Postnatal Protein Intake as a Determinant of Skeletal Muscle Structure and Function in Mice—A Pilot Study
Abstract
1. Introduction
2. Results
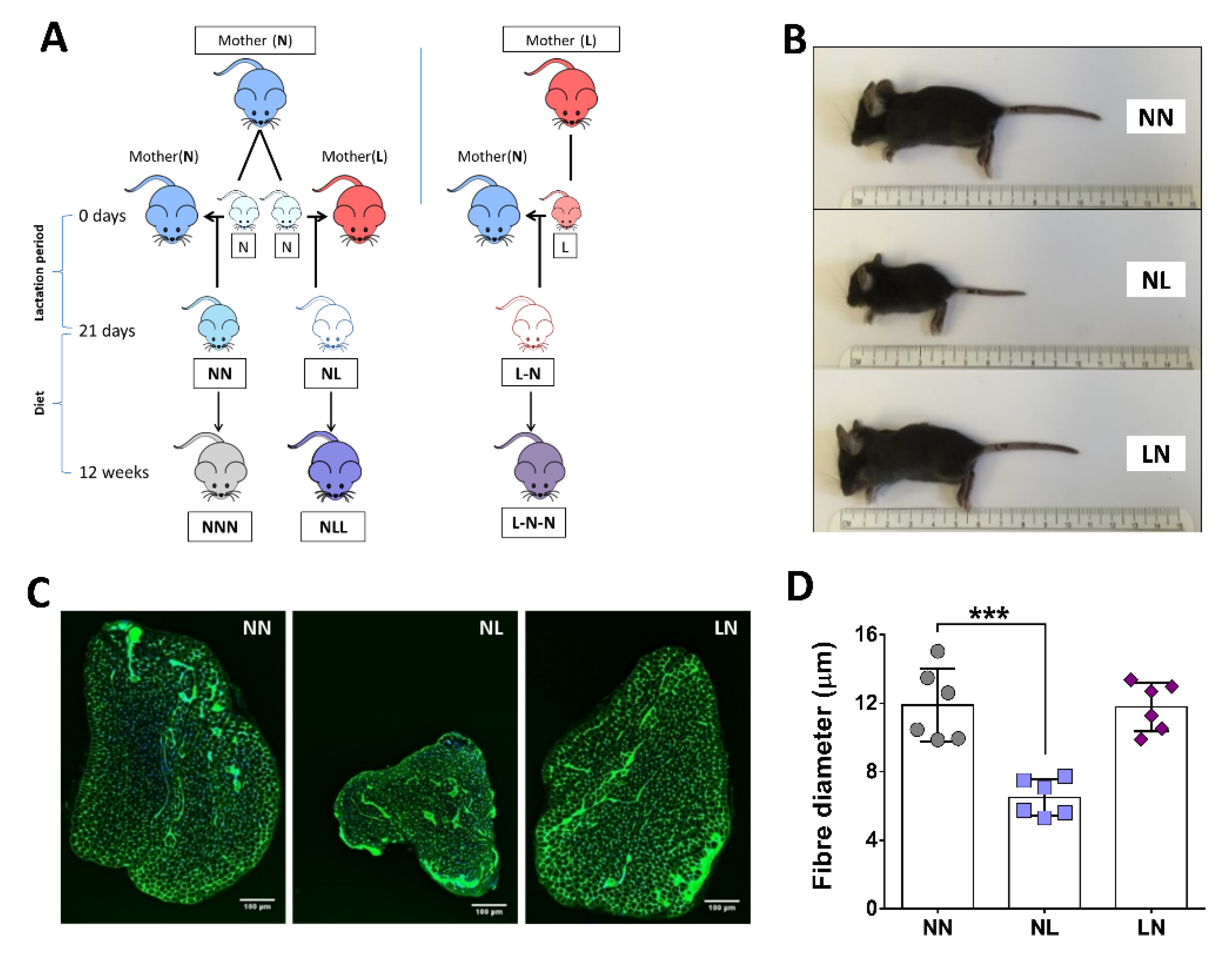
2.1. The Effect of Dietary Pre- or Postnatal Protein Restriction on Body and Muscle Characteristics of 21-Days Old Mice
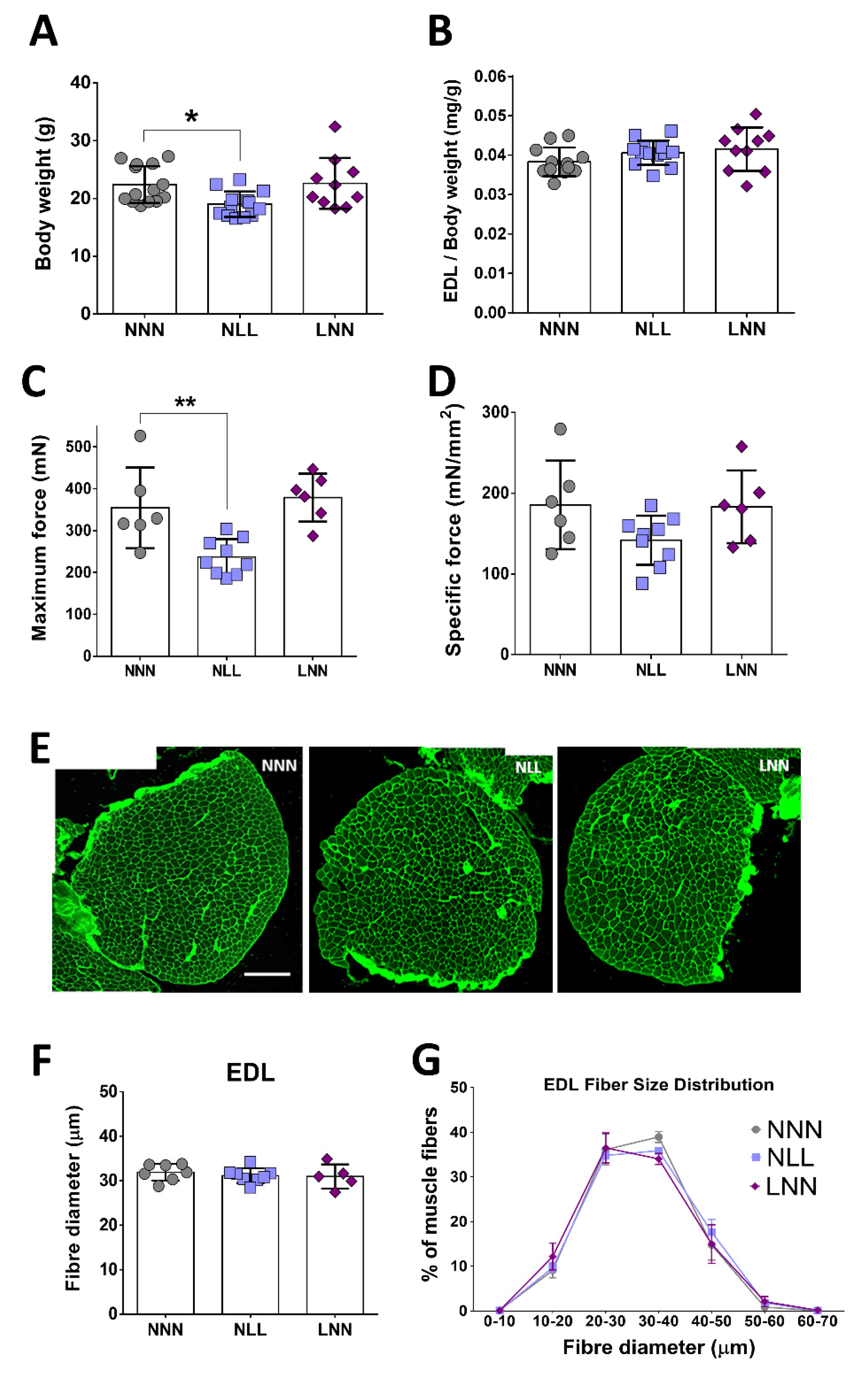
2.2. The Effect of Protein-Deficient Diet Pre- or -Postnatally on Muscle Weight, Total Body Weight and EDL Forces of 12-Week Old Mice
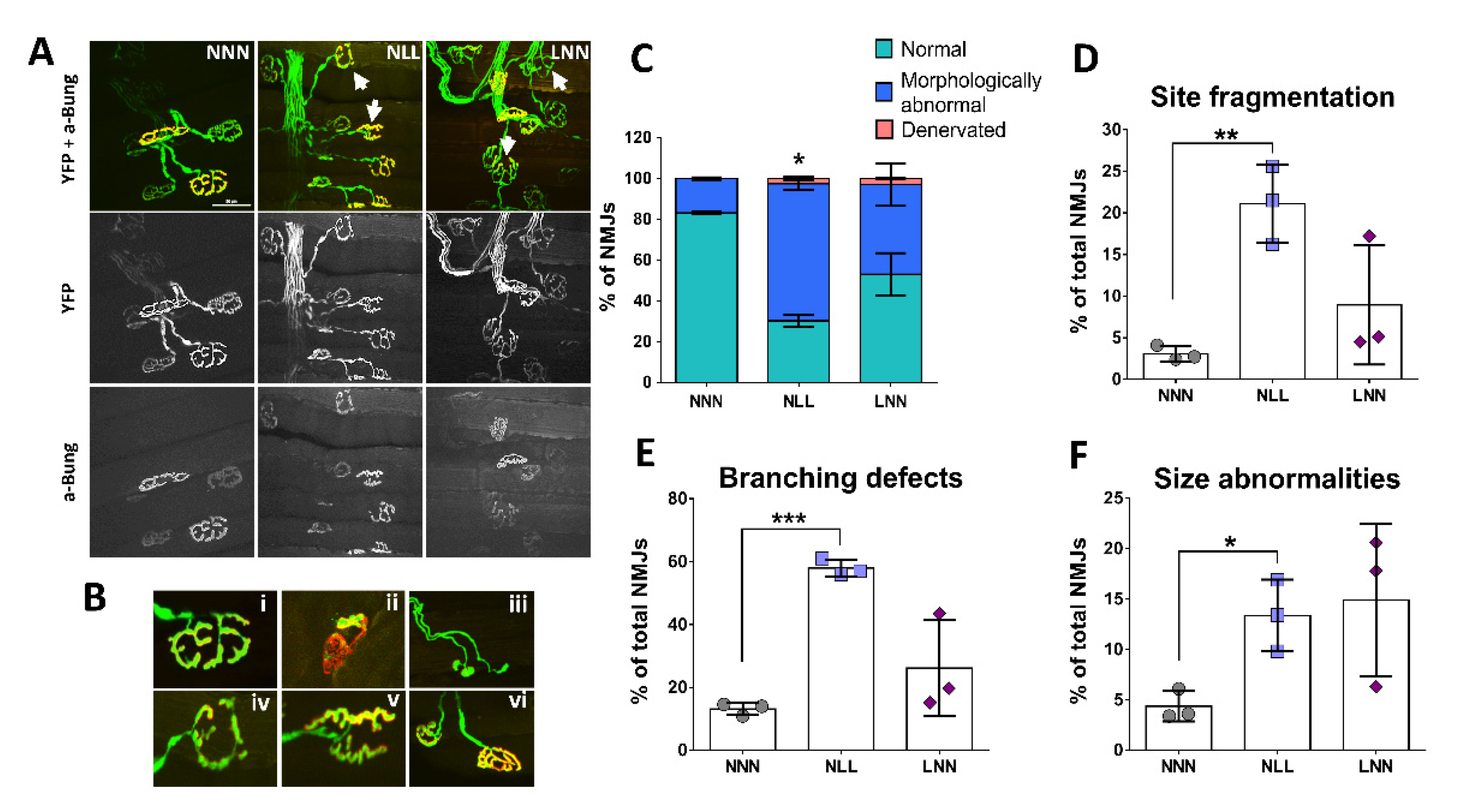
2.3. Assessment of NMJ Structural Integrity
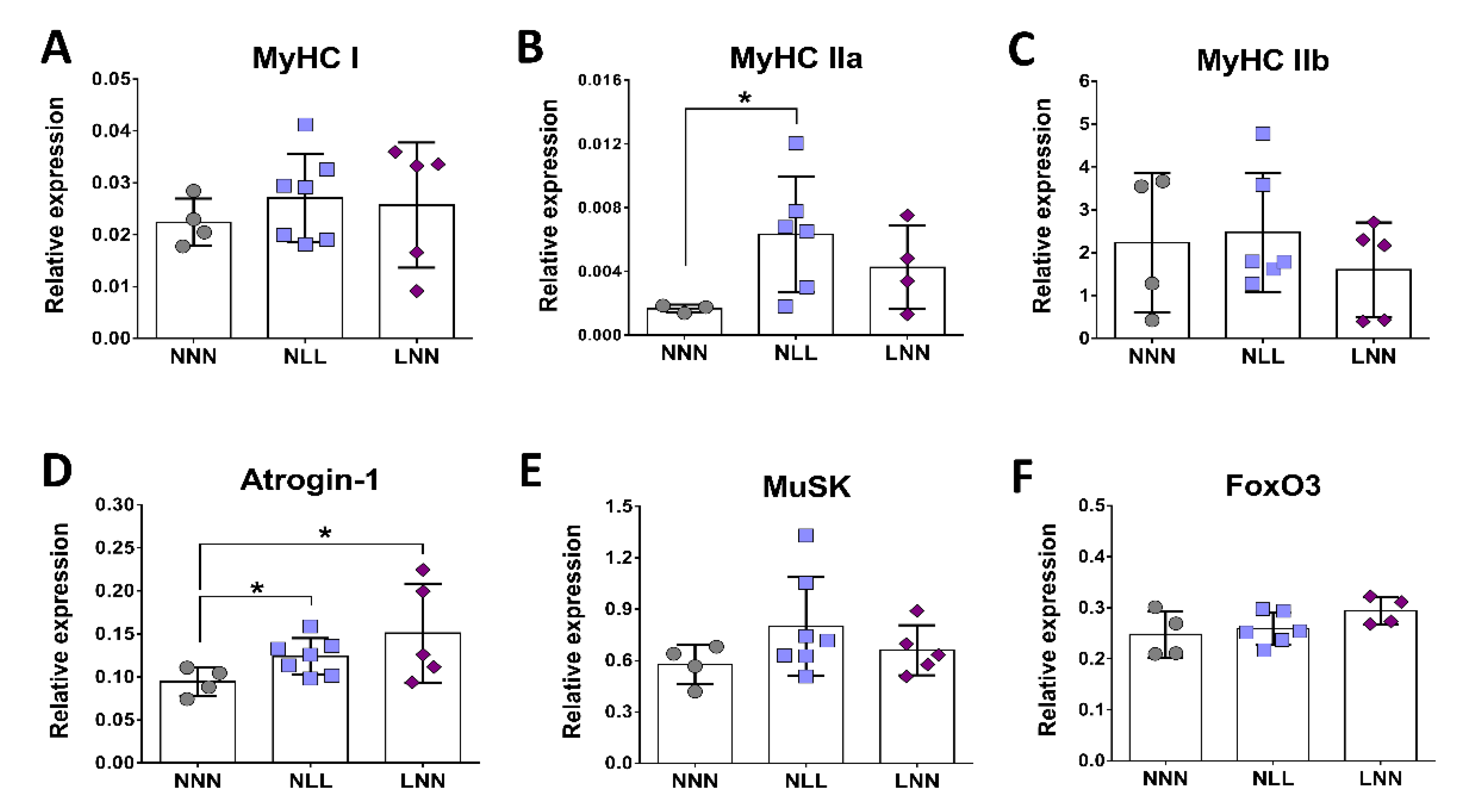
2.4. Gene Expression Analysis of Marker Genes for Muscle Fibre Isoforms, Muscle Atrophy and NMJ Formation
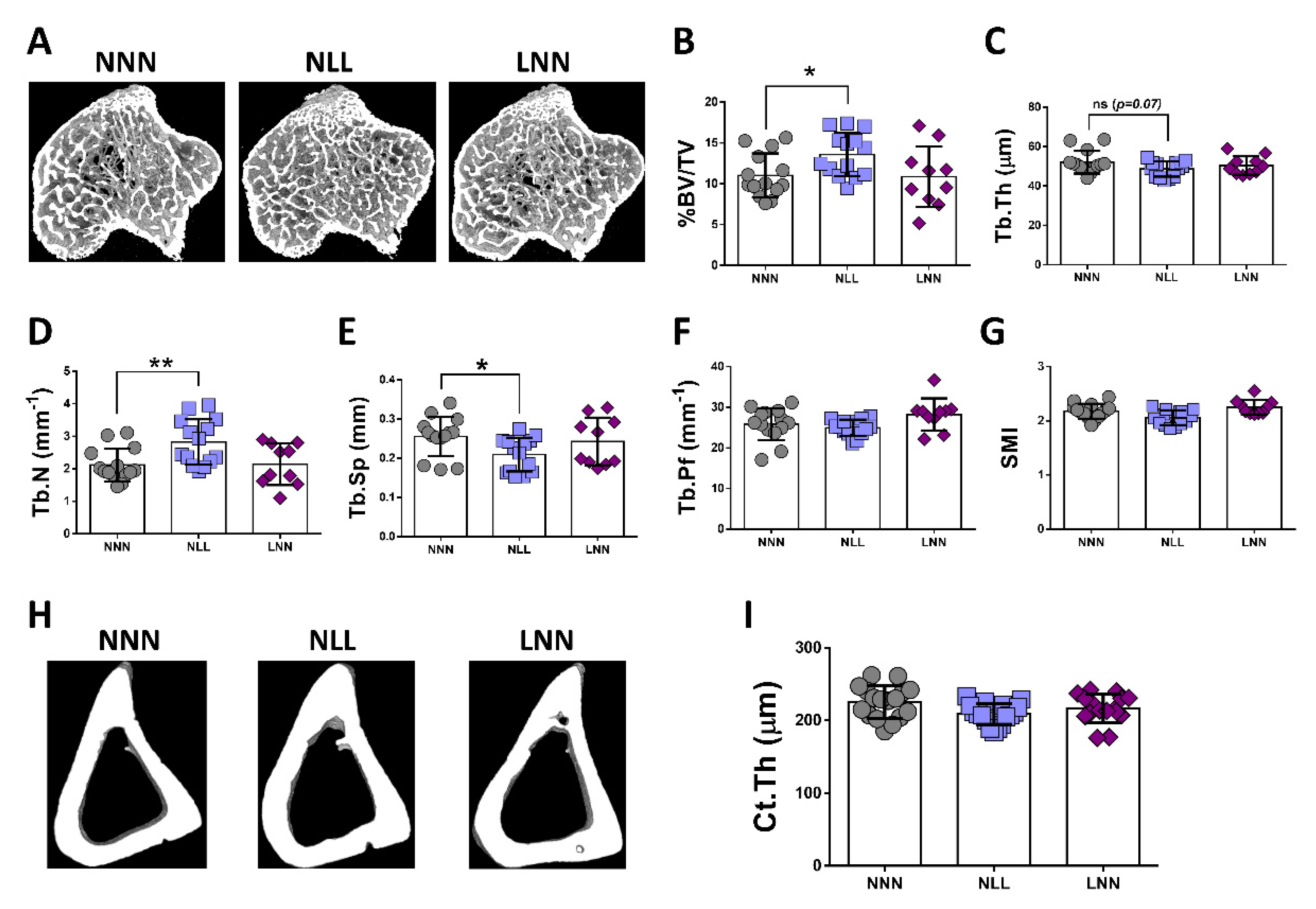
2.5. The Effects of Protein Restriction on Bone during Adulthood
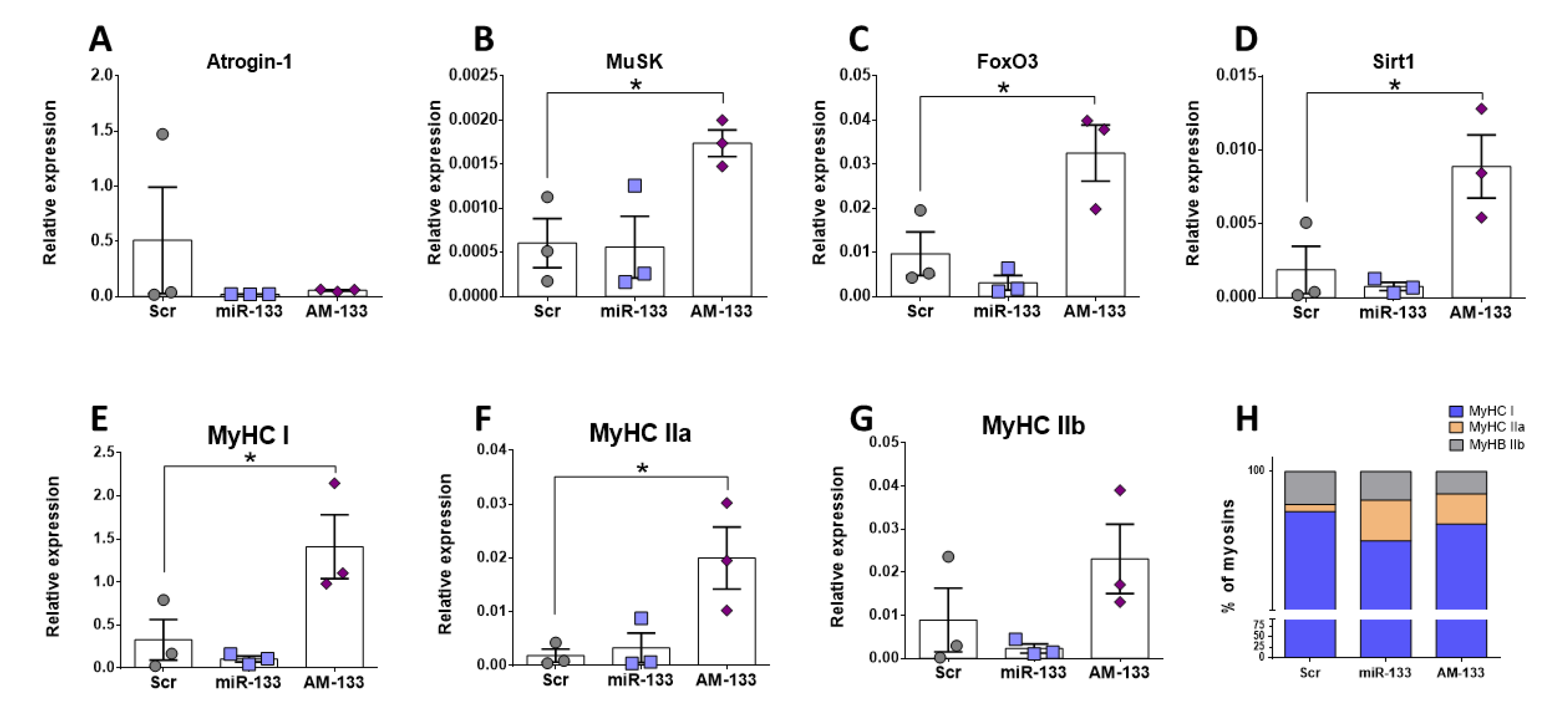
2.6. Role of miR-133 in Skeletal Muscle In Vivo and In Vitro
2.7. miR-133 Regulates Expression of Myosins and Genes Associated with Hypertrophy Pathways
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Groups
4.2. EDL Force Measurement
4.3. Histological Analyses
4.4. Cell Culture, Transfection, Cytotoxicity Assay and Immunostaining
4.5. Sample Preparation and RNA Extraction
4.6. cDNA Synthesis and RT-qPCR
4.7. Micro-Computed Tomography (microCT)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Bortz, W.M., 2nd. A conceptual framework of frailty: A review. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M283–M288. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, S.; Walston, J.D. Frailty in older adults: Insights and interventions. Clevel. Clin. J. Med. 2005, 72, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Leeuwenburgh, C.; Lauretani, F.; Onder, G.; Bandinelli, S.; Maraldi, C.; Guralnik, J.M.; Pahor, M.; Ferrucci, L. Frailty syndrome and skeletal muscle: Results from the Invecchiare in Chianti study. Am. J. Clin. Nutr. 2006, 83, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Lees, H.A.; Wohlgemuth, S.E.; Leeuwenburgh, C. Sarcopenia of aging: Underlying cellular mechanisms and protection by calorie restriction. Biofactors 2009, 35, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.R.; Luo, K.; Deng, X.; Xu, L.; Wang, R.R.; Ji, P. Effect of sarcopenia in predicting postoperative mortality in emergency laparotomy: A systematic review and meta-analysis. World J. Emerg. Surg. 2022, 17, 36. [Google Scholar] [CrossRef]
