Association of Antihypertensive Effects of Esaxerenone with the Internal Sodium Balance in Dahl Salt-Sensitive Hypertensive Rats
Abstract
1. Introduction
2. Results
2.1. Esaxerenone Treatment Reduces BP in HSD-Fed DSS Rats
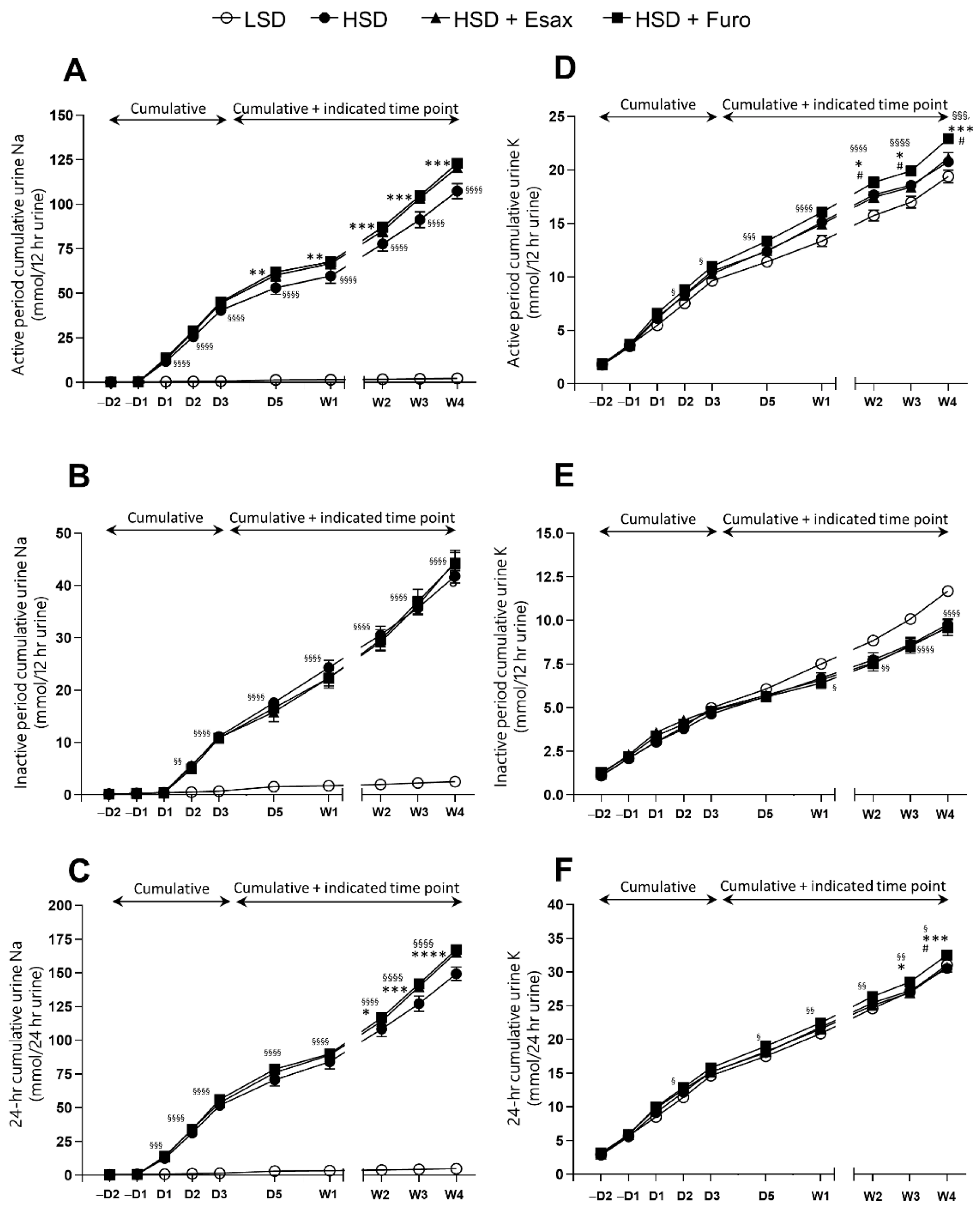
2.2. Esaxerenone Treatment Decreases Urinary Sodium Excretion
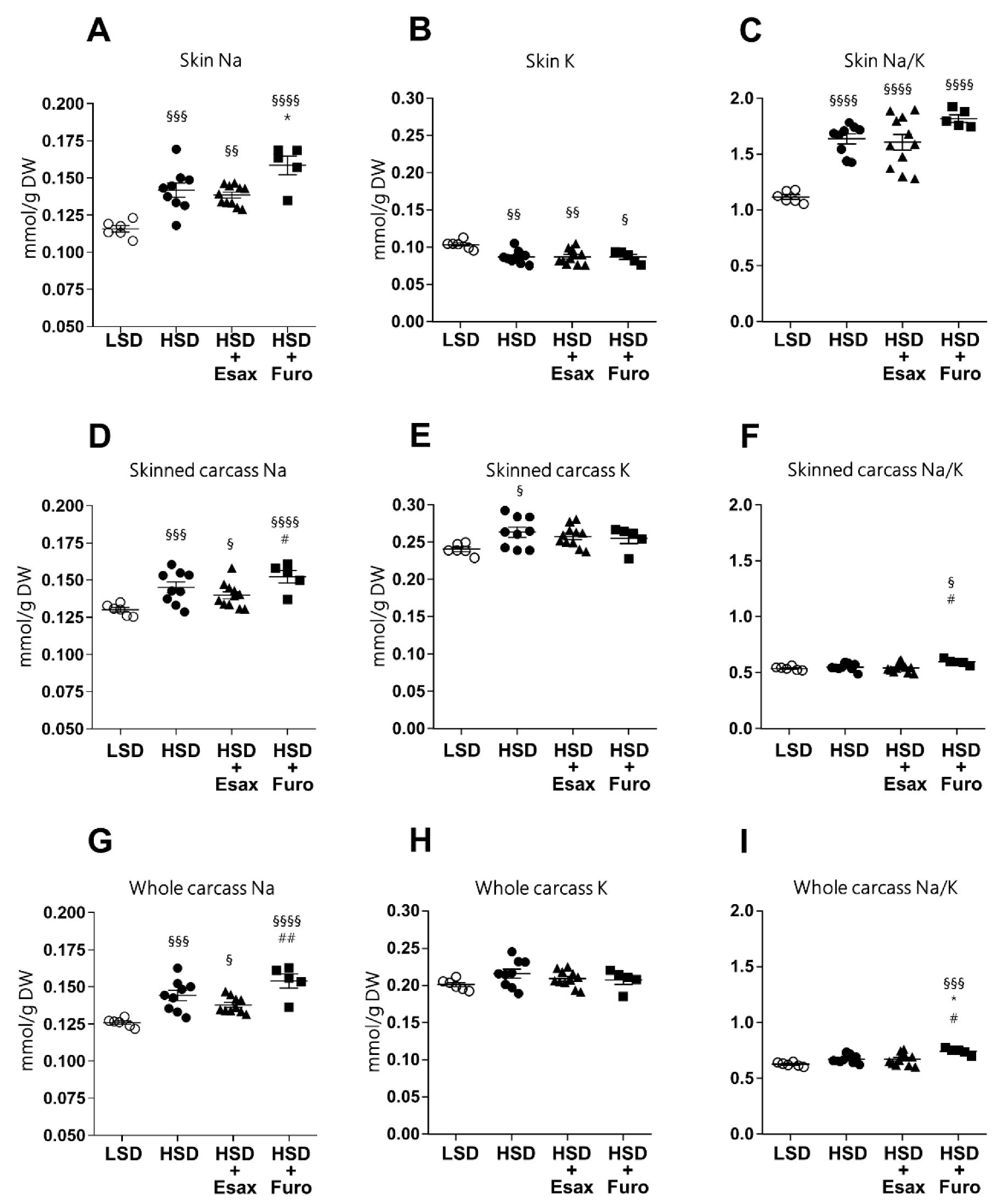
2.3. Esaxerenone Treatment Tends to Reduce Skin and Carcass Sodium Concentrations
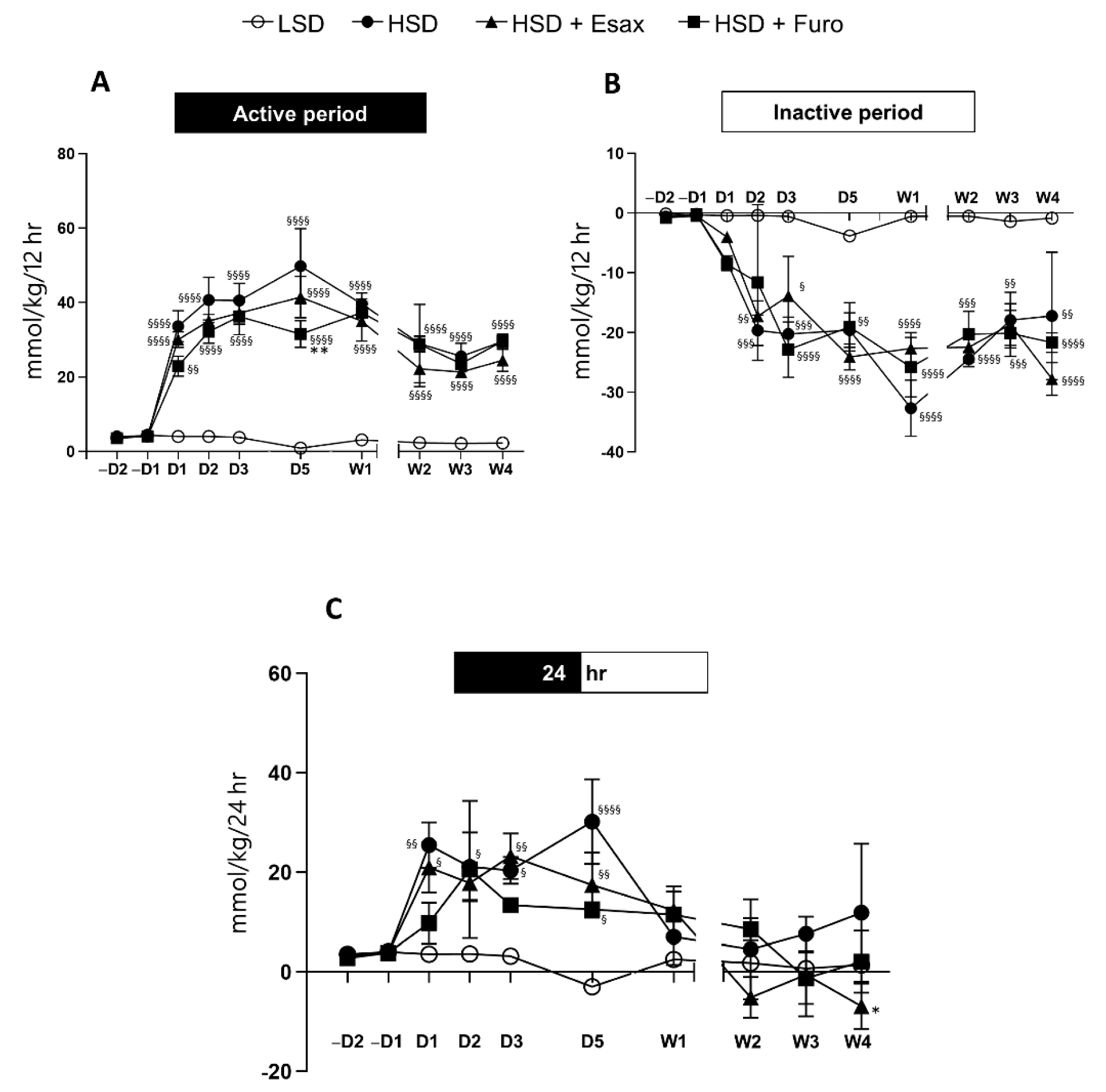
2.4. Esaxerenone Treatment Decreases the Sodium Balance in the Active Period
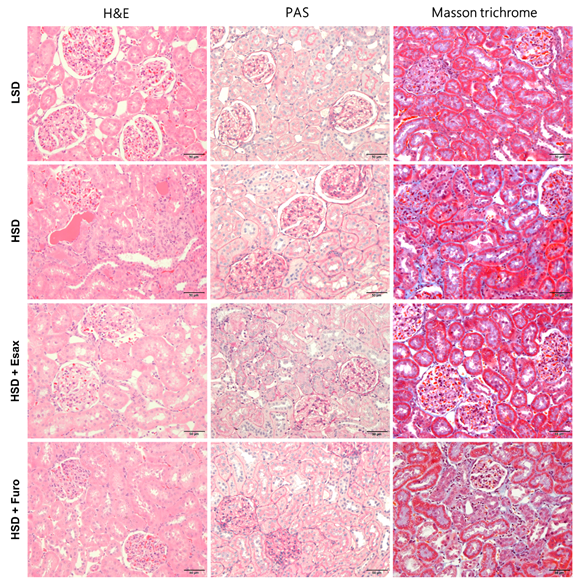
2.5. Esaxerenone Treatment Improves Renal Histology in HSD-Fed DSS Rats
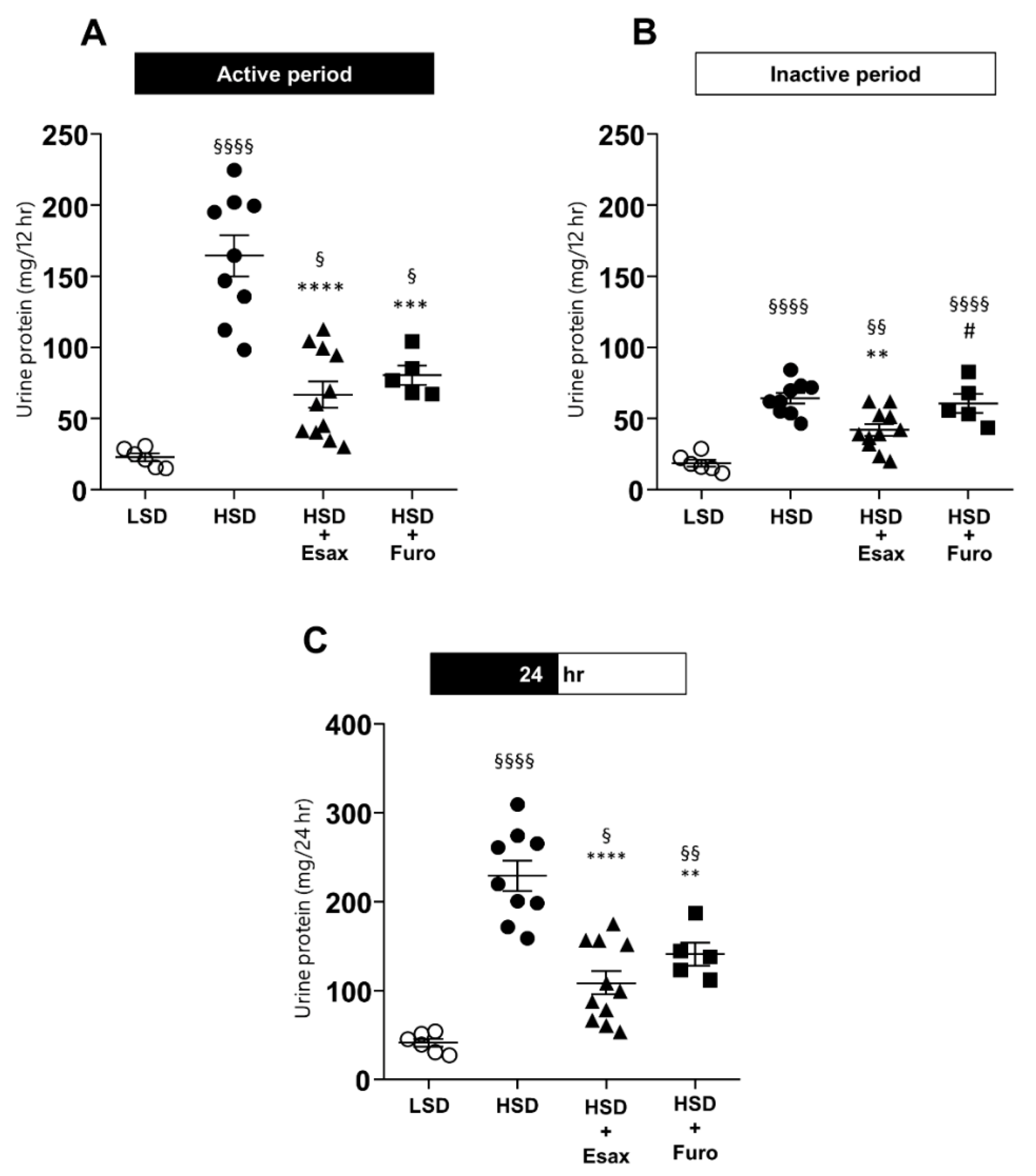
2.6. Esaxerenone Treatment Reduces Urinary Protein Excretion
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Drugs and Modified Chow
4.3. Protocols
4.3.1. Protocol I: BP Measurement with a Radiotelemetry System
4.3.2. Protocol II: Analysis of Sodium Homeostasis
Urine Electrolytes, Protein, and Osmolality
Skin and Carcass Water Content, and Electrolytes
4.3.3. Protocol III: Renal Histological Examination
Histological Analysis
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rethy, L.; Shah, N.S.; Paparello, J.J.; Lloyd-Jones, D.M.; Khan, S.S. Trends in Hypertension-Related Cardiovascular Mortality in the United States, 2000 to 2018. Hypertension 2020, 76, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Delea, C.S.; Bartter, F.C.; Smith, H. The effect of high-sodium and low-sodium intakes on blood pressure and other related variables in human subjects with idiopathic hypertension. Am. J. Med. 1978, 64, 193–198. [Google Scholar] [CrossRef]
