1. Introduction
Vascular targeting with precision thrombosis is an approach aimed at occlusion and destruction of pathological vasculature in tumors and brain arteriovenous malformations (AVMs) [
1,
2,
3]. This approach requires the identification of discriminatory biomarkers at the luminal surface of the pathological endothelium for use as molecular targets for the focused delivery of thrombogenic agents. To date, various endogenous markers have been identified on tumor vasculature and investigated in mouse tumor infarction models (reviewed in [
1]). However, limitations to endogenous biomarker discovery in brain arteriovenous malformations (AVMs) have led to the use of ionizing radiation as a priming agent for target generation in this context [
4,
5,
6,
7,
8,
9,
10,
11,
12,
13].
Stereotactic radiosurgery can deliver a focused dose of radiation to clustered vessels in an AVM nidus, allowing precise target expression for radiation-guided drug delivery [
14]. Radiation damage sets off a cascade of cellular events in endothelial cells that can alter the molecules or proteins at the cell surface (surfaceome) [
4,
5,
15,
16]. Surface exposure of common inflammatory markers such as phosphatidylserine (PS), vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1) have been previously investigated as radiation-stimulated targets in an AVM animal model [
6,
7,
13,
17]. However, their frequent exposure in response to other biological stimuli may reduce targeting specificity. Interestingly, canonical autophagic pathways that normally process damaged molecules via the lysosome appear to become overwhelmed after radiation exposure, leading to the non-canonical transport of intracellular proteins to the extracellular space or cell surface [
18]. Functionally, this may represent a survival mechanism for the removal of toxic proteins or a signal (exposure of “self” antigens) to alert the immune system to the presence of stressed or damaged cells [
19]. Regardless, these normally intracellular proteins may represent highly discriminatory markers for targeting on the surface of radiation-primed vasculature, given their absence on the surface of healthy vasculature and in vessels exposed to other biological stimuli. One such protein is the E2 subunit of pyruvate dehydrogenase (PDCE2). This mitochondrial protein can translocate from intracellular compartments to the endothelial surface in response to high doses of radiation via this unconventional pathway [
4,
18]. The lack of surface exposure of PDCE2 in healthy tissues may make this an ideal target. To date, ectopic PDCE2 exposure has only been described in primary biliary cirrhosis, an autoimmune disorder causing the inflammation and destruction of the intrahepatic ducts [
20,
21,
22].
The primary objective of this study was to assess PDCE2 as a molecular target for radiation-guided delivery of pro-thrombotic agents. A vascular targeting agent (VTA) was designed to deliver the pro-coagulant thrombin to PDCE2 exposed at the endothelial surface after irradiation. An anti-PDCE2 antibody was chemically linked to thrombin to create a “coaguligand” as VTA [