- Han, D.S.; Wu, W.T.; Hsu, P.C.; Chang, H.C.; Huang, K.C.; Chang, K.V. Sarcopenia Is Associated With Increased Risks of Rotator Cuff Tendon Diseases Among Community-Dwelling Elders: A Cross-Sectional Quantitative Ultrasound Study. Front. Med. 2021, 8, 630009. [Google Scholar] [CrossRef]
- Sayer, A.A.; Cooper, C. Fetal programming of body composition and musculoskeletal development. Early Hum. Dev. 2005, 81, 735–744. [Google Scholar] [CrossRef]
- Sayer, A.A.; Syddall, H.E.; Gilbody, H.J.; Dennison, E.M.; Cooper, C. Does sarcopenia originate in early life? Findings from the Hertfordshire cohort study. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, M930–M934. [Google Scholar] [CrossRef]
- Brown, L.D. Endocrine regulation of fetal skeletal muscle growth: Impact on future metabolic health. J. Endocrinol. 2014, 221, R13–R29. [Google Scholar] [CrossRef]
- Martin-Gronert, M.S.; Tarry-Adkins, J.L.; Cripps, R.L.; Chen, J.H.; Ozanne, S.E. Maternal protein restriction leads to early life alterations in the expression of key molecules involved in the aging process in rat offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R494–R500. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, J.H.; Martin-Gronert, M.S.; Tarry-Adkins, J.; Ozanne, S.E. Maternal protein restriction affects postnatal growth and the expression of key proteins involved in lifespan regulation in mice. PLoS ONE 2009, 4, e4950. [Google Scholar] [CrossRef] [PubMed]
- Mallinson, J.E.; Sculley, D.V.; Craigon, J.; Plant, R.; Langley-Evans, S.C.; Brameld, J.M. Fetal exposure to a maternal low-protein diet during mid-gestation results in muscle-specific effects on fibre type composition in young rats. Br. J. Nutr. 2007, 98, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Toscano, A.E.; Manhaes-de-Castro, R.; Canon, F. Effect of a low-protein diet during pregnancy on skeletal muscle mechanical properties of offspring rats. Nutrition 2008, 24, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Cabeco, L.C.; Budri, P.E.; Baroni, M.; Castan, E.P.; Carani, F.R.; de Souza, P.A.T.; Boer, P.A.; Matheus, S.M.M.; Dal-Pai-Silva, M. Maternal protein restriction induce skeletal muscle changes without altering the MRFs MyoD and myogenin expression in offspring. J. Mol. Histol. 2012, 43, 461–471. [Google Scholar] [CrossRef]
