Impacts of Glucose-Dependent Insulinotropic Polypeptide on Orthodontic Tooth Movement-Induced Bone Remodeling
Abstract
:1. Introduction
2. Results
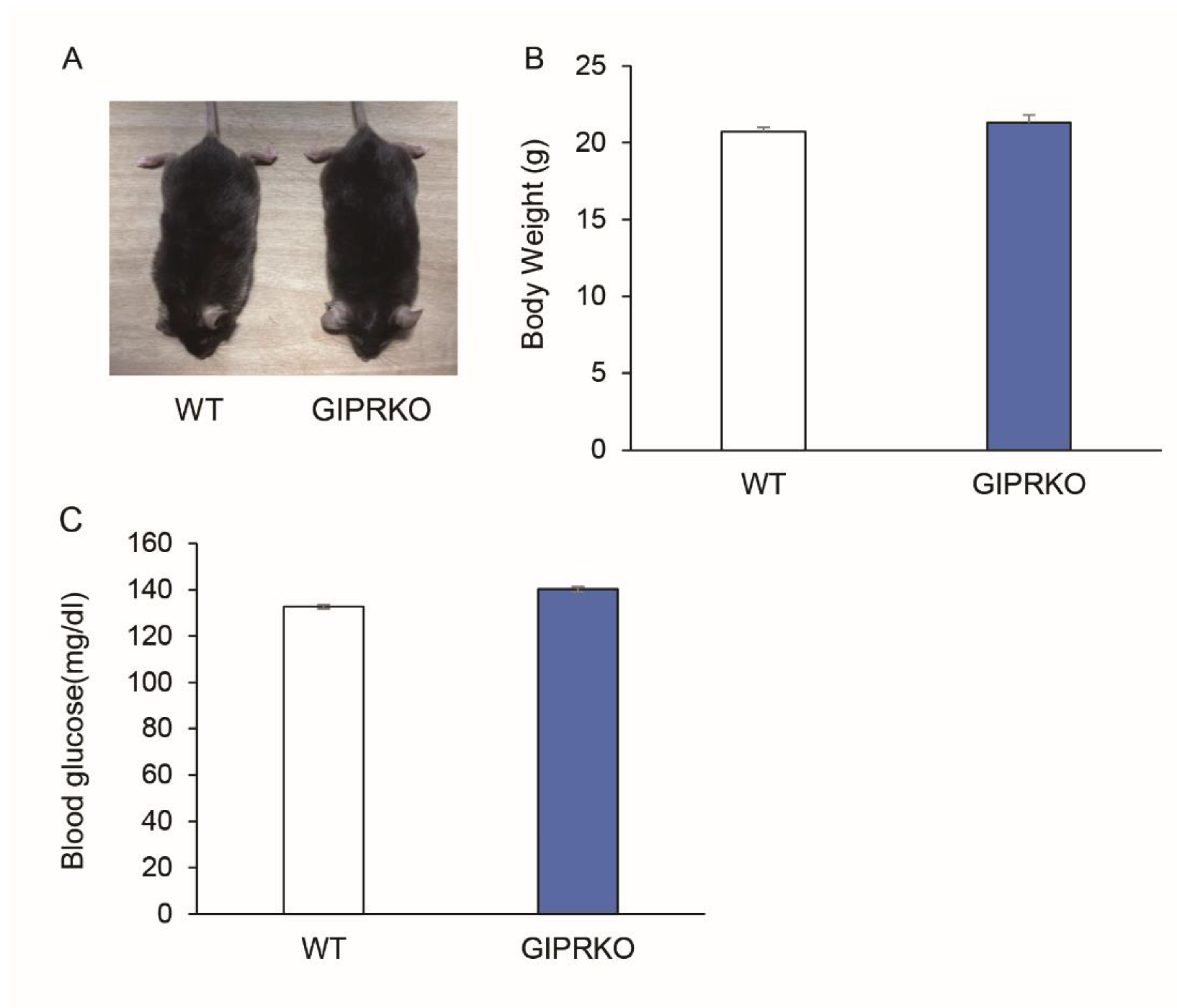
2.1. Animal Characteristics
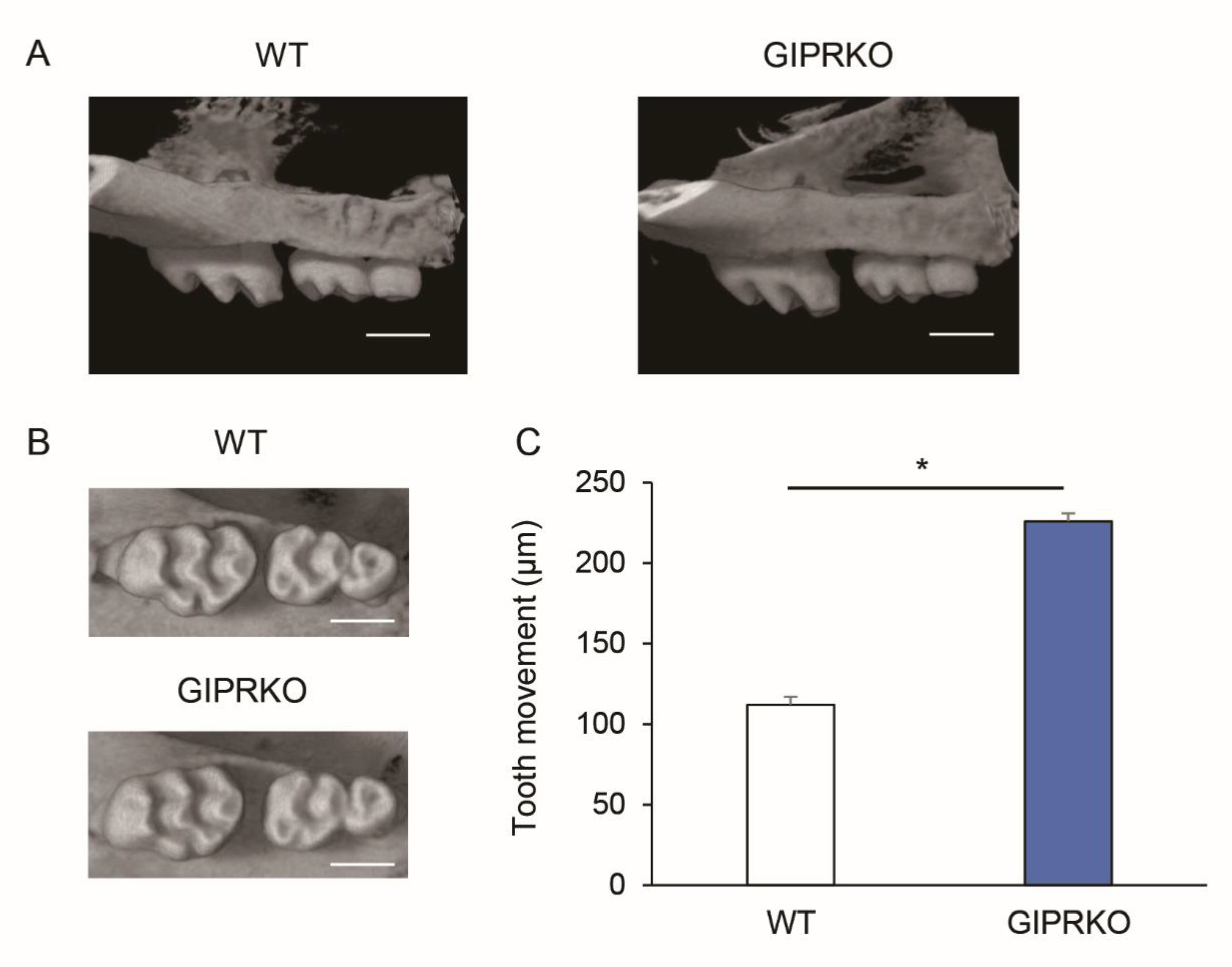
2.2. Evaluation of Orthodontic Tooth Movement
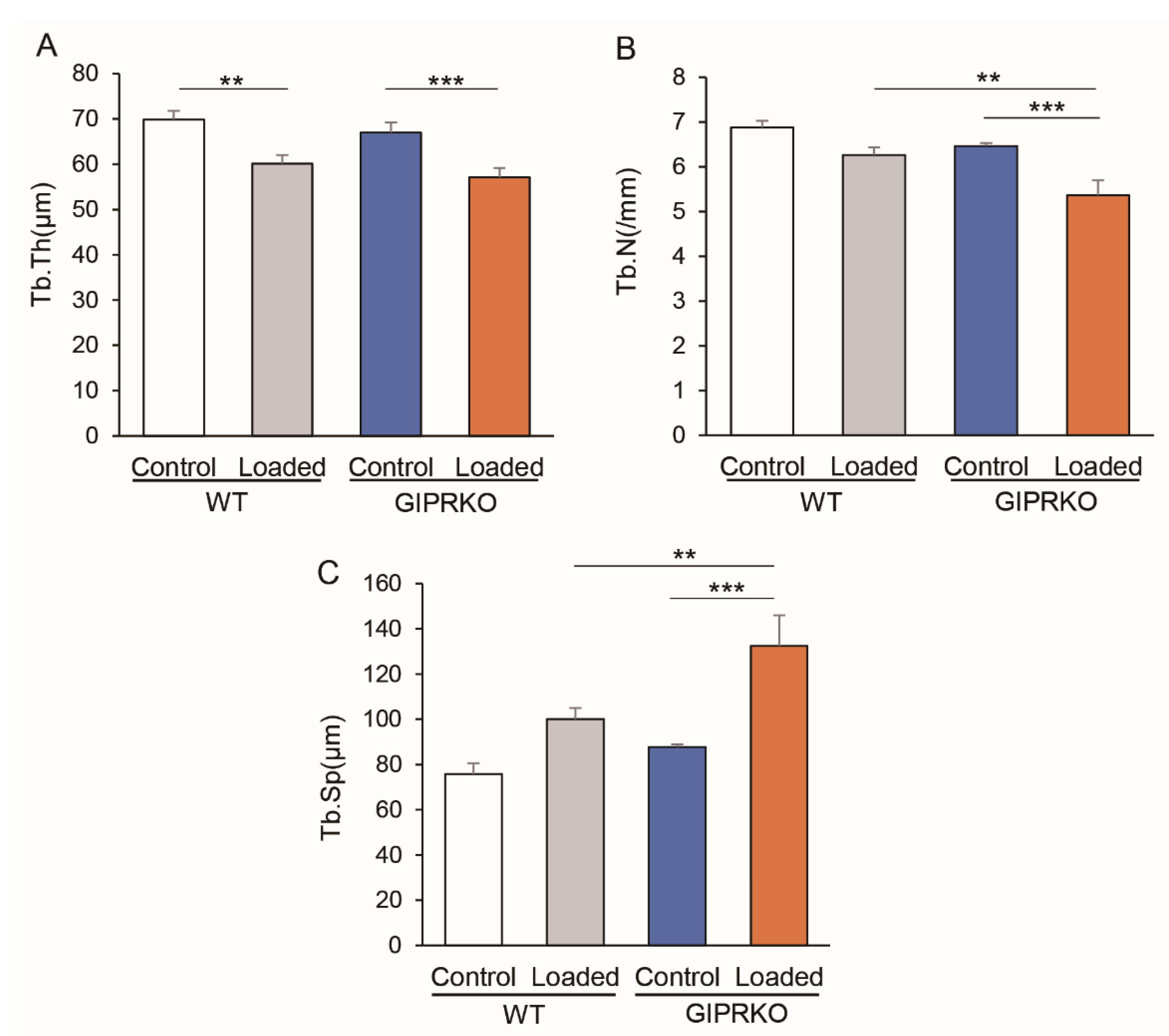
2.3. Residual Alveolar Bone Amount and Trabecular Structure
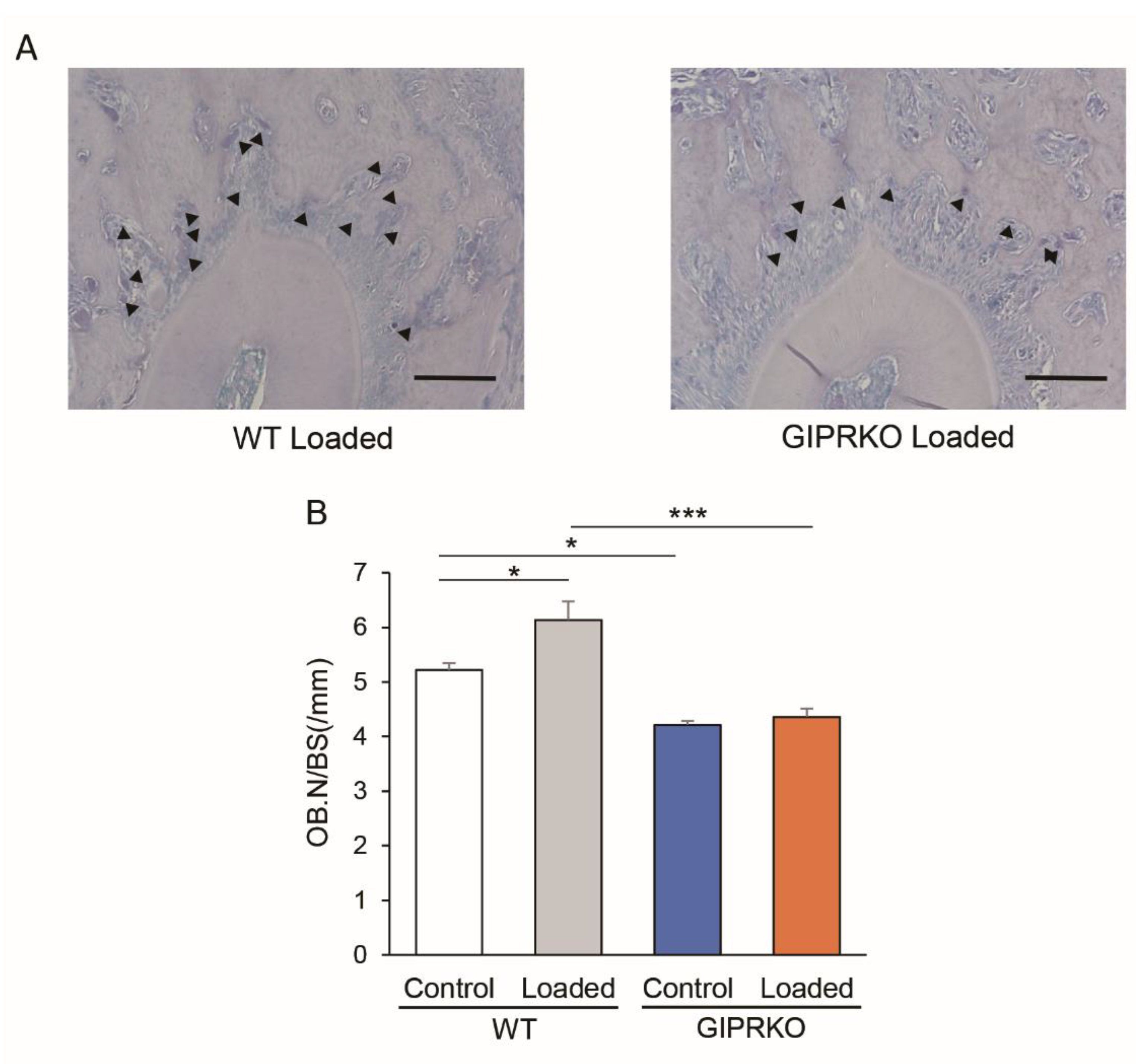
2.4. Osteoclast Formation by Orthodontic Tooth Movement
2.5. Osteoblast Formation by Orthodontic Tooth Movement
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Orthodontic Tooth Movement
4.3. Microcomputer Tomography
4.4. Histopathological Observation
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Datta, H.K.; Ng, W.F.; Walker, J.A.; Tuck, S.P.; Varanasi, S.S. The cell biology of bone metabolism. J. Clin. Pathol. 2008, 61, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, H.; Kimura, K.; Ishida, M.; Sugisawa, H.; Kohara, H.; Yoshimatsu, M.; Takano-Yamamoto, T. Effect of cytokines on osteoclast formation and bone resorption during mechanical force loading of the periodontal membrane. Sci. World J. 2014, 2014, 617032. [Google Scholar] [CrossRef] [PubMed]
