Biochemical Hazards during Three Phases of Assisted Reproductive Technology: Repercussions Associated with Epigenesis and Imprinting
Abstract
:1. Introduction
- Preparation for and controlled ovarian hyperstimulation (COH).
- Methylation processes during in vitro development and culture of preimplantation embryos.
- Long-term effects of unmetabolized folic acid (UMFA) during embryogenesis on imprinting “tags”: these are usually sex-specific chemical marks that silence or activate genes. Abnormally high intakes of folic acid (FA) generally used to supplement ART pregnancies lead to accumulation of UMFA, which contributes further to the dysregulation of these three phases.
2. Imprinting and Epigenesis
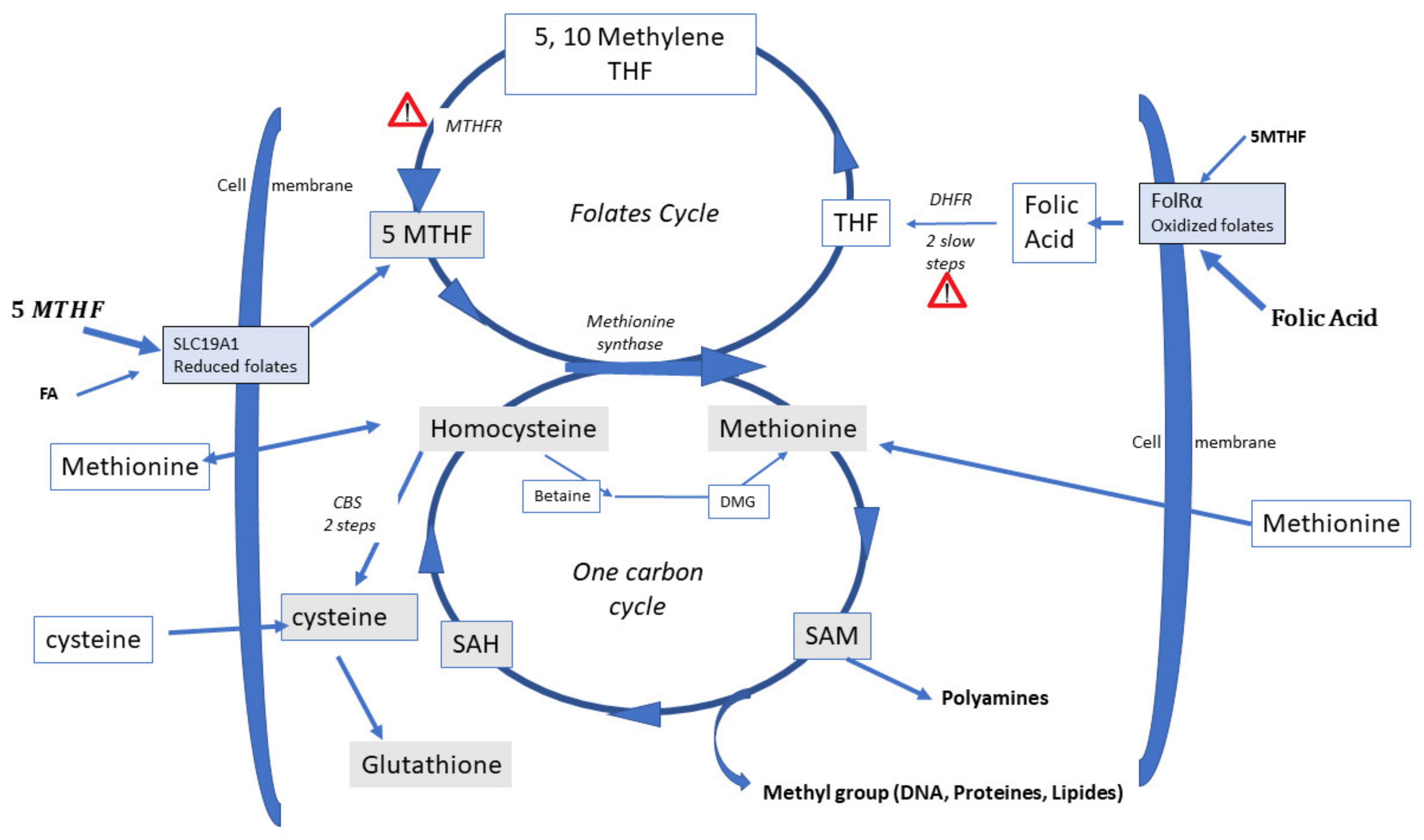
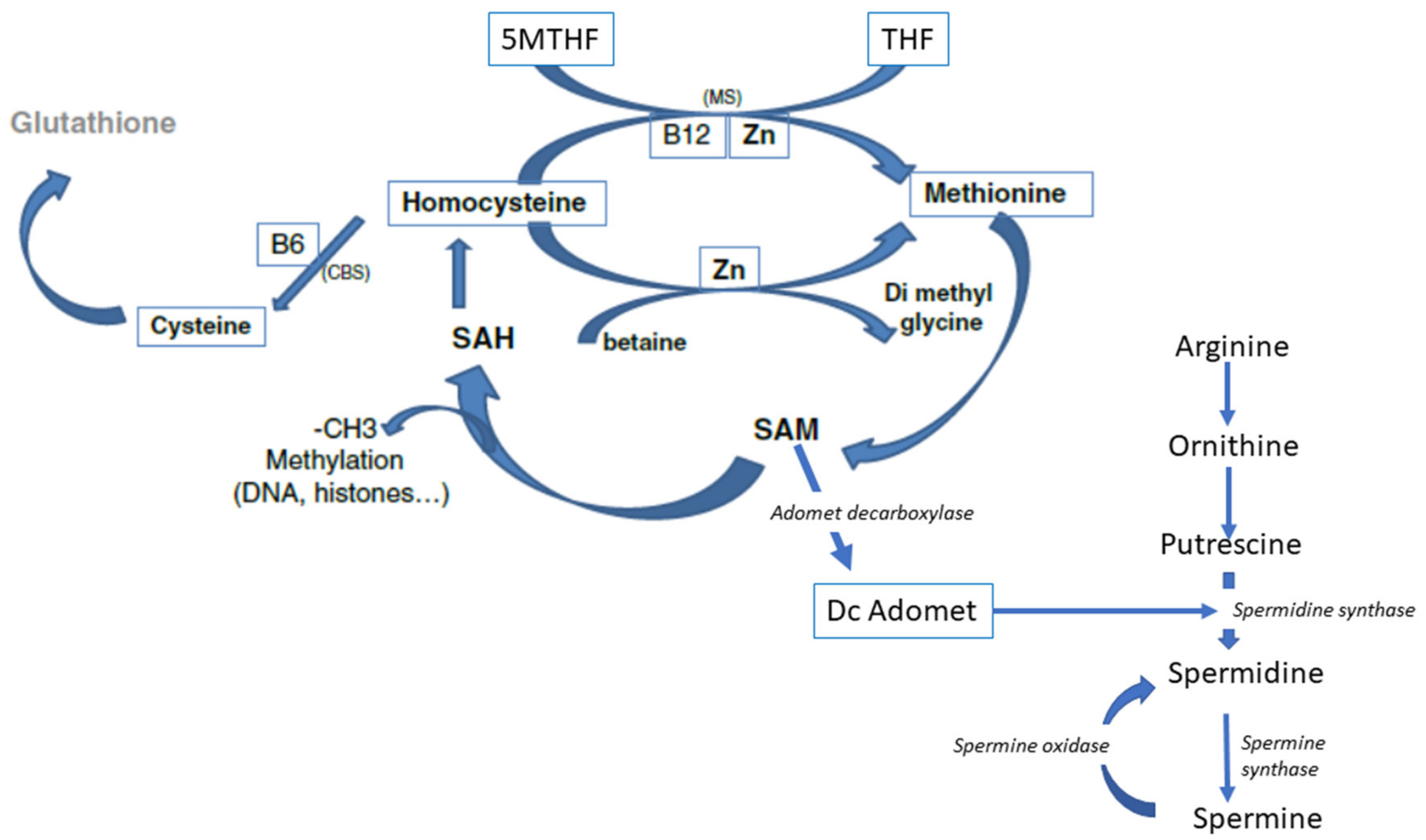