- Costello, P.M.; Hollis, L.J.; Cripps, R.L.; Bearpark, N.; Patel, H.P.; Sayer, A.A.; Cooper, C.; Hanson, M.A.; Ozanne, S.E.; Green, L.R. Lower maternal body condition during pregnancy affects skeletal muscle structure and glut-4 protein levels but not glucose tolerance in mature adult sheep. Reprod. Sci. 2013, 20, 1144–1155. [Google Scholar] [CrossRef]
- Confortim, H.D.; Jeronimo, L.C.; Centenaro, L.A.; Pinheiro, P.F.F.; Brancalhao, R.M.; Matheus, S.M.M.; Torrejais, M.M. Effects of aging and maternal protein restriction on the muscle fibers morphology and neuromuscular junctions of rats after nutritional recovery. Micron 2015, 71, 7–13. [Google Scholar] [CrossRef]
- Cooper, C.; Eriksson, J.G.; Forsen, T.; Osmond, C.; Tuomilehto, J.; Barker, D.J. Maternal height, childhood growth and risk of hip fracture in later life: A longitudinal study. Osteoporos. Int. 2001, 12, 623–629. [Google Scholar] [CrossRef]
- Javaid, M.K.; Eriksson, J.G.; Kajantie, E.; Forsen, T.; Osmond, C.; Barker, D.J.; Cooper, C. Growth in childhood predicts hip fracture risk in later life. Osteoporos. Int. 2011, 22, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.M.; Sereda, N.H.; Hoffman, M.L.; Valley, E.V.; Crenshaw, T.D.; Park, Y.K.; Lee, J.Y.; Zinn, S.A.; Govoni, K.E. Effects of Poor Maternal Nutrition during Gestation on Bone Development and Mesenchymal Stem Cell Activity in Offspring. PLoS ONE 2016, 11, e0168382. [Google Scholar] [CrossRef]
- Lanham, S.A.; Bertram, C.; Cooper, C.; Oreffo, R.O. Animal models of maternal nutrition and altered offspring bone structure--bone development across the lifecourse. Eur. Cell Mater. 2011, 22, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Mehta, G.; Roach, H.I.; Langley-Evans, S.; Taylor, P.; Reading, I.; Oreffo, R.O.; Aihie-Sayer, A.; Clarke, N.M.; Cooper, C. Intrauterine exposure to a maternal low protein diet reduces adult bone mass and alters growth plate morphology in rats. Calcif. Tissue Int. 2002, 71, 493–498. [Google Scholar] [CrossRef]
- Ambros, V. microRNAs: Tiny regulators with great potential. Cell 2001, 107, 823–826. [Google Scholar] [CrossRef]
- Lagos-Quintana, M.; Rauhut, R.; Yalcin, A.; Meyer, J.; Lendeckel, W.; Tuschl, T. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 2002, 12, 735–739. [Google Scholar] [CrossRef]