- Seriki, A.S. Role of the Kidneys in the Regulation of Intra-and Extra-Renal Blood Pressure. Ann. Clin. Hypertens. 2018, 2, 48–58. [Google Scholar] [CrossRef]
- Hall, J.E.; Guyton, A.C.; Coleman, T.G.; Mizelle, H.L.; Woods, L.L. Regulation of arterial pressure: Role of pressure natriuresis and diuresis. Fed. Proc. 1986, 45, 2897–2903. [Google Scholar]
- Titze, J.; Krause, H.; Hecht, H.; Dietsch, P.; Rittweger, J.; Lang, R.; Kirsch, K.A.; Hilgers, K.F. Reduced osmotically inactive Na storage capacity and hypertension in the Dahl model. Am. J. Physiol. Physiol. 2002, 283, F134–F141. [Google Scholar] [CrossRef] [PubMed]
- Kimura, G.; Dohi, Y.; Fukuda, M. Salt sensitivity and circadian rhythm of blood pressure: The keys to connect CKD with cardiovascular events. Hypertens. Res. 2010, 33, 515–520. [Google Scholar] [CrossRef]
- Boggia, J.; Li, Y.; Thijs, L.; Hansen, T.W.; Kikuya, M.; Björklund-Bodegård, K.; Richart, T.; Ohkubo, T.; Kuznetsova, T.; Torp-Pedersen, C.; et al. Prognostic accuracy of day versus night ambulatory blood pressure: A cohort study. Lancet 2007, 370, 1219–1229. [Google Scholar] [CrossRef]
- Iwahori, T.; Ueshima, H.; Torii, S.; Saito, Y.; Kondo, K.; Tanaka-Mizuno, S.; Arima, H.; Miura, K. Diurnal variation of urinary sodium-to-potassium ratio in free-living Japanese individuals. Hypertens. Res. 2017, 40, 658–664. [Google Scholar] [CrossRef]
- Bankir, L.; Bochud, M.; Maillard, M.; Bovet, P.; Gabriel, A.; Burnier, M. Nighttime blood pressure and nocturnal dipping are associated with daytime urinary sodium excretion in african subjects. Hypertension 2008, 51, 891–898. [Google Scholar] [CrossRef]
