Dimethyl Fumarate-Loaded Transethosomes: A Formulative Study and Preliminary Ex Vivo and In Vivo Evaluation
Abstract
:1. Introduction
2. Results
2.1. Preparation of Transethosomes
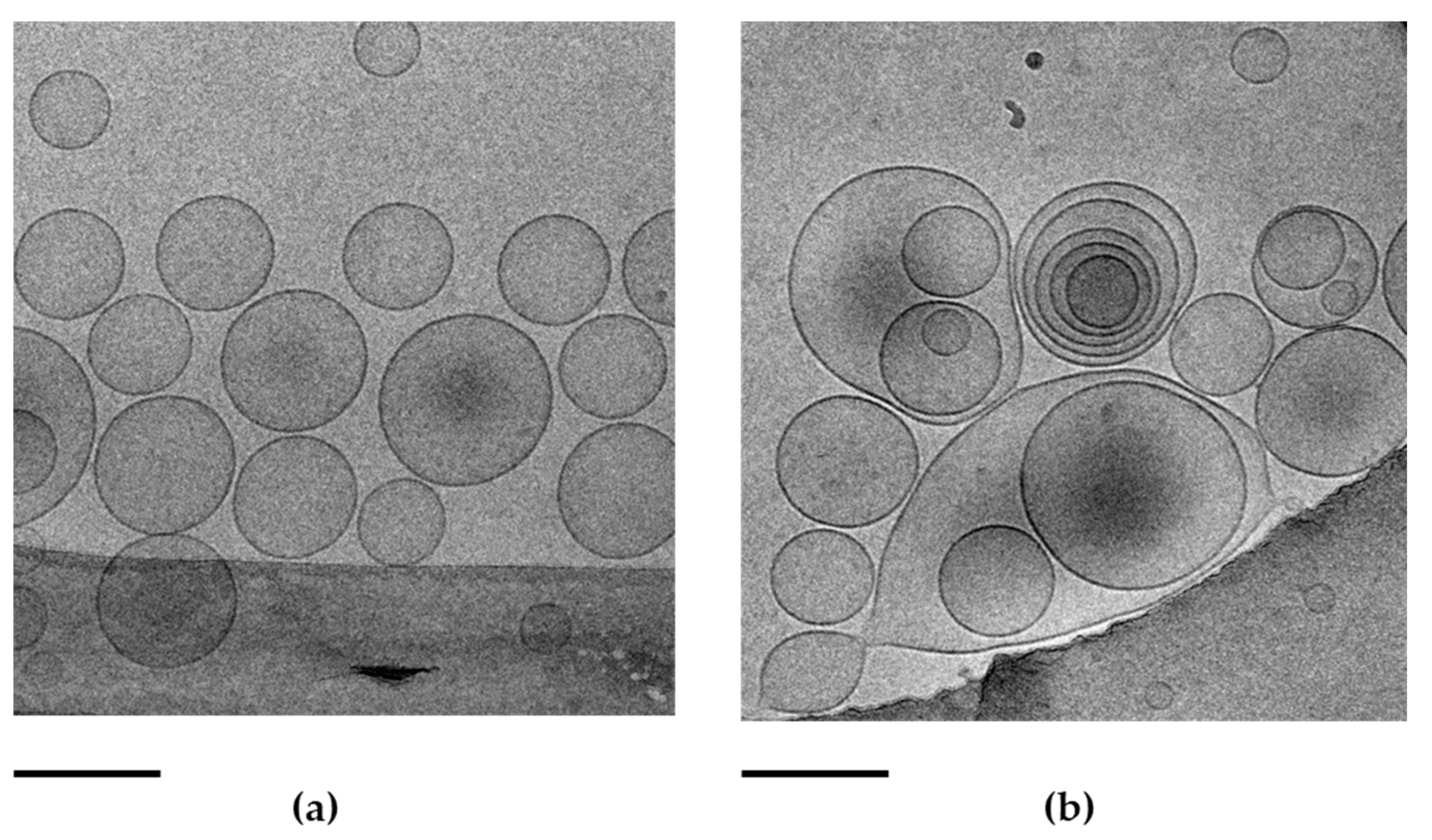
2.2. Morphology of Transethosomes
2.3. Size Distribution of Transethosomes
2.4. DMF Entrapment Capacity (EC)
2.5. Stability Evaluation
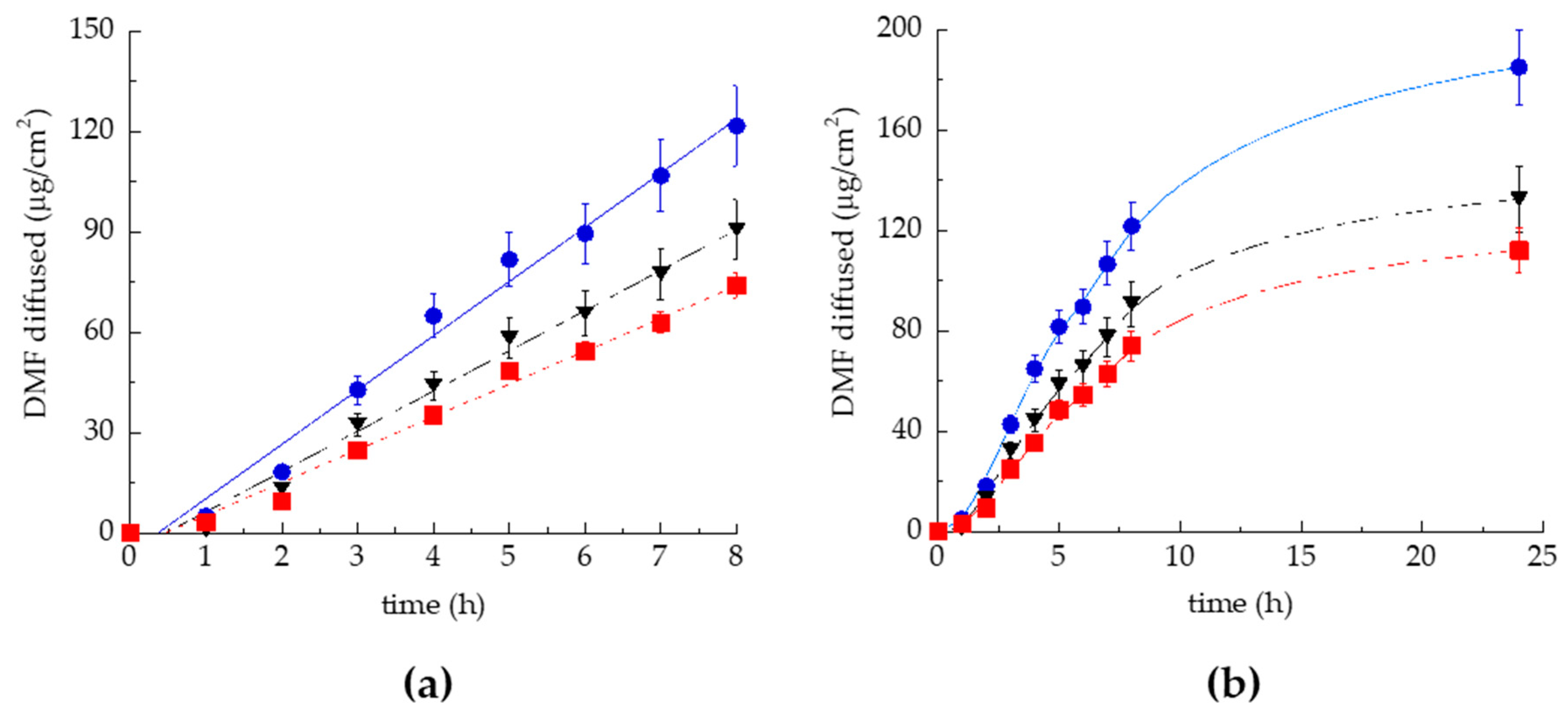
2.6. In Vitro Release Test (IVRT)
2.7. In Vitro Permeation Test (IVPT)
2.8. Cell Viability
2.9. Scratch Assay
2.10. Transmission Electron Microscopy
2.11. TET0.9-DMF50 Formulation
2.12. TET Gel Formulation
2.13. Patch Test
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Transethosome Preparation
4.3. Photon Correlation Spectroscopy (PCS)
4.4. Cryo-Transmission Electron Microscopy (Cryo-TEM)
4.5. Evaluation of DMF Entrapment Capacity in Transethosome
4.6. Franz Cell Diffusion Experiments
4.6.1. In Vitro Release Test (IVRT)
4.6.2. In Vitro Skin Permeation Test (IVPT)
4.7. HPLC Analysis
4.8. Biological Activity Studies
4.8.1. Cytotoxicity Study
4.8.2. Scratch Assay
4.9. Transmission Electron Microscopy (TEM) Analyses
4.10. Preparation of TET Gel
4.11. Patch Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Broughton, G.; Janis, J.E.; Attinger, C.E. Wound healing: An overview. Plast. Reconstr. Surg. 2006, 117, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Greenhalgh, D.G. Wound healing and diabetes mellitus. Clin. Plast. Surg. 2003, 30, 37–45. [Google Scholar] [CrossRef]
- Ting, M. Wound healing and peripheral vascular disease. Crit Care Nurs. Clin. North Am. 1991, 3, 515–523. [Google Scholar] [CrossRef]
- Mrowietz, U.; Barker, J.; Boehncke, W.H.; Iversen, L.; Kirby, B.; Naldi, L.; Reich, K.; Tanew, A.; van de Kerkhof, P.C.M.; Warren, R.B. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: A European expert consensus. J. Eur. Acad. Dermatology Venereol. 2018, 32, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Reszke, R.; Szepietowski, J.C. A safety evaluation of dimethyl fumarate in moderate-to-severe psoriasis. Expert Opin. Drug Saf. 2020, 19, 373–380. [Google Scholar] [CrossRef]