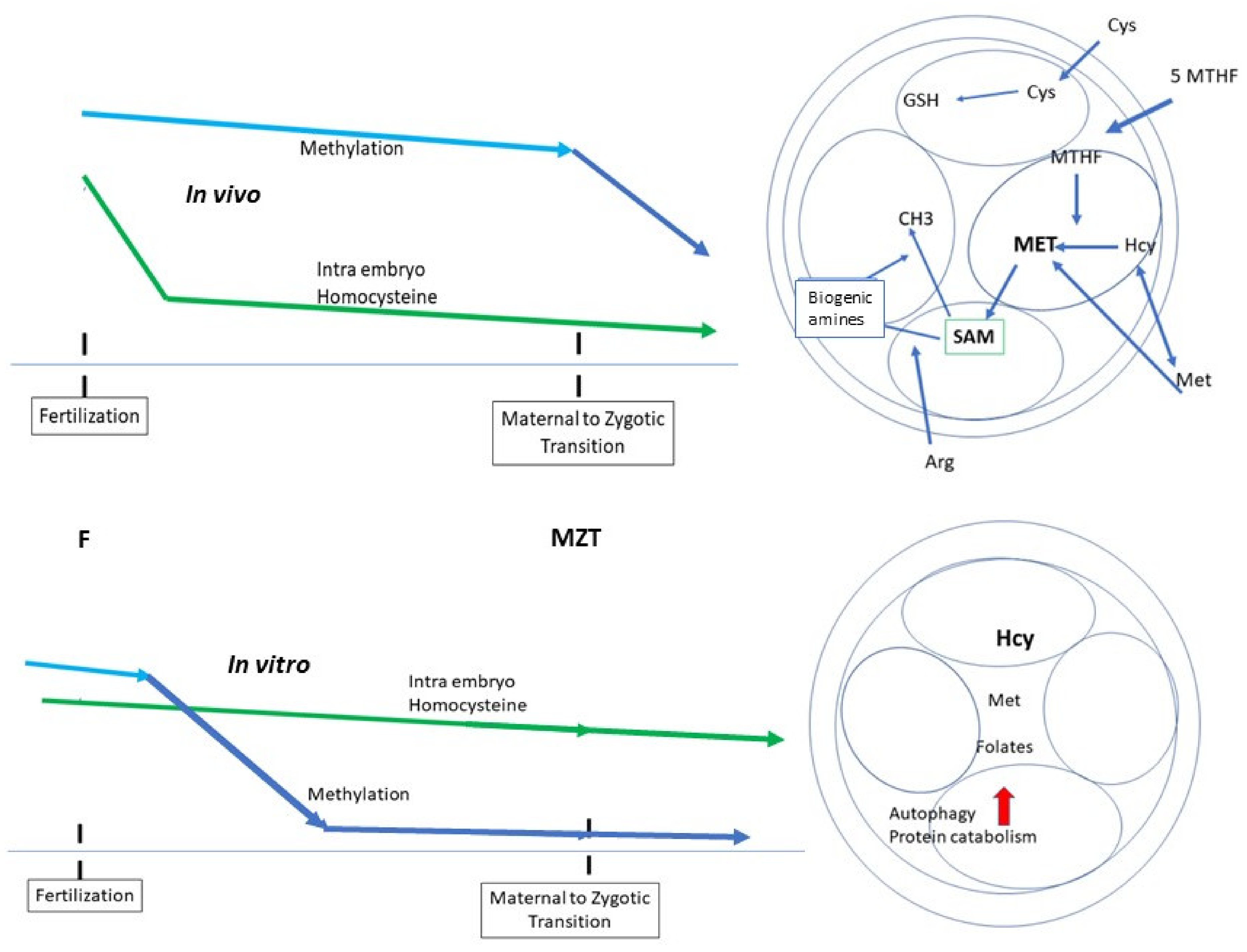
3. Biochemistry of Methylation
4. Homocysteine Recycling
Three Different Pathways can Recycle Hcy
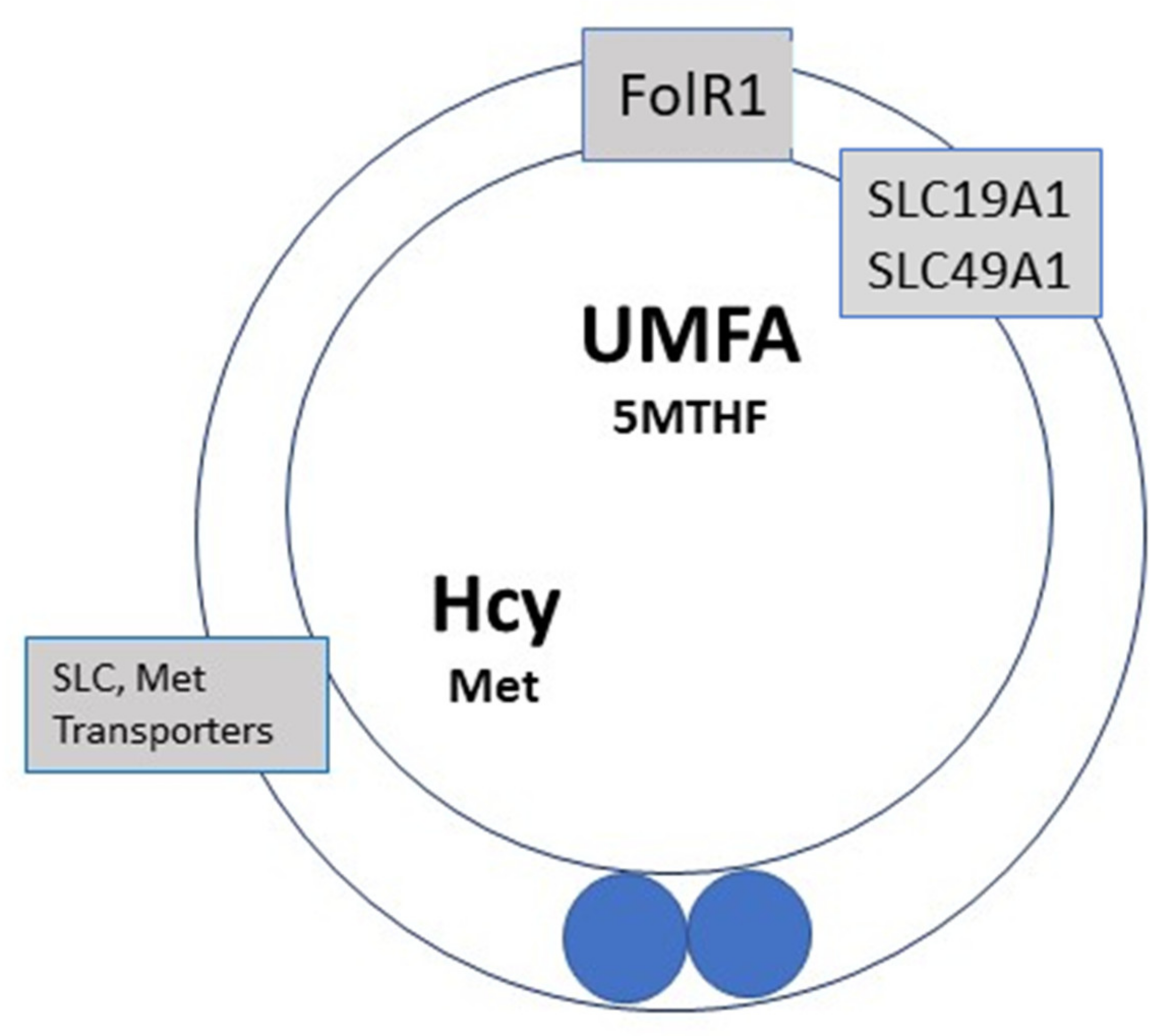
- Recycling to methionine via interaction with 5-Methyl Tetrahydrofolate (5-MTHF, a component of the folate cycle) and methionine synthase (MS, zinc, and vitamin B12-dependent). Cellular transport of 5-MTHF is carried out by two systems: SLC (solute carrier) 19A1, also known as Reduced Folate Carrier (RFC), which has a poor affinity for folic acid. Folate receptor alpha (FolR1) has a much higher affinity for folic acid; it binds folates and internalizes them by endocytosis [16].
- Hcy can be recycled by the enzyme Cystathionine Beta Synthase (CBS), leading to the formation of cysteine and then glutathione; all the enzymes involved in glutathione metabolism are present and active in oocytes and preimplantation embryos. This is an important feature, as glutathione protects the epigenetic marking from de-methylation due to undue oxidative stress, thus preserving redox homeostasis [17,18]. The cystathionine beta synthase pathway also generates hydrogen sulfide (H2S), which is important in cell signaling and in post-translational modifications [19]. The CBS pathway, however, is poorly expressed in the preimplantation embryo [20] and in the placenta [21,22].
- A third mechanism, available primarily in the liver, uses betaine homocysteine to recycle homocysteine to methionine: this pathway is also poorly expressed in the human preimplantation embryo and placenta. This pathway is active in the mouse embryo.