- Chen, J.F.; Mandel, E.M.; Thomson, J.M.; Wu, Q.; Callis, T.E.; Hammond, S.M.; Conlon, F.L.; Wang, D.Z. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 2006, 38, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Chen, J.F.; Wang, D.Z. Transgenic overexpression of miR-133a in skeletal muscle. BMC Musculoskelet. Disord. 2011, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Valdez, G.; Heyer, M.P.; Feng, G.; Sanes, J.R. The role of muscle microRNAs in repairing the neuromuscular junction. PLoS ONE 2014, 9, e93140. [Google Scholar] [CrossRef]
- McCarthy, J.J.; Mula, J.; Miyazaki, M.; Erfani, R.; Garrison, K.; Farooqui, A.B.; Srikuea, R.; Lawson, B.A.; Grimes, B.; Keller, C.; et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 2011, 138, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Goljanek-Whysall, K.; Pais, H.; Rathjen, T.; Sweetman, D.; Dalmay, T.; Munsterberg, A. Regulation of multiple target genes by miR-1 and miR-206 is pivotal for C2C12 myoblast differentiation. J. Cell Sci. 2012, 125, 3590–3600. [Google Scholar] [CrossRef]
- Rao, P.K.; Missiaglia, E.; Shields, L.; Hyde, G.; Yuan, B.; Shepherd, C.J.; Shipley, J.; Lodish, H.F. Distinct roles for miR-1 and miR-133a in the proliferation and differentiation of rhabdomyosarcoma cells. FASEB J. 2010, 24, 3427–3437. [Google Scholar] [CrossRef]
- Soliman, A.; De Sanctis, V.; Elalaily, R. Nutrition and pubertal development. Indian J. Endocrinol. Metab. 2014, 18, S39–S47. [Google Scholar] [CrossRef] [PubMed]
- O’Tierney, P.F.; Barker, D.J.; Osmond, C.; Kajantie, E.; Eriksson, J.G. Duration of breast-feeding and adiposity in adult life. J. Nutr. 2009, 139, 422S–425S. [Google Scholar] [CrossRef] [PubMed]
- Augusto, V.; Padovani, C.R.; Eduardo, G.; Campos, R. Skeletal muscule fiber types in C57BL6J mice. J. Morphol. Sci. 2017, 21, 2. [Google Scholar]
- Doherty, T.J. Invited review: Aging and sarcopenia. J. Appl. Physiol. 2003, 95, 1717–1727. [Google Scholar] [CrossRef]
- Chan, S.; Head, S.I. Age- and gender-related changes in contractile properties of non-atrophied EDL muscle. PLoS ONE 2010, 5, e12345. [Google Scholar] [CrossRef]
- Barany, M. ATPase activity of myosin correlated with speed of muscle shortening. J. Gen. Physiol. 1967, 50, 197–218. [Google Scholar] [CrossRef]
- Sheth, K.A.; Iyer, C.C.; Wier, C.G.; Crum, A.E.; Bratasz, A.; Kolb, S.J.; Clark, B.C.; Burghes, A.H.M.; Arnold, W.D. Muscle strength and size are associated with motor unit connectivity in aged mice. Neurobiol. Aging 2018, 67, 128–136. [Google Scholar] [CrossRef]
