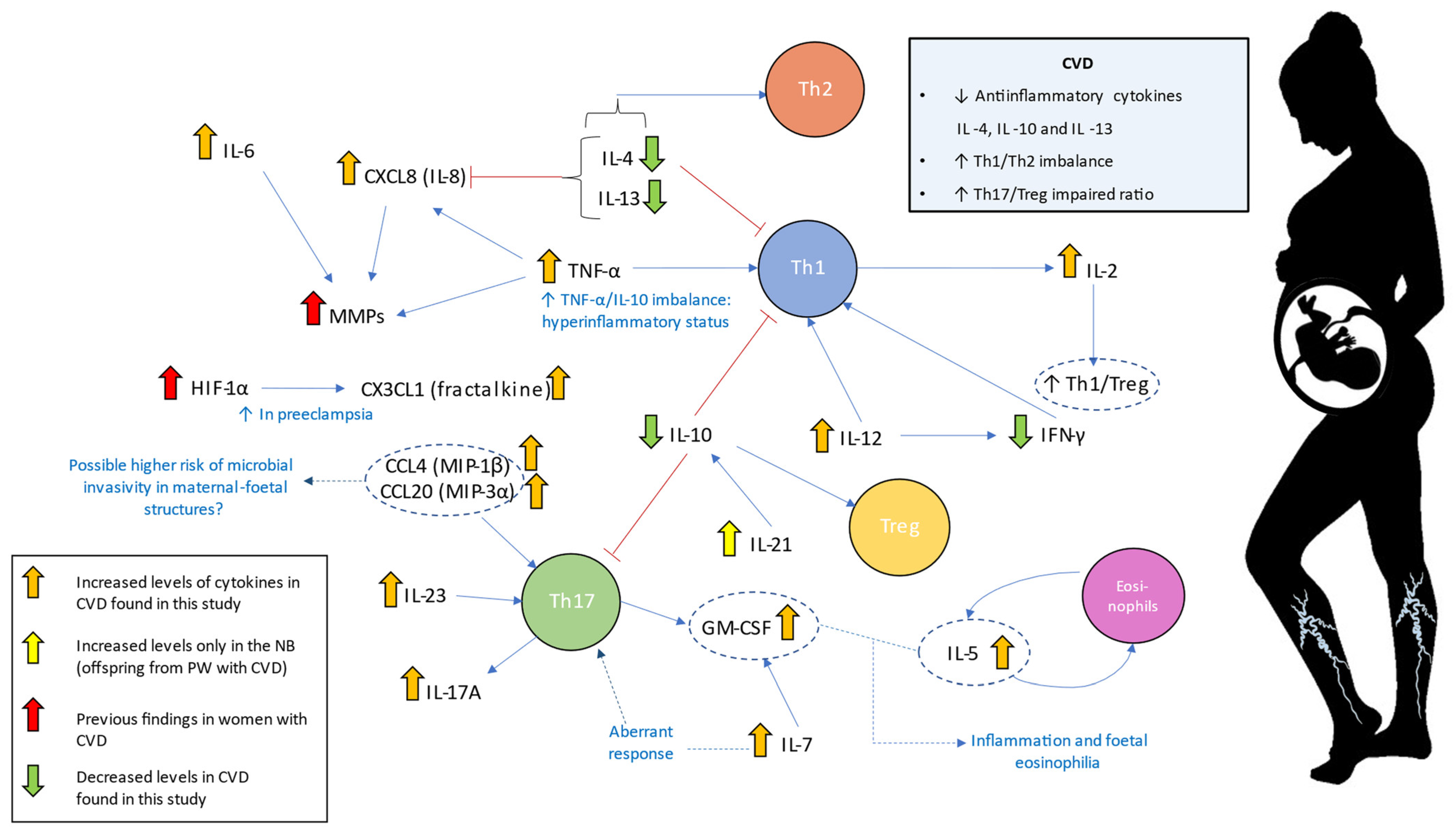
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Determination of Inflammatory Status
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Youn, Y.J.; Lee, J. Chronic Venous Insufficiency and Varicose Veins of the Lower Extremities. Korean J. Intern. Med. 2019, 34, 269–283. [Google Scholar] [CrossRef]
- Raffetto, J.D.; Mannello, F. Pathophysiology of Chronic Venous Disease. Int. Angiol. 2014, 33, 212–221. [Google Scholar] [PubMed]
- Vlajinac, H.D.; Radak, D.J.; Marinković, J.M.; Maksimović, M.Ž. Risk Factors for Chronic Venous Disease. Phlebology 2012, 27, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Homs-Romero, E.; Romero-Collado, A.; Verdú, J.; Blanch, J.; Rascón-Hernán, C.; Martí-Lluch, R. Validity of Chronic Venous Disease Diagnoses and Epidemiology Using Validated Electronic Health Records From Primary Care: A Real-World Data Analysis. J. Nurs. Scholarsh. 2021, 53, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Zolotukhin, I.A.; Seliverstov, E.I.; Shevtsov, Y.N.; Avakiants, I.P.; Nikishkov, A.S.; Tatarintsev, A.M.; Kirienko, A.I. Prevalence and Risk Factors for Chronic Venous Disease in the General Russian Population. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.; Fraile-Martínez, O.; García-Montero, C.; Álvarez-Mon, M.; Chaowen, C.; Ruiz-Grande, F.; Pekarek, L.; Monserrat, J.; Asúnsolo, A.; García-Honduvilla, N.; et al. Understanding Chronic Venous Disease: A Critical Overview of Its Pathophysiology and Medical Management. J. Clin. Med. 2021, 10, 3239. [Google Scholar] [CrossRef] [PubMed]
- Morton, A. Physiological Changes and Cardiovascular Investigations in Pregnancy. Heart Lung Circ. 2021, 30, e6–e15. [Google Scholar] [CrossRef]
- Ropacka-Lesiak, M.; Jaroslaw, K.; Bręborowicz, G. Pregnancy-Dependent Blood Flow Velocity Changes in Lower Extremities Veins in Venous Insufficiency. Ginekol. Pol. 2015, 86, 659–665. [Google Scholar] [CrossRef]
- Taylor, J.; Hicks, C.W.; Heller, J.A. The Hemodynamic Effects of Pregnancy on the Lower Extremity Venous System. J. Vasc. Surg. Venous Lymphat. Disord. 2018, 6, 246–255. [Google Scholar] [CrossRef]
- Lohr, J.M.; Bush, R.L. Venous Disease in Women: Epidemiology, Manifestations, and Treatment. J. Vasc. Surg. 2013, 57, 37S–45S. [Google Scholar] [CrossRef]
- NH, T. Physiologic and Hemodynamic Changes During Pregnancy. AACN Adv. Crit. Care 2018, 29, 273–283. [Google Scholar] [CrossRef]
- Labropoulos, N. How Does Chronic Venous Disease Progress from the First Symptoms to the Advanced Stages? A Review. Adv. Ther. 2019, 36, 13–19. [Google Scholar] [CrossRef]
- Ortega, M.A.; Saez, M.Á.; Asúnsolo, Á.; Romero, B.; Bravo, C.; Coca, S.; Sainz, F.; Álvarez-Mon, M.; Buján, J.; García-Honduvilla, N. Upregulation of VEGF and PEDF in Placentas of Women with Lower Extremity Venous Insufficiency during Pregnancy and Its Implication in Villous Calcification. BioMed Res. Int. 2019, 2019, 5320902. [Google Scholar] [CrossRef]
- Ortega, M.A.; Romero, B.; Asúnsolo, Á.; Martínez-Vivero, C.; Sainz, F.; Bravo, C.; de León-Luis, J.; Álvarez-Mon, M.; Buján, J.; García-Honduvilla, N. Pregnancy-Associated Venous Insufficiency Course with Placental and Systemic Oxidative Stress. J. Cell. Mol. Med. 2020, 24, 4157–4170. [Google Scholar] [CrossRef]
- Ortega, M.A.; Saez, M.A.; Fraile-Martínez, O.; Asúnsolo, Á.; Pekarek, L.; Bravo, C.; Coca, S.; Sainz, F.; Álvarez-Mon, M.; Buján, J.; et al. Increased Angiogenesis and Lymphangiogenesis in the Placental Villi of Women with Chronic Venous Disease during Pregnancy. Int. J. Mol. Sci. 2020, 21, 2487. [Google Scholar] [CrossRef]
- García-Honduvilla, N.; Ortega, M.A.; Asúnsolo, Á.; Álvarez-Rocha, M.J.; Romero, B.; de León-Luis, J.; Álvarez-Mon, M.; Buján, J. Placentas from Women with Pregnancy-Associated Venous Insufficiency Show Villi Damage with Evidence of Hypoxic Cellular Stress. Hum. Pathol. 2018, 77, 45–53. [Google Scholar] [CrossRef]
- Chaiworapongsa, T.; Chaemsaithong, P.; Yeo, L.; Romero, R. Pre-Eclampsia Part 1: Current Understanding of Its Pathophysiology. Nat. Rev. Nephrol. 2014, 10, 466–480. [Google Scholar] [CrossRef]
- Ortega, M.A.; Chaowen, C.; Fraile-Martinez, O.; García-Montero, C.; Saez, M.A.; Cruza, I.; Pereda-Cerquella, C.; Alvarez-Mon, M.A.; Guijarro, L.G.; Fatych, Y.; et al. Chronic Venous Disease in Pregnant Women Causes an Increase in ILK in the Placental Villi Associated with a Decrease in E-Cadherin. J. Pers. Med. 2022, 12, 277. [Google Scholar] [CrossRef]
