Human Umbilical Cord Lining-Derived Epithelial Cells: A Potential Source of Non-Native Epithelial Cells That Accelerate Healing in a Porcine Cutaneous Wound Model
Abstract
:1. Introduction
2. Results
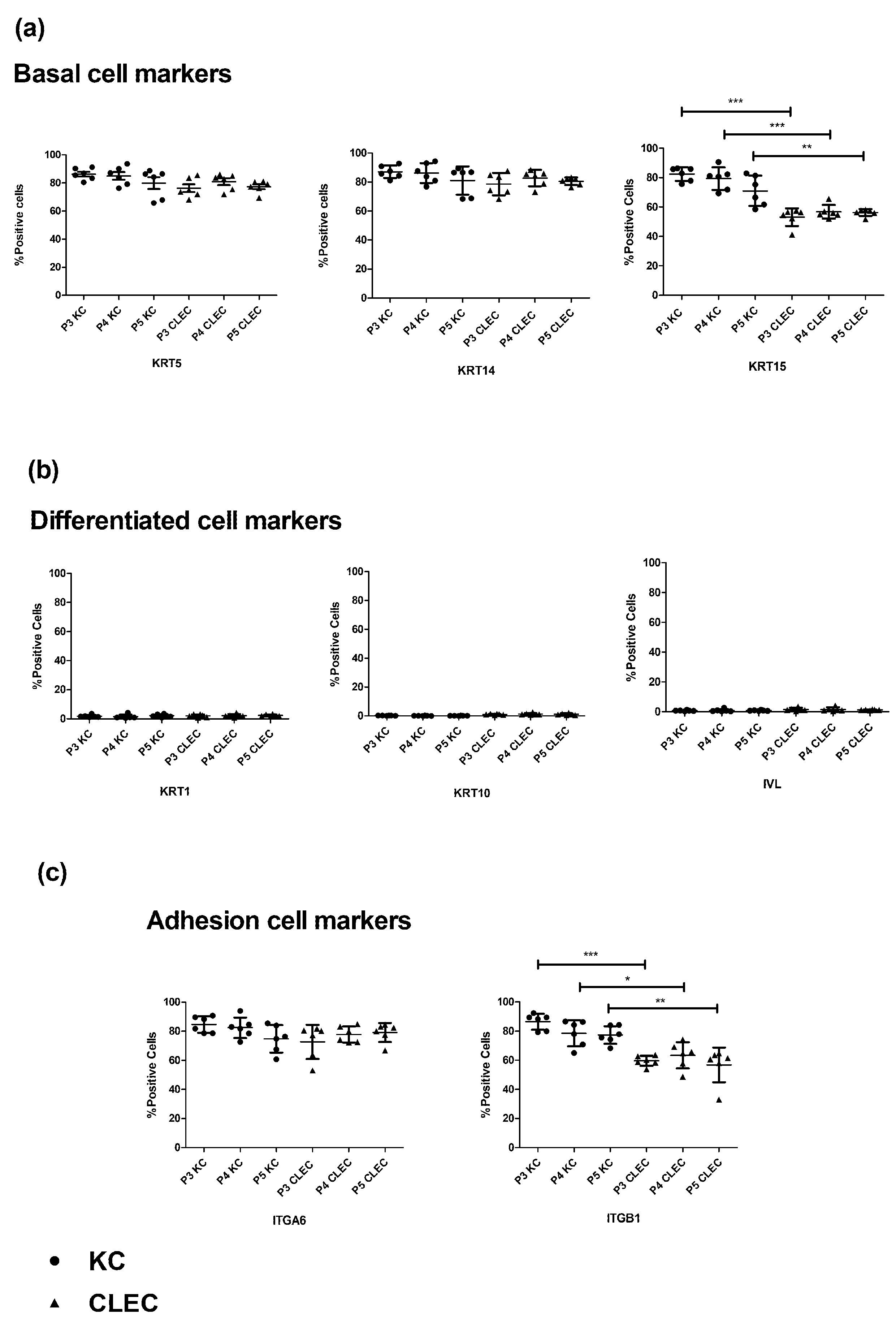
2.1. Immunophenotypic Profile of CLECs in Comparison to KCs
2.2. Stratification of CLECs Compared to KCs in Organotypic Cultures
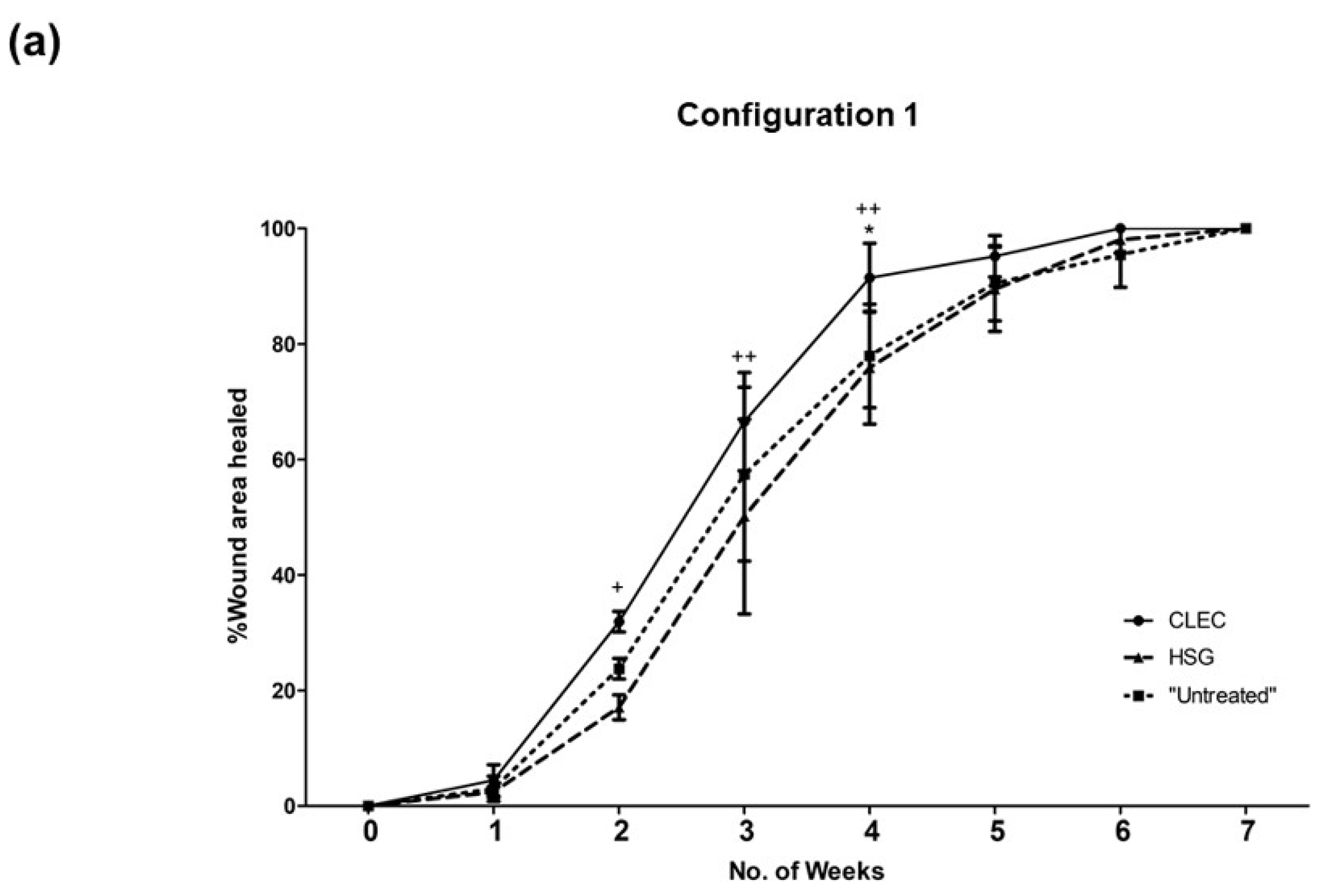
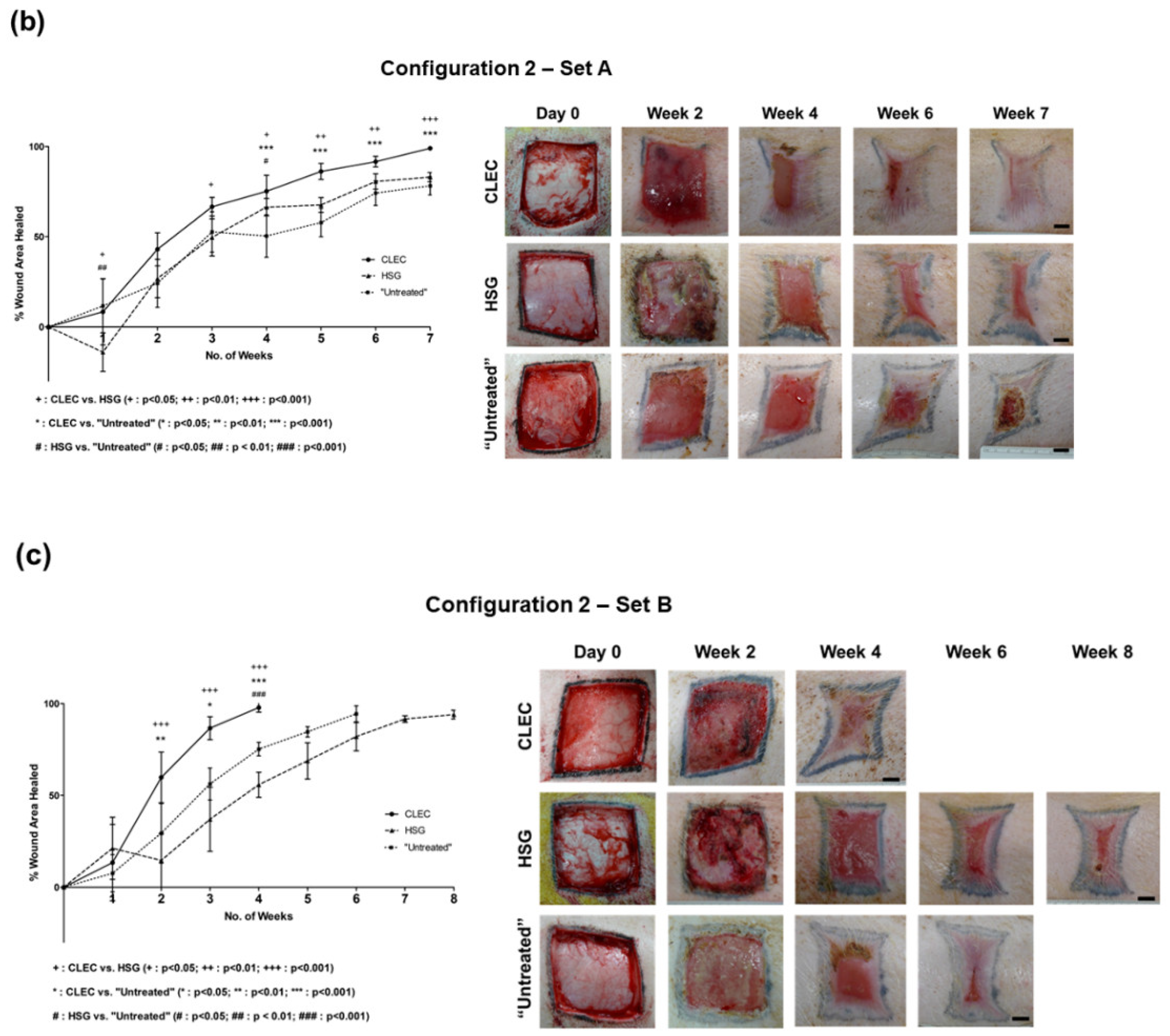
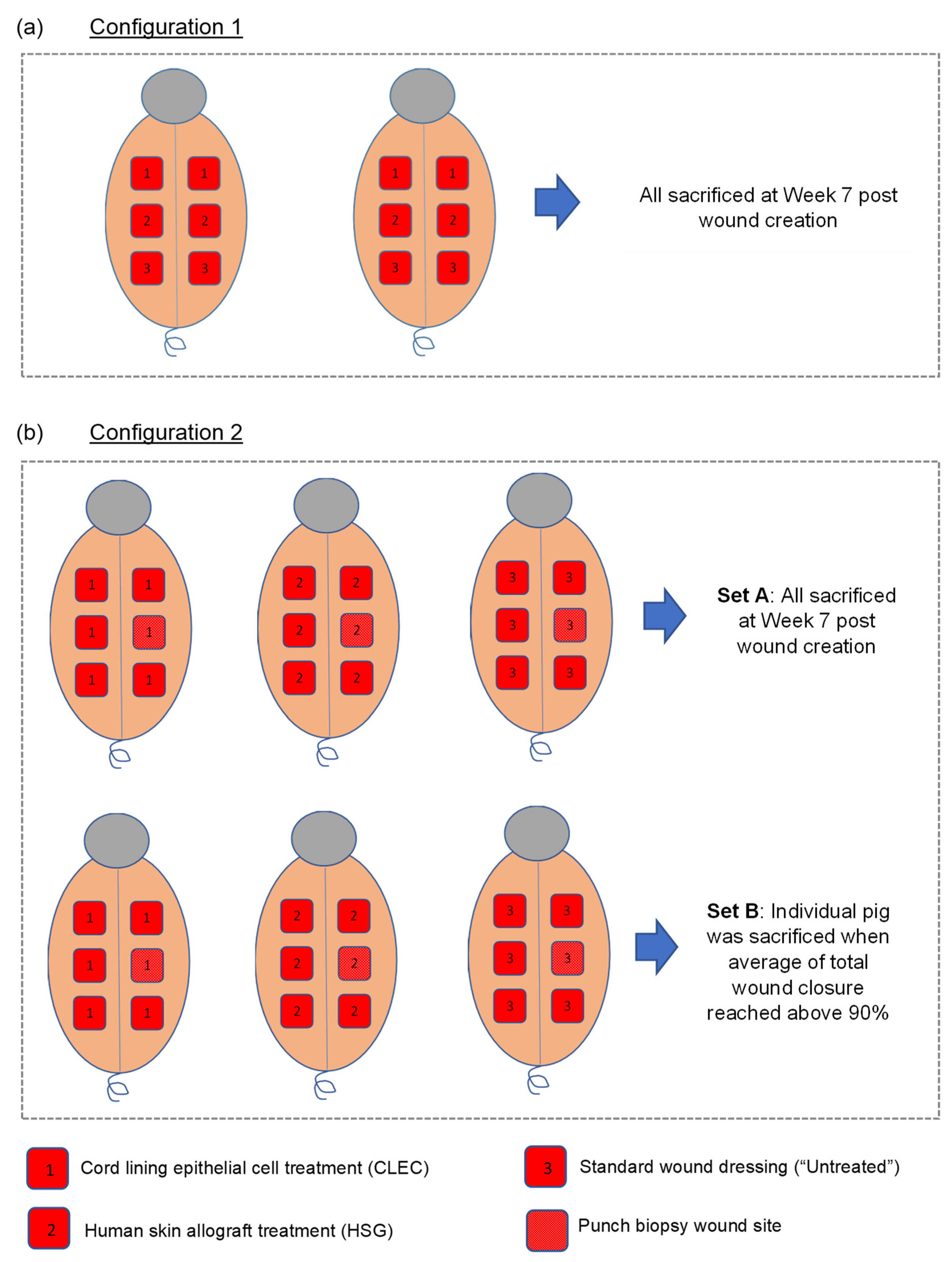
2.3. Wound Healing Performance of CLECs in Comparison to Human Skin Grafts (HSGs) in a Porcine Excisional Wound Model
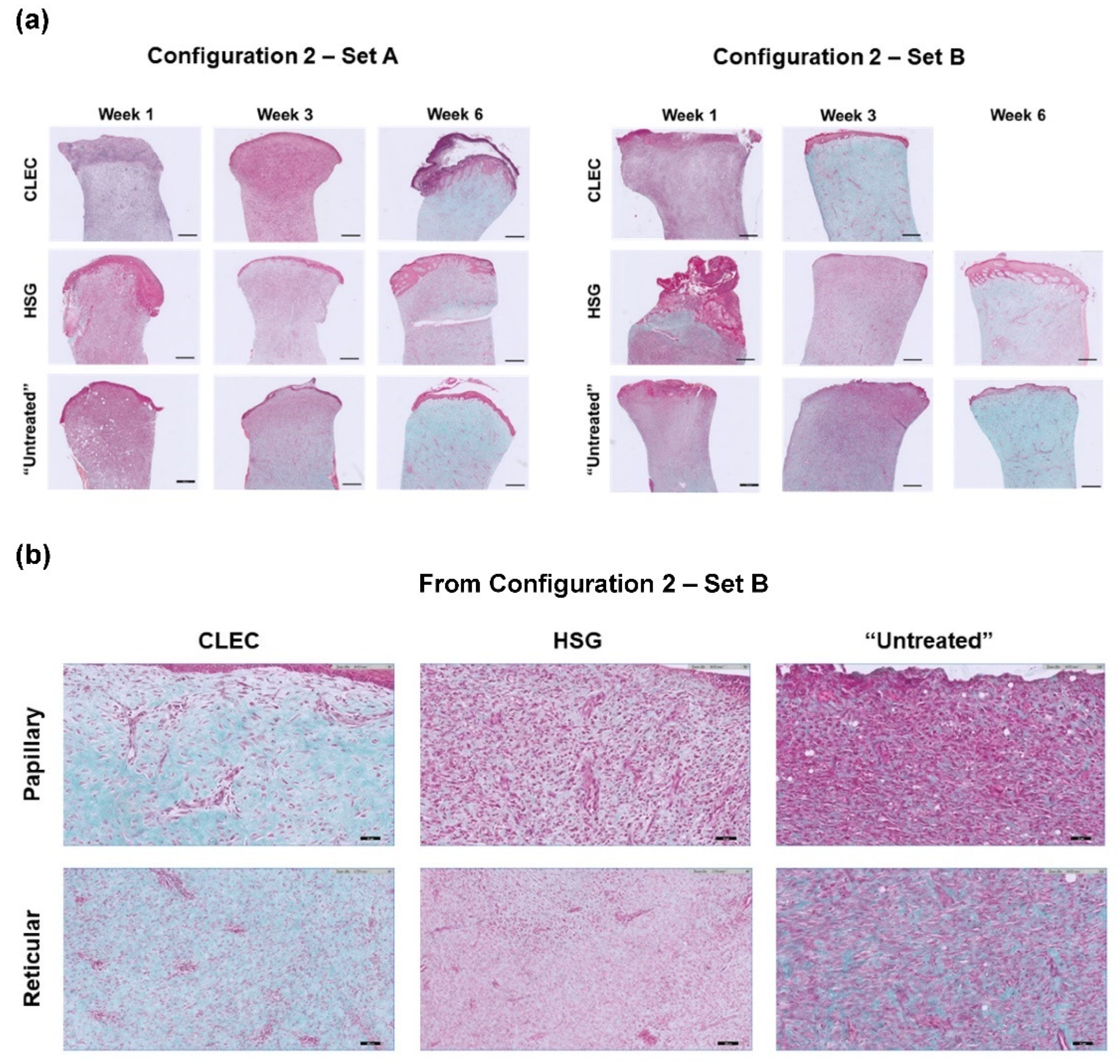
2.4. Histological Assessment of the Wounds after Respective Treatments
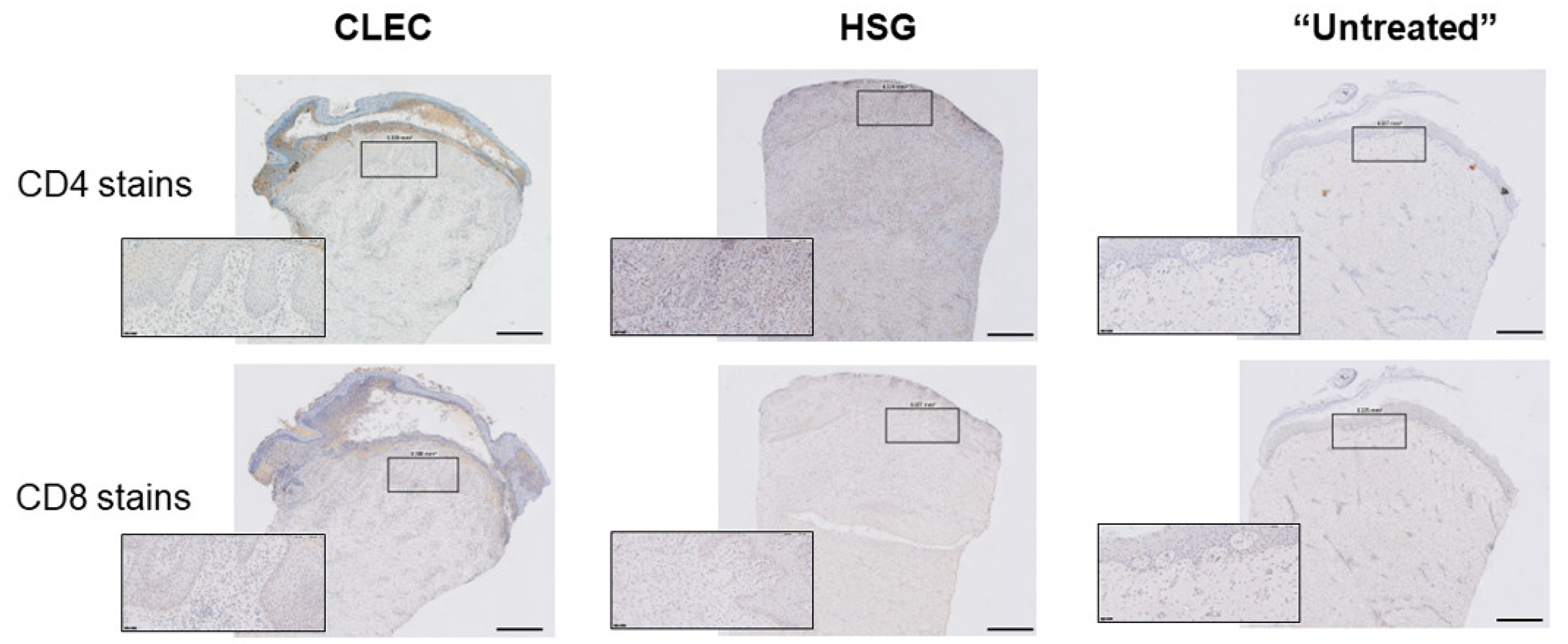
2.5. Evaluation of Systemic Host Response through Time-Course Profiling of Probed Inflammatory Cytokines in Porcine Serum with Respective Wound Treatments
3. Discussion
3.1. Expression Levels of KRT15 and ITGB1 Were Found to Be Lower in CLECs Compared to KCs in Flow Cytometry
3.2. Some Basal Cells Markers of the Human Epidermis Were Found to Be Expressed in Both the Basal and the Suprabasal Layers of CLEC-Generated Epithelium
3.3. CLEC Treatment Accelerated Wound Closure Compared to HSG Treatment
3.4. Use of CLECs and HSGs Elicited High Levels of Local and Systemic Immune Response in the Animals during the First Week but These Effects Tapered off More Quickly in the CLEC-Treated Group
4. Materials and Methods
4.1. Isolation and Culture of Human Cord Lining Epithelial Cells (CLECs)
4.2. Isolation and Culture of Human Skin Keratinocytes (KCs)
4.3. Flow Cytometry (FC) and Analysis of Data
