Activation of cAMP Signaling in Response to α-Phellandrene Promotes Vascular Endothelial Growth Factor Levels and Proliferation in Human Dermal Papilla Cells
Abstract
:1. Introduction
2. Results
2.1. α-Phellandrene Increases cAMP Levels in DPCs
2.2. α-Phellandrene Promotes DPC Proliferation
2.3. cAMP Accumulation by α-Phellandrene Leads to the Stimulation of Downstream PKA/CREB Signaling in DPCs
2.4. α-Phellandrene Promotes Cell Proliferation via the cAMP Signaling Pathway
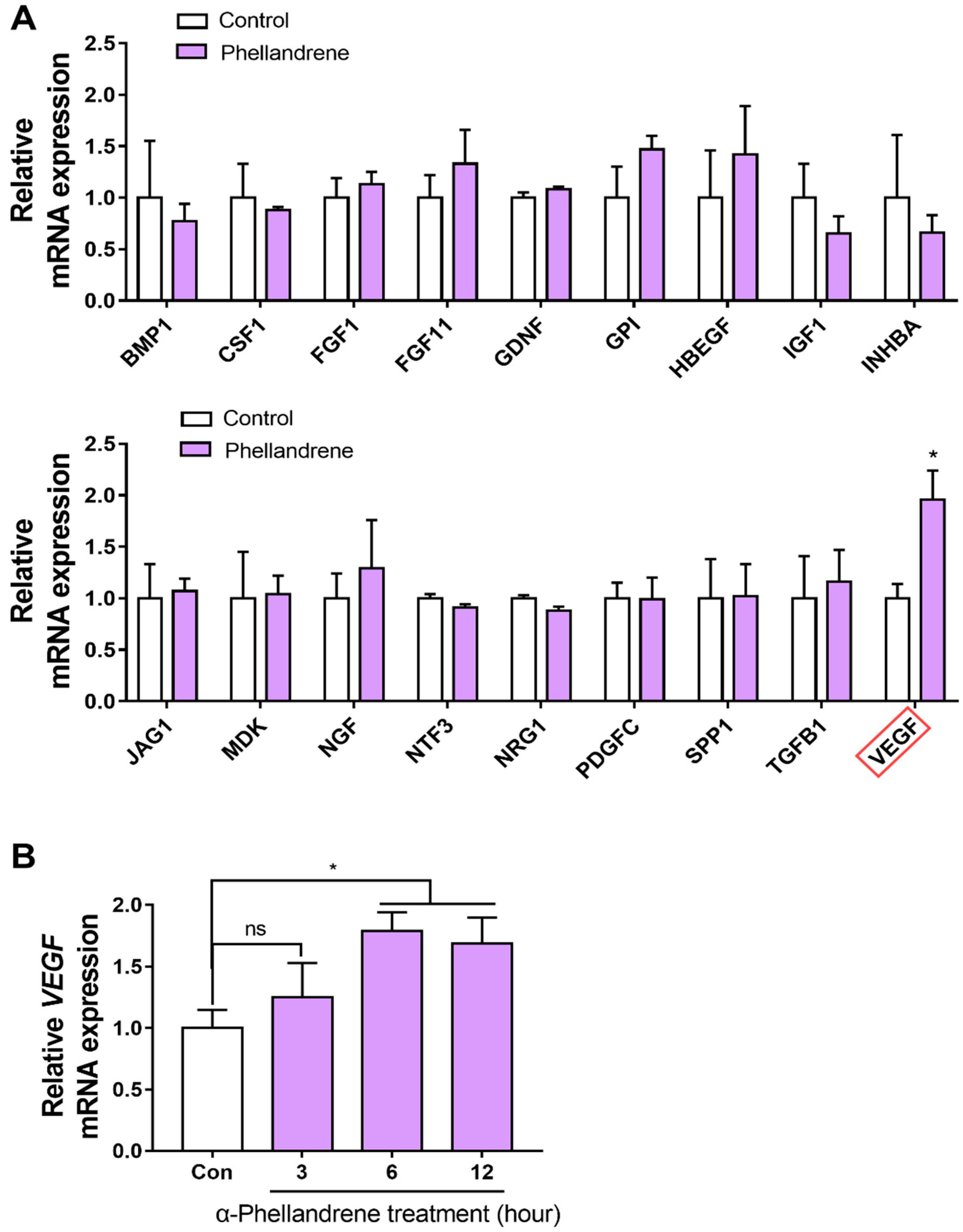
2.5. α-Phellandrene Increases VEGF Gene Expression in DPCs
2.6. The Inhibition of the cAMP Pathway Blocks the Effect of α-Phellandrene on VEGF Production
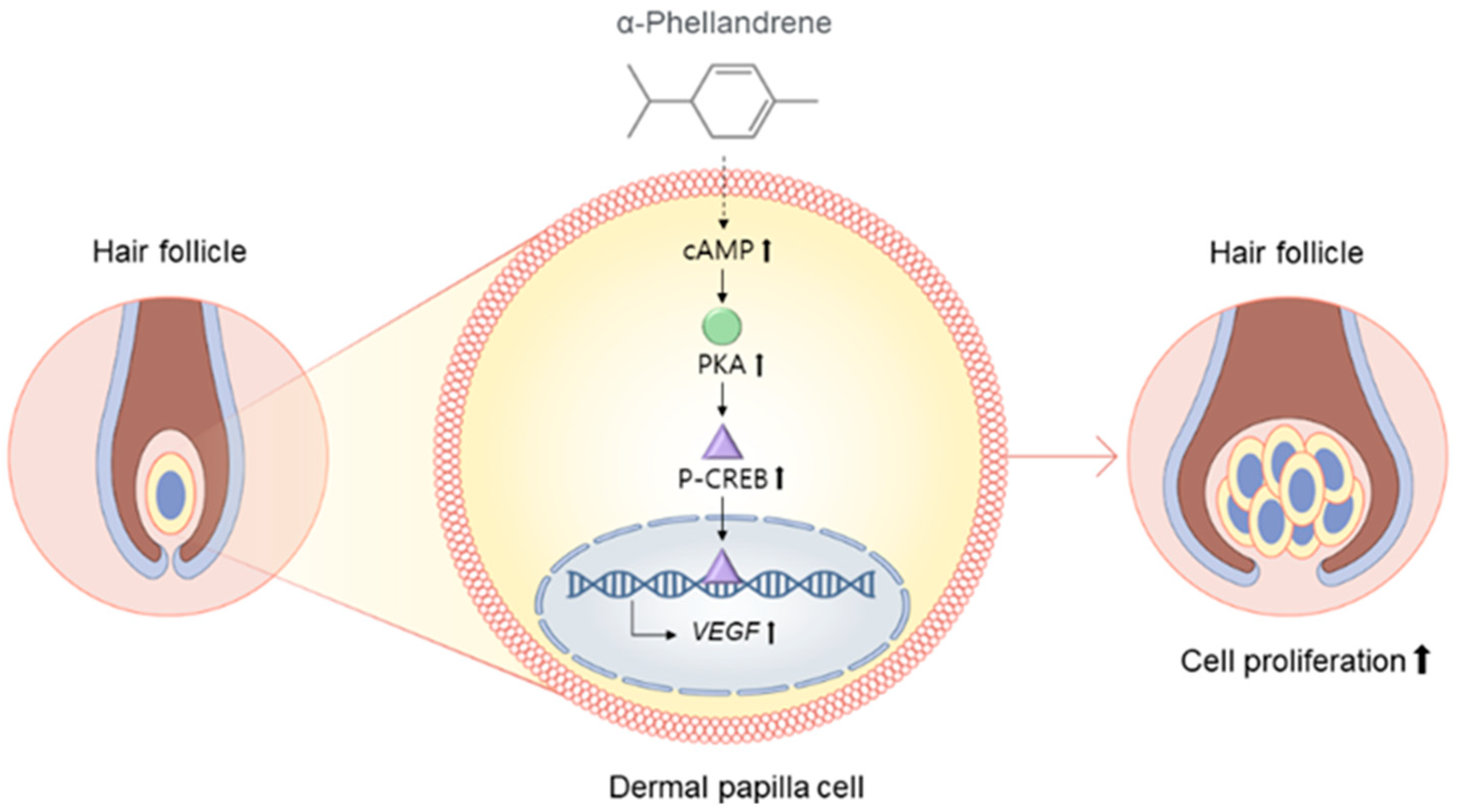
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. cAMP Measurement
4.3. WST-1 Assay
4.4. Lactate Dehydrogenase (LDH) Assay
4.5. RNA Isolation, Complementary DNA (cDNA) Synthesis, and Quantitative Polymerase Chain Reaction (qPCR)
4.6. Western Blot
4.7. Quantification of VEGF Levels
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Driskell, R.R.; Clavel, C.; Rendl, M.; Watt, F.M. Hair follicle dermal papilla cells at a glance. J. Cell Sci. 2011, 124, 1179–1182. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.L.; Gangadaran, P.; Bak, S.S.; Oh, J.M.; Kalimuthu, S.; Lee, H.W.; Baek, S.H.; Zhu, L.; Sung, Y.K.; Jeong, S.Y. Extracellular vesicles derived from MSCs activates dermal papilla cell in vitro and promotes hair follicle conversion from telogen to anagen in mice. Sci. Rep. 2017, 7, 15560. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Kobayashi, K.; Hama, T.; Murakami, K.; Ogawa, R. Standardized scalp massage results in increased hair thickness by inducing stretching forces to dermal papilla cells in the subcutaneous tissue. Eplasty 2016, 16, e8. [Google Scholar]
- Itami, S.; Kurata, S.; Takayasu, S. Androgen induction of follicular epithelial cell growth is mediated via insulin-like growth factor-I from dermal papilla cells. Biochem. Biophys. Res. Commun. 1995, 212, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Nan, W.; Wang, S.; Zhang, T.; Si, H.; Wang, D.; Yang, F.; Li, G. Epidermal growth factor promotes proliferation of dermal papilla cells via Notch signaling pathway. Biochimie 2016, 127, 10–18. [Google Scholar] [CrossRef]