- Palomero, J.; Vasilaki, A.; Pye, D.; McArdle, A.; Jackson, M.J. Aging increases the oxidation of dichlorohydrofluorescein in single isolated skeletal muscle fibers at rest, but not during contractions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R351–R358. [Google Scholar] [CrossRef]
- Short, K.R.; Bigelow, M.L.; Kahl, J.; Singh, R.; Coenen-Schimke, J.; Raghavakaimal, S.; Nair, K.S. Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. USA 2005, 102, 5618–5623. [Google Scholar] [CrossRef]
- Jackman, R.W.; Kandarian, S.C. The molecular basis of skeletal muscle atrophy. Am. J. Physiol. Cell. Physiol. 2004, 287, C834–C843. [Google Scholar] [CrossRef]
- Lyons, P.R.; Slater, C.R. Structure and function of the neuromuscular junction in young adult mdx mice. J. Neurocytol. 1991, 20, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Pratt, S.J.P.; Shah, S.B.; Ward, C.W.; Kerr, J.P.; Stains, J.P.; Lovering, R.M. Recovery of altered neuromuscular junction morphology and muscle function in mdx mice after injury. Cell. Mol. Life Sci. 2015, 72, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Pratt, S.J.P.; Valencia, A.P.; Le, G.K.; Shah, S.B.; Lovering, R.M. Pre- and postsynaptic changes in the neuromuscular junction in dystrophic mice. Front. Physiol. 2015, 6, 252. [Google Scholar] [CrossRef]
- Vasilaki, A.; Pollock, N.; Giakoumaki, I.; Goljanek-Whysall, K.; Sakellariou, G.K.; Pearson, T.; Kayani, A.; Jackson, M.J.; McArdle, A. The effect of lengthening contractions on neuromuscular junction structure in adult and old mice. Age 2016, 38, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Taetzsch, T.; Shapiro, D.; Eldosougi, R.; Myers, T.; Settlage, R.E.; Valdez, G. The microRNA miR-133b functions to slow Duchenne muscular dystrophy pathogenesis. J. Physiol. 2021, 599, 171–192. [Google Scholar] [CrossRef]
- Valdez, G.; Tapia, J.C.; Kang, H.; Clemenson, G.D., Jr.; Gage, F.H.; Lichtman, J.W.; Sanes, J.R. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc. Natl. Acad. Sci. USA 2010, 107, 14863–14868. [Google Scholar] [CrossRef]
- Tintignac, L.A.; Brenner, H.R.; Ruegg, M.A. Mechanisms Regulating Neuromuscular Junction Development and Function and Causes of Muscle Wasting. Physiol. Rev. 2015, 95, 809–852. [Google Scholar] [CrossRef] [PubMed]
- Slater, C.R. Postnatal maturation of nerve-muscle junctions in hindlimb muscles of the mouse. Dev. Biol. 1982, 94, 11–22. [Google Scholar] [CrossRef]