- Ayuzawa, N.; Fujita, T. The Mineralocorticoid Receptor in Salt-Sensitive Hypertension and Renal Injury. J. Am. Soc. Nephrol. 2021, 32, 279–289. [Google Scholar] [CrossRef]
- Shibata, S.; Mu, S.; Kawarazaki, H.; Muraoka, K.; Ishizawa, K.-I.; Yoshida, S.; Kawarazaki, W.; Takeuchi, M.; Ayuzawa, N.; Miyoshi, J.; et al. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor–dependent pathway. J. Clin. Investig. 2011, 121, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y. Effects of eplerenone, a selective mineralocorticoid receptor antagonist, on clinical and experimental salt-sensitive hypertension. Hypertens. Res. 2009, 32, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Bayorh, M.A.; Mann, G.; Walton, M.; Eatman, D. Effects of enalapril, tempol, and eplerenone on salt-induced hypertension in dahl salt-sensitive rats. Clin. Exp. Hypertens. 2006, 28, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Hood, S.J.; Taylor, K.P.; Ashby, M.J.; Brown, M.J. The spironolactone, amiloride, losartan, and thiazide (salt) double-blind crossover trial in patients with low-renin hypertension and elevated aldosterone-renin ratio. Circulation 2007, 116, 268–275. [Google Scholar] [CrossRef]
- Sato, A.; Saruta, T.; Funder, J.W. Combination Therapy with Aldosterone Blockade and Renin-Angiotensin Inhibitors Confers Organ Protection. Hypertens. Res. 2006, 29, 211–216. [Google Scholar] [CrossRef]
- Young, M.J.; Kanki, M.; Karthigan, N.; Konstandopoulos, P. The Role of the Mineralocorticoid Receptor and Mineralocorticoid Receptor–Directed Therapies in Heart Failure. Endocrinology 2021, 162, bqab105. [Google Scholar] [CrossRef]
