Biomarker Associations in Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage
Abstract
:1. Introduction
2. Results
2.1. Patients’ Characteristics
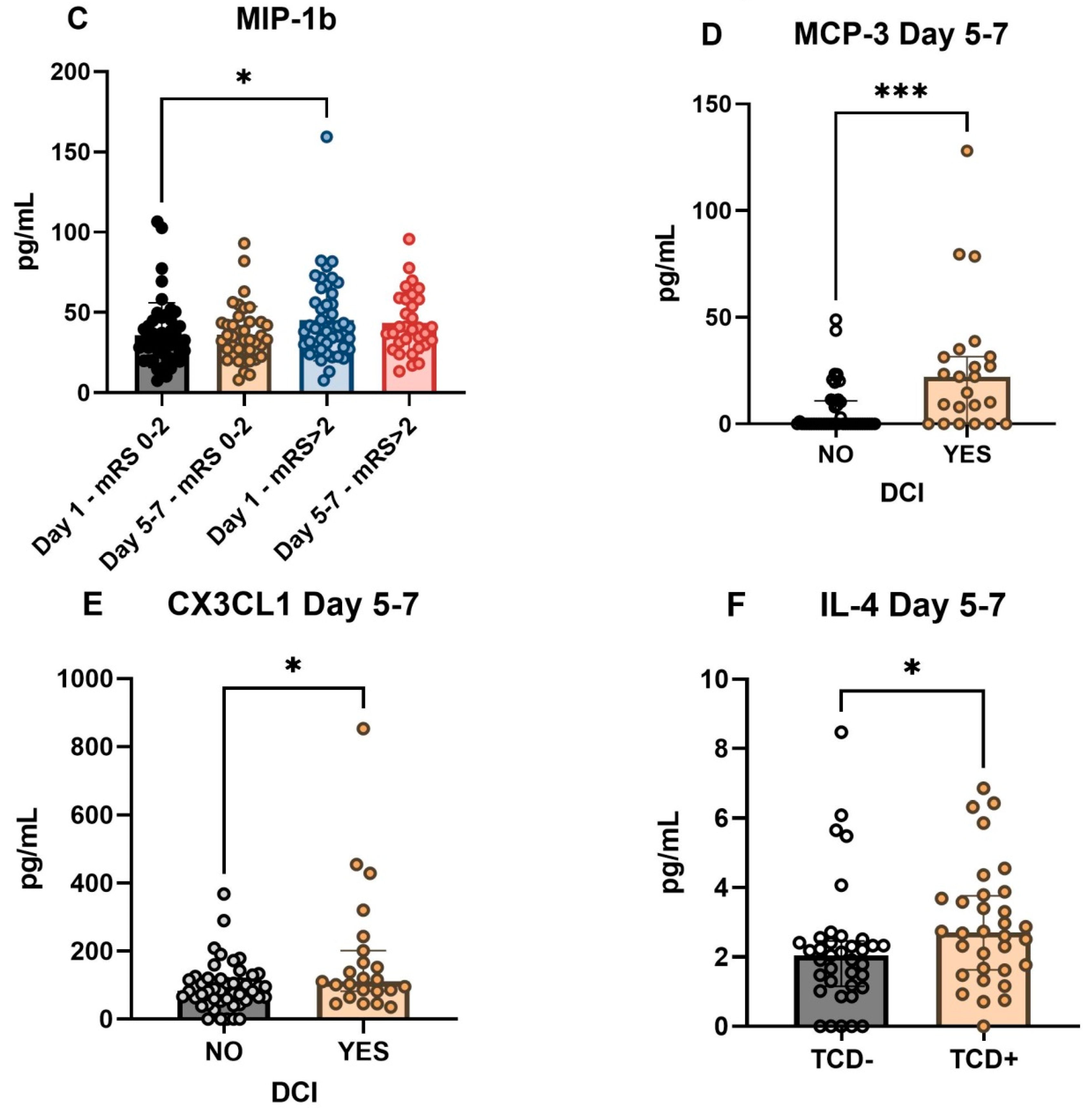
2.2. Cytokines Associated with DCI and Functional Outcome
2.3. Clinical Variables Associated with DCI and Day 30 Functional Outcome
2.4. Correlations between Biomarkers in aSAH Patients
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Clinical Definitions
4.3. Sampling and Laboratory Analysis
4.4. Statistical Analysis
4.5. Ethical Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Rooij, N.K.; Linn, F.H.H.; Van Der Plas, J.A.; Algra, A.; Rinkel, G.J.E. Incidence of subarachnoid haemorrhage: A systematic review with emphasis on region, age, gender and time trends. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Velat, G.J.; Kimball, M.M.; Mocco, J.; Hoh, B.L. Vasospasm after Aneurysmal Subarachnoid Hemorrhage: Review of Randomized Controlled Trials and Meta-Analyses in the Literature. World Neurosurg. 2011, 76, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Alaraj, A.; Charbel, F.T.; Amin-Hanjani, S. Peri-operative measures for treatment and prevention of cerebral vasospasm following subarachnoid hemorrhage. Neurol. Res. 2009, 31, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Dodd, W.S.; Laurent, D.; Dumont, A.S.; Hasan, D.M.; Jabbour, P.M.; Starke, R.M.; Hosaka, K.; Polifka, A.J.; Hoh, B.L.; Chalouhi, N. Pathophysiology of Delayed Cerebral Ischemia after Subarachnoid Hemorrhage: A Review. J. Am. Heart Assoc. 2021, 10, e021845. [Google Scholar] [CrossRef]
- Sehba, F.A.; Pluta, R.M.; Zhang, J.H. Metamorphosis of Subarachnoid Hemorrhage Research: From Delayed Vasospasm to Early Brain Injury. Mol. Neurobiol. 2011, 43, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, R.L. Delayed neurological deterioration after subarachnoid haemorrhage. Nat. Rev. Neurol. 2014, 10, 44–58. [Google Scholar] [CrossRef]
- Vergouwen, M.D.; Vermeulen, M.; van Gijn, J.; Rinkel, G.J.; Wijdicks, E.F.; Muizelaar, J.P.; Mendelow, A.D.; Juvela, S.; Yonas, H.; Terbrugge, K.G.; et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: Proposal of a multidisciplinary research group. Stroke 2010, 41, 2391–2395. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Tawab, M.; Hasan, A.A.; Ahmed, M.A.; Seif, H.M.A.; Yousif, H.A. Prognostic factors of delayed cerebral ischemia after subarachnoid hemorrhage including CT perfusion: A prospective cohort study. Egypt. J. Radiol. Nucl. Med. 2020, 51, 61. [Google Scholar] [CrossRef]
- Koenig, H.M.; Chen, J.; Sieg, E.P. Delayed Cerebral Ischemia: Is Prevention Better than Treatment? J. Neurosurg. Anesthesiol. 2021, 33, 191–192. [Google Scholar] [CrossRef]