- Shi, L.; Fu, A.K.; Ip, N.Y. Molecular mechanisms underlying maturation and maintenance of the vertebrate neuromuscular junction. Trends Neurosci. 2012, 35, 441–453. [Google Scholar] [CrossRef]
- Balicegordon, R.J.; Lichtman, J.W. Invivo Observations of Presynaptic and Postsynaptic Changes during the Transition from Multiple to Single Innervation at Developing Neuromuscular-Junctions. J. Neurosci. 1993, 13, 834–855. [Google Scholar] [CrossRef]
- Marques, M.J.; Conchello, J.A.; Lichtman, J.W. From plaque to pretzel: Fold formation and acetylcholine receptor loss at the developing neuromuscular junction. J. Neurosci. 2000, 20, 3663–3675. [Google Scholar] [CrossRef]
- Willadt, S.; Nash, M.; Slater, C.R. Age-related fragmentation of the motor endplate is not associated with impaired neuromuscular transmission in the mouse diaphragm. Sci. Rep. 2016, 6, 24849. [Google Scholar] [CrossRef]
- Tu, H.; Zhang, D.; Corrick, R.M.; Muelleman, R.L.; Wadman, M.C.; Li, Y.L. Morphological Regeneration and Functional Recovery of Neuromuscular Junctions after Tourniquet-Induced Injuries in Mouse Hindlimb. Front. Physiol. 2017, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Oreffo, R.O.; Lashbrooke, B.; Roach, H.I.; Clarke, N.M.; Cooper, C. Maternal protein deficiency affects mesenchymal stem cell activity in the developing offspring. Bone 2003, 33, 100–107. [Google Scholar] [CrossRef]
- Lanham, S.A.; Roberts, C.; Cooper, C.; Oreffo, R.O. Intrauterine programming of bone. Part 1: Alteration of the osteogenic environment. Osteoporos. Int. 2008, 19, 147–156. [Google Scholar] [CrossRef]
- Lanham, S.A.; Roberts, C.; Perry, M.J.; Cooper, C.; Oreffo, R.O. Intrauterine programming of bone. Part 2: Alteration of skeletal structure. Osteoporos. Int. 2008, 19, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Agbulut, O.; Noirez, P.; Beaumont, F.; Butler-Browne, G. Myosin heavy chain isoforms in postnatal muscle development of mice. Biol. Cell. 2003, 95, 399–406. [Google Scholar] [CrossRef]
- Rhee, H.S.; Lucas, C.A.; Hoh, J.F.Y. Fiber types in rat laryngeal muscles and their transformations after denervation and reinnervation. J. Histochem. Cytochem. 2004, 52, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Acakpo-Satchivi, L.J.; Edelmann, W.; Sartorius, C.; Lu, B.D.; Wahr, P.A.; Watkins, S.C.; Metzger, J.M.; Leinwand, L.; Kucherlapati, R. Growth and muscle defects in mice lacking adult myosin heavy chain genes. J. Cell Biol. 1997, 139, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, C.A.; Lu, B.D.; Acakpo-Satchivi, L.; Jacobsen, R.P.; Byrnes, W.C.; Leinwand, L.A. Myosin heavy chains IIa and IId are functionally distinct in the mouse. J. Cell Biol. 1998, 141, 943–953. [Google Scholar] [CrossRef]