- Iino, M.; Ehama, R.; Nakazawa, Y.; Iwabuchi, T.; Ogo, M.; Tajima, M.; Arase, S. Adenosine stimulates fibroblast growth factor-7 gene expression via adenosine A2b receptor signaling in dermal papilla cells. J. Investig. Dermatol. 2007, 127, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Ami, S.; Rata, S.; Noda, T.; Kayasu, S. Interaction between dermal papilla cells and follicular epithelial cells in vitro: Effect of androgen. Br. J. Dermatol. 1995, 132, 527–532. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Gao, Y.; Liu, X.; Bai, T.; Li, M.; Li, L.; Chi, G.; Xu, H.; Liu, F. Maintenance of high proliferation and multipotent potential of human hair follicle-derived mesenchymal stem cells by growth factors. Int. J. Mol. Med. 2013, 31, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Lee, M.J.; Lee, W.-Y.; Pyo, J.; Shin, M.-S.; Hwang, G.S.; Shin, D.; Kim, C.E.; Park, E.-S.; Kang, K.S. Hair Growth Stimulation Effect of Centipeda minima Extract: Identification of Active Compounds and Anagen-Activating Signaling Pathways. Biomolecules 2021, 11, 976. [Google Scholar] [CrossRef] [PubMed]
- Madaan, A.; Verma, R.; Singh, A.T.; Jaggi, M. Review of hair follicle dermal papilla cells as in vitro screening model for hair growth. Int. J. Cosmet. Sci. 2018, 40, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Bassino, E.; Gasparri, F.; Giannini, V.; Munaron, L. Paracrine crosstalk between human hair follicle dermal papilla cells and microvascular endothelial cells. Exp. Dermatol. 2015, 24, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.; Lee, S.; Kim, S.; Park, D.; Jung, E. Effect of sinapic acid on hair growth promoting in human hair follicle dermal papilla cells via Akt activation. Arch. Dermatol. Res. 2017, 309, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Kang, J.-I.; Hyun, J.W.; Koh, Y.S.; Kang, J.-H.; Hyun, C.-G.; Yoon, K.-S.; Lee, K.S.; Lee, C.M.; Kim, T.Y. Myristoleic Acid Promotes Anagen Signaling by Autophagy through Activating Wnt/β-Catenin and ERK Pathways in Dermal Papilla Cells. Biomol. Ther. 2021, 29, 211. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-I.; Choi, Y.K.; Koh, Y.-S.; Hyun, J.-W.; Kang, J.-H.; Lee, K.S.; Lee, C.M.; Yoo, E.-S.; Kang, H.-K. Vanillic Acid Stimulates Anagen Signaling via the PI3K/Akt/β-Catenin Pathway in Dermal Papilla Cells. Biomol. Ther. 2020, 28, 354. [Google Scholar] [CrossRef] [PubMed]
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schiöth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017, 16, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Hauser, A.S.; Chavali, S.; Masuho, I.; Jahn, L.J.; Martemyanov, K.A.; Gloriam, D.E.; Babu, M.M. Pharmacogenomics of GPCR drug targets. Cell 2018, 172, 41–54. [Google Scholar] [CrossRef]