4.4. Reconstruction of Epithelium on De-Epithelialized Dermis (Organotypic Culture)
4.5. Porcine Full Thickness Excisional Wound Model
4.6. Wound Healing Analysis
4.7. Histological Staining and Analysis
4.8. Milliplex Cytokine Array
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirsch, T.; Rothoeft, T.; Teig, N.; Bauer, J.W.; Pellegrini, G.; De Rosa, L.; Scaglione, D.; Reichelt, J.; Klausegger, A.; Kneisz, D.; et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature 2017, 551, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Williams, F.N.; Herndon, D.N.; Hawkins, H.K.; Lee, J.O.; Cox, R.A.; Kulp, G.A.; Finnerty, C.C.; Chinkes, D.L.; Jeschke, M.G. The leading causes of death after burn injury in a single pediatric burn center. Crit. Care 2009, 13, R183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Avignon, L.C.; Hogan, B.K.; Murray, C.K.; Loo, F.L.; Hospenthal, D.R.; Cancio, L.C.; Kim, S.H.; Renz, E.M.; Barillo, D.; Holcomb, J.B.; et al. Contribution of bacterial and viral infections to attributable mortality in patients with severe burns: An autopsy series. Burn. J. Int. Soc. Burn Inj. 2010, 36, 773–779. [Google Scholar] [CrossRef]
- Chim, H.; Tan, B.H.; Song, C. Five-year review of infections in a burn intensive care unit: High incidence of Acinetobacter baumannii in a tropical climate. Burn. J. Int. Soc. Burn Inj. 2007, 33, 1008–1014. [Google Scholar] [CrossRef]
- Song, C.T.; Hwee, J.; Song, C.; Tan, B.K.; Chong, S.J. Burns infection profile of Singapore: Prevalence of multidrug-resistant Acinetobacter baumannii and the role of blood cultures. Burn. Trauma 2016, 4, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krueger, J.G.; Lin, A.N.; Leong, I.; Carter, D.M. Junctional epidermolysis bullosa keratinocytes in culture display adhesive, structural, and functional abnormalities. J. Investig. Dermatol. 1991, 97, 849–861. [Google Scholar] [CrossRef] [Green Version]