- Blair, H.A. Dimethyl Fumarate: A Review in Relapsing-Remitting MS. Drugs 2019, 79, 1965–1976. [Google Scholar] [CrossRef]
- Nicolay, J.P.; Albrecht, J.D.; Assaf, C.; Dippel, E.; Stadler, R.; Wehkamp, U.; Wobser, M.; Guelow, K.; Goerdt, S.; Krammer, P.H. Dimethyl fumarate (DMF) therapy in CTCL: Results from a clinical phase II study. Eur. J. Cancer 2021, 156, S21–S22. [Google Scholar] [CrossRef]
- Ghods, A.; Glick, R.; Braun, D.; Feinstein, D. Beneficial actions of the anti-inflammatory dimethyl fumarate in glioblastomas. Surg. Neurol. Int. 2013, 4, 160. [Google Scholar] [CrossRef]
- Li, Y.; Ma, F.; Li, H.; Song, Y.; Zhang, H.; Jiang, Z.; Wu, H. Dimethyl fumarate accelerates wound healing under diabetic conditions. J. Mol. Endocrinol. 2018, 61, 163–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco-Fernandez, B.; Castaño, O.; Mateos-Timoneda, M.Á.; Engel, E.; Pérez-Amodio, S. Nanotechnology Approaches in Chronic Wound Healing. Adv. Wound Care 2021, 10, 234–256. [Google Scholar] [CrossRef] [PubMed]
- Yudaev, P.; Mezhuev, Y.; Chistyakov, E. Nanoparticle-Containing Wound Dressing: Antimicrobial and Healing Effects. Gels 2022, 8, 329. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Ghosh, B.; Mukhopadhyay, M. Development of nanotechnology for advancement and application in wound healing: A review. IET Nanobiotechnology 2019, 13, 778–785. [Google Scholar] [CrossRef]
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674. [Google Scholar] [CrossRef]
- Chhibber, S.; Kaur, J.; Kaur, S. Liposome entrapment of bacteriophages improves wound healing in a diabetic mouse MRSA infection. Front. Microbiol. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A.; Kumar, N.; Singh, P.; Uttam, T.; Bhoj, S.; Singh, R. In vivo therapeutic efficacy of Curcuma longa extract loaded ethosomes on wound healing. Vet. Res. Commun. 2022, 1–17. [Google Scholar] [CrossRef]