- Gomes, M.D.; Lecker, S.H.; Jagoe, R.T.; Navon, A.; Goldberg, A.L. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc. Natl. Acad. Sci. USA 2001, 98, 14440–14445. [Google Scholar] [CrossRef] [PubMed]
- Edstrom, E.; Altun, M.; Hagglund, M.; Ulfhake, B. Atrogin-1/MAFbx and MuRF1 are downregulated in aging-related loss of skeletal muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Hesser, B.A.; Henschel, O.; Witzemann, V. Synapse disassembly and formation of new synapses in postnatal muscle upon conditional inactivation of MuSK. Mol. Cell Neurosci. 2006, 31, 470–480. [Google Scholar] [CrossRef] [PubMed]
- DeChiara, T.M.; Bowen, D.C.; Valenzuela, D.M.; Simmons, M.V.; Poueymirou, W.T.; Thomas, S.; Kinetz, E.; Compton, D.L.; Rojas, E.; Park, J.S.; et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell 1996, 85, 501–512. [Google Scholar] [CrossRef]
- Missias, A.C.; Chu, G.C.; Klocke, B.J.; Sanes, J.R.; Merlie, J.P. Maturation of the acetylcholine receptor in skeletal muscle: Regulation of the AChR gamma-to-epsilon switch. Dev. Biol. 1996, 179, 223–238. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, J. MicroRNAs in skeletal myogenesis. Cell Cycle 2011, 10, 441–448. [Google Scholar] [CrossRef]
- Liu, N.; Bezprozvannaya, S.; Shelton, J.M.; Frisard, M.I.; Hulver, M.W.; McMillan, R.P.; Wu, Y.R.; Voelker, K.A.; Grange, R.W.; Richardson, J.A.; et al. Mice lacking microRNA 133a develop dynamin 2-dependent centronuclear myopathy. J. Clin. Investig. 2011, 121, 3258–3268. [Google Scholar] [CrossRef]
- Spangenburg, E.E.; Booth, F.W. Molecular regulation of individual skeletal muscle fibre types. Acta Physiol. Scand. 2003, 178, 413–424. [Google Scholar] [CrossRef]
- Soriano-Arroquia, A.; Clegg, P.D.; Molloy, A.P.; Goljanek-Whysall, K. Preparation and Culture of Myogenic Precursor Cells/Primary Myoblasts from Skeletal Muscle of Adult and Aged Humans. J. Vis. Exp. 2017, 16, e55047. [Google Scholar] [CrossRef]
- Goljanek-Whysall, K.; Mok, G.F.; Alrefaei, A.F.; Kennerley, N.; Wheeler, G.N.; Munsterberg, A. myomiR-dependent switching of BAF60 variant incorporation into Brg1 chromatin remodeling complexes during embryo myogenesis. Development 2014, 141, 3378–3387. [Google Scholar] [CrossRef]