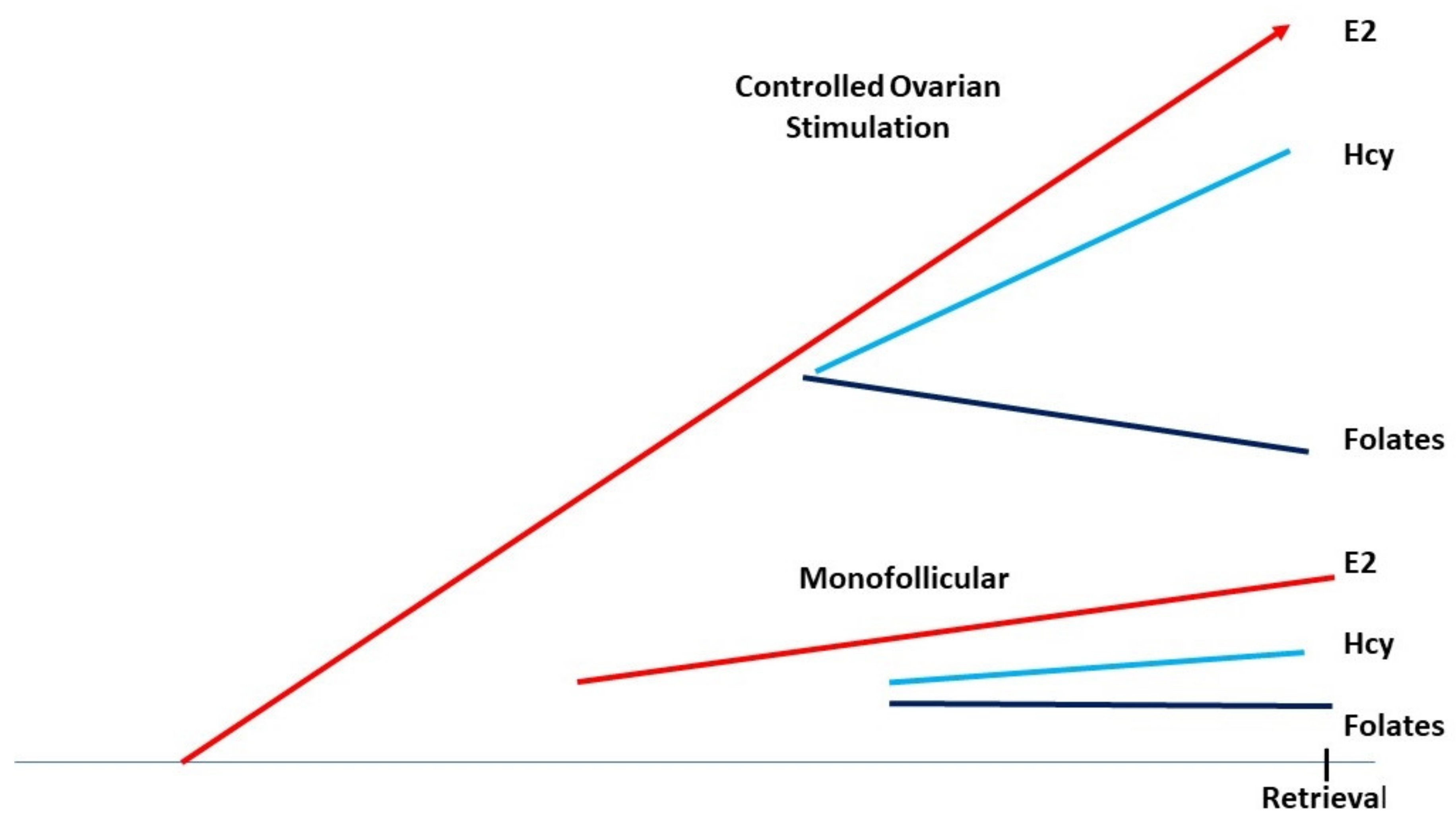
5. Controlled Ovarian Hyperstimulation, Folates, Methionine, and Homocysteine
6. Early-Stage Embryo Culture, from Fertilization to Genomic Activation
6.1. Cysteine/Cystine Requirement
6.2. Methionine Availability Is Crucial
6.3. Arginine and Polyamines
6.4. The Folates
7. Impact on ART
- The culture system itself does not allow appropriate methylation/epigenetic marking.
- An increase in the rate/speed of embryo development, sometimes considered as a standard of embryo quality, is due to a defective imprint methylation procedure [49]. The timescale necessary for 2 biochemical processes, translation of the stored mRNAs and epigenetic marking, must be respected.