- Takahashi, M.; Ubukata, O.; Homma, T.; Asoh, Y.; Honzumi, M.; Hayashi, N.; Saito, K.; Tsuruoka, H.; Aoki, K.; Hanzawa, H. Crystal structure of the mineralocorticoid receptor ligand-binding domain in complex with a potent and selective nonsteroidal blocker, esaxerenone (CS-3150). FEBS Lett. 2020, 594, 1615–1623. [Google Scholar] [CrossRef]
- Wan, N.; Rahman, A.; Nishiyama, A. Esaxerenone, a novel nonsteroidal mineralocorticoid receptor blocker (MRB) in hypertension and chronic kidney disease. J. Hum. Hypertens. 2021, 35, 148–156. [Google Scholar] [CrossRef]
- Ito, S.; Itoh, H.; Rakugi, H.; Okuda, Y.; Yoshimura, M.; Yamakawa, S. Double-Blind Randomized Phase 3 Study Comparing Esaxerenone (CS-3150) and Eplerenone in Patients With Essential Hypertension (ESAX-HTN Study). Hypertension 2020, 75, 51–58. [Google Scholar] [CrossRef]
- Rakugi, H.; Ito, S.; Itoh, H.; Okuda, Y.; Yamakawa, S. Long-term phase 3 study of esaxerenone as mono or combination therapy with other antihypertensive drugs in patients with essential hypertension. Hypertens. Res. 2019, 42, 1932–1941. [Google Scholar] [CrossRef]
- Rahman, A.; Sawano, T.; Sen, A.; Hossain, A.; Jahan, N.; Kobara, H.; Masaki, T.; Kosaka, S.; Kitada, K.; Nakano, D.; et al. Cardioprotective Effects of a Nonsteroidal Mineralocorticoid Receptor Blocker, Esaxerenone, in Dahl Salt-Sensitive Hypertensive Rats. Int. J. Mol. Sci. 2021, 22, 2069. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guan, Y.; Kobori, H.; Morishita, A.; Kobara, H.; Masaki, T.; Nakano, D.; Nishiyama, A. Effects of the novel nonsteroidal mineralocorticoid receptor blocker, esaxerenone (CS-3150), on blood pressure and urinary angiotensinogen in low-renin Dahl salt-sensitive hypertensive rats. Hypertens. Res. 2018, 42, 769–778. [Google Scholar] [CrossRef]