- Fredriksson, J.M.; Lindquist, J.M.; Bronnikov, G.E.; Nedergaard, J. Norepinephrine induces vascular endothelial growth factor gene expression in brown adipocytes through a β-adrenoreceptor/cAMP/protein kinase A pathway involving Src but independently of Erk1/2. J. Biol. Chem. 2000, 275, 13802–13811. [Google Scholar] [CrossRef]
- Nuamnaichati, N.; Sato, V.H.; Moongkarndi, P.; Parichatikanond, W.; Mangmool, S. Sustained β-AR stimulation induces synthesis and secretion of growth factors in cardiac myocytes that affect on cardiac fibroblast activation. Life Sci. 2018, 193, 257–269. [Google Scholar] [CrossRef]
- Verhoeckx, K.C.; Doornbos, R.P.; Witkamp, R.F.; van der Greef, J.; Rodenburg, R.J. Beta-adrenergic receptor agonists induce the release of granulocyte chemotactic protein-2, oncostatin M, and vascular endothelial growth factor from macrophages. Int. Immunopharmacol. 2006, 6, 419636. [Google Scholar] [CrossRef]
- Park, S.; Kang, W.; Choi, D.; Son, B.; Park, T. Nonanal Stimulates Growth Factors via Cyclic Adenosine Monophosphate (cAMP) Signaling in Human Hair Follicle Dermal Papilla Cells. Int. J. Mol. Sci. 2020, 21, 8054. [Google Scholar] [CrossRef]
- Tong, T.; Kim, M.; Park, T. α-Cedrene, a Newly Identified Ligand of MOR23, Increases Skeletal Muscle Mass and Strength. Mol. Nutr. Food Res. 2018, 62, 1800173. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yu, L.; Fan, J.; Wan, B.; Jiang, T.; Yin, J.; Huang, Y.; Li, Q.; Yin, G.; Hu, Z. Endogenous parathyroid hormone promotes fracture healing by increasing expression of BMPR2 through cAMP/PKA/CREB pathway in mice. Cell. Physiol. Biochem. 2017, 42, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-H.; Chae, B.-C.; Kim, H.-A.; Seo, G.-Y.; Seo, D.-W.; Chun, G.-T.; Yie, S.-W.; Eom, S.-H.; Kim, P.-H. The PKA/CREB pathway is closely involved in VEGF expression in mouse macrophages. Mol. Cells (Springer Sci. Bus. Media BV) 2007, 23, 23–29. [Google Scholar]
- Saraswat, A.; Kumar, B. Minoxidil vs. finasteride in the treatment of men with androgenetic alopecia. Arch. Dermatol. 2003, 139, 1219–1221. [Google Scholar] [CrossRef]
- Adil, A.; Godwin, M. The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2017, 77, 136–141. [Google Scholar] [CrossRef]
- Hu, R.; Xu, F.; Sheng, Y.; Qi, S.; Han, Y.; Miao, Y.; Rui, W.; Yang, Q. Combined treatment with oral finasteride and topical minoxidil in male androgenetic alopecia: A randomized and comparative study in Chinese patients. Dermatol. Ther. 2015, 28, 303–308. [Google Scholar] [CrossRef]