- Mombeiny, R.; Tavakol, S.; Kazemi, M.; Mehdizadeh, M.; Hasanzadeh, A.; Babaahmadi, K.M.; Abedi, A.; Keyhanvar, P. Anti-inflammatory ethosomal nanoformulation in combination with iontophoresis in chronic wound healing: An ex vivo study. IET Nanobiotechnology 2021, 15, 710–718. [Google Scholar] [CrossRef]
- Natsheh, H.; Vettorato, E.; Touitou, E. Ethosomes for Dermal Administration of Natural Active Molecules. Curr Pharm Des. 2019, 25, 2338–2348. [Google Scholar] [CrossRef]
- Touitou, E.; Natsheh, H. Topical administration of drugs incorporated in carriers containing phospholipid soft vesicles for the treatment of skin medical conditions. Pharmaceutics 2021, 13, 2129. [Google Scholar] [CrossRef]
- Shen, L.N.; Zhang, Y.T.; Wang, Q.; Xu, L.; Feng, N.P. Enhanced in vitro and in vivo skin deposition of apigenin delivered using ethosomes. Int. J. Pharm. 2014, 460, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Subheet, J.; Tiwary, A.K.; Sapra, B.; Jain, N.K. Formulation and evaluation of ethosomes for transdermal delivery of lamivudine. World J. Pharm. Res. 2007, 8, E111. [Google Scholar] [CrossRef] [Green Version]
- Kumar Mishra, K.; Deep Kaur, C.; Verma, S.; Kumar Sahu, A.; Kumar Dash, D.; Kashyap, P.; Prasad Mishra, S. Transethosomes and Nanoethosomes: Recent Approach on Transdermal Drug Delivery System. Nanomedicines 2019. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Utreja, P. Transethosomes of Econazole Nitrate for Transdermal Delivery: Development, In-vitro Characterization, and Ex-vivo Assessment. Pharm Nanotechnol. 2018, 6, 171–179. [Google Scholar] [CrossRef]
- Chen, Z.X.; Li, B.; Liu, T.; Wang, X.; Zhu, Y.; Wang, L.; Wang, X.H.; Niu, X.; Xiao, Y.; Sun, Q. Evaluation of paeonol-loaded transethosomes as transdermal delivery carriers. Eur. J. Pharm. Sci. 2017, 99, 240–245. [Google Scholar] [CrossRef]