- Vlachogiannis, P.; Hillered, L.; Enblad, P.; Ronne-Engström, E. Temporal patterns of inflammation-related proteins measured in the cerebrospinal fluid of patients with aneurysmal subarachnoid hemorrhage using multiplex Proximity Extension Assay technology. PLoS ONE 2022, 17, e0263460. [Google Scholar] [CrossRef]
- Lieschke, S.; Zechmeister, B.; Haupt, M.; Zheng, X.; Jin, F.; Hein, K.; Weber, M.S.; Hermann, D.M.; Bähr, M.; Kilic, E.; et al. CCL11 Differentially Affects Post-Stroke Brain Injury and Neuroregenerationin Mice Depending on Age. Cells 2019, 9, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Pan, X.-F.; Liu, G.-D.; Liu, Z.-H.; Zhang, C.; Chen, T.; Wang, Y.-H. FGF-2 suppresses neuronal autophagy by regulating the PI3K/Akt pathway in subarachnoid hemorrhage. Brain Res. Bull. 2021, 173, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Krafft, P.R.; Ma, Q.; Rolland, W.B.; Caner, B.; Lekic, T.; Manaenko, A.; Le, M.; Tang, J.; Zhang, J.H. Fibroblast growth factors preserve blood-brain barrier integrity through RhoA inhibition after intracerebral hemorrhage in mice. Neurobiol. Dis. 2012, 46, 204–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarozzo, G.; Campanella, M.; Ghiani, M.; Bulfone, A.; Beltramo, M. Expression of fractalkine and its receptor, CX3CR1, in response to ischaemia-reperfusion brain injury in the rat. Eur. J. Neurosci. 2002, 15, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, X.; Dong, T.; Gao, F.; Li, Z.; Ma, Z. Expression of interferon regulatory factor 4 and inflammation in secondary injury of intracerebral haemorrhage. Folia Neuropathol. 2021, 59, 291–297. [Google Scholar] [CrossRef]
- Li, X.; Lin, S.; Chen, X.; Huang, W.; Li, Q.; Zhang, H.; Chen, X.; Yang, S.; Jin, K.; Shao, B. The Prognostic Value of Serum Cytokines in Patients with Acute Ischemic Stroke. Aging Dis. 2019, 10, 544–556. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.-H.; Savarraj, J.; Parsha, K.; Hergenroeder, G.W.; Chang, T.R.; Kim, D.H.; Kitagawa, R.S.; Blackburn, S.L.; Choi, H.A. Inflammation in delayed ischemia and functional outcomes after subarachnoid hemorrhage. J. Neuroinflamm. 2019, 16, 1–10. [Google Scholar] [CrossRef]
- Niwa, A.; Osuka, K.; Nakura, T.; Matsuo, N.; Watabe, T.; Takayasu, M. Interleukin-6, MCP-1, IP-10, and MIG are sequentially expressed in cerebrospinal fluid after subarachnoid hemorrhage. J. Neuroinflamm. 2016, 13, 217. [Google Scholar] [CrossRef] [Green Version]
- Rancan, M.; Bye, N.; Otto, V.I.; Trentz, O.; Kossmann, T.; Frentzel, S.; Morganti-Kossmann, M.C. The Chemokine Fractalkine in Patients with Severe Traumatic Brain Injury and a Mouse Model of Closed Head Injury. J. Cereb. Blood Flow Metab. 2004, 24, 1110–1118. [Google Scholar] [CrossRef] [Green Version]