- Lima, D.F.; Brandão, M.S.; Moura, J.B.; Leitão, J.M.; Carvalho, F.A.; Miúra, L.M.; Leite, J.R.; Sousa, D.P.; Almeida, F.R. Antinociceptive activity of the monoterpene α-phellandrene in rodents: Possible mechanisms of action. J. Pharm. Pharmacol. 2012, 64, 283–292. [Google Scholar] [CrossRef]
- Siqueira, H.D.A.S.; Neto, B.S.; Sousa, D.P.; Gomes, B.S.; da Silva, F.V.; Cunha, F.V.; Wanderley, C.W.; Pinheiro, G.; Cândido, A.G.; Wong, D.V. α-Phellandrene, a cyclic monoterpene, attenuates inflammatory response through neutrophil migration inhibition and mast cell degranulation. Life Sci. 2016, 160, 27–33. [Google Scholar] [CrossRef]
- İşcan, G.; Kırımer, N.; Demirci, F.; Demirci, B.; Noma, Y.; Başer, K.H.C. Biotransformation of (−)-(R)-α-phellandrene: Antimicrobial activity of its major metabolite. Chem. Biodivers. 2012, 9, 1525–1532. [Google Scholar] [CrossRef]
- De Christo Scherer, M.M.; Marques, F.M.; Figueira, M.M.; Peisino, M.C.O.; Schmitt, E.F.P.; Kondratyuk, T.P.; Endringer, D.C.; Scherer, R.; Fronza, M. Wound healing activity of terpinolene and α-phellandrene by attenuating inflammation and oxidative stress in vitro. J. Tissue Viability 2019, 28, 94–99. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, W.; Zhang, H.-M.; Xie, G.-Y.; Miao, Y.-R.; Xia, M.; Guo, A.-Y. hTFtarget: A comprehensive database for regulations of human transcription factors and their targets. Genom. Proteom. Bioinform. 2020, 18, 120–128. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Halmos, T.; Suba, I. The physiological role of growth hormone and insulin-like growth factors. Orv. Hetil. 2019, 160, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Freitas, I.; Owen, B.M. Metabolic roles of endocrine fibroblast growth factors. Curr. Opin. Pharmacol. 2015, 25, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Goodman, L.V.; Ledbetter, S.R. Secretion of stromelysin by cultured dermal papilla cells: Differential regulation by growth factors and functional role in mitogen-induced cell proliferation. J. Cell. Physiol. 1992, 151, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Heyneman, C.A.; Lawless-Liday, C.; Wall, G.C. Oral versus topical NSAIDs in rheumatic diseases. Drugs 2000, 60, 555–574. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.-Y.; Liu, P.-F.; Huang, C.-M. Decreasing systemic toxicity via transdermal delivery of anticancer drugs. Curr. Drug Metab. 2008, 9, 592–597. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.D.; Meinardi, M.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. Viewp. 2000, 9, 165–169. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Choi, D.; Park, T. Decanal Protects against UVB-Induced Photoaging in Human Dermal Fibroblasts via the cAMP Pathway. Nutrients 2020, 12, 1214. [Google Scholar] [CrossRef]