- Ramani, T.; Auletta, C.S.; Weinstock, D.; Mounho-Zamora, B.; Ryan, P.C.; Salcedo, T.W.; Bannish, G. Cytokines: The Good, the Bad, and the Deadly. Int. J. Toxicol. 2015, 34, 355–365. [Google Scholar] [CrossRef]
- Feldmann, M. Many Cytokines Are Very Useful Therapeutic Targets in Disease. J. Clin. Investig. 2008, 118, 3533–3536. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Aggarwal, R.; Jain, A.K.; Mittal, P.; Kohli, M.; Jawanjal, P.; Rath, G. Association of Pro- and Anti-Inflammatory Cytokines in Preeclampsia. J. Clin. Lab. Anal. 2019, 33, e22834. [Google Scholar] [CrossRef]
- Raghupathy, R.; Kalinka, J. Cytokine Imbalance in Pregnancy Complications and Its Modulation. Front. Biosci. 2008, 13, 985–994. [Google Scholar] [CrossRef]
- Yockey, L.J.; Iwasaki, A. Interferons and Proinflammatory Cytokines in Pregnancy and Fetal Development. Immunity 2018, 49, 397–412. [Google Scholar] [CrossRef]
- Velez, D.R.; Fortunato, S.J.; Morgan, N.; Edwards, T.L.; Lombardi, S.J.; Williams, S.M.; Menon, R. Patterns of Cytokine Profiles Differ with Pregnancy Outcome and Ethnicity. Hum. Reprod. 2008, 23, 1902–1909. [Google Scholar] [CrossRef]
- Hernández-Trejo, M.; Montoya-Estrada, A.; Torres-Ramos, Y.; Espejel-Núñez, A.; Guzmán-Grenfell, A.; Morales-Hernández, R.; Tolentino-Dolores, M.; Laresgoiti-Servitje, E. Oxidative Stress Biomarkers and Their Relationship with Cytokine Concentrations in Overweight/Obese Pregnant Women and Their Neonates. BMC Immunol. 2017, 18, 3. [Google Scholar] [CrossRef]
- Agarwal, S.; Karmaus, W.; Davis, S.; Gangur, V. Immune Markers in Breast Milk and Fetal and Maternal Body Fluids: A Systematic Review of Perinatal Concentrations. J. Hum. Lact. 2011, 27, 171–186. [Google Scholar] [CrossRef]
- Conrad, K.P.; Miles, T.M.; Benyo, D.F. Circulating Levels of Immunoreactive Cytokines in Women with Preeclampsia. Am. J. Reprod. Immunol. 1998, 40, 102–111. [Google Scholar] [CrossRef]
- Szarka, A.; Rigó, J.; Lázár, L.; Beko, G.; Molvarec, A. Circulating Cytokines, Chemokines and Adhesion Molecules in Normal Pregnancy and Preeclampsia Determined by Multiplex Suspension Array. BMC Immunol. 2010, 11, 59. [Google Scholar] [CrossRef]
- Lattimer, C.R.; Kalodiki, E.; Geroulakos, G.; Hoppensteadt, D.; Fareed, J. Are Inflammatory Biomarkers Increased in Varicose Vein Blood? Clin. Appl. Thromb. Hemost. 2016, 22, 656–664. [Google Scholar] [CrossRef]
- Ligi, D.; Croce, L.; Mannello, F. Chronic Venous Disorders: The Dangerous, the Good, and the Diverse. Int. J. Mol. Sci. 2018, 19, 2544. [Google Scholar] [CrossRef] [PubMed]
- Grudzinska, E.; Lekstan, A.; Szliszka, E.; Czuba, Z.P. Cytokines Produced by Lymphocytes in the Incompetent Great Saphenous Vein. Mediat. Inflamm. 2018, 2018, 7161346. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Sánchez-Trujillo, L.; Bravo, C.; Fraile-Martinez, O.; García-Montero, C.; Saez, M.A.; Alvarez-Mon, M.A.; Sainz, F.; Alvarez-Mon, M.; Bujan, J.; et al. Newborns of Mothers with Venous Disease during Pregnancy Show Increased Levels of Lipid Peroxidation and Markers of Oxidative Stress and Hypoxia in the Umbilical Cord. Antioxidants 2021, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.; Fraile-Martínez, O.; Saez, M.; Álvarez-Mon, M.; Gómez-Lahoz, A.M.; Bravo, C.; Luis, J.A.D.L.; Sainz, F.; Coca, S.; Asúnsolo; et al. Abnormal Proinflammatory and Stressor Environmental with Increased the Regulatory Cellular IGF-1/PAPP-A/STC and Wnt-1/β-Catenin Canonical Pathway in Placenta of Women with Chronic Venous Disease during Pregnancy. Int. J. Med. Sci. 2021, 18, 2814–2827. [Google Scholar] [CrossRef]
- Ortega, M.A.; Asúnsolo, Á.; Fraile-Martínez, O.; Sainz, F.; Saez, M.A.; Bravo, C.; De León-Luis, J.A.; Alvarez-Mon, M.A.; Coca, S.; Álvarez-Mon, M.; et al. An Increase in Elastogenic Components in the Placental Villi of Women with Chronic Venous Disease during Pregnancy Is Associated with Decreased EGFL7 Expression Level. Mol. Med. Rep. 2021, 24, 556. [Google Scholar] [CrossRef]
- Ortega, M.A.; Saez, M.A.; Sainz, F.; Fraile-Martínez, O.; García-Gallego, S.; Pekarek, L.; Bravo, C.; Coca, S.; Álvarez-Mon, M.; Buján, J.; et al. Lipidomic Profiling of Chorionic Villi in the Placentas of Women with Chronic Venous Disease. Int. J. Med. Sci. 2020, 17, 2790–2798. [Google Scholar] [CrossRef]