- Albash, R.; Abdelbary, A.A.; Refai, H.; El-Nabarawi, M.A. Use of transethosomes for enhancing the transdermal delivery of olmesartan medoxomil: In vitro, ex vivo, and in vivo evaluation. Int. J. Nanomedicine 2019, 14, 1953–1968. [Google Scholar] [CrossRef] [Green Version]
- Sguizzato, M.; Ferrara, F.; Mariani, P.; Pepe, A.; Cortesi, R.; Huang, N.; Simelière, F.; Boldrini, P.; Baldisserotto, A.; Valacchi, G.; et al. “Plurethosome” as vesicular system for cutaneous administration of mangiferin: Formulative study and 3D skin tissue evaluation. Pharmaceutics 2021, 13, 1124. [Google Scholar] [CrossRef]
- Sguizzato, M.; Ferrara, F.; Hallan, S.S.; Baldisserotto, A.; Drechsler, M.; Malatesta, M.; Costanzo, M.; Cortesi, R.; Puglia, C.; Valacchi, G.; et al. Ethosomes and transethosomes for mangiferin transdermal delivery. Antioxidants 2021, 10, 768. [Google Scholar] [CrossRef]
- Ferrara, F.; Benedusi, M.; Sguizzato, M.; Cortesi, R.; Baldisserotto, A.; Buzzi, R.; Valacchi, G.; Esposito, E. Ethosomes and Transethosomes as Cutaneous Delivery Systems for Quercetin: A Preliminary Study on Melanoma Cells. Pharmaceutics 2022, 14, 1038. [Google Scholar] [CrossRef]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® synthetic membrane: Permeability comparison to human cadaver skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef]
- European Medicines Agency. Draft Guideline on quality and equivalence of topical products. In Nanomedicines [Internet]; Farrukh, M.A., Ed.; IntechOpen: London, UK, 2019; Volume 44, pp. 1–36. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-quality-equivalence-topical-products_en.pdf (accessed on 20 April 2022).
- The United States Pharmacopeial Convention. <1724> Semisolid Drug Products/General Information Semisolid Drug Products—Performance Tests. In The United States Pharmacopeia 37; The United States Pharmacopeial Convention: Rockville, MD, USA, 2014; pp. 1273–1284.