- Morley, S.M.; D’Alessandro, M.; Sexton, C.; Rugg, E.L.; Navsaria, H.; Shemanko, C.S.; Huber, M.; Hohl, D.; Heagerty, A.I.; Leigh, I.M.; et al. Generation and characterization of epidermolysis bullosa simplex cell lines: Scratch assays show faster migration with disruptive keratin mutations. Br. J. Dermatol. 2003, 149, 46–58. [Google Scholar] [CrossRef]
- Zhou, Y.; Gan, S.U.; Lin, G.; Lim, Y.T.; Masilamani, J.; Mustafa, F.B.; Phua, M.L.; Rivino, L.; Phan, T.T.; Lee, K.O.; et al. Characterization of human umbilical cord lining-derived epithelial cells and transplantation potential. Cell Transplant. 2011, 20, 1827–1841. [Google Scholar] [CrossRef]
- Sivalingam, J.; Krishnan, S.; Ng, W.H.; Lee, S.S.; Phan, T.T.; Kon, O.L. Biosafety assessment of site-directed transgene integration in human umbilical cord-lining cells. Mol. Ther. J. Am. Soc. Gene Ther. 2010, 18, 1346–1356. [Google Scholar] [CrossRef]
- Gubar, O.S.; Rodnichenko, A.E.; Vasyliev, R.G.; Zlatska, A.V.; Zubov, D.O. Postnatal extra-embryonic tissues as a source of multiple cell types for regenerative medicine applications. Exp. Oncol. 2017, 39, 186–190. [Google Scholar] [CrossRef]
- Hayward, C.J.; Fradette, J.; Galbraith, T.; Remy, M.; Guignard, R.; Gauvin, R.; Germain, L.; Auger, F.A. Harvesting the potential of the human umbilical cord: Isolation and characterisation of four cell types for tissue engineering applications. Cells Tissues