- Myatt, L. Placental Adaptive Responses and Fetal Programming. J. Physiol. 2006, 572, 25. [Google Scholar] [CrossRef]
- Fajersztajn, L.; Veras, M.M. Hypoxia: From Placental Development to Fetal Programming. Birth Defects Res. 2017, 109, 1377–1385. [Google Scholar] [CrossRef]
- Konkel, L. Lasting Impact of an Ephemeral Organ: The Role of the Placenta in Fetal Programming. Environ. Health Perspect. 2016, 124, A124–A129. [Google Scholar] [CrossRef]
- Loppnow, H. Cytokines: Classification, Receptors, Mechanisms of Action. Internist 2001, 42, 13–27. [Google Scholar] [CrossRef]
- Justiz Vaillant, A.A.; Qurie, A. Interleukin; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Zaretsky, M.V.; Alexander, J.M.; Byrd, W.; Bawdon, R.E. Transfer of Inflammatory Cytokines across the Placenta. Obstet. Gynecol. 2004, 103, 546–550. [Google Scholar] [CrossRef]
- Uciechowski, P.; Dempke, W.C.M. Interleukin-6: A Masterplayer in the Cytokine Network. Oncology 2020, 98, 131–137. [Google Scholar] [CrossRef]
- Xiao, J.P.; Yin, Y.X.; Gao, Y.F.; Lau, S.; Shen, F.; Zhao, M.; Chen, Q. The Increased Maternal Serum Levels of IL-6 Are Associated with the Severity and Onset of Preeclampsia. Cytokine 2012, 60, 856–860. [Google Scholar] [CrossRef]
- Martinez-Portilla, R.J.; Hawkins-Villarreal, A.; Alvarez-Ponce, P.; Chinolla-Arellano, Z.L.; Moreno-Espinosa, A.L.; Sandoval-Mejia, A.L.; Moreno-Uribe, N. Maternal Serum Interleukin-6: A Non-Invasive Predictor of Histological Chorioamnionitis in Women with Preterm-Prelabor Rupture of Membranes. Fetal Diagn. Ther. 2019, 45, 168–175. [Google Scholar] [CrossRef]
- Deon, D.; Ahmed, S.; Tai, K.; Scaletta, N.; Herrero, C.; Lee, I.-H.; Krause, A.; Ivashkiv, L.B. Cross-Talk between IL-1 and IL-6 Signaling Pathways in Rheumatoid Arthritis Synovial Fibroblasts. J. Immunol. 2001, 167, 5395–5403. [Google Scholar] [CrossRef]
- Chen, L.M.; Liu, B.; Zhao, H.B.; Stone, P.; Chen, Q.; Chamley, L. IL-6, TNFα and TGFβ Promote Nonapoptotic Trophoblast Deportation and Subsequently Causes Endothelial Cell Activation. Placenta 2010, 31, 75–80. [Google Scholar] [CrossRef]
- Rocha, G.; Proença, E.; Guedes, A.; Carvalho, C.; Areias, A.; Ramos, J.P.; Rodrigues, T.; Guimarães, H. Cord Blood Levels of IL-6, IL-8 and IL-10 May Be Early Predictors of Bronchopulmonary Dysplasia in Preterm Newborns Small for Gestational Age. Dis. Markers 2012, 33, 51–60. [Google Scholar] [CrossRef]
- Jonsson, Y.; Rubèr, M.; Matthiesen, L.; Berg, G.; Nieminen, K.; Sharma, S.; Ernerudh, J.; Ekerfelt, C. Cytokine Mapping of Sera from Women with Preeclampsia and Normal Pregnancies. J. Reprod Immunol. 2006, 70, 83–91. [Google Scholar] [CrossRef]
- Huang, Q.; Jin, X.; Li, P.; Zheng, Z.; Jiang, Y.; Liu, H. Elevated Inflammatory Mediators from the Maternal-Fetal Interface to Fetal Circulation during Labor. Cytokine 2021, 148, 155707. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Ding, X.; Duan, B.; Li, L.; Wang, X. Serum Levels of TNF-α and IL-6 Are Associated with Pregnancy-Induced Hypertension. Reprod. Sci. 2016, 23, 1402–1408. [Google Scholar] [CrossRef]
- Carpentier, P.A.; Dingman, A.L.; Palmer, T.D. Placental TNF-α Signaling in Illness-Induced Complications of Pregnancy. Am. J. Pathol. 2011, 178, 2802–2810. [Google Scholar] [CrossRef] [PubMed]
- Stefańska, K.; Zieliński, M.; Jankowiak, M.; Zamkowska, D.; Sakowska, J.; Adamski, P.; Jassem-Bobowicz, J.; Piekarska, K.; Leszczyńska, K.; Świątkowska-Stodulska, R.; et al. Cytokine Imprint in Preeclampsia. Front. Immunol. 2021, 12, 667841. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, A.M.; Jang, S.; Salgame, P. Signaling from Cytokine Receptors That Affect Th1 Responses. Front. Biosci. 2002, 7, 1247–1254. [Google Scholar] [CrossRef]
- Gaffen, S.L.; Liu, K.D. Overview of Interleukin-2 Function, Production and Clinical Applications. Cytokine 2004, 28, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Leonard, W.J. Biology of IL-2 and Its Therapeutic Modulation: Mechanisms and Strategies. J. Leukoc. Biol. 2018, 103, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Trotta, E.; Simeonov, D.R.; Marson, A.; Bluestone, J.A. Revisiting IL-2: Biology and Therapeutic Prospects. Sci. Immunol. 2018, 3, eaat1482. [Google Scholar] [CrossRef] [PubMed]