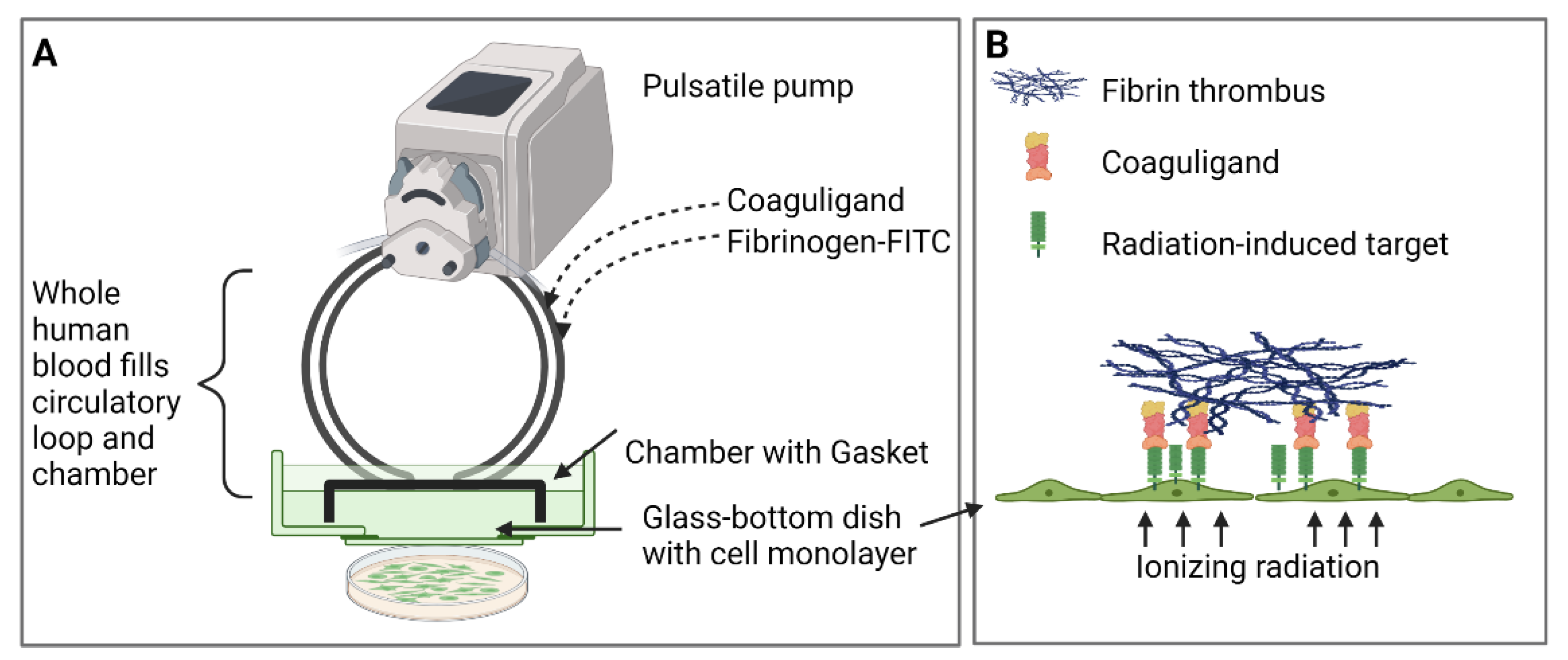
3]. VTAs were tested in vitro for focused thrombosis induction using a parallel-plate flow system where whole human blood is circulated in a pulsatile manner over irradiated endothelial monolayers. This system is an economical and ethical way to assess VTA specificity and efficacy before translation to animal models and has been used successfully to assess other radiation-induced markers and associated pro-thrombotic VTAs [
11,
12]. Using this system, we demonstrate that the endothelial surface expression of PDCE2 after 15–25 Gy of radiation is sufficient to bind targeting antibodies under a high flow and confirm that the antibody-directed delivery of thrombotic effectors to ectopic PDCE2 can induce selective thrombosis on irradiated human endothelial cells.
3. Discussion
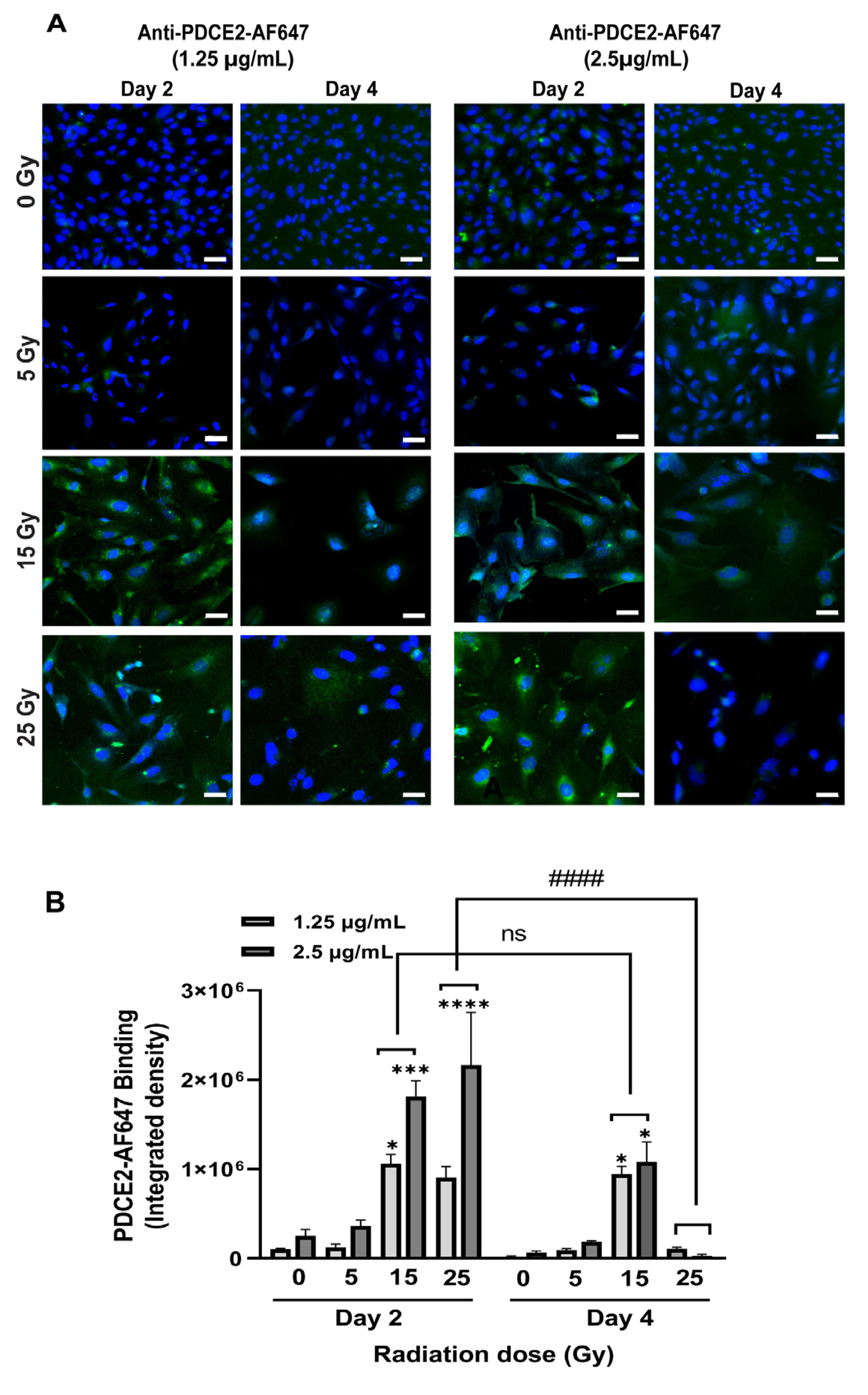
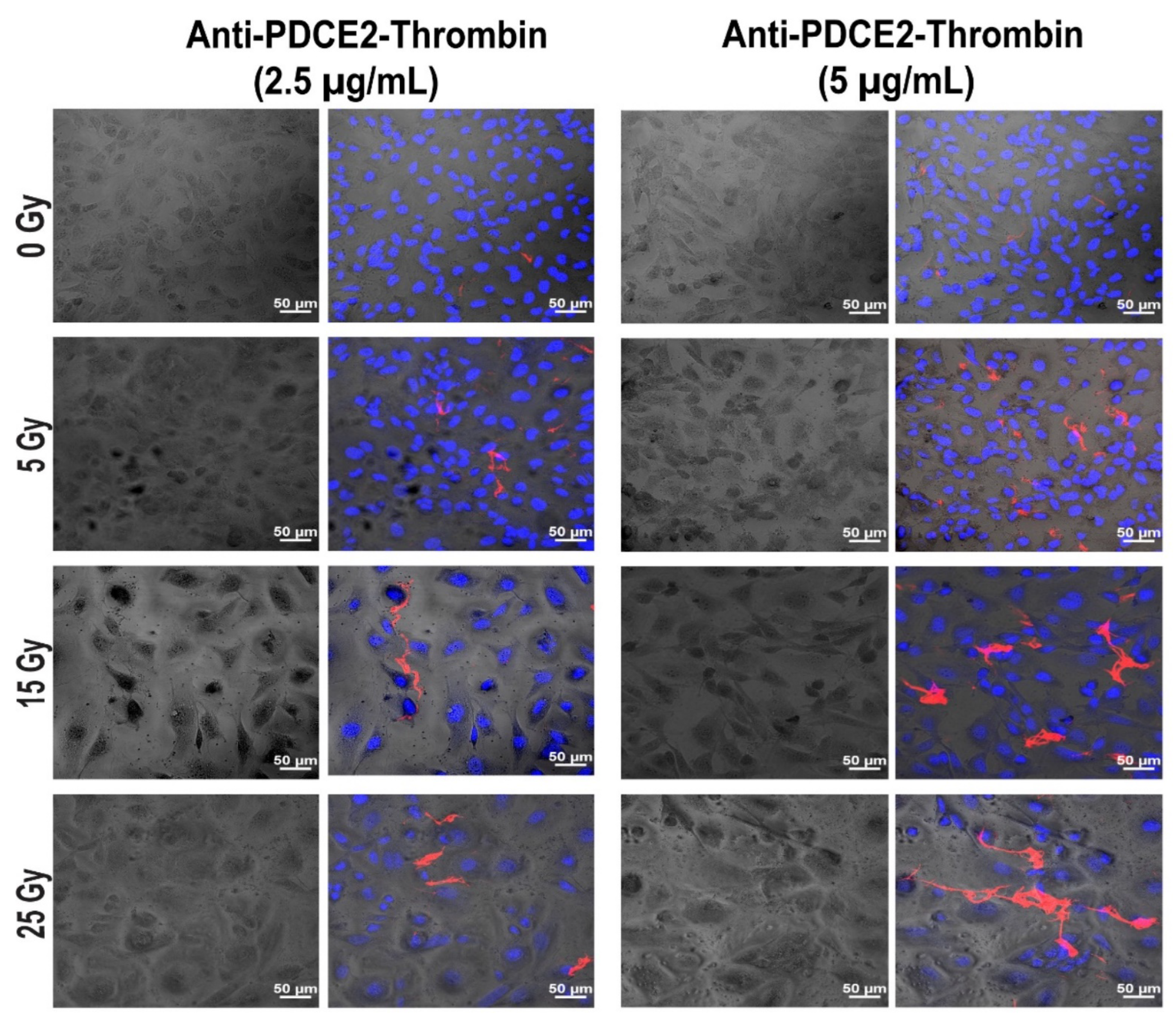
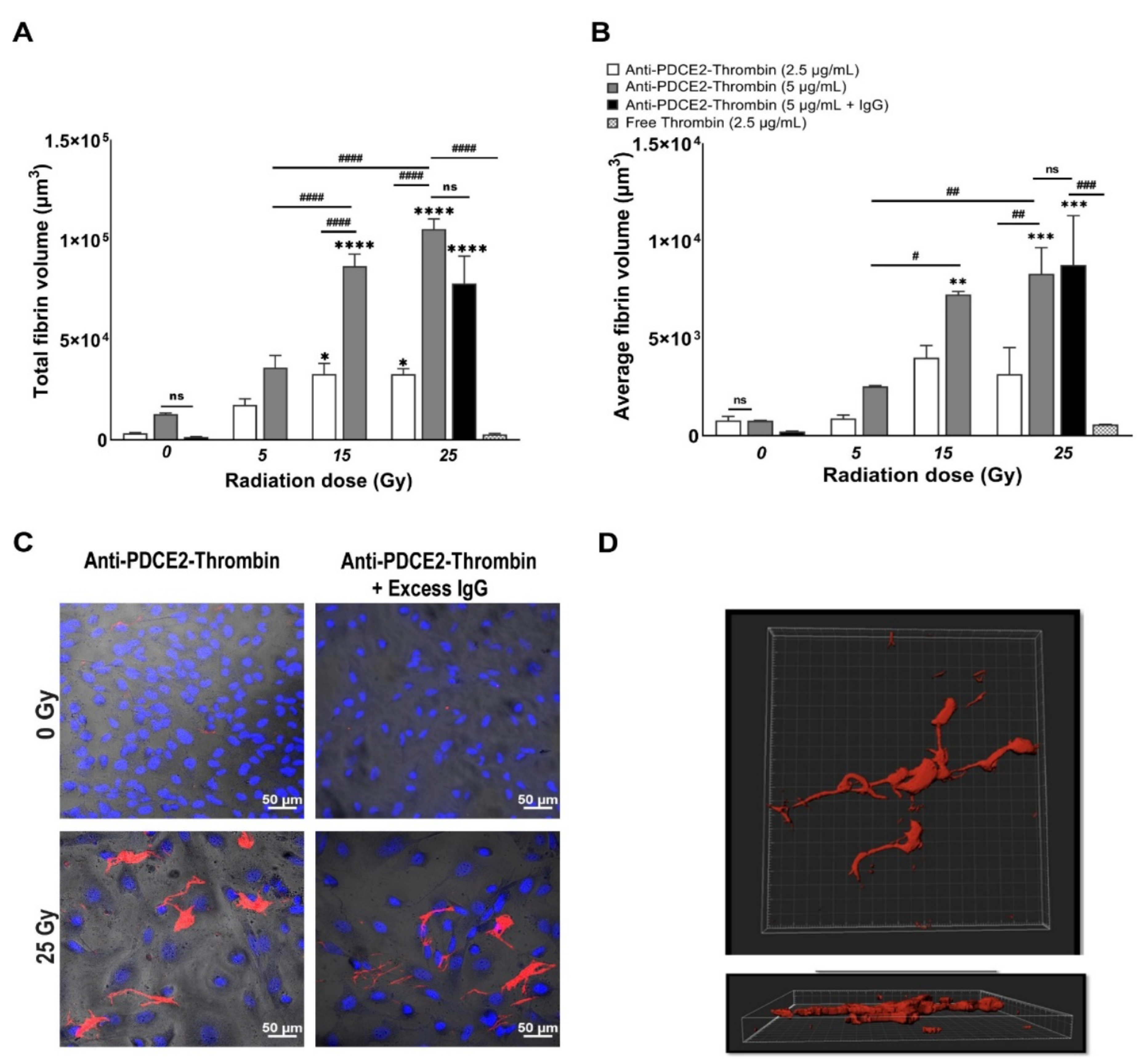
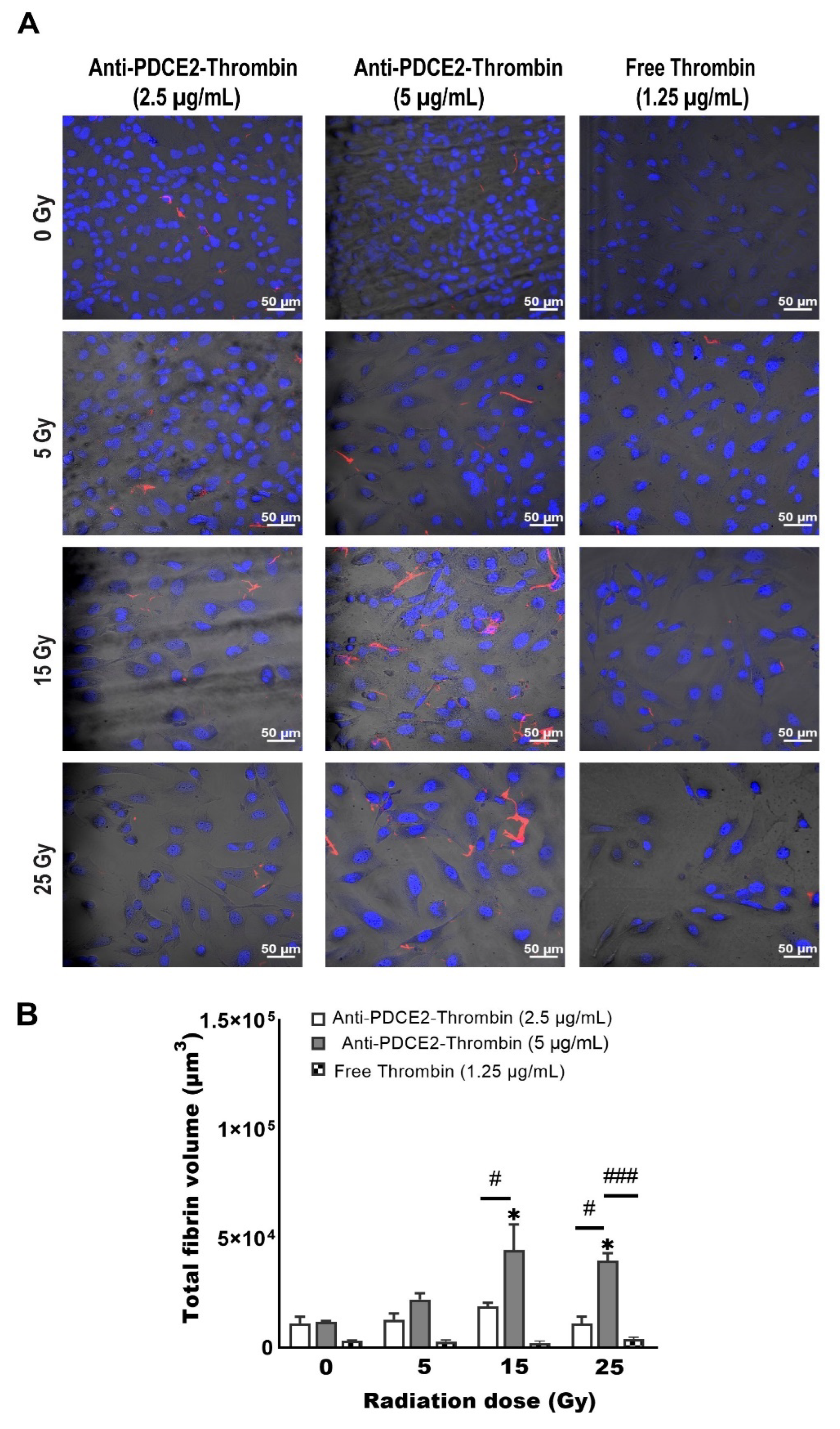
In this study, a parallel-plate flow system was employed to systematically examine cell surface PDCE2 exposure and test an anti-PDCE2-thrombin coaguligand as a VTA for selective and stable thrombosis on irradiated cells. The PDCE2-targeting VTA provided acceptable activity in stimulating clot formation on irradiated human endothelial cells with whole blood circulating under flow conditions. Using this strategy, the following major findings have been concluded: (1) PDCE2 