Organs 2013, 197, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, M.; Ikeda, S.; Suga, Y.; Ogawa, H. Expression of cytokeratins and cornified cell envelope-associated proteins in umbilical cord epithelium: A comparative study of the umbilical cord, amniotic epithelia and fetal skin. J. Investig. Dermatol. 2000, 115, 133–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruetze, M.; Gallinat, S.; Lim, I.J.; Chow, E.; Phan, T.T.; Staeb, F.; Wenck, H.; Deppert, W.; Knott, A. Common features of umbilical cord epithelial cells and epidermal keratinocytes. J. Dermatol. Sci. 2008, 50, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.J.; Huang, L.; Leung, T.Y.; Burd, A. A study of the immune properties of human umbilical cord lining epithelial cells. Cytotherapy 2014, 16, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Garcés, V.; Harvat, M.; Molina Aguilar, P.; Ferrández Izquierdo, A.; Ruiz-Saurí, A. Comparative measurement of collagen bundle orientation by Fourier analysis and semiquantitative evaluation: Reliability and agreement in Masson’s trichrome, Picrosirius red and confocal microscopy techniques. J. Microsc. 2017, 267, 130–142. [Google Scholar] [CrossRef]
- Richters, C.D.; Hoekstra, M.J.; du Pont, J.S.; Kreis, R.W.; Kamperdijk, E.W. Immunology of skin transplantation. Clin. Dermatol. 2005, 23, 338–342. [Google Scholar] [CrossRef]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, H.; et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef] [Green Version]