- Vilardaga, J.-P.; Jean-Alphonse, F.G.; Gardella, T.J. Endosomal generation of cAMP in GPCR signaling. Nat. Chem. Biol. 2014, 10, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Filardo, E.J.; Quinn, J.A.; Frackelton, A.R., Jr.; Bland, K.I. Estrogen action via the G protein-coupled receptor, GPR30: Stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol. Endocrinol. 2002, 16, 70–84. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Awale, M.; Reymond, J.-L. The polypharmacology browser: A web-based multi-fingerprint target prediction tool using ChEMBL bioactivity data. J. Chem. 2017, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Awale, M.; Reymond, J.-L. Polypharmacology browser PPB2: Target prediction combining nearest neighbors with machine learning. J. Chem. Inf. Model. 2018, 59, 10–17. [Google Scholar] [CrossRef]
- Grant, M.B.; Davis, M.I.; Caballero, S.; Feoktistov, I.; Biaggioni, I.; Belardinelli, L. Proliferation, migration, and ERK activation in human retinal endothelial cells through A2B adenosine receptor stimulation. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2068–2073. [Google Scholar]
- Turner, N.A.; Porter, K.E.; Smith, W.H.; White, H.L.; Ball, S.G.; Balmforth, A.J. Chronic β2-adrenergic receptor stimulation increases proliferation of human cardiac fibroblasts via an autocrine mechanism. Cardiovasc. Res. 2003, 57, 784–792. [Google Scholar] [CrossRef]
- Coronas, V.; Bantubungi, K.; Fombonne, J.; Krantic, S.; Schiffmann, S.N.; Roger, M. Dopamine D3 receptor stimulation promotes the proliferation of cells derived from the post-natal subventricular zone. J. Neurochem. 2004, 91, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, U.; Patel, H.H.; Lai, N.C.; Aroonsakool, N.; Roth, D.M.; Insel, P.A. The cyclic AMP effector Epac integrates pro- and anti-fibrotic signals. Proc. Natl. Acad. Sci. USA 2008, 105, 6386–6391. [Google Scholar] [CrossRef] [PubMed]
- Perez-Aso, M.; Fernandez, P.; Mediero, A.; Chan, E.S.; Cronstein, B.N. Adenosine 2A receptor promotes collagen production by human fibroblasts via pathways involving cyclic AMP and AKT but independent of Smad2/3. FASEB J. 2014, 28, 802–812. [Google Scholar] [CrossRef]