antibody can be effectively bound to the surface of irradiated cells and maintained under flow conditions, confirming cell surface ectopic expression of this target on human cells in response to high radiation doses (15–25 Gy); (2) the developed anti-PDCE2-thrombin coaguligand can specifically and selectively target externalized PDCE2 and maintain the enzymatic activity of thrombin; and (3) the combination of ectopic PDCE2 expression level and targeted thrombin activity is sufficient to induce significant thrombosis on irradiated cells with minimal off-target effects on non-irradiated endothelial cells and minimal thrombosis in the presence of free, non-targeted thrombin. These data support the hypothesis that ectopic PDCE2 exposure induced in response to ionizing radiation provides a putative discriminatory target for radiation-guided drug delivery, and that the accumulation of PDCE2-targeted thrombogens can overcome endothelial defenses to induce focused thrombosis on pathological vasculature.
The parallel-plate flow system was previously established for the in vitro testing of externalized phosphatidylserine (PS) and alpha-B-crystallin (CRYAB) as radiation-induced surface markers [
11,
12]. This dynamic system represents an ethical and economical approach for the rapid assessment of multiple VTA constructs prior to pre-clinical animal studies. It allows the assessment of VTA binding and thrombosis induction under pulsatile flow, conditions more closely replicating physiological parameters important for thrombus