8. Salient Points Surrounding the Importance of Methylation Processes during In Vitro Culture
9. High Doses of FA during Pregnancy, Unmetabolized Folic Acid (UMFA), and the Impact on Embryo Health
10. Discussion and Conclusions
10.1. Controlled Ovarian Stimulation in the Presence of Abnormally High Doses of FA
10.2. Duration of In Vitro Culture
10.3. Folic Acid Supplementation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faddy, M.J.; Gosden, M.D.; Gosden, R.G. A demographic projection of the contribution of assisted reproductive technologies to world population growth. Reprod. Biomed. Online 2018, 36, 455–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fauser, B.C. Towards the global coverage of a unified registry of IVF outcomes. Reprod. Biomed. Online 2019, 38, 133–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calhaz-Jorge, C.; De Geyter, C.H.; Kupka, M.S.; Wyns, C.; Mocanu, E.; Motrenko, T.; Scaravelli, G.; Smeenk, J.; Vidakovic, S.; Goossens, V. Survey on ART and IUI: Legislation, regulation, funding and registries in European countries: The European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum. Reprod. Open 2020, 2020, hoz044. [Google Scholar] [CrossRef] [PubMed]
- De Geyter, C.; Wyns, C.; Calhaz-Jorge, C.; de Mouzon, J.; Ferraretti, A.P.; Kupka, M.; Nyboe Andersen, A.; Nygren, K.G.; Goossens, V. 20 years of the European IVF-monitoring Consortium registry: What have we learned? A comparison with registries from two other regions. Hum. Reprod. 2020, 35, 2832–2849. [Google Scholar] [CrossRef] [PubMed]
- Choux, C.; Binquet, C.; Carmignac, V.; Bruno, C.; Chapusot, C.; Barberet, J.; Lamotte, M.; Sagot, P.; Bourc’his, D.; Fauque, P. The epigenetic control of transposable elements and imprinted genes in newborns is affected by the mode of conception: ART versus spontaneous conception without underlying infertility. Hum. Reprod. 2018, 33, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Hiura, H.; Okae, H.; Miyauchi, N.; Sato, F.; Sato, A.; Van De Pette, M.; John, R.M.; Kagami, M.; Nakai, K.; Soejima, H.; et al. Characterization of DNA methylation errors in patients with disorders conceived by assisted reproduction technologies. Hum. Reprod. 2012, 8, 2541–2548. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Ghosh, J.; Mainigi, M.; Turan, N.; Weinerman, R.; Truongcao, M.; Coutifaris, C.; Sapienza, C. DNA methylation differences between in vitro and in vivo conceived children are associated with ART procedures rather than infertility. Clin. Epigenetics 2015, 7, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Katari, S.; Turan, N.; Bibikova, M.; Erinle, O.; Chalian, R.; Foster, M.; Gaughan, J.P.; Coutifaris, C.; Sapienza, C. DNA methylation and gene expression differences in children conceived in vitro or in vivo. Hum. Mol. Genet. 2009, 18, 3769–3778. [Google Scholar] [CrossRef]
- Sciorio, R.; El Haji, N. Epigenetic risks of medically assisted reproduction. J. Clin. Med. 2022, 11, 2151. [Google Scholar] [CrossRef]
- Håberg, S.E.; Page, C.M.; Lee, Y.; Nustad, H.E.; Magnus, M.C.; Haftorn, K.L.; Carlsen, E.; Denault, W.R.P.; Bohlin, J.; Jugessur, A.; et al. DNA methylation in newborns conceived by assisted reproductive technology. Nat. Commun. 2022, 13, 1896. [Google Scholar] [CrossRef]
- Hattori, H.; Hiura, H.; Kitamura, A.; Miyauchi, N.; Kobayashi, N.; Takahashi, S.; Okae, H.; Kyono, K.; Kagami, M.; Ogata, T.; et al. Association of four imprinting disorders and ART. Clin. Epigenetics 2019, 11, 21. [Google Scholar] [CrossRef] [Green Version]
- Lazaraviciute, G.; Kauser, M.; Bhattacharya, S.; Haggarty, P.; Bhattacharya, S. A systematic review and meta-analysis of DNA methylation levels and imprinting disorders in children conceived by IVF/ICSI compared with children conceived spontaneously. Hum. Reprod. Update 2014, 20, 840–852. [Google Scholar] [CrossRef] [Green Version]
- Menezo, Y.; Servy, E. Advanced Paternal Age and Endocrine Disruptors: Two Causes of Psychiatric Disorders in Children, with DNA Methylation Dys-Regulation as a Common Biochemical Mechanism 2017 in Psychiatric Disorders. pp. 1–12. Available online: www.smgebooks.com (accessed on 20 September 2017).
- Menezo, Y.J.; Elder, K.; Dale, B. Link Between Increased Prevalence of Autism Spectrum Disorder Syndromes and Oxidative Stress, DNA Methylation, and Imprinting: The Impact of the Environment. JAMA Pediatr. 2015, 169, 1066–1067. [Google Scholar] [CrossRef] [Green Version]
- Menezo, Y.; Khatchadourian, C.; Gharib, A.; Hamidi, J.; Greenland, T.; Sarda, N. Regulation of S-adenosyl methionine synthesis in the mouse embryo. Life Sci. 1989, 44, 1601–1609. [Google Scholar] [CrossRef]
- Kelemen, L.E. The role of folate receptor alpha in cancer development, progression and treatment: Cause, consequence or innocent bystander? Int. J. Cancer 2006, 119, 243–250. [Google Scholar] [CrossRef]
- Menezo, Y.; Dale, B.; Elder, K. Modulating oxidative stress and epigenetic homeostasis in preimplantation IVF embryos. Zygote 2022, 30, 149–158. [Google Scholar] [CrossRef]
- Leese, H.J.; McKeegan, P.J.; Sturmey, R.G. Amino Acids and the Early Mammalian Embryo: Origin, Fate, Function and Life-Long Legacy. Int. J. Environ. Res. Public Health 2021, 18, 9874. [Google Scholar] [CrossRef]