- Lauro, C.; Catalano, M.; Trettel, F.; Limatola, C. Fractalkine in the nervous system: Neuroprotective or neurotoxic molecule? Ann. N. Y. Acad. Sci. 2015, 1351, 141–148. [Google Scholar] [CrossRef]
- Zheng, Z.V.; Wong, K.C.G. Microglial activation and polarization after subarachnoid hemorrhage. Neuroimmunol. Neuroinflamm. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Zhang, J.; Hu, X.; Zhang, L.; Mao, L.; Jiang, X.; Liou, A.K.-F.; Leak, R.; Gao, Y.; Chen, J. Microglia/Macrophage Polarization Dynamics in White Matter after Traumatic Brain Injury. J. Cereb. Blood Flow Metab. 2013, 33, 1864–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cipriani, R.; Villa, P.; Chece, G.; Lauro, C.; Paladini, A.; Micotti, E.; Perego, C.; de Simoni, M.G.; Fredholm, B.B.; Eusebi, F.; et al. CX3CL1 Is Neuroprotective in Permanent Focal Cerebral Ischemia in Rodents. J. Neurosci. 2011, 31, 16327–16335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawelec, P.; Ziemka-Nalecz, M.; Sypecka, J.; Zalewska, T. The Impact of the CX3CL1/CX3CR1 Axis in Neurological Disorders. Cells 2020, 9, 2277. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jiang, M.; Li, H.; Wang, Y.; Shen, H.; Li, X.; Zhang, Y.; Wu, J.; Yu, Z.; Chen, G. CX3CL1/CX3CR1 axis attenuates early brain injury via promoting the delivery of exosomal microRNA-124 from neuron to microglia after subarachnoid hemorrhage. J. Neuroinflamm. 2020, 17, 209. [Google Scholar] [CrossRef]
- Zanier, E.R.; Marchesi, F.; Ortolano, F.; Perego, C.; Arabian, M.; Zoerle, T.; Sammali, E.; Pischiutta, F.; De Simoni, M.-G. Fractalkine Receptor Deficiency Is Associated with Early Protection but Late Worsening of Outcome following Brain Trauma in Mice. J. Neurotrauma 2016, 33, 1060–1072. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.H.; Kellner, C.P.; Hahn, D.K.; Desantis, B.M.; Musabbir, M.; Starke, R.M.; Rynkowski, M.; Komotar, R.J.; Otten, M.L.; Sciacca, R.; et al. Monocyte chemoattractant protein-1 predicts outcome and vasospasm following aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2008, 109, 38–43. [Google Scholar] [CrossRef]
- Lu, H.; Shi, J.-X.; Chen, H.-L.; Hang, C.-H.; Wang, H.-D.; Yin, H.-X. Expression of monocyte chemoattractant protein-1 in the cerebral artery after experimental subarachnoid hemorrhage. Brain Res. 2009, 1262, 73–80. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Yaish-Ohad, S.; Sarau, H.M.; Barone, F.C.; Feuerstein, G.Z. Molecular cloning and expression of the rat monocyte chemotactic protein-3 gene: A possible role in stroke. Mol. Brain Res. 1999, 71, 304–312. [Google Scholar] [CrossRef]