- Fainboim, L.; Arruvito, L. Mechanisms Involved in the Expansion of Tregs during Pregnancy: Role of IL-2/STAT5 Signalling. J. Reprod. Immunol. 2011, 88, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Hadinedoushan, H.; Mirahmadian, M.; Aflatounian, A. Increased Natural Killer Cell Cytotoxicity and IL-2 Production in Recurrent Spontaneous Abortion. Am. J. Reprod. Immunol. 2007, 58, 409–414. [Google Scholar] [CrossRef]
- Vignali, D.A.A.; Kuchroo, V.K. IL-12 Family Cytokines: Immunological Playmakers. Nat. Immunol. 2012, 13, 722–728. [Google Scholar] [CrossRef]
- Becker, C.; Wirtz, S.; Neurath, M.F. Stepwise Regulation of TH1 Responses in Autoimmunity: IL-12-Related Cytokines and Their Receptors. Inflamm. Bowel Dis. 2005, 11, 755–764. [Google Scholar] [CrossRef]
- Perricone, C.; de Carolis, C.; Perricone, R. Pregnancy and Autoimmunity: A Common Problem. Best Pract. Res. Clin. Rheumatol. 2012, 26, 47–60. [Google Scholar] [CrossRef]
- Murphy, S.P.; Tayade, C.; Ashkar, A.A.; Hatta, K.; Zhang, J.; Croy, B.A. Interferon Gamma in Successful Pregnancies. Biol. Reprod. 2009, 80, 848–859. [Google Scholar] [CrossRef]
- Scott, M.E.; Kubin, M.; Kohl, S. High Level Interleukin-12 Production, but Diminished Interferon-γ Production, by Cord Blood Mononuclear Cells. Pediatric Res. 1997, 41, 547–553. [Google Scholar] [CrossRef]
- Giurgescu, C.; Sanguanklin, N.; Engeland, C.G.; White-Traut, R.C.; Park, C.; Mathews, H.L.; Janusek, L.W. Relationships among Psychosocial Factors, Biomarkers, Preeclampsia, and Preterm Birth in African American Women: A Pilot. Appl. Nurs. Res. 2015, 28, e1–e6. [Google Scholar] [CrossRef]
- Saraiva, M.; O’Garra, A. The Regulation of IL-10 Production by Immune Cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Wang, X.; Wong, K.; Ouyang, W.; Rutz, S. Targeting IL-10 Family Cytokines for the Treatment of Human Diseases. Cold Spring Harb. Perspect. Biol. 2019, 11, a028548. [Google Scholar] [CrossRef]
- Brogin Moreli, J.; Cirino Ruocco, A.M.; Vernini, J.M.; Rudge, M.V.C.; Calderon, I.M.P. Interleukin 10 and Tumor Necrosis Factor-Alpha in Pregnancy: Aspects of Interest in Clinical Obstetrics. ISRN Obstet. Gynecol. 2012, 2012, 230742. [Google Scholar] [CrossRef]
- Chatterjee, P.; Chiasson, V.L.; Bounds, K.R.; Mitchell, B.M. Regulation of the Anti-Inflammatory Cytokines Interleukin-4 and Interleukin-10 during Pregnancy. Front. Immunol. 2014, 5, 253. [Google Scholar] [CrossRef]
- Junttila, I.S. Tuning the Cytokine Responses: An Update on Interleukin (IL)-4 and IL-13 Receptor Complexes. Front. Immunol. 2018, 9, 888. [Google Scholar] [CrossRef]
- Chatterjee, P.; Kopriva, S.E.; Chiasson, V.L.; Young, K.J.; Tobin, R.P.; Newell-Rogers, K.; Mitchell, B.M. Interleukin-4 Deficiency Inducesmild Preeclampsia Inmice. J. Hypertens. 2013, 31, 1414–1423. [Google Scholar] [CrossRef]
- Daneva, A.; Hadži-Lega, M.; Stefanovic, M. Correlation of the System of Cytokines in Moderate and Severe Preeclampsia. Clin. Exp. Obs. Gynecol. 2016, 43, 220–224. [Google Scholar] [CrossRef]
- Englich, B.; Herberth, G.; Rolle-Kampczyk, U.; Trump, S.; Röder, S.; Borte, M.; Stangl, G.I.; von Bergen, M.; Lehmann, I.; Junge, K.M. Maternal Cytokine Status May Prime the Metabolic Profile and Increase Risk of Obesity in Children. Int. J. Obes. 2017, 41, 1440–1446. [Google Scholar] [CrossRef]
- Sykes, L.; MacIntyre, D.A.; Yap, X.J.; Teoh, T.G.; Bennett, P.R. The Th1:Th2 Dichotomy of Pregnancy and Preterm Labour. Mediat. Inflamm. 2012, 2012, 12. [Google Scholar] [CrossRef]
- Zenobia, C.; Hajishengallis, G. Basic Biology and Role of Interleukin-17 in Immunity and Inflammation. Periodontol. 2000 2015, 69, 142–159. [Google Scholar] [CrossRef]
- Eghbal-Fard, S.; Yousefi, M.; Heydarlou, H.; Ahmadi, M.; Taghavi, S.; Movasaghpour, A.; Jadidi-Niaragh, F.; Yousefi, B.; Dolati, S.; Hojjat-Farsangi, M.; et al. The Imbalance of Th17/Treg Axis Involved in the Pathogenesis of Preeclampsia. J. Cell. Physiol. 2019, 234, 5106–5116. [Google Scholar] [CrossRef]
- Poordast, T.; Najib, F.; Baharlou, R.; Bijani, A.; Alamdarloo, S.; Poordast, A. Assessment of T Helper 17-Associated Cytokines in Third Trimester of Pregnancy. Iran. J. Immunol. 2017, 14, 172–179. [Google Scholar]
- Darmochwal-Kolarz, D.; Michalak, M.; Kolarz, B.; Przegalinska-Kalamucka, M.; Bojarska-Junak, A.; Sliwa, D.; Oleszczuk, J. The Role of Interleukin-17, Interleukin-23, and Transforming Growth Factor- β in Pregnancy Complicated by Placental Insufficiency. BioMed Res. Int. 2017, 2017, 6904325. [Google Scholar] [CrossRef]