- Ng, S.F.; Rouse, J.; Sanderson, D.; Eccleston, G. A Comparative study of transmembrane diffusion and permeation of ibuprofen across synthetic membranes using franz diffusion cells. Pharmaceutics 2010, 2, 209–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costanzo, M.; Esposito, E.; Sguizzato, M.; Lacavalla, M.A.; Drechsler, M.; Valacchi, G.; Zancanaro, C.; Malatesta, M. Formulative study and intracellular fate evaluation of ethosomes and transethosomes for vitamin D3 delivery. Int. J. Mol. Sci. 2021, 22, 5341. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, R. The Neglected Role of the Surfactant Tail Self-Assembly. Langmuir 2002, 18, 31–38. [Google Scholar] [CrossRef]
- Šegota, S.; Težak, D. Spontaneous formation of vesicles. Adv. Colloid Interface Sci. 2006, 121, 51–75. [Google Scholar] [CrossRef]
- Paolino, D.; Lucania, G.; Mardente, D.; Alhaique, F.; Fresta, M. Ethosomes for skin delivery of ammonium glycyrrhizinate: In vitro percutaneous permeation through human skin and in vivo anti-inflammatory activity on human volunteers. J. Control. Release 2005, 106, 99–110. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, D.; Zhang, L.; Lin, Q.; Ren, W.; Zhang, J.; Nadeem, L.; Xu, G. Dual Effects of N,N-dimethylformamide on Cell Proliferation and Apoptosis in Breast Cancer. Dose-Response 2017, 15, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Sguizzato, M.; Valacchi, G.; Pecorelli, A.; Boldrini, P.; Simelière, F.; Huang, N.; Cortesi, R.; Esposito, E. Gallic acid loaded poloxamer gel as new adjuvant strategy for melanoma: A preliminary study. Colloids Surf. B Biointerfaces 2020, 185, 110613. [Google Scholar] [CrossRef]
- Pecora, R. Dynamic light scattering measurement of nanometer particles in liquids. J. Nanoparticle Res. 2000, 2, 123–131. [Google Scholar] [CrossRef]
- Hallan, S.S.; Sguizzato, M.; Mariani, P.; Cortesi, R.; Huang, N.; Simelière, F.; Marchetti, N.; Drechsler, M.; Ruzgas, T.; Esposito, E. Design and characterization of ethosomes for transdermal delivery of caffeic acid. Pharmaceutics 2020, 12, 740. [Google Scholar] [CrossRef]
- Le Guyader, G.; Do, B.; Vieillard, V.; Andrieux, K.; Paul, M. Comparison of the in vitro and ex vivo permeation of existing topical formulations used in the treatment of facial angiofibroma and characterization of the variations observed. Pharmaceutics 2020, 12, 1060. [Google Scholar] [CrossRef] [PubMed]
- Sguizzato, M.; Mariani, P.; Spinozzi, F.; Benedusi, M.; Cervellati, F.; Cortesi, R.; Drechsler, M.; Prieux, R.; Valacchi, G.; Esposito, E. Ethosomes for coenzyme Q10 cutaneous administration: From design to 3D skin tissue evaluation. Antioxidants 2020, 9, 485. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Pecorelli, A.; Mencarelli, M.; Carbotti, P.; Fortino, V. Rottlerin: A multifaced regulator of keratinocyte cell cycle. Exp. Dermatol. 2009, 18, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Muresan, M.X.; Sticozzi, C.; Belmonte, G.; Cervellati, F.; Ferrara, F.; Mary Ann, L.; Valacchi, G. SR-B1 involvement in keratinocytes in vitro wound closure. Arch. Biochem. Biophys. 2018, 658, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.K.; Cohen, C.; De Fraissinette, A.D.B.; Ponec, M.; Whittle, E.; Fentem, J.H. Non-animal testing strategies for assessment of the skin corrosion and skin irritation potential of ingredients and finished products. Food Chem. Toxicol. 2002, 40, 573–592. [Google Scholar] [CrossRef]
- Nohynek, G.J.; Antignac, E.; Re, T.; Toutain, H. Safety assessment of personal care products/cosmetics and their ingredients. Toxicol. Appl. Pharmacol. 2010, 243, 239–259. [Google Scholar] [CrossRef]
- SCCNFP/0245/99; Opinion Concerning Basic Criteria of the Protocols for the Skin Compatibility Testing of Potentially Cutaneous Irritant Cosmetic Ingredients or Mixtures of Ingredients on Human Volunteers. European Commission: Bruxelles, Belgium, 1999; pp. 1–5. Available online: https://ec.europa.eu/health/archive/ph_risk/committees/sccp/documents/out101_en.pdf (accessed on 25 April 2022).