formation that cannot be replicated in static models. This system also allows the pre-assessment of targets and VTAs in a model more relevant to the human context (human cells, human blood). In line with earlier studies, we found that maximum thrombosis in response to PDCE2-targeting VTAs occurred at the higher 15 and 25 Gy doses [
11,
12]. Clinical median marginal dosage required to achieve AVM closure using stereotactic surgery with minimal adverse radiation effects is typically 20 Gy (19–22 Gy), close to the upper 25 Gy limit used here [
24,
25,
26], so target expression is achievable within clinically relevant dose ranges. The potential ability to stimulate the expression of these targets and thrombosis at lower marginal radiation doses (e.g., 15 Gy), as observed here, is an important result. SRS suffers from significantly reduced obliteration rates at marginal doses of 15–16 Gy (60–70%), while higher doses of radiation increase the risk of late adverse effects and clinical complications, limiting the application of radiosurgery, particularly for complete obliteration of large AVMs [
25,
27]. In the clinic, this targeting approach could potentially allow lowering of the therapeutic radiation dose without a significant reduction in obliteration rates. Use of a lower dose may also increase the therapeutic window for PDCE2 targeting, as PDCE2 expression had greater durability (sustained expression) at the 15 Gy dose in this study relative to the 25 Gy dose. Further investigation of this phenomenon is warranted.