- Elkassar, N.; Gress, R.E. An Overview of IL-7 Biology and Its Use in Immunotherapy. J. Immunotoxicol. 2010, 7, 1–7. [Google Scholar] [CrossRef]
- Wu, L.; Li, J.; Xu, H.L.; Xu, B.; Tong, X.H.; Kwak-Kim, J.; Liu, Y.S. IL-7/IL-7R Signaling Pathway Might Play a Role in Recurrent Pregnancy Losses by Increasing Inflammatory Th17 Cells and Decreasing Treg Cells. Am. J. Reprod. Immunol. 2016, 76, 454–464. [Google Scholar] [CrossRef]
- Leonard, W.J.; Wan, C.K. IL-21 Signaling in Immunity. F1000Research 2016, 5, 224. [Google Scholar] [CrossRef]
- Doganci, A.; Birkholz, J.; Gehring, S.; Puhl, A.G.; Zepp, F.; Meyer, C.U. In the Presence of IL-21 Human Cord Blood T Cells Differentiate to IL-10-Producing Th1 but Not Th17 or Th2 Cells. Int. Immunol. 2013, 25, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Radonjic-Hoesli, S.; Valent, P.; Klion, A.D.; Wechsler, M.E.; Simon, H.U. Novel Targeted Therapies for Eosinophil-Associated Diseases and Allergy. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 633–656. [Google Scholar] [CrossRef] [PubMed]
- Griseri, T.; Arnold, I.C.; Pearson, C.; Krausgruber, T.; Schiering, C.; Franchini, F.; Schulthess, J.; McKenzie, B.S.; Crocker, P.R.; Powrie, F. Granulocyte Macrophage Colony-Stimulating Factor-Activated Eosinophils Promote Interleukin-23 Driven Chronic Colitis. Immunity 2015, 43, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, G.A.; Yacoub, M.R.; Ripa, M.; Mannina, D.; Cariddi, A.; Saporiti, N.; Ciceri, F.; Castagna, A.; Colombo, G.; Dagna, L. Eosinophils from Physiology to Disease: A Comprehensive Review. Biomed. Res. Int 2018, 2018, 9095275. [Google Scholar] [CrossRef]
- Spencer, L.A.; Szela, C.T.; Perez, S.A.C.; Kirchhoffer, C.L.; Neves, J.S.; Radke, A.L.; Weller, P.F. Human Eosinophils Constitutively Express Multiple Th1, Th2, and Immunoregulatory Cytokines That Are Secreted Rapidly and Differentially. J. Leukoc. Biol. 2008, 85, 117–123. [Google Scholar] [CrossRef]
- Romero, R.; Kusanovic, J.P.; Gomez, R.; Lamont, R.; Bytautiene, E.; Garfield, R.E.; Mittal, P.; Hassan, S.S.; Yeo, L. The Clinical Significance of Eosinophils in the Amniotic Fluid in Preterm Labor. J. Matern. Fetal Neonatal Med. 2010, 23, 320–329. [Google Scholar] [CrossRef]
- Lebold, K.M.; Drake, M.G.; Hales-Beck, L.B.; Fryer, A.D.; Jacoby, D.B. IL-5 Exposure in Utero Increases Lung Nerve Density and Airway Reactivity in Adult Offspring. Am. J. Respir. Cell Mol. Biol. 2020, 62, 493–502. [Google Scholar] [CrossRef]
- Stone, W.L.; Leavitt, L.; Varacallo, M. Excerpt. In Physiology, Growth Factor; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Duvallet, E.; Semerano, L.; Assier, E.; Falgarone, G.; Boissier, M.C. Interleukin-23: A Key Cytokine in Inflammatory Diseases. Ann. Med. 2011, 43, 503–511. [Google Scholar] [CrossRef]
- Grifka-Walk, H.M.; Giles, D.A.; Segal, B.M. IL-12-Polarized Th1 Cells Produce GM-CSF and Induce EAE Independent of IL-23. Eur. J. Immunol. 2015, 45, 2780–2786. [Google Scholar] [CrossRef]
- Herndler-Brandstetter, D.; Flavell, R.A. Producing GM-CSF: A Unique T Helper Subset? Cell Res. 2014, 24, 1379–1380. [Google Scholar] [CrossRef]
- Petrina, M.; Martin, J.; Basta, S. Granulocyte Macrophage Colony-Stimulating Factor Has Come of Age: From a Vaccine Adjuvant to Antiviral Immunotherapy. Cytokine Growth Factor Rev. 2021, 59, 101–110. [Google Scholar] [CrossRef]
- Robertson, S.A. GM-CSF Regulation of Embryo Development and Pregnancy. Cytokine Growth Factor Rev. 2007, 18, 287–298. [Google Scholar] [CrossRef]
- Perricone, R.; De Carolis, C.; Giacomelli, R.; Guarino, M.D.; De Sanctis, G.; Fontana, L. GM-CSF and Pregnancy: Evidence of Significantly Reduced Blood Concentrations in Unexplained Recurrent Abortion Efficiently Reverted by Intravenous Immunoglobulin Treatment. Am. J. Reprod. Immunol. 2003, 50, 232–237. [Google Scholar] [CrossRef]
- Sjöblom, C.; Roberts, C.T.; Wikland, M.; Robertson, S.A. Granulocyte-Macrophage Colony-Stimulating Factor Alleviates Adverse Consequences of Embryo Culture on Fetal Growth Trajectory and Placental Morphogenesis. Endocrinology 2005, 146, 2142–2153. [Google Scholar] [CrossRef]
- Huang, S.J.; Zenclussen, A.C.; Chen, C.P.; Basar, M.; Yang, H.; Arcuri, F.; Li, M.; Kocamaz, E.; Buchwalder, L.; Rahman, M.; et al. The Implication of Aberrant GM-CSF Expression in Decidual Cells in the Pathogenesis of Preeclampsia. Am. J. Pathol. 2010, 177, 2472–2482. [Google Scholar] [CrossRef]