- Arai, K.; Tsuruoka, H.; Homma, T. CS-3150, a novel non-steroidal mineralocorticoid receptor antagonist, prevents hypertension and cardiorenal injury in Dahl salt-sensitive hypertensive rats. Eur. J. Pharmacol. 2015, 769, 266–273. [Google Scholar] [CrossRef]
- Arai, K.; Homma, T.; Morikawa, Y.; Ubukata, N.; Tsuruoka, H.; Aoki, K.; Ishikawa, H.; Mizuno, M.; Sada, T. Pharmacological profile of CS-3150, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist. Eur. J. Pharmacol. 2015, 761, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Sufiun, A.; Rahman, A.; Rafiq, K.; Fujisawa, Y.; Nakano, D.; Kobara, H.; Masaki, T.; Nishiyama, A. Association of a Disrupted Dipping Pattern of Blood Pressure with Progression of Renal Injury during the Development of Salt-Dependent Hypertension in Rats. Int. J. Mol. Sci. 2020, 21, 2248. [Google Scholar] [CrossRef] [PubMed]
- Kario, K.; Ito, S.; Itoh, H.; Rakugi, H.; Okuda, Y.; Yoshimura, M.; Yamakawa, S. Effect of the Nonsteroidal Mineralocorticoid Receptor Blocker, Esaxerenone, on Nocturnal Hypertension: A Post Hoc Analysis of the ESAX-HTN Study. Am. J. Hypertens. 2021, 34, 540–551. [Google Scholar] [CrossRef]
- Staessen, J.; Broughton, P.M.G.; Fletcher, A.E.; Markowe, H.L.J.; Marmot, M.G.; Rose, G.; Semmence, A.; Shipley, M.J.; Bulpitt, C.J. The assessment of the relationship between blood pressure and sodium intake using whole-day, daytime and overnight urine collections. J. Hypertens. 