- Dahinden, C.A.; Geiser, T.; Brunner, T.; Von Tscharner, V.; Caput, D.; Ferrara, P.; Minty, A.; Baggiolini, M. Monocyte chemotactic protein 3 is a most effective basophil- and eosinophil-activating chemokine. J. Exp. Med. 1994, 179, 751–756. [Google Scholar] [CrossRef] [Green Version]
- Ransohoff, R.M.; Hamilton, T.A.; Tani, M.; Stoler, M.H.; Shick, H.E.; Major, J.A.; Estes, M.L.; Thomas, D.M.; Tuohy, V.K. Astrocyte expression of mRNA encoding cytokines IP-10 and JE/MCP-1 in experimental autoimmune encephalomyelitis. FASEB J. 1993, 7, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Lahrtz, F.; Piali, L.; Nadal, D.; Pfister, H.-W.; Spanaus, K.-S.; Baggiolini, M.; Fontana, A. Chemotactic activity on mononuclear cells in the cerebrospinal fluid of patients with viral meningitis is mediated by interferon-γ inducible protein-10 and monocyte chemotactic protein-1. Eur. J. Immunol. 1997, 27, 2484–2489. [Google Scholar] [CrossRef] [PubMed]
- Righy, C.; Turon, R.; De Freitas, G.; Japiassú, A.M.; Neto, H.C.D.C.F.; Bozza, M.; Oliveira, M.F.; Bozza, F.A. Hemoglobin metabolism by-products are associated with an inflammatory response in patients with hemorrhagic stroke. Subprodutos do metabolismo da hemoglobina se associam com respostainflamatóriaempacientes com acidente vascular cerebral hemorrágico. Rev. Bras. Ter. Intensiv. 2018, 30, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Loetscher, P.; Seitz, M.; Clark-Lewis, I.; Baggiolini, M.; Moser, B. Monocyte chemotactic proteins MCP-1, MCP-2, and MCP-3 are major attractants for human CD4+ and CD8+ T lymphocytes. FASEB J. 1994, 8, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Taub, D.D.; Proost, P.; Murphy, W.J.; Anver, M.; Longo, D.L.; Van Damme, J.; Oppenheim, J.J. Monocyte chemotactic protein-1 (MCP-1), -2, and -3 are chemotactic for human T lymphocytes. J. Clin. Investig. 1995, 95, 1370–1376. [Google Scholar] [CrossRef] [Green Version]
- Carrera, E.; Schmidt, J.M.; Oddo, M.; Fernandez, L.; Claassen, J.; Seder, D.; Lee, K.; Badjatia, N.; Connolly, E.S., Jr.; Mayer, S.A. Transcranial Doppler for predicting delayed cerebral ischemia after subarachnoid hemorrhage. Neurosurgery 2009, 65, 316–324. [Google Scholar] [CrossRef]
- Chang, J.J.; Triano, M.; Corbin, M.J.; Desale, S.; Liu, A.-H.; Felbaum, D.R.; Mai, J.C.; Armonda, R.A.; Aulisi, E.F. Transcranial Doppler velocity and associations with delayed cerebral ischemia in aneurysmal subarachnoid Hemorrhage. J. Neurol. Sci. 2020, 415, 116934. [Google Scholar] [CrossRef]
- Papaioannou, V.E.; Budohoski, K.P.; Placek, M.M.; Czosnyka, Z.; Smielewski, P.; Czosnyka, M. Association of transcranial Doppler blood flow velocity slow waves with delayed cerebral ischemia in patients suffering from subarachnoid hemorrhage: A retrospective study. Intensiv. Care Med. Exp. 2021, 9, 11. [Google Scholar] [CrossRef]