- Kanakis, I.; Alameddine, M.; Scalabrin, M.; van’t Hof, R.J.; Liloglou, T.; Ozanne, S.E.; Goljanek-Whysall, K.; Vasilaki, A. Low protein intake during reproduction compromises the recovery of lactation-induced bone loss in female mouse dams without affecting skeletal muscles. FASEB J. 2020, 34, 11844–11859. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, I.; Liu, K.; Poulet, B.; Javaheri, B.; van’t Hof, R.J.; Pitsillides, A.A.; Bou-Gharios, G. Targeted Inhibition of Aggrecanases Prevents Articular Cartilage Degradation and Augments Bone Mass in the STR/Ort Mouse Model of Spontaneous Osteoarthritis. Arthritis Rheumatol. 2019, 71, 571–582. [Google Scholar] [CrossRef] [PubMed]
- van’t Hof, R.J. Analysis of bone architecture in rodents using microcomputed tomography. Methods Mol. Biol. 2012, 816, 461–476. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giakoumaki, I.; Pollock, N.; Aljuaid, T.; Sannicandro, A.J.; Alameddine, M.; Owen, E.; Myrtziou, I.; Ozanne, S.E.; Kanakis, I.; Goljanek-Whysall, K.; et al. Postnatal Protein Intake as a Determinant of Skeletal Muscle Structure and Function in Mice—A Pilot Study. Int. J. Mol. Sci. 2022, 23, 8815. https://doi.org/10.3390/ijms23158815
Giakoumaki I, Pollock N, Aljuaid T, Sannicandro AJ, Alameddine M, Owen E, Myrtziou I, Ozanne SE, Kanakis I, Goljanek-Whysall K, et al. Postnatal Protein Intake as a Determinant of Skeletal Muscle Structure and Function in Mice—A Pilot Study. International Journal of Molecular Sciences. 2022; 23(15):8815. https://doi.org/10.3390/ijms23158815
Chicago/Turabian StyleGiakoumaki, Ifigeneia, Natalie Pollock, Turki Aljuaid, Anthony J. Sannicandro, Moussira Alameddine, Euan Owen, Ioanna Myrtziou, Susan E. Ozanne, Ioannis Kanakis, Katarzyna Goljanek-Whysall, and et al. 2022. "Postnatal Protein Intake as a Determinant of Skeletal Muscle Structure and Function in Mice—A Pilot Study" International Journal of Molecular Sciences 23, no. 15: 8815. https://doi.org/10.3390/ijms23158815
APA StyleGiakoumaki, I., Pollock, N., Aljuaid, T., Sannicandro, A. J., Alameddine, M., Owen, E., Myrtziou, I., Ozanne, S. E., Kanakis, I., Goljanek-Whysall, K., & Vasilaki, A. (2022). Postnatal Protein Intake as a Determinant of Skeletal Muscle Structure and Function in Mice—A Pilot Study. International Journal of Molecular Sciences, 23(15), 8815. https://doi.org/10.3390/ijms23158815