- Fuglsang-Nielsen, R.; Rakvaag, E.; Vestergaard, P.; Hartmann, B.; Holst, J.J.; Hermansen, K.; Gregersen, S.; Starup-Linde, J. Consumption of nutrients and insulin resistance suppress markers of bone turnover in subjects with abdominal obesity. Bone 2020, 133, 115230. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Wang, N.; Feng, X.; Bi, L. Periodontal ligament remodeling and alveolar bone resorption during orthodontic tooth movement in rats with diabetes. Diabetes Technol. Ther. 2010, 12, 65–73. [Google Scholar] [CrossRef]
- Reichert, C.; Deschner, J.; Jäger, A. Influence of diabetes mellitus on the development and treatment of malocclusions—A case report with literature review. J. Orofac. Orthop. 2009, 70, 160–175. [Google Scholar] [CrossRef] [PubMed]
- Verna, C.; Dalstra, M.; Melsen, B. The rate and the type of orthodontic tooth movement is influenced by bone turnover in a rat model. Eur. J. Orthod. 2000, 22, 343–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the international diabetes federation diabetes atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- Courties, A.; Sellam, J. Osteoarthritis and type 2 diabetes mellitus: What are the links? Diabetes Res. Clin. Pract. 2016, 122, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Sözen, T.; Başaran, N.; Tınazlı, M.; Özışık, L. Musculoskeletal problems in diabetes mellitus. Eur. J. Rheumatol. 2018, 5, 258–265. [Google Scholar] [CrossRef]
- Williams, M.F.; London, D.A.; Husni, E.M.; Navaneethan, S.; Kashyap, S.R. Type 2 diabetes and osteoarthritis: A systematic review and meta-analysis. J. Diabetes Complicat. 2016, 30, 944–950. [Google Scholar] [CrossRef]