- Snider, S.B.; Migdady, I.; LaRose, S.L.; Mckeown, M.E.; Regenhardt, R.W.; Lai, P.M.R.; Vaitkevicius, H.; Du, R. Transcranial-Doppler-Measured Vasospasm Severity is Associated with Delayed Cerebral Infarction after Subarachnoid Hemorrhage. Neurocrit. Care 2022, 36, 815–821. [Google Scholar] [CrossRef]
- Al-Tamimi, Y.Z.; Bhargava, D.; Orsi, N.M.; Teraifi, A.; Cummings, M.; Ekbote, U.V.; Quinn, A.C.; Homer-Vanniasinkam, S.; Ross, S. Compartmentalisation of the inflammatory response following aneurysmal subarachnoid haemorrhage. Cytokine 2019, 123, 154778. [Google Scholar] [CrossRef]
- Eyang, J.; Eding, S.; Ehuang, W.; Eqichuan, Z.; Huang, S.; Ezhang, Y.; Zhuge, Q. Interleukin-4 Ameliorates the Functional Recovery of Intracerebral Hemorrhage through the Alternative Activation of Microglia/Macrophage. Front. Neurosci. 2016, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Enkhjargal, B.; Travis, Z.D.; Ocak, U.; Tang, J.; Suzuki, H.; Zhang, J.H. FGF-2 Attenuates Neuronal Apoptosis via FGFR3/PI3k/Akt Signaling Pathway after Subarachnoid Hemorrhage. Mol. Neurobiol. 2019, 56, 8203–8219. [Google Scholar] [CrossRef] [PubMed]
- Akhter, R.; Shao, Y.; Formica, S.; Khrestian, M.; Bekris, L.M. TREM2 alters the phagocytic, apoptotic and inflammatory response to Aβ42 in HMC3 cells. Mol. Immunol. 2021, 131, 171–179. [Google Scholar] [CrossRef]
- Grosset, D.G.; Straiton, J.; du Trevou, M.; Bullock, R. Prediction of symptomatic vasospasm after subarachnoid hemorrhage by rapidly increasing transcranial Doppler velocity and cerebral blood flow changes. Stroke 1992, 23, 674–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frontera, J.A.; Fernandez, A.; Schmidt, J.M.; Claassen, J.; Wartenberg, K.E.; Badjatia, N.; Connolly, E.S.; Mayer, S. A Defining vasospasm after subarachnoid hemorrhage: What is the most clinically relevant definition? Stroke 2009, 40, 1963–1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Number of Patients with Aneurysmal SAH, n = 112 | ||
|---|---|---|
| Age | (years, mean ± SD) | 57 ± 13 |
| Female | (N, %) | 69 (61.6%) |
| Hypertension | (N, %) | 49 (43.8%) |
| Diabetes | (N, %) | 11 (9.8%) |
| Smoking | (N, %) | 12 (10.7%) |
| Aneurysm location | ||
| (N, %) | 16 (14.3%) |
| (N, %) | 22 (19.6%) |
| (N, %) | 31 (27.7%) |
| (N, %) | 13 (11.6%) |
| (N, %) | 14 (12.5%) |
| (N, %) | 16 (14.3%) |
| WFNS | ||
| (N, %) | 38 (33.9%) |
| (N, %) | 24 (21.4%) |
| (N, %) | 8 (7.1%) |
| (N, %) | 14 (12.5%) |
| (N, %) | 28 (25%) |
| modified Fischer grade | ||
| (N, %) | 1 (0.9%) |
| (N, %) | 18 (16,1%) |
| (N, %) | 57 (50.9%) |
| (N, %) | 36 (32.1%) |