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Regulators of the transsulfuration pathway. Br. J. Pharmacol. 2019, 176, 583–593. [Google Scholar] [CrossRef]
- Ménézo, Y.; Lichtblau, I.; Elder, K. New insights into human pre-implantation metabolism in vivo and in vitro. J. Assist. Reprod. Genet. 2013, 30, 293–303. [Google Scholar] [CrossRef] [Green Version]
- Tsitsiou, E.; Sibley, C.P.; D’Souza, S.W.; Catanescu, O.; Jacobsen, D.W. Homocysteine is transported by the microvillous plasma membrane of human placenta. J. Inherit. Metab. Dis. 2011, 34, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Solanky, N.; Jimenez, A.R.; D’Souza, S.; Sibley, C.; Glazier, J. Expression of folate transporters in human placenta and implications for homocysteine metabolism. Placenta 2010, 31, 134–143. [Google Scholar] [CrossRef]
- Duthie, S.; Narayanan, S.; Brand, G.; Pirie, L.; Grant, G. Impact of folate deficiency on DNA stability. J. Nutr. 2002, 132 (Suppl. 8), 2444S–2449S. [Google Scholar] [CrossRef]
- Enciso, M.; Sarasa, J.; Xanthopoulou, L.; Bristow, S.; Bowles, M.; Fragouli, E.; Delhanty, J.; Wells, D. Polymorphisms in the MTHFR gene influence embryo viability and the incidence of aneuploidy. Hum. Genet. 2016, 135, 555–568. [Google Scholar] [CrossRef]
- Benkhalifa, M.; Montjean, D.; Cohen-Bacrie, P.; Ménézo, Y. Imprinting: RNA expression for homocysteine recycling in the human oocyte. Fertil. Steril. 2010, 93, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Boxmeer, J.C.; Brouns, R.M.; Lindemans, J.; Steegers, E.A.; Martini, E. Preconception folic acid treatment affects the microenvironment of the maturing oocyte in humans. Fertil. Steril. 2008, 89, 1766–1770. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.W.; Ayling, J.E. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc. Natl. Acad. Sci. USA 2009, 106, 15424–15429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menezo, Y.; Elder, K.; Clement, A.; Clement, P. Folic Acid, Folinic Acid, 5 Methyl TetraHydroFolate Supplementation for Mutations That Affect Epigenesis through the Folate and One-Carbon Cycles. Biomolecules 2022, 12, 197. [Google Scholar] [CrossRef]
- Boxmeer, J.C.; Macklon, N.S.; Lindemans, J.; Beckers, N.G.; Eijkemans, M.J.; Laven, J.S.; Steegers, E.A.; Steegers-Theunissen, R.P. IVF outcomes are associated with biomarkers of the homocysteine pathway in monofollicular fluid. Hum. Reprod. 2009, 24, 1059–1066. [Google Scholar] [CrossRef]
- Szymański, W.; Kazdepka-Ziemińska, A. Effect of homocysteine concentration in follicular fluid on a degree of oocyte maturity. Ginekol. Pol. 2003, 74, 1392–1396. [Google Scholar]
- Berker, B.; Kaya, C.; Aytac, R.; Satiroglu, H. Homocysteine concentrations in follicular fluid are associated with poor oocyte and embryo qualities in polycystic ovary syndrome patients undergoing assisted reproduction. Hum. Reprod. 2009, 24, 2293–2302. [Google Scholar] [CrossRef] [Green Version]
- Razi, Y.; Eftekhar, M.; Fesahat, F.; Dehghani Firouzabadi, R.; Razi, N.; Sabour, M.; Razi, M.H. Concentrations of homocysteine in follicular fluid and embryo quality and oocyte maturity in infertile women: A prospective cohort. J. Obstet. Gynaecol. 2021, 41, 588–593. [Google Scholar] [CrossRef]
- Navarrete-Muñoz, E.M.; Valera-Gran, D.; Garcia-de-la-Hera, M.; Gonzalez-Palacios, S.; Riaño, I.; Murcia, M.; Lertxundi, A.; Guxens, M.; Tardón, A.; Amiano, P.; et al. INMA Project. High doses of folic acid in the periconceptional period and risk of low weight for gestational age at birth in a population-based cohort study. Eur. J. Nutr. 2019, 58, 241–251. [Google Scholar] [CrossRef]
- Valera-Gran, D.; Navarrete-Muñoz, E.M.; De La Hera, M.G.; Fernández-Somoano, A.; Tardón, A.; Ibarluzea, J.; Balluerka, N.; Murcia, M.; González-Safont, L.; Romaguera, D.; et al. Effect of maternal high dosages of folic acid supplements on neurocognitive development in children at 4-5 y of age: The prospective birth cohort Infancia y Medio Ambiente (INMA) study. Am. J. Clin. Nutr. 2017, 106, 878–887. [Google Scholar] [CrossRef] [Green Version]
- Ly, L.; Chan, D.; Landry, M.; Angle, C.; Martel, J.; Trasler, J. impact of mothers’ early life exposure to low or high folate on progeny outcome and DNA methylation patterns. Environ. Epigenet. 2020, 6, dvaa018. [Google Scholar] [CrossRef]
- Barroso, M.; Handy, D.E.; Castro, R. The link between Hyperhomocysteinemia and Hypomethylation: Implication for cardiovascular disease. J. Inborn Errors Metab. Screen. 2017, 5, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Menezo, Y.J.; Silvestris, E.; Dale, B.; Elder, K. Oxidative stress and alterations in DNA methylation: Two sides of the same coin in reproduction. Reprod. Biomed. Online 2016, 33, 668–683. [Google Scholar] [CrossRef] [Green Version]
- Smith, Z.D.; Chan, M.M.; Humm, K.C.; Karnik, R.; Mekhoubad, S.; Regev, A.; Eggan, K.; Meissner, A. DNA methylation dynamics of the human pre-implantation embryo. Nature 2014, 511, 611–615. [Google Scholar] [CrossRef] [Green Version]
- Croteau, S.; Menezo, Y. Methylation in fertilized and parthenogenetic preimplantation mouse embryos. Zygote 1994, 2, 47–52. [Google Scholar] [CrossRef]