In contrast to the thrombosis observed at 15 and 25 Gy doses of radiation, thrombosis was not significantly induced at the lowest radiation dose employed when targeting either PDCE2, in this study, or CRYAB, previously [
12]. Limited binding at 5 Gy could reduce the chance of off-target thrombus formation following coaguligand presentation both in the systemic, non-irradiated vasculature, as well as in adjacent vessels that may receive a minimal dose. Interestingly, a PS-targeting VTA was observed to be significantly more effective at this 5 Gy dose in the same parallel-plate flow system [
11]. PS is an abundant phospholipid that flips from the inner surface of the plasma membrane to the outer surface in response to a variety of biological stimuli, including radiation, and is most often observed in apoptotic cells [
28,
29,
30]. The higher efficacy may reflect greater levels of PS exposure at the surface relative to PDCE2 and CRYAB or, alternatively, could reflect the relative affinity of each ligand for their respective targets [
1]. A recent in vivo investigation of PS targeting in a rat AVM model revealed that both irradiated and non-irradiated vasculature could be thrombosed with a high affinity PS-targeting VTA [
17]. Off-target thrombosis in this instance could be minimized with reduced VTA dosing; however, the possibility of unintended thrombotic occlusion caused by non-specific PS expression in off-target vasculature must be addressed in the future optimization of this target. Future studies could examine lower affinity PS ligands to assess whether specificity could be improved. For the targeting of intracellular proteins such as PDCE2 and CRYAB, lower basal levels of expression after radiation relative to PS may lower the risk of off-target binding but may also reduce in vivo efficacy. Overall, the current study supports advancement of this target and associated VTA to pre-clinical animal studies to assess in vivo efficacy and specificity. This target could be examined in animal models of brain AVMs as previously described [
17] and in mouse models of cancer where radiation was first described to enhance tumor vascular targeting [
31].
One limitation of the parallel-plate flow system used here is the volume of blood used for each independent experiment, which necessitates the constant recruitment of donors. This slows progress when multiple targets and VTAs are to be analyzed. Hence, a second goal of this study was to establish whether a static in vitro model of thrombosis induction could be developed as an efficient method of VTA assessment before the hemodynamic flow studies. A static Petri dish assay allowed a reduction in the amount of blood used to one-sixth of the original volume (2 mL vs. 12 mL). Although this model was not as sensitive as the flow system to differentiate radiation dose and coaguligand concentrations, it demonstrated similar trends: the highest level of thrombosis was detected at 15 and 25 Gy with a higher coaguligand dose; no fibrin clot formation was found with an equivalent activity dose of free thrombin or on non-irradiated cells. When compared to the parallel-plate flow findings, the amount of clot identified using the static model was considerably lower; however, this was not unexpected given that cells had less access to the clotting factors, fibrinogen and VTA and given the lower blood volume used. Reduced incubation duration (10 vs. 15 min) may also have influenced fibrin deposition. Lower levels of thrombosis may also indicate how shear stress in the flow system contributes to the formation and maturation of the clot (e.g., from platelet activation), which was not fulfilled via the static model [
32]. Overall, this static assay offers some advantages over the flow model, such as reduced blood volume, coaguligand consumption and experimental time. While the need for confocal imaging is still laborious and time-consuming, this static Petri dish model could be used to increase the productivity of preliminary screening where multiple radiation-induced targets and VTAs are assessed simultaneously.
The static multi-well model proved not as sensitive and reproducible as the Petri dish system to differentiate radiation dose and coaguligand concentrations and cannot be used as a single technique to monitor fibrinogen deposition with the current set-up. Several technical issues were encountered with this model. The handling of viscous blood samples in small volumes led to pipetting inaccuracy, while in the presence of high clot formation, removing loosely bound blood after incubation was problematic as increased washing caused greater a detachment of cells (and clots) from the surface, negatively affecting accuracy. Further optimization of this approach is necessary before use as a high-throughput assay; however, the promising trend observed with the microplate fluorescent reading suggests feasibility with further methodological development.
In conclusion, the lack of surface localization of mitochondrial PDCE2 on normal healthy endothelial cells, and the increased surface expression established here in response to radiation, supports further investigation of PDCE2 as a discriminatory surface marker for vascular targeting in radiation primed tissues. Overall, these data indicate that using a lower radiation dose (15 Gy versus 25 Gy) in the clinic could provide a larger therapeutic window for VTA administration with PDCE2 as a target and allow for more specific targeting without off-target binding and thrombosis. The static and parallel-plate flow systems described here provide economical and ethical models for rapid comparisons of multiple radiation-stimulated targets and VTA constructs prior to pre-clinical evaluation in animal models. The data gained from these model systems support the advancement of this target and associated VTA to pre-clinical studies to examine precision thrombosis in both irradiated tumor and AVM animal models.