| Glasgow coma scale, on admission | median, IQR | 13 (6–15) |
| Neutrophile-lymphocyte ratio, on admission | median, IQR | 5.9 (4–10) |
| C-reactive protein, on admission | median, IQR | 13 (4–61) |
| Creatinine, on admission | median, IQR | 61 (50–72) |
| Extraventricular drainage | (N, %) | 53 (47.3%) |
| Infection, CSF | (N, %) | 7 (6.3%) |
| Infection, systemic | (N, %) | 18 (16.1%) |
| Infection, CSF + systemic | (N, %) | 5 (4.5%) |
| Mechanical ventilation | (N, %) | 50 (44.6%) |
| Decompressive craniotomy | (N, %) | 14 (12.5%) |
| Lumbal drainage | (N, %) | 14 (12.5%) |
| Delayed cerebral ischemia | (N, %) | 32 (29.1%) |
| Angiographic vasospasm | (N, %) | 28 (28.3%) |
| Transcranial Doppler positivity | (N, %) | 41 (41.8%) |
| Ischemiclesion on MRI | (N, %) | 16 (15.8%) |
| Favorable outcome on Day 30 (mRS = 0–2) | (N, %) | 58 (51.8%) |
| In-hospital death | (N, %) | 15 (13.4%) |
| DCI during Hospitalization | mRSScore at Day 30 | |||
|---|---|---|---|---|
| DCI − (n = 78 (71%)) vs. DCI + (n = 32 [29%]) | Unfavorable Outcome (mRS ≥ 3, n = 54 (48.2%)) vs. Favorable Outcome (mRS ≤ 2, n = 58 (51.8%)) | |||
| Cytokines | Day 1 | Day 5–7 | Day 1 | Day 5–7 |
| Eotaxin | - | - | - | - |
| FGF-2 | - | - | - | - |
| FLT-3L | - | - | - | - |
| CX3CL1 | - | H * | - | - |
| IL-1b | - | - | - | - |
| IL-4 | - | - | - | - |
| IP-10 | - | - | H * | - |
| MCP-3 | - | H ** | H * | - |
| MIP-1b | - | - | H * | - |
| Total | 0 | 2 | 3 | 0 |
| No DCI (n= 78) | Group A: DCI before T2 (n = 7) | Group B: DCI after T2 (n = 25) | p-Value (between A and B) | |
|---|---|---|---|---|
| MCP-3 T2, pg/mL, median (IQR) | 0 (0–11) | 22 (8–27) | 18 (0–32) | 0.857 |
| CX3CL1 T2, pg/mL, median (IQR) | 83 (58–119) | 116 (103–138) | 106 (65–243) | 0.691 |
| Variable | DCI | p-Value | Functional Outcome at Day 30 | p-Value | ||
|---|---|---|---|---|---|---|
| DCI (n = 32) | No-DCI (n = 78) | Unfavorable (n = 54) | Favorable (n = 58) | |||
| Age (years, mean ± SD) | 54.8 ± 11 | 57.9 ± 14 | 0.223 | 61.8 ± 12 | 52.6 ± 12 | <0.001 |
| Female, N (%) | 17 (53%) | 50 (64%) | 0.284 | 29 (53.7%) | 40 (69%) | 0.097 |
| Hypertension, n (%) | 11 (34.4%) | 37 (47.4%) | 0.210 | 28 (51.9%) | 21 (36.2%) | 0.095 |
| Diabetes, n (%) | 3 (9.4%) | 8 (10.3%) | 0.889 | 10 (18.5%) | 1 (1.7%) | 0.003 |
| Smoking, n (%) | 2 (6.3%) | 10 (12.8%) | 0.315 | 4 (7.4%) | 8 (13.8%) | 0.275 |
| WFNS, median (IQR) | 3 (1–5) | 2 (1–4) | 0.412 | 4 (3–5) | 1 (1–2) | <0.001 |
| modified Fischer grade, median (IQR) | 3 (2–4) | 3 (2–4) | 1.000 | 4 (3–4) | 2 (1–3) | <0.001 |
| Glasgow coma scale, median (IQR) | 9 (5–14) | 14 (10–15) | 0.02 | 6 (3–12) | 14 (13–15) | <0.001 |
| Neutrophile-lymphocyte ratio, median (IQR) | 7 (5–10) | 5 (3–11) | 0.092 | 7 (4–12) | 5.3 (3–8) | 0.054 |