1991, 9, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Uzu, T.; Ishikawa, K.; Fujii, T.; Nakamura, S.; Inenaga, T.; Kimura, G. Sodium Restriction Shifts Circadian Rhythm of Blood Pressure From Nondipper to Dipper in Essential Hypertension. Circulation 1997, 96, 1859–1862. [Google Scholar] [CrossRef]
- Kintscher, U.; Bakris, G.L.; Kolkhof, P. Novel non-steroidal mineralocorticoid receptor antagonists in cardiorenal disease. Br. J. Pharmacol. 2022, 179, 3220–3234. [Google Scholar] [CrossRef]
- Farmer, C.K.T.; Goldsmith, D.J.A.; Cox, J.; Dallyn, P.; Kingswood, J.C.; Sharpstone, P. An investigation of the effect of advancing uraemia, renal replacement therapy and renal transplantation on blood pressure diurnal variability. Nephrol. Dial. Transplant. 1997, 12, 2301–2307. [Google Scholar] [CrossRef][Green Version]
- Haruhara, K.; Tsuboi, N.; Koike, K.; Fukui, A.; Miyazaki, Y.; Kawamura, T.; Ogura, M.; Yokoo, T. Renal histopathological findings in relation to ambulatory blood pressure in chronic kidney disease patients. Hypertens. Res. 2015, 38, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, J.; Yamamoto, K.; Mano, T.; Sakata, Y.; Nishio, M.; Ohtani, T.; Hori, M.; Miwa, T.; Masuyama, T. Different effects of long- and short-acting loop diuretics on survival rate in Dahl high-salt heart failure model rats. Cardiovasc. Res. 2005, 68, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Kittikulsuth, W.; Fujisawa, Y.; Sufiun, A.; Rafiq, K.; Hitomi, H.; Nakano, D.; Sohara, E.; Uchida, S.; Nishiyama, A. Effects of diuretics on sodium-dependent glucose cotransporter 2 inhibitor-induced changes in blood pressure in obese rats suffering from the metabolic syndrome. J. Hypertens. 