- Utheim, T.P.; Utheim, O.A.; Khan, Q.E.; Sehic, A. Culture of Oral Mucosal Epithelial Cells for the Purpose of Treating Limbal Stem Cell Deficiency. J. Funct. Biomater. 2016, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Gallant-Behm, C.L.; Hart, D.A. Genetic analysis of skin wound healing and scarring in a porcine model. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2006, 14, 46–54. [Google Scholar] [CrossRef]
- Huang, L.; Wong, Y.P.; Gu, H.; Cai, Y.J.; Ho, Y.; Wang, C.C.; Leung, T.Y.; Burd, A. Stem cell-like properties of human umbilical cord lining epithelial cells and the potential for epidermal reconstitution. Cytotherapy 2011, 13, 145–155. [Google Scholar] [CrossRef]
- Webb, A.; Li, A.; Kaur, P. Location and phenotype of human adult keratinocyte stem cells of the skin. Differ. Res. Biol. Divers. 2004, 72, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; Teh, M.T.; Mackenzie, I.C.; Waseem, A. Keratin k15 as a biomarker of epidermal stem cells. Int. J. Mol. Sci. 2013, 14, 19385–19398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seaton, M.; Hocking, A.; Gibran, N.S. Porcine models of cutaneous wound healing. ILAR J. 2015, 56, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.; Burd, A. “Xenograft” dressing in the treatment of burns. Clin. Dermatol. 2005, 23, 419–423. [Google Scholar] [CrossRef]
- Chua, A.; Song, C.; Chai, A.; Chan, L.; Tan, K.C. The impact of skin banking and the use of its cadaveric skin allografts for severe burn victims in Singapore. Burn. J. Int. Soc. Burn Inj. 2004, 30, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mehta, N.D.; Zhao, Y.; DiPietro, L.A. Absence of CD4 or CD8 lymphocytes changes infiltration of inflammatory cells and profiles of cytokine expression in skin wounds, but does not impair healing. Exp Derm. 2014, 23, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Tjin, M.S.; Chua, A.W.C.; Tryggvason, K. Chemically defined and xenogeneic-free culture method for human epidermal keratinocytes on laminin-based matrices. Nat. Protoc. 2020, 15, 694–711. [Google Scholar] [CrossRef]