| C-reactive protein, median (IQR) | 24 (5–75) | 9.5 (3–43) | 0.104 | 41 (9–89) | 6.8 (3–17) | <0.001 |
| Creatinine, median (IQR) | 61 (50–72) | 60 (50–72) | 0.744 | 63 (50–76) | 59 (50–67) | 0.122 |
| Extraventricular drainage, n (%) | 18 (56.3%) | 33 (42.3%) | 0.183 | 41 (75.9%) | 12 (20.7%) | <0.001 |
| Mechanical ventilation, n (%) | 19 (59.4%) | 29 (37.2%) | 0.033 | 43 (79.6%) | 7 (12.1%) | <0.001 |
| Decompressive craniotomy, n (%) | 8 (25%) | 5 (6.4%) | 0.006 | 11 (20.4%) | 3 (5.2%) | 0.015 |
| Angiographic vasospasm, n (%) | 27 (84.4%) | 1 (1.5%) | <0.001 | 22 (52.4%) | 6 (10.5%) | <0.001 |
| Transcranial Doppler positivity, N (%) | 30 (96.8%) | 11 (16.4%) | <0.001 | 23 (54.8%) | 18 (32.1%) | 0.025 |
| Ischemic lesion on MRI, N (%) | 16 (50%) | 0 (0%) | <0.001 | 16 (35.6%) | 0 (0%) | <0.001 |
| No Infection | Infection during Hospitalization | |||||
|---|---|---|---|---|---|---|
| No DCI | DCI | p-Value | No DCI | DCI | p-Value | |
| MCP-3 T2, pg/mL, median (IQR) | 0 (0–11) | 12 (0–32) | 0.025 | 0 (0–8) | 23 (9–27) | 0.004 |
| CX3CL1 T2, pg/mL, median (IQR) | 82 (53–118) | 102 (46–201) | 0.221 | 94 (60–179) | 116 (95–166) | 0.152 |
| B | Wald | Sig. | Exp(B) | |
|---|---|---|---|---|
| MCP-3 T2 | 0.045 | 5.221 | 0.022 | 1.046 |
| GCS on admission | −0.031 | −0.062 | 0.803 | 0.97 |
| Mechanical Ventilation | −0.954 | 0.638 | 0.424 | 0.385 |
| Gender | −0.974 | 2.496 | 0.114 | 0.378 |
| Age | −0.026 | 1.062 | 0.303 | 0.974 |
| Constant | 1.593 | 0.922 | 0.337 | 4.917 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spantler, D.; Molnar, T.; Simon, D.; Berki, T.; Buki, A.; Schwarcz, A.; Csecsei, P. Biomarker Associations in Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage. Int. J. Mol. Sci. 2022, 23, 8789. https://doi.org/10.3390/ijms23158789
Spantler D, Molnar T, Simon D, Berki T, Buki A, Schwarcz A, Csecsei P. Biomarker Associations in Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage. International Journal of Molecular Sciences. 2022; 23(15):8789. https://doi.org/10.3390/ijms23158789
Chicago/Turabian StyleSpantler, Dora, Tihamer Molnar, Diana Simon, Timea Berki, Andras Buki, Attila Schwarcz, and Peter Csecsei. 2022. "Biomarker Associations in Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage" International Journal of Molecular Sciences 23, no. 15: 8789. https://doi.org/10.3390/ijms23158789
APA StyleSpantler, D., Molnar, T., Simon, D., Berki, T., Buki, A., Schwarcz, A., & Csecsei, P. (2022). Biomarker Associations in Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage. International Journal of Molecular Sciences, 23(15), 8789. https://doi.org/10.3390/ijms23158789