- Hughes, C.E.; Nibbs, R.J.B. A Guide to Chemokines and Their Receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef]
- Sokol, C.L.; Luster, A.D. The Chemokine System in Innate Immunity. Cold Spring Harb. Perspect. Biol. 2015, 7, a016303. [Google Scholar] [CrossRef]
- Palomino, D.C.; Arolina, T.; Marti, L.C. Avalheiro Chemokines and Immunity. Einstein 2015, 13, 469–473. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Laresgoiti-Servitje, E.; Olson, D.M.; Estrada-Gutiérrez, G.; Vadillo-Ortega, F. The Role of Chemokines in Term and Premature Rupture of the Fetal Membranes: A Review1. Biol. Reprod. 2010, 82, 809–814. [Google Scholar] [CrossRef]
- Hannan, N.J.; Salamonsen, L.A. Role of Chemokines in the Endometrium and in Embryo Implantation. Curr. Opin. Obstet. Gynecol. 2007, 19, 266–272. [Google Scholar] [CrossRef]
- Spence, T.; Allsopp, P.J.; Yeates, A.J.; Mulhern, M.S.; Strain, J.J.; McSorley, E.M. Maternal Serum Cytokine Concentrations in Healthy Pregnancy and Preeclampsia. J. Pregnancy 2021, 2021, 6649608. [Google Scholar] [CrossRef]
- Bonecchi, R.; Facchetti, F.; Dusi, S.; Luini, W.; Lissandrini, D.; Simmelink, M.; Locati, M.; Bernasconi, S.; Allavena, P.; Brandt, E.; et al. Induction of Functional IL-8 Receptors by IL-4 and IL-13 in Human Monocytes. J. Immunol. 2000, 164, 3862–3869. [Google Scholar] [CrossRef]
- Osawa, Y.; Nagaki, M.; Banno, Y.; Brenner, D.A.; Asano, T.; Nozawa, Y.; Moriwaki, H.; Nakashima, S. Tumor Necrosis Factor Alpha-Induced Interleukin-8 Production via NF-ΚB and Phosphatidylinositol 3-Kinase/Akt Pathways Inhibits Cell Apoptosis in Human Hepatocytes. Infect. Immun. 2002, 70, 6294–6301. [Google Scholar] [CrossRef]
- Ortega, M.A.; Asúnsolo, Á.; Álvarez-Rocha, M.J.; Romero, B.; de León-Luis, J.; Álvarez-Mon, M.; Buján, J.; García-Honduvilla, N. Remodelling of Collagen Fibres in the Placentas of Women with Venous Insufficiency during Pregnancy. Histol. Histopathol. 2018, 33, 567–576. [Google Scholar] [CrossRef]
- Huang, S.J.; Chen, C.P.; Buchwalder, L.; Yu, Y.C.; Piao, L.; Huang, C.Y.; Schatz, F.; Lockwood, C.J. Regulation of CX3CL1 Expression in Human First-Trimester Decidual Cells: Implications for Preeclampsia. Reprod. Sci. 2019, 26, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Usta, A.; Turan, G.; Sancakli Usta, C.; Avci, E.; Adali, E. Placental Fractalkine Immunoreactivity in Preeclampsia and Its Correlation with Histopathological Changes in the Placenta and Adverse Pregnancy Outcomes. J. Matern. Fetal Neonatal Med. 2020, 33, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, G.; Pyzlak, M.; Pankiewicz, K.; Szczerba, E.; Stangret, A.; Szukiewicz, D.; Skoda, M.; Bierła, J.; Cukrowska, B.; Fijałkowska, A. The Potential Association between a New Angiogenic Marker Fractalkine and a Placental Vascularization in Preeclampsia. Arch. Gynecol. Obs. 2021, 304, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Szukiewicz, D.; Kochanowski, J.; Pyzlak, M.; Szewczyk, G.; Stangret, A.; Mittal, T.K. Fractalkine (CX3CL1) and Its Receptor CX3CR1 May Contribute to Increased Angiogenesis in Diabetic Placenta. Mediat. Inflamm. 2013, 2013, 437576. [Google Scholar] [CrossRef] [PubMed]
- Du, M.R.; Wang, S.C.; Li, D.J. The Integrative Roles of Chemokines at the Maternal-Fetal Interface in Early Pregnancy. Cell. Mol. Immunol. 2014, 11, 438–448. [Google Scholar] [CrossRef]
- Chaisavaneeyakorn, S.; Moore, J.M.; Mirel, L.; Othoro, C.; Otieno, J.; Chaiyaroj, S.C.; Shi, Y.P.; Nahlen, B.L.; Lal, A.A.; Udhayakumar, V. Levels of Macrophage Inflammatory Protein 1α-(MIP-1α) and MIP-1β in Intervillous Blood Plasma Samples from Women with Placental Malaria and Human Immunodeficiency Virus Infection. Clin. Diagn. Lab. Immunol. 2003, 10, 631–636. [Google Scholar] [CrossRef]
- Hannan, N.J.; Jones, R.L.; White, C.A.; Salamonsen, L.A. The Chemokines, CX3CL1, CCL14, and CCL4, Promote Human Trophoblast Migration at the Feto-Maternal Interface. Biol. Reprod. 2006, 74, 896–904. [Google Scholar] [CrossRef]
- Schutyser, E.; Struyf, S.; Van Damme, J. The CC Chemokine CCL20 and Its Receptor CCR6. Cytokine Growth Factor Rev. 2003, 14, 409–426. [Google Scholar] [CrossRef]
- Li, Q.; Laumonnier, Y.; Syrovets, T.; Simmet, T. Recruitment of CCR6-Expressing Th17 Cells by CCL20 Secreted from Plasmin-Stimulated Macrophages. Acta Biochim. Biophys. Sin. 2013, 45, 593–600. [Google Scholar] [CrossRef]