- Parker, D.; Ferreri, K.; Nakajima, T.; LaMorte, V.; Evans, R.; Koerber, S.; Hoeger, C.; Montminy, M. Phosphorylation of CREB at Ser-133 induces complex formation with CREB-binding protein via a direct mechanism. Mol. Cell. Biol. 1996, 16, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Kasper, L.H.; Lerach, S.; Jeevan, T.; Brindle, P.K. Individual CREB-target genes dictate usage of distinct cAMP-responsive coactivation mechanisms. EMBO J. 2007, 26, 2890–2903. [Google Scholar] [CrossRef] [PubMed]
- Siu, Y.T.; Jin, D.Y. CREB− a real culprit in oncogenesis. FEBS J. 2007, 274, 3224–3232. [Google Scholar] [CrossRef] [PubMed]
- Cha-Molstad, H.; Keller, D.M.; Yochum, G.S.; Impey, S.; Goodman, R.H. Cell-type-specific binding of the transcription factor CREB to the cAMP-response element. Proc. Natl. Acad. Sci. USA 2004, 101, 13572–13577. [Google Scholar] [CrossRef] [PubMed]
- Martianov, I.; Choukrallah, M.-A.; Krebs, A.; Ye, T.; Legras, S.; Rijkers, E.; Van Ijcken, W.; Jost, B.; Sassone-Corsi, P.; Davidson, I. Cell-specific occupancy of an extended repertoire of CREM and CREB binding loci in male germ cells. BMC Genom. 2010, 11, 530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Odom, D.T.; Koo, S.-H.; Conkright, M.D.; Canettieri, G.; Best, J.; Chen, H.; Jenner, R.; Herbolsheimer, E.; Jacobsen, E. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 4459–4464. [Google Scholar] [CrossRef] [PubMed]
- Lachgar, S.; Moukadiri, H.; Jonca, F.; Charveron, M.; Bouhaddioui, N.; Gall, Y.; Bonafe, J.L.; Plouët, J. Vascular endothelial growth factor is an autocrine growth factor for hair dermal papilla cells. J. Investig. Dermatol. 1996, 106, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Brown, L.F.; Detmar, M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J. Clin. Investig. 2001, 107, 409–417. [Google Scholar] [CrossRef]
- Li, W.; Man, X.-Y.; Li, C.-M.; Chen, J.-Q.; Zhou, J.; Cai, S.-Q.; Lu, Z.-F.; Zheng, M. VEGF induces proliferation of human hair follicle dermal papilla cells through VEGFR-2-mediated activation of ERK. Exp. Cell Res. 2012, 318, 1633–1640. [Google Scholar] [CrossRef]
- Lee, E.; Choi, E.J.; Kim, J.; Hwang, Y.; Kim, C.D.; Lee, M.; Roh, S.; Kim, Y.; Han, I.; Kang, S. Malva verticillata seed extracts upregulate the Wnt pathway in human dermal papilla cells. Int. J. Cosmet. Sci. 2016, 38, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Junlatat, J.; Sripanidkulchai, B. Hair Growth-Promoting Effect of Carthamus tinctorius Floret Extract. Phytother. Res. 2014, 28, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Rho, S.-S.; Park, S.