- Tjin, M.S.; Chua, A.W.C.; Moreno-Moral, A.; Chong, L.Y.; Tang, P.Y.; Harmston, N.P.; Cai, Z.; Petretto, E.; Tan, B.K.; Tryggvason, K. Biologically relevant laminin as chemically defined and fully human platform for human epidermal keratinocyte culture. Nat. Commun. 2018, 9, 4432. [Google Scholar] [CrossRef] [Green Version]
- Kirk, R.G.W. Recovering The Principles of Humane Experimental Technique: The 3Rs and the Human Essence of Animal Research. Sci. Technol. Hum. Values 2018, 43, 622–648. [Google Scholar] [CrossRef] [Green Version]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008, 2008, pdb.prot4986. [Google Scholar] [CrossRef]
- Jones, P.H.; Watt, F.M. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 1993, 73, 713–724. [Google Scholar] [CrossRef]
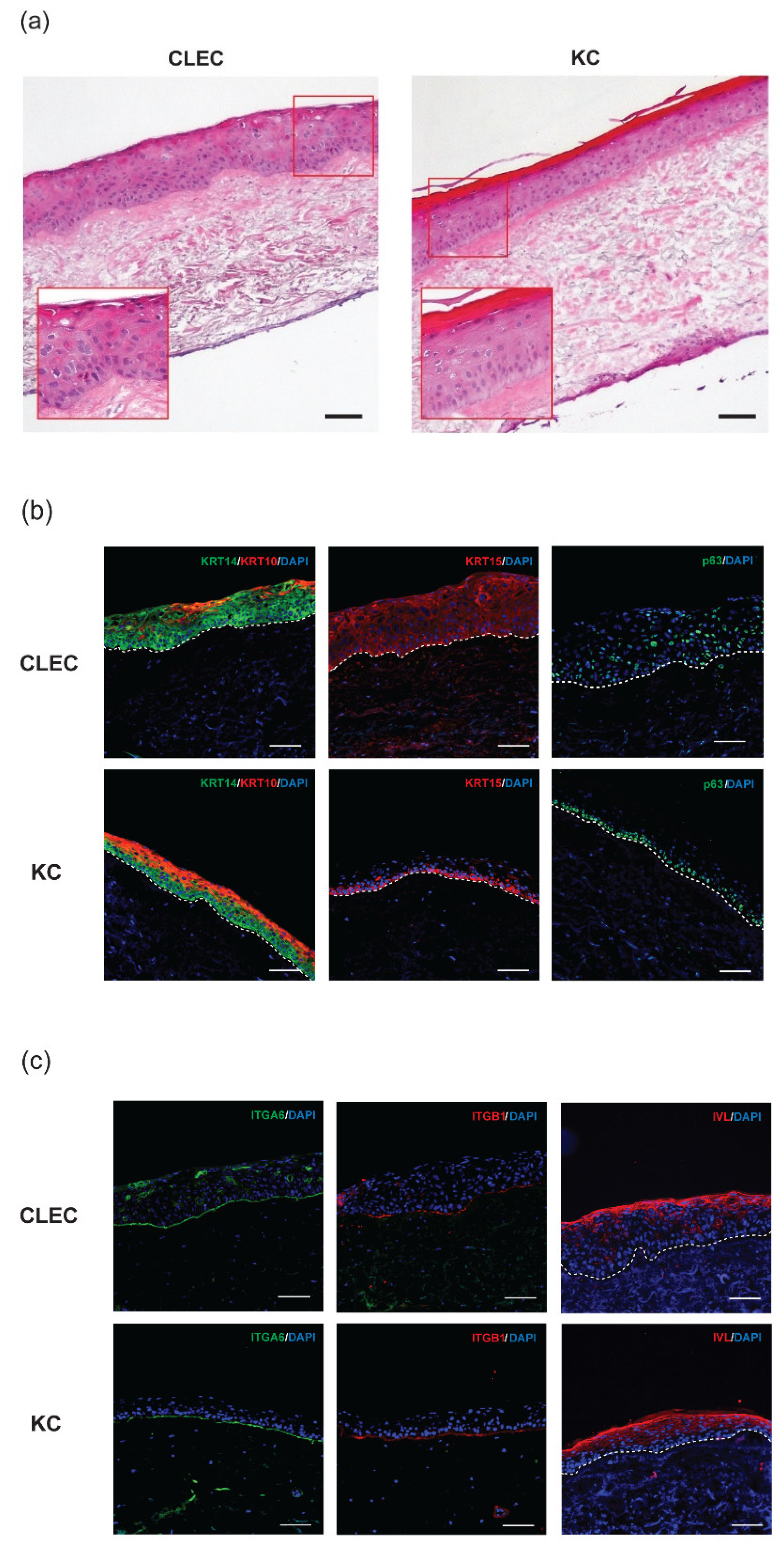
| Protein Marker | CLEC | KC |
|---|---|---|
| Keratin 10 (KRT10) | sb (+) | sb (++) |
| Keratin 14 (KRT14) | bl (++), sb (++) | bl (++) |
| Keratin 15 (KRT15) | bl (++), sb (++) | bl (++) |
| Integrin alpha-6 (ITGA6) | bm (++) | bm (++) |
| Integrin beta-1 (ITGB1) | bm (++) | bm (++) |
| Involucrin (IVL) | usb (++) | usb (++) |
| p63 | bl (+), sb (+) | bm (++) |
| Configuration 2—Set A | Configuration 2—Set B | |||||
|---|---|---|---|---|---|---|
| Week | 1 | 3 | 6 | 1 | 3 | 6 |
| CLEC treatment | ||||||
| Papillary | - | - | + | - | + | NA |
| Recticular | - | + | + | + | + | NA |
| HSG treatment | ||||||