- Hamill, N.; Romero, R.; Gotsch, F.; Pedro Kusanovic, J.; Edwin, S.; Erez, O.; Gabor Than, N.; Mittal, P.; Espinoza, J.; Friel, L.A.; et al. Exodus-1 (CCL20): Evidence for the Participation of This Chemokine in Spontaneous Labor at Term, Preterm Labor, and Intrauterine Infection. J. Perinat. Med. 2008, 36, 217–227. [Google Scholar] [CrossRef]
| Significantly Altered Cytokines | ||||||
|---|---|---|---|---|---|---|
| Cytokines | Original Designation | Abbreviatures | Targets and Functions | Pregnancy-Induced CVD | Previous Studies and Possible Implications | References |
| Interleukins | Interleukin-6 | IL-6 | A major proinflammatory cytokine. Synergic effects with TNF-α. B-cell differentiation and stimulation of acute phase proteins. | PW: ↑ ** NB: - | Increased maternal IL-6 levels have been related to the development and severity of different pregnancy-associated complications. High levels of IL-6 in the umbilical cord have been associated with the requirement of oxygen at 36 weeks of post-menstrual age in small for gestational age newborns. | [44,45,48] |
| Interleukin-12 | IL-12 | Involved in pathogenic Th1 responses and IFN-γ production. It causes inhibition of IL-2 and interferon gamma. | PW: ↑ *** NB: ↑ *** | High IL-12 and low IFN-γ were observed in mononuclear cord blood cells. Th2-type response has been associated with pregnancy complications such as recurrent spontaneous abortion, obstetric complications, and poor pregnancy outcomes. | [41,62,64] | |
| Interleukin-10 | IL-10 | Anti-inflammatory cytokine Diminish Th1 responses and induce T reg activity. | PW: ↓ * NB: ↓ * | IL-10 and IL-4 reduction is associated with a plethora of pregnancy-related disorders, including infertility, spontaneous abortion, preterm birth, fetal growth restriction, pre-eclampsia, gestational hypertension | [68,69] | |
| Interleukin-13 | IL-13 | Anti-inflammatory effects acting synergically with IL-4 to promote Th2 responses | PW: ↓ *** NB: ↓ *** | Maternal levels of IL-4 and IL-13 were directly correlated with a decreased risk of NB for developing overweight in 1–2 years old | [73] | |
| Interleukin-2 | IL-2 | Pleiotropic effects on multiple immune populations. At high levels, it induces Th1 responses | PW: ↑ *** NB: ↑ *** | IL-2 dysregulation may negatively affect Treg expansion during pregnancy. Increased levels of IL-2 have been related to higher NK cytotoxicity, which has been proposed as a risk factor for human recurrent abortions. | [59,79] | |
| Interleukin-7 | IL-7 | Involved in T-cell development and homeostasis. B-cell proliferation. | PW: ↑ *** NB: ↑ *** | During pregnancy, IL-7 promotes an aberrant Th17 response with Treg reductions. Also, IL-7 could affect fetal neurons producing cortical and behavioral abnormalities. | [80] | |
| Interleukin-4 | IL-4 | Anti-inflammatory effects. IL-4 is a central inductor of Th2 responses and Th1 inhibition | PW: ↓ *** NB: ↓ * | IL-10 and IL-4 reduction are associated with a plethora of pregnancy-related disorders, including infertility, spontaneous miscarriage, preterm birth, fetal growth restriction, pre-eclampsia, gestational hypertension. Low maternal levels of IL-4 have been positively correlated with an increased risk of NB for developing overweight during childhood. | [69,73] | |
| Interleukin-5 | IL-5 | Together with GM-CSF and IL-3, they are “eosinopoietins” because of their ability to induce eosinophils proliferation and activation | PW: ↑ *** NB: ↑ ** | An altered eosinophilic activity might be a clinical risk of preterm labor In utero exposition to IL-5 result in fetal eosinophilia and is a developmental origin of airway hyperreactivity | [87,88] | |
| Interleukin-17A | IL-17A | Along with IL-23, it mediates Th17 responses. Involved in the development of many inflammatory diseases | PW: ↑ *** NB: ↑ *** | Studies in women with pre-eclampsia show increased IL-17A levels alone or in combination with IL-23 | [76,77,78] | |
| Interleukin-21 | IL-21 | Inductor of Th17 responses | PW: - NB: ↑ * | In cord blood cells, it may induce IL-10 production | [82] | |
| Interleukin-23 | IL-23 | Along with IL-17A, it mediates Th17 responses. | PW: ↑ *** NB: ↑ *** | Studies in women with pre-eclampsia show increased IL-17A in combination with IL-23 | [76] | |