- Inagaki, N.; Seino, Y.; Takeda, J.; Yano, H.; Yamada, Y.; Bell, G.I.; Eddy, R.L.; Fukushima, Y.; Byers, M.G.; Shows, T.B.; et al. Gastric inhibitory polypeptide: Structure and chromosomal localization of the human gene. Mol. Endocrinol. 1989, 3, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Seino, Y.; Fukushima, M.; Yabe, D. Gip and glp-1, the two incretin hormones: Similarities and differences. J. Diabetes Investig. 2010, 1, 8–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: Glp-1 and gip. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Campbell, J.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013, 17, 819–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogi, Y.; Nagashima, M.; Terasaki, M.; Nohtomi, K.; Watanabe, T.; Hirano, T. Glucose-dependent insulinotropic polypeptide prevents the progression of macrophage-driven atherosclerosis in diabetic apolipoprotein e-null mice. PLoS ONE 2012, 7, e35683. [Google Scholar] [CrossRef]
- Seino, Y.; Yabe, D. Glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1: Incretin actions beyond the pancreas. J. Diabetes Investig. 2013, 4, 108–130. [Google Scholar] [CrossRef] [Green Version]
- Tsukiyama, K.; Yamada, Y.; Yamada, C.; Harada, N.; Kawasaki, Y.; Ogura, M.; Bessho, K.; Li, M.; Amizuka, N.; Sato, M.; et al. Gastric inhibitory polypeptide as an endogenous factor promoting new bone formation after food ingestion. Mol. Endocrinol. 2006, 20, 1644–1651. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Q.; Itokawa, T.; Sridhar, S.; Ding, K.H.; Xie, D.; Kang, B.; Bollag, W.B.; Bollag, R.J.; Hamrick, M.; Insogna, K.; et al. Effects of glucose-dependent insulinotropic peptide on osteoclast function. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E543–E548. [Google Scholar] [CrossRef]
- Wise, G.; King, G. Mechanisms of tooth eruption and orthodontic tooth movement. J. Dental Res. 2008, 87, 414–434. [Google Scholar] [CrossRef] [Green Version]
- Murrell, E.; Yen, E.; Johnson, R. Vascular changes in the periodontal ligament after removal of orthodontic forces. Am. J. Orthod. Dentofac. Orthop. 1996, 110, 280–286. [Google Scholar] [CrossRef]
- Rody Jr, W.J.; King, G.J.; Gu, G. Osteoclast recruitment to sites of compression in orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 2001, 120, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Holland, R.; Bain, C.; Utreja, A. Osteoblast differentiation during orthodontic tooth movement. Orthod. Craniofac. Res. 2019, 22, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Uribe, F.; Kalajzic, Z.; Bibko, J.; Nanda, R.; Olson, C.; Rowe, D.; Wadhwa, S. Early effects of orthodontic forces on osteoblast differentiation in a novel mouse organ culture model. Angle Orthod. 