4. Materials and Methods
4.1. Cell Culture and Irradiation
The immortalized human cerebral microvascular endothelial cell line, hCMEC/D3 (CELLutions Biosystems Inc., Burlington, ON, Canada), was cultured in a complete medium consisting of Endothelial Basal Medium-2 (EBM-2) (Lonza, Basel, Switzerland), fetal bovine serum (5%), penicillin-streptomycin (1%), HEPES (10 mM) (Thermo Fisher, Waltham, MA, USA) and human basic fibroblast growth factor (1 ng/mL) (Sigma-Aldrich, St. Louis, MI, USA), maintained at 37 °C carbon dioxide 5% in humidified 95% air [
18]. All cells between passages 11 and 25 were sub-cultured by trypsin-EDTA solution (Sigma-Aldrich). Trypan blue staining via an automated cell counter (Thermo Fisher) determined the viability and number of cells. All dishes were pre-coated with 100 μg/mL rat tail collagen IV (In Vitro Technologies, Sydney, NSW, Australia). One mL of collagen diluted in autoclaved water was added to each Petri dish, followed by at least 1 h incubation and was then washed three times with phosphate-buffered saline (PBS). Cells were seeded at 1 × 10
5 cells/mL in pre-coated 35 mm glass-bottom Petri dishes (MatTek Corporation, Ashland, MA, USA) containing 2 mL complete EBM-2 medium, which was replaced with fresh medium every other day. Confluent cells (100%) were irradiated (0–25 Gy) by a linear accelerator (LINAC) (Crawley, UK) at Macquarie University Hospital (Sydney, Australia) as previously described [
11,
12]. Sham- irradiated cells were treated identically but without radiation.
4.2. Fluorescent Antibody Conjugate Preparation
Labelled anti-PDCE2 antibody was prepared by conjugating Alexa Fluor 647 (AF-647) (Thermo Fisher) to a PDCE2 mouse monoclonal antibody (sc-166899, Santa Cruz, CA, USA). Briefly, 100 µg of antibody was purified using NAb Protein G Spin Columns (Thermofisher) to eliminate gelatin. Purified anti-PDCE2 antibody (1 mg/mL) was resuspended in sodium bicarbonate (pH 8.0) before mixing with dye in dimethyl sulfoxide. The mixture was kept for 1 h at room temperature with gentle agitation. The absorbance of antibodies and dye was measured via NanoDrop (Thermo Fisher). The degree of labelling (DOL) was determined as 1.2.
4.3. Antibody–Thrombin Coaguligand Preparation
Click-&-Go™ Lys-to-Lys Protein-Protein Conjugation Kit (Click Chemistry Tools, Scottsdale, USA) was used to conjugate purified anti-PDCE2 antibody (166899, Santa Cruz, CA, USA) and human thrombin (Jomar Life Research, Scoresby, VIC, Australia). Based on the manufacturer’s protocol, 121 µL thrombin (3.57 mg/mL) and 100 μL anti- PDCE2 antibody were labelled with a 20-fold molar excess of TCO and Tetrazine reagents, respectively. The labelled anti-PDCE2 and thrombin were mixed and kept at room temperature for 1 h. The efficiency of the conjugation was evaluated by SDS-PAGE. The ratio of conjugation measured via NIH Image J (Bethesda, Maryland, USA) [
33] was 60%.
4.4. Thrombin Activity Assay
The enzymatic activity of thrombin was compared before and after the conjugation process using a fluorometric thrombin activity assay (SensoLyte 520, Ana Spec, Fremont, CA, USA). To examine the activity level, 10 μL of free thrombin and conjugated thrombin were diluted in 40 μL buffer and assayed for excitation/absorbance at 485/535 nm. The total thrombogenic activity of the coaguligand was determined to be approximately 61% of the original thrombin (equivalent to 1940 NIH units/mg).
4.5. Human Blood Collection
Human blood was collected with informed consent from healthy volunteers. The research was authorized by the Macquarie University Human Research Ethics Committee (approval number HREC: 5201837496785). Healthy adult volunteers were recruited in this study with an age range of 25–58 years. Written consent was obtained from all volunteers. A volume of 20 mL was taken intravenously based on phlebotomy guidelines. The blood was collected into 4.5 mL anti-coagulation CTAD tubes (BD Bioscience, Sydney, NSW, Australia). The initial 3–5 mL of blood was discarded to avoid contamination with tissue factor or thrombin [
34]. The collected blood was kept at room temperature up to 30 min before applying it to the flow system.
4.6. Parallel-Plate Flow System Set-Up and Operation
The parallel-plate flow system was set up as originally described [
11,
12]. All flow system parts were assembled after rinsing with distilled warm water based on Series 1400 Pulsatile Blood Pumps User’s Manual (Harvard Apparatus, Cambridge, MA, USA). The average shear stress of 3.1 dynes/cm
2 and constant flow rate were achieved by setting a rate adjustment of 38 strokes/min, stroke volume of 2.4 mL/min and circulation time of 15 min. These flow conditions were previously established to simulate in vivo experiments while preserving the confluence of cells during blood circulation with minimum cell lifting [
11]. Approximately 11–12 mL of whole human blood was recalcified by adding 5 mM calcium chloride and 10 mM magnesium chloride in a final volume of 1:10 dilution to the blood to restore the coagulation cascade. FITC-labelled fibrinogen (Molecular Innovations, Novi, MI, USA) with a final concentration of 130 µg/mL was incorporated into the whole blood immediately before circulation to visualize and measure fibrin clot formation. The mixture of recalcified, labelled blood (~12 mL) was injected into the enclosed circulation slowly but constantly to prevent blood bubble formation. In the next step, a collagen-coated 35-mm glass Petri dish with confluent cells following different doses of radiation were fitted in the gasket and connected to the pulsatile circulation tubes and vacuum inlet. The system was started with an optimized forwarding mode to avoid backward negative pressure. The anti-PDCE2-thrombin coaguligand (final concentration of 2.5 and 5 µg/mL) or non-specific IgG (final concentration of 12.5 µg/mL) was injected into the circulation immediately after starting the blood circulation. After 15 min of flow circulation, cells were disconnected from the pulsatile system and gently washed twice with PBS, followed by ethanol fixation (50% in PBS, 20 min), Hoechst staining (5 µg/mL, 5 min) and mounting for confocal imaging. Additionally, independent experiments were performed using non-conjugated free thrombin at a final concentration of 2.5 µg/mL (equivalent activity to 10 µg/mL of coaguligand based on percent reduction (39%) in thrombin activity post-conjugation and the size differential between the VTA (~180 kDa) and free thrombin (~30 kDa)).