| Papillary | - | - | + | - | - | + |
| Recticular | - | - | + | - | - | + |
| “Untreated” | ||||||
| Papillary | - | - | + | - | - | + |
| Recticular | - | + | + | + | + | + |
| For CD4+ T cells | ||||||||
| Week | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| CLEC treatment | ||||||||
| Set A | +++ | + | + | ++ | - | + | + | NA |
| Set B | +++ | + | - | - | NA | NA | NA | NA |
| HSG treatment | ||||||||
| Set A | +++ | + | + | - | + | ++ | ++ | NA |
| Set B | ++ | ++ | + | ++ | ++ | ++ | - | - |
| “Untreated” | ||||||||
| Set A | + | ++ | + | + | ++ | - | + | NA |
| Set B | +++ | + | + | + | - | + | NA | NA |
| For CD8+ T cells | ||||||||
| Week | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| CLEC treatment | ||||||||
| Set A | ++ | ++ | ++ | + | + | + | + | NA |
| Set B | ++ | ++ | ++ | ++ | NA | NA | NA | NA |
| HSG treatment | ||||||||
| Set A | ++ | ++ | ++ | ++ | ++ | + | + | NA |
| Set B | + | ++ | ++ | ++ | ++ | ++ | ++ | + |
| “Untreated” | ||||||||
| Set A | + | ++ | ++ | ++ | ++ | ++ | ++ | NA |
| Set B | ++ | ++ | ++ | ++ | + | + | NA | NA |
| CLEC treatment | ||||||||
| Day 4 | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | |
| IFN-γ | + | |||||||
| IL-8 | ++++ | +++ | ||||||
| TNF-α | + | |||||||
| HSG treatment | ||||||||
| Day 4 | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | |
| IFN-γ | + | |||||||
| IL-1β | ++ | + | ||||||
| IL-2 | + | |||||||
| IL-6 | + | |||||||
| IL-8 | ++++ | +++ | ++++ | ++++ | +++ | |||
| IL-10 | + | + | ||||||
| IL-12 | + | + | ||||||
| IL-18 | + | + | ||||||
| TNF-α | +++ | +++ | +++ | ++ | ||||
| “Untreated” | ||||||||
| Day 4 | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | |
| IL-10 | + | + | + | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kua, J.E.H.; Siow, C.W.; Lim, W.K.; Masilamani, J.; Tjin, M.S.; Yeong, J.; Lim, T.K.H.; Phan, T.T.; Chua, A.W.C. Human Umbilical Cord Lining-Derived Epithelial Cells: A Potential Source of Non-Native Epithelial Cells That Accelerate Healing in a Porcine Cutaneous Wound Model. Int. J. Mol. Sci. 2022, 23, 8918. https://doi.org/10.3390/ijms23168918
Kua JEH, Siow CW, Lim WK, Masilamani J, Tjin MS, Yeong J, Lim TKH, Phan TT, Chua AWC. Human Umbilical Cord Lining-Derived Epithelial Cells: A Potential Source of Non-Native Epithelial Cells That Accelerate Healing in a Porcine Cutaneous Wound Model. International Journal of Molecular Sciences. 2022; 23(16):8918. https://doi.org/10.3390/ijms23168918
Chicago/Turabian StyleKua, Jonah Ee Hsiang, Chun Wei Siow, Wee Keng Lim, Jeyakumar Masilamani, Monica Suryana Tjin, Joe Yeong, Tony Kiat Hon Lim, Toan Thang Phan, and Alvin Wen Choong Chua. 2022. "Human Umbilical Cord Lining-Derived Epithelial Cells: A Potential Source of Non-Native Epithelial Cells That Accelerate Healing in a Porcine Cutaneous Wound Model" International Journal of Molecular Sciences 23, no. 16: 8918. https://doi.org/10.3390/ijms23168918
APA StyleKua, J. E. H., Siow, C. W., Lim, W. K., Masilamani, J., Tjin, M. S., Yeong, J., Lim, T. K. H., Phan, T. T., & Chua, A. W. C. (2022). Human Umbilical Cord Lining-Derived Epithelial Cells: A Potential Source of Non-Native Epithelial Cells That Accelerate Healing in a Porcine Cutaneous Wound Model. International Journal of Molecular Sciences, 23(16), 8918. https://doi.org/10.3390/ijms23168918