2011, 81, 284–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollag, R.J.; Zhong, Q.; Phillips, P.; Min, L.; Zhong, L.; Cameron, R.; Mulloy, A.L.; Rasmussen, H.; Qin, F.; Ding, K.H.; et al. Osteoblast-derived cells express functional glucose-dependent insulinotropic peptide receptors. Endocrinology 2000, 141, 1228–1235. [Google Scholar] [CrossRef]
- Skov-Jeppesen, K.; Hepp, N.; Oeke, J.; Hansen, M.S.; Jafari, A.; Svane, M.S.; Balenga, N.; Olson, J.A., Jr.; Frost, M.; Kassem, M.; et al. The antiresorptive effect of gip, but not glp-2, is preserved in patients with hypoparathyroidism-a randomized crossover study. J. Bone Miner. Res. 2021, 36, 1448–1458. [Google Scholar] [CrossRef]
- Mieczkowska, A.; Bouvard, B.; Chappard, D.; Mabilleau, G. Glucose-dependent insulinotropic polypeptide (gip) directly affects collagen fibril diameter and collagen cross-linking in osteoblast cultures. Bone 2015, 74, 29–36. [Google Scholar] [CrossRef]
- Ren, Y.; Maltha, J.C.; Kuijpers-Jagtman, A.M. The rat as a model for orthodontic tooth movement—a critical review and a proposed solution. Eur. J. Orthod. 2004, 26, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Sprogar, S.; Vaupotic, T.; Cör, A.; Drevenšek, M.; Drevenšek, G. The endothelin system mediates bone modeling in the late stage of orthodontic tooth movement in rats. Bone 2008, 43, 740–747. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamauchi, T.; Miyabe, M.; Nakamura, N.; Ito, M.; Sekiya, T.; Kanada, S.; Hoshino, R.; Matsubara, T.; Miyazawa, K.; Goto, S.; et al. Impacts of Glucose-Dependent Insulinotropic Polypeptide on Orthodontic Tooth Movement-Induced Bone Remodeling. Int. J. Mol. Sci. 2022, 23, 8922. https://doi.org/10.3390/ijms23168922
Yamauchi T, Miyabe M, Nakamura N, Ito M, Sekiya T, Kanada S, Hoshino R, Matsubara T, Miyazawa K, Goto S, et al. Impacts of Glucose-Dependent Insulinotropic Polypeptide on Orthodontic Tooth Movement-Induced Bone Remodeling. International Journal of Molecular Sciences. 2022; 23(16):8922. https://doi.org/10.3390/ijms23168922
Chicago/Turabian StyleYamauchi, Taisuke, Megumi Miyabe, Nobuhisa Nakamura, Mizuho Ito, Takeo Sekiya, Saki Kanada, Rina Hoshino, Tatsuaki Matsubara, Ken Miyazawa, Shigemi Goto, and et al. 2022. "Impacts of Glucose-Dependent Insulinotropic Polypeptide on Orthodontic Tooth Movement-Induced Bone Remodeling" International Journal of Molecular Sciences 23, no. 16: 8922. https://doi.org/10.3390/ijms23168922
APA StyleYamauchi, T., Miyabe, M., Nakamura, N., Ito, M., Sekiya, T., Kanada, S., Hoshino, R., Matsubara, T., Miyazawa, K., Goto, S., & Naruse, K. (2022). Impacts of Glucose-Dependent Insulinotropic Polypeptide on Orthodontic Tooth Movement-Induced Bone Remodeling. International Journal of Molecular Sciences, 23(16), 8922. https://doi.org/10.3390/ijms23168922