- Okamoto, Y.; Yoshida, N.; Suzuki, T.; Shimozawa, N.; Asami, M.; Matsuda, T.; Kojima, N.; Perry, A.C.F.; Takada, T. DNA methylation dynamics in mouse preimplantation embryos revealed by mass spectrometry. Sci. Rep. 2016, 6, 19134. [Google Scholar] [CrossRef] [Green Version]
- Fulka, H.; Mrazek, M.; Tepla, O.; Fulka, J., Jr. DNA methylation pattern in human zygotes and developing embryos. Reproduction 2004, 128, 703–708. [Google Scholar] [CrossRef]
- Sun, H.; Kang, J.; Su, J.; Zhang, J.; Zhang, L.; Liu, X.; Zhang, J.; Wang, F.; Lu, Z.; Xing, X.; et al. Methionine adenosyltransferase 2A regulates mouse zygotic genome activation and morula to blastocyst transition. Biol. Reprod. 2019, 100, 601–617. [Google Scholar] [CrossRef]
- Chen, L.; Xu, T.; Qiu, Y.; Liu, N.; Ke, X.; Fang, L.; Yan, J.; Zhu, D. Homocysteine induced a calcium-mediated disruption of mitochondrial function and dynamics in endothelial cells. J. Biochem. Mol. Toxicol. 2021, 35, e22737. [Google Scholar] [CrossRef]
- Lu, S.; Hoestje, S.M.; Choo, E.; Epner, D.E. Induction of caspase dependant and independant apoptosis in response to methionine restriction. Int. J. Oncol. 2003, 22, 415–420. [Google Scholar]
- Khatchadourian, C.; Guillaud, J.; Menezo, Y. interactions in glycine and methionine uptake, conversion and incorporation into proteins in the preimplantation mouse embryo. Zygote 1994, 2, 301–306. [Google Scholar] [CrossRef]
- Pey, A.L.; Majtan, T.; Sanchez-Ruiz, J.M.; Kraus, J.P. Human cystathionine beta-synthase (CBS) contains two classes of binding sites for S-adenosylmethionine (SAM): Complex regulation of CBS activity and stability by SAM. Biochem. J. 2013, 449, 109–121. [Google Scholar] [CrossRef] [Green Version]
- Soda, K. Polyamine Metabolism and Gene Methylation in Conjunction with One-Carbon Metabolism. Int. J. Mol. Sci. 2018, 19, 3106. [Google Scholar] [CrossRef] [Green Version]
- Market-Velker, B.A.; Fernandes, A.D.; Mann, M.R. Side-by-side comparison of five commercial media systems in a mouse model: Suboptimal in vitro culture interferes with imprint maintenance. Biol. Reprod. 2010, 83, 938–950. [Google Scholar] [CrossRef] [Green Version]
- Market Velker, B.A.; Denomme, M.M.; Mann, M.R. Loss of genomic imprinting in mouse embryos with fast rates of preimplantation development in culture. Biol. Reprod. 2012, 86, 1–16. [Google Scholar] [CrossRef]
- Gardner, D.K.; Lane, M. Culture and selection of viable blastocysts: A feasible proposition for human IVF? Hum. Reprod. Update 1997, 3, 367–382. [Google Scholar] [CrossRef]
- Lane, M.; Gardner, D.K. Nonessential amino acids and glutamine decrease the time of the first three cleavage divisions and increase compaction of mouse zygotes in vitro. J. Assist. Reprod. Genet. 1997, 14, 398–403. [Google Scholar] [CrossRef] [Green Version]
- Lane, M.; Gardner, D.K. Differential regulation of mouse embryo development and viability by amino acids. J. Reprod. Fertil. 1997, 109, 153–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, M.; Hooper, K.; Gardner, D.K. Effect of essential amino acids on mouse embryo viability and ammonium production. J. Assist. Reprod. Genet. 2001, 18, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Morbeck, D.E.; Krisher, R.L.; Herrick, J.R.; Baumann, N.A.; Matern, D. Composition of commercial media used for human embryo culture. Fertil. Steril. 2014, 102, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Clare, C.E.; Pestinger, V.; Kwong, W.Y.; Tutt, D.A.R.; Xu, J.; Byrne, H.M.; Barrett, D.A.; Emes, R.D.; Sinclair, K.D. Interspecific Variation in One-Carbon Metabolism within the Ovarian Follicle, Oocyte, and Preimplantation Embryo: Consequences for Epigenetic Programming of DNA Methylation. Int. J. Mol. Sci. 2021, 22, 1838. [Google Scholar] [CrossRef]
- Young, L.E.; Sinclair, K.D.; Wilmut, I. Large offspring syndrome in cattle and sheep. Rev. Reprod. 1998, 3, 155–163. [Google Scholar] [CrossRef]
- Chen, Z.; Hagen, D.E.; Elsik, C.G.; Ji, T.; Morris, C.J. Characterization of global loss of imprinting in fetal overgrowth syndrome induced by assisted reproduction. Proc. Natl. Acad. Sci. USA 2015, 112, 4618–4623. [Google Scholar] [CrossRef] [Green Version]
- Martín-Romero, F.J.; Miguel-Lasobras, E.M.; Domínguez-Arroyo, J.A.; González-Carrera, E.; Alvarez, I.S. Contribution of culture media to oxidative stress and its effect on human oocytes. Reprod. Biomed. Online 2008, 17, 652–661. [Google Scholar] [CrossRef]
- Kaplan, P.; Tatarkova, Z.; Sivonova, M.; Racay, P.; Lehotsky, J. Homocysteine and Mitochondria in Cardiovascular and Cerebrovascular Systems. Int. J. Mol. Sci. 2020, 21, 7698. [Google Scholar] [CrossRef]
- Ménézo, Y.; Elder, K. Epigenetic remodeling of chromatin in human ART: Addressing deficiencies in culture media. J. Assist. Reprod. Genet. 2020, 37, 1781–1788. [Google Scholar] [CrossRef]
- Herrick, J.R.; Lyons, S.M.; Greene-Ermisch, A.F.; Broeckling, C.D.; Schoolcraft, W.B.; Krisher, R.L. A carnivore embryo’s perspective on essential amino acids and ammonium in culture medium: Effects on the development of feline embryos. Biol. Reprod. 2018, 99, 1070–1081. [Google Scholar] [CrossRef]
- Kalmbach, R.D.; Choumenkovitch, S.F.; Troen, A.M.; D’Agostino, R.; Jacques, P.F.; Selhub, J. Circulating folic acid in plasma: Relation to folic acid fortification. Am. J. Clin. Nutr. 2008, 88, 763–768. [Google Scholar] [CrossRef] [Green Version]