2016, 34, 893–906. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hattori, M.; Rahman, A.; Kidoguchi, S.; Jahan, N.; Fujisawa, Y.; Morisawa, N.; Ohsaki, H.; Kobara, H.; Masaki, T.; Hossain, A.; et al. Association of Antihypertensive Effects of Esaxerenone with the Internal Sodium Balance in Dahl Salt-Sensitive Hypertensive Rats. Int. J. Mol. Sci. 2022, 23, 8915. https://doi.org/10.3390/ijms23168915
Hattori M, Rahman A, Kidoguchi S, Jahan N, Fujisawa Y, Morisawa N, Ohsaki H, Kobara H, Masaki T, Hossain A, et al. Association of Antihypertensive Effects of Esaxerenone with the Internal Sodium Balance in Dahl Salt-Sensitive Hypertensive Rats. International Journal of Molecular Sciences. 2022; 23(16):8915. https://doi.org/10.3390/ijms23168915
Chicago/Turabian StyleHattori, Mai, Asadur Rahman, Satoshi Kidoguchi, Nourin Jahan, Yoshihide Fujisawa, Norihiko Morisawa, Hiroyuki Ohsaki, Hideki Kobara, Tsutomu Masaki, Akram Hossain, and et al. 2022. "Association of Antihypertensive Effects of Esaxerenone with the Internal Sodium Balance in Dahl Salt-Sensitive Hypertensive Rats" International Journal of Molecular Sciences 23, no. 16: 8915. https://doi.org/10.3390/ijms23168915
APA StyleHattori, M., Rahman, A., Kidoguchi, S., Jahan, N., Fujisawa, Y., Morisawa, N., Ohsaki, H., Kobara, H., Masaki, T., Hossain, A., Steeve, A., & Nishiyama, A. (2022). Association of Antihypertensive Effects of Esaxerenone with the Internal Sodium Balance in Dahl Salt-Sensitive Hypertensive Rats. International Journal of Molecular Sciences, 23(16), 8915. https://doi.org/10.3390/ijms23168915