- Elsayed, M.M.A.; Abdallah, O.Y.; Naggar, V.F.; Khalafallah, N.M. Deformable liposomes and ethosomes: Mechanism of enhanced skin delivery. Int. J. Pharm. 2006, 322, 60–66. [Google Scholar] [CrossRef]






| Formulation | PC 1 % w/w | Ethanol % w/w | TW80 2 % w/w | DMF 3 % w/w | Water % w/w |
|---|---|---|---|---|---|
| TET0.9 | 0.89 | 28.81 | 0.3 | - | 70 |
| TET2.7 | 2.69 | 27.01 | 0.3 | - | 70 |
| TET0.9-DMF | 0.89 | 28.76 | 0.3 | 0.05 | 70 |
| TET2.7-DMF | 2.69 | 26.96 | 0.3 | 0.05 | 70 |
| Formulation | Z-Average (nm) | Typical Intensity Distribution (nm) | Dispersity Index |
|---|---|---|---|
| TET0.9 | 144.20 ± 13.13 | 146.2 (100 %) | 0.14 ± 0.02 |
| TET2.7 | 350.40 ± 23.60 | 724.1 (84%) 166.6 (16 %) | 0.23 ± 0.05 |
| TET0.9-DMF | 130.25 ± 4.50 | 157.2 (99.6%) 36.15 (0.7%) | 0.13 ± 0.01 |
| TET2.7-DMF | 344.20 ± 17.02 | 484 (97%) 4781 (3%) | 0.17 ± 0.03 |
| IVRT Parameters | TET0.9-DMF | TET2.7-DMF | SOL-DMF |
|---|---|---|---|
| RDMF 1 ± s.d. (mg/cm2/h) | 85.28 ± 3.4 | 63.07 ± 1.9 | 64.18 ± 1.6 |
| Tlag 2 ± s.d. (h) | 0.41 ± 0.02 | 0.51 ± 0.10 | 0.30 ± 0.05 |
| ADMF 3 ± s.d. (mg/cm2) | 175.86 ± 8.2 | 140.12 ± 10.4 | 209.39 ± 12.0 |
| MDMF 4 ± s.d. (mg) | 10.0 ± 2.5 | 22.5 ± 7.1 | 15.0 ± 3.2 |
| IVPT Parameters | TET0.9-DMF | TET2.7-DMF | SOL-DMF |
|---|---|---|---|
| Jss 1 (mg cm−2 h−1) | 16.24 ± 0.7 | 9.82 ± 0.8 | 12.05 ± 0.4 |
| Tlag 2 ± s.d. (h) | 0.53 ± 0.01 | 0.42 ± 0.03 | 0.55 ± 0.02 |
| Kp 3 (cm h−1 10−3) | 32.48 ± 1.4 | 19.64 ± 1.6 | 24.1 ± 0.8 |
| D 4 (cm h−1) × 10−3 | 0.28 ± 0.1 | 0.35 ± 0.2 | 0.27 ± 0.1 |
| P 5 membrane/vehicle | 104.4 ± 5.4 | 50.5 ± 6.0 | 80.33 ± 3.0 |
| ADMF 6 (mg cm−2) | 185.2 ± 10.5 | 112.2 ± 6.8 | 132.6 ± 9.2 |
| MDMF 7 (mg cm−2) | 25.2 ± 5.2 | 32.2 ± 6.7 | 40.4 ± 5.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrara, F.; Benedusi, M.; Cervellati, F.; Sguizzato, M.; Montesi, L.; Bondi, A.; Drechsler, M.; Pula, W.; Valacchi, G.; Esposito, E. Dimethyl Fumarate-Loaded Transethosomes: A Formulative Study and Preliminary Ex Vivo and In Vivo Evaluation. Int. J. Mol. Sci. 2022, 23, 8756. https://doi.org/10.3390/ijms23158756
Ferrara F, Benedusi M, Cervellati F, Sguizzato M, Montesi L, Bondi A, Drechsler M, Pula W, Valacchi G, Esposito E. Dimethyl Fumarate-Loaded Transethosomes: A Formulative Study and Preliminary Ex Vivo and In Vivo Evaluation. International Journal of Molecular Sciences. 2022; 23(15):8756. https://doi.org/10.3390/ijms23158756
Chicago/Turabian StyleFerrara, Francesca, Mascia Benedusi, Franco Cervellati, Maddalena Sguizzato, Leda Montesi, Agnese