-J.; Hwang, S.-L.; Lee, M.-H.; Kim, C.D.; Lee, I.-H.; Chang, S.-Y.; Rang, M.-J. The hair growth promoting effect of Asiasari radix extract and its molecular regulation. J. Dermatol. Sci. 2005, 38, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Messenger, A.; Rundegren, J. Minoxidil: Mechanisms of action on hair growth. Br. J. Dermatol. 2004, 150, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Stork, P.J.; Schmitt, J.M. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol. 2002, 12, 258–266. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Pelling, J.C.; Ramaswamy, N.T.; Eppler, J.W.; Wallace, D.P.; Nagao, S.; Rome, L.A.; Sullivan, L.P.; Grantham, J.J. cAMP stimulates the in vitro proliferation of renal cyst epithelial cells by activating the extracellular signal-regulated kinase pathway. Kidney Int. 2000, 57, 1460–1471. [Google Scholar] [CrossRef] [PubMed]
- Hänel, K.H.; Cornelissen, C.; Lüscher, B.; Baron, J.M. Cytokines and the skin barrier. Int. J. Mol. Sci. 2013, 14, 6720–6745. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, E.; Soto-Heredero, G.; Mittelbrunn, M. The role of extracellular vesicles in cutaneous remodeling and hair follicle dynamics. Int. J. Mol. Sci. 2019, 20, 2758. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, W.; Park, S.; Choi, D.; Son, B.; Park, T. Activation of cAMP Signaling in Response to α-Phellandrene Promotes Vascular Endothelial Growth Factor Levels and Proliferation in Human Dermal Papilla Cells. Int. J. Mol. Sci. 2022, 23, 8959. https://doi.org/10.3390/ijms23168959
Kang W, Park S, Choi D, Son B, Park T. Activation of cAMP Signaling in Response to α-Phellandrene Promotes Vascular Endothelial Growth Factor Levels and Proliferation in Human Dermal Papilla Cells. International Journal of Molecular Sciences. 2022; 23(16):8959. https://doi.org/10.3390/ijms23168959
Chicago/Turabian StyleKang, Wesuk, Soyoon Park, Dabin Choi, Bomin Son, and Taesun Park. 2022. "Activation of cAMP Signaling in Response to α-Phellandrene Promotes Vascular Endothelial Growth Factor Levels and Proliferation in Human Dermal Papilla Cells" International Journal of Molecular Sciences 23, no. 16: 8959. https://doi.org/10.3390/ijms23168959
APA StyleKang, W., Park, S., Choi, D., Son, B., & Park, T. (2022). Activation of cAMP Signaling in Response to α-Phellandrene Promotes Vascular Endothelial Growth Factor Levels and Proliferation in Human Dermal Papilla Cells. International Journal of Molecular Sciences, 23(16), 8959. https://doi.org/10.3390/ijms23168959