| Tumor necrosis factor | Tumor necrosis factor-α | TNF-α | Proinflammatory cytokine that coordinates Th1 responses | PW: ↑ * NB: ↑ *** | High levels of TNF-α alone or with increased IL-6 and low IL-10 are related to pregnancy hypertensive disorders and other complications. | [24,47,51,52] |
| Interferons | Type II interferon gamma | IFN-γ | Proinflammatory cytokine that coordinates Th1 responses | PW: ↓ *** NB: ↓ *** | Low IFN-γ levels were detected in women with pre-eclampsia and blood cord despite high IL-12 levels. | [64,65] |
| Colony-stimulating factors | Granulocyte-macrophage colony-stimulating factor or colony-stimulating factor 2 | GM-CSF (CSF-2) | Participates in Th1 and Th17 responses Together with IL-5 and IL-3, they are “eosinopoietins” because of their ability to induce eosinophils proliferation and activation | PW: ↑ * NB: ↑ ** | Reduced levels of this cytokine were related to recurrent miscarriage, placental dysfunction, and abnormal fetal growth. Increased levels of this cytokine might be implicated in the pathogenesis of pre-eclampsia. An altered eosinophilic activity might be a clinical risk of preterm labor | [62,87,96,97] |
| Chemokines | Fractalkine or chemokine (C-X3-C motif) ligand 1 | CX3CL1 | Chemoatractive properties. Upregulated by hypoxia | PW: ↑ *** NB: ↑ ** | Overexpression of this cytokine is related to poor pregnancy outcomes such as pre-eclampsia and gestational diabetes | [108,110] |
| Chemokine (CXC motif) ligand-8 or Interleukin-8 | CXCL8 (IL-8) | Neutrophils recruitment. Involved in Th1 responses and inhibited by Th2 cytokines (IL-4 and IL-13) | PW: ↑ NB: ↑ | Some studies have found a positive correlation between maternal IL-8 levels and the risk of mental disorders in adulthood offspring. IL-8 induces matrix remodeling in placental tissue. Elevated levels of cord blood IL-8 have been associated with pre-eclampsia and moderate-severe bronchopulmonary dysplasia in newborns | [48,101,103] | |
| Macrophage inflammatory protein-1β or Chemokine (C-C motif) ligand 4 | MIP-1β (CCL4) | Chemoattractive molecule of T lymphocites, dendritic cells, monocytes, and NKs; HIV correceptor | PW: ↑ *** NB: ↑ *** | Increased levels of this molecule appear to be indicative of active infections during pregnancy. Together with fractalkine, it is a central component in maternal-fetal dialogue | [112,113] | |
| Macrophage inflammatory protein-3α or chemokine (C-C motif) ligand 20 | MIP-3α (CCL20) | Chemotactic and antimicrobial activity; associated with Th17 polarization and inflammation | PW: ↑ *** NB: ↑ *** | The presence of this cytokine in the amniotic fluid is a marker of infection or inflammation affecting the amniotic cavity. It remains to be elucidated is correlation with serum levels | [111,116] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, M.A.; Gómez-Lahoz, A.M.; Sánchez-Trujillo, L.; Fraile-Martinez, O.; García-Montero, C.; Guijarro, L.G.; Bravo, C.; De Leon-Luis, J.A.; Saz, J.V.; Bujan, J.; et al. Chronic Venous Disease during Pregnancy Causes a Systematic Increase in Maternal and Fetal Proinflammatory Markers. Int. J. Mol. Sci. 2022, 23, 8976. https://doi.org/10.3390/ijms23168976
Ortega MA, Gómez-Lahoz AM, Sánchez-Trujillo L, Fraile-Martinez O, García-Montero C, Guijarro LG, Bravo C, De Leon-Luis JA, Saz JV, Bujan J, et al. Chronic Venous Disease during Pregnancy Causes a Systematic Increase in Maternal and Fetal Proinflammatory Markers. International Journal of Molecular Sciences. 2022; 23(16):8976. https://doi.org/10.3390/ijms23168976
Chicago/Turabian StyleOrtega, Miguel A., Ana M. Gómez-Lahoz, Lara Sánchez-Trujillo, Oscar Fraile-Martinez, Cielo García-Montero, Luis G. Guijarro, Coral Bravo, Juan A. De Leon-Luis, Jose V. Saz, Julia Bujan, and et al. 2022. "Chronic Venous Disease during Pregnancy Causes a Systematic Increase in Maternal and Fetal Proinflammatory Markers" International Journal of Molecular Sciences 23, no. 16: 8976. https://doi.org/10.3390/ijms23168976
APA StyleOrtega, M. A., Gómez-Lahoz, A. M., Sánchez-Trujillo, L., Fraile-Martinez, O., García-Montero, C., Guijarro, L. G., Bravo, C., De Leon-Luis, J. A., Saz, J. V., Bujan, J., García-Honduvilla, N., Monserrat, J., & Alvarez-Mon, M. (2022). Chronic Venous Disease during Pregnancy Causes a Systematic Increase in Maternal and Fetal Proinflammatory Markers. International Journal of Molecular Sciences, 23(16), 8976. https://doi.org/10.3390/ijms23168976