- Plumptre, L.; Masih, S.P.; Ly, A.; Aufreiter, S.; Sohn, K.J. High concentrations of folate and unmetabolized folic acid in a cohort of pregnant Canadian women and umbilical cord blood. Am. J. Clin. Nutr. 2015, 102, 848–857. [Google Scholar] [CrossRef]
- Pfeiffer, C.M.; Sternberg, M.R.; Fazili, Z.; Yetley, E.A.; Lacher, D.A.; Bailey, R.L.; Johnson, C.L. Unmetabolized folic acid is detected in nearly all serum samples from US children, adolescents, and adults. J. Nutr. 2015, 145, 520–531. [Google Scholar] [CrossRef] [Green Version]
- Kubo, Y.; Fukuoka, H.; Kawabata, T.; Shoji, K.; Mori, C.; Sakurai, K.; Nishikawa, M.; Ohkubo, T.; Oshida, K.; Yanagisawa, N.; et al. Distribution of 5-Methyltetrahydrofolate and Folic Acid Levels in Maternal and Cord Blood Serum: Longitudinal Evaluation of Japanese Pregnant Women. Nutrients 2020, 12, 1633. [Google Scholar] [CrossRef]
- Škovierová, H.; Vidomanová, E.; Mahmood, S.; Sopková, J.; Drgová, A.; Červeňová, T.; Halašová, E.; Lehotský, J. The Molecular and Cellular Effect of Homocysteine Metabolism Imbalance on Human Health. Int. J. Mol. Sci. 2016, 17, 1733. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.D.; Refsum, H.; Bottiglieri, T.; Fenech, M.; Hooshmand, B.; McCaddon, A.; Miller, J.W.; Rosenberg, I.H.; Obeid, R. Homocysteine and Dementia: An International Consensus Statement. J. Alzheimers Dis. 2018, 62, 561–570. [Google Scholar] [CrossRef] [Green Version]
- Steinfeld, R.; Grapp, M.; Kraetzner, R.; Dreha-Kulaczewski, S.; Helms, G.; Dechent, P.; Wevers, R.; Grosso, S.; Gärtner, J. Folate receptor alpha defect causes cerebral folate transport deficiency: A treatable neurodegenerative disorder associated with disturbed myelin metabolism. Am. J. Hum. Genet. 2009, 85, 354–363. [Google Scholar] [CrossRef] [Green Version]
- Menezo, Y.; Clement, P.; Elder, K. Are UMFA (un-metabolized folic acid) and endocrine disruptor chemicals (EDCs) co-responsible for sperm degradation ? An epigenetic/methylation perspective. Andrologia 2022, 11, e14400. [Google Scholar] [CrossRef]
- Tang, Z.R.; Xu, X.L.; Deng, S.L.; Lian, Z.X.; Yu, K. Oestrogenic endocrine disruptors in the placenta and the fetus. Int. J. Mol. Sci. 2020, 21, 1519. [Google Scholar] [CrossRef] [Green Version]
- Manikkam, M.; Tracey, R.; Guerrero-Bosagna, C.; Skinner, M.K. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS ONE 2013, 8, e55387. [Google Scholar] [CrossRef]
- Tanner EMHallerbäck, M.U.; Wikström, S.; Lindh, C.; Kiviranta, H. Early prenatal exposure to suspected endocrine disruptor mixtures is associated with lower IQ at age seven. Environ. Int. 2020, 134, 105185. [Google Scholar] [CrossRef]
- Lucaccioni, L.; Trevisani, V.; Passini, E.; Righi, B.; Plessi, C. Perinatal Exposure to Phthalates: From Endocrine to Neurodevelopment Effects. Int. J. Mol. Sci. 2021, 22, 4063. [Google Scholar] [CrossRef]
- Sunde, A.; Brison, D.; Dumoulin, J.; Harper, J.; Lundin, K.; Magli, M.C.; Van den Abbeel, E.; Veiga, A. Time to take human embryo culture seriously. Hum. Reprod. 2016, 31, 2174–2182. [Google Scholar] [CrossRef] [Green Version]
- Rubini, E.; Snoek, K.M.; Schoenmakers, S.; Willemsen, S.P.; Sinclair, K.D.; Rousian, M.; Steegers-Theunissen, R.P.M. First Trimester Maternal Homocysteine and Embryonic and Fetal Growth: The Rotterdam Periconception Cohort. Nutrients 2022, 14, 1129. [Google Scholar] [CrossRef]
- Dvoran, M.; Nemcova, L.; Kalous, J. An Interplay between Epigenetics and Translation in Oocyte Maturation and Embryo Development: Assisted Reproduction Perspective. Biomedicines 2022, 10, 1689. [Google Scholar] [CrossRef]
| Vitrolife | Sage | COOK | In Vitro Care | Origio | IVF Online | Cooper | Irvine | |
|---|---|---|---|---|---|---|---|---|
| Medium | G1 | QACM | SICM | IVC1 | Seq Fert/Cleav * | Global | Universal IVF | CSC |
| Cys | 0 | 0 | 2 | 0 | 0 | 52 | 0 | 46 |
| Met | 0 | 0 | 4 | 0 | 0 | 51 | 0 | 53 |
| Arg | 0 | 0 | 25 | 0 | 0 | 328 | 0 | 281 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menezo, Y.; Elder, K.; Clement, P.; Clement, A.; Patrizio, P. Biochemical Hazards during Three Phases of Assisted Reproductive Technology: Repercussions Associated with Epigenesis and Imprinting. Int. J. Mol. Sci. 2022, 23, 8916. https://doi.org/10.3390/ijms23168916
Menezo Y, Elder K, Clement P, Clement A, Patrizio P. Biochemical Hazards during Three Phases of Assisted Reproductive Technology: Repercussions Associated with Epigenesis and Imprinting. International Journal of Molecular Sciences. 2022; 23(16):8916. https://doi.org/10.3390/ijms23168916
Chicago/Turabian StyleMenezo, Yves, Kay Elder, Patrice Clement, Arthur Clement, and Pasquale Patrizio. 2022. "Biochemical Hazards during Three Phases of Assisted Reproductive Technology: Repercussions Associated with Epigenesis and Imprinting" International Journal of Molecular Sciences 23, no. 16: 8916. https://doi.org/10.3390/ijms23168916
APA StyleMenezo, Y., Elder, K., Clement, P., Clement, A., & Patrizio, P. (2022). Biochemical Hazards during Three Phases of Assisted Reproductive Technology: Repercussions Associated with Epigenesis and Imprinting. International Journal of Molecular Sciences, 23(16), 8916. https://doi.org/10.3390/ijms23168916