4.7. Confocal Microscopy
Z-stack images were taken using a Zeiss LSM880 Confocal Microscope (Jena, Germany) with First/Last operational mode. The range and number of Z-stacks were adjusted based on FITC staining (8 slides). Images were acquired from three channels: bright field, ex358 nm/em461 nm for Hoechst (nuclei), and ex 495/em519 nm for FITC (fibrin clot) imaging. Two-dimensional confocal images were taken using two channels (ex358 nm/em461 nm, ex650 nm/em665 nm) for visualizing DAPI and AF-647 (PDCE2).
4.8. 3D and 2D Image Analysis
Thrombus volume was measured via 3D reconstruction of the Z-stack image using Bitplane (IMARIS software version 9) as previously described [
11,
12]. Briefly, a new surface was added to the Z-stack image via “add a surface” toolbar icon. The image contrast and brightness were adjusted, and the image was displayed in Blend mode. FITC was selected as the desired channel with absolute intensity set for thresholding. A threshold was manually adjusted to render all the fibrin structures. Finally, the volume of rendered thrombus was measured under the Statistics Tab. 2D images were analyzed with Zen 2.5 (Blue edition) software using identical settings between controls and irradiated cells (Carl Zeiss Microscopy, Germany). For analysis of AF-647 tagged antibody binding, the integrated density was determined per field of view using NIH Image J [
33] after adjusting the threshold.
4.9. Static Models
4.9.1. Static Petri-Dish Model
Human endothelial cells (hCMEC/D3) were cultured in 35 mm glass-bottom pre-coated Petri dish and irradiated at 0, 5, 15 or 25 Gy, as per the flow study method. At day 2 post-irradiation, 2 mL of fresh human blood, FITC-labelled fibrinogen and anti-PDCE2-thrombin conjugate (2.5 and 5 µg/mL) or free thrombin (1.25 µg/mL; an equivalent enzyme activity to 5 µg/mL conjugate) was added directly to the cells for 5, 10 or 15 min at room temperature. Cells were then thoroughly washed, fixed and stained before confocal imaging, 3D reconstruction and IMARIS analysis.
4.9.2. Static Multi-Well Model
Human endothelial cells (hCMEC/D3) were cultured in 96 flat-bottom, black-well microplates (Corning, New York, NY, USA) pre-coated with 100 μg/mL rat tail collagen. Cells were seeded at 0.5 × 10
4 cells/mL in a final volume of 250 μL of complete EBM2 medium per well. Fully confluent cells were irradiated at 25 Gy or sham-irradiated. After 2 days, a mixture of 200 µL of recalcified whole human blood containing FITC-fibrinogen (130 µg/mL) with VTA (0, 2.5, 5 or 10 µg/mL in normal saline) was added by a multichannel pipettor to each well. To evaluate the reproducibility of the assay, at least a triplicate of each group was tested with the same blood donor for irradiated and sham-irradiated cells. After 10 min of incubation, plates were inverted and tapped to remove excess blood. Cells were thoroughly washed three times with 500 µL PBS 1× and fixed with ethanol (50%
v/
v) in PBS. The level of deposited fibrin in each well was first assessed via fluorescence microscopy (EVOS Cell Imaging Systems, Thermo Fisher, Massachusetts, USA) and the corresponding integrated density per field of view determined using NIH Image J [
33]. In addition, the FITC signal of deposited fibrinogen was read by PHERAstar microplate reader (BMG Labtech; Offenburg, Germany). The protocol was set up with excitation wavelength at 485 nm and emission at 520 nm. For each experiment, the focal height and gain adjustment were performed before reading. Single and well-scanning measurements including matrix, orbital and spiral were tested to establish the best scan mode. A single center bottom reading was consequently selected as a detection mode. To prevent and minimize auto-fluorescent signals (e.g., from the empty microplate, over-confluent cells and irradiated cells) and to reduce intra-experimental bias, values were first corrected to the blank (empty wells) and then divided by the average of the negative control wells (no coaguligand) for each plate separately to give final integrated FITC intensity values.
4.10. Statistical Analysis
Data analyses were performed using Prism 8.4.3 (GraphPad Software Inc., CA, USA). Values represent mean ± SEM of at least three independent experiments unless otherwise stated. Two-way ANOVA was employed to calculate statistical differences with Tukey’s post-hoc analysis. p-value was set to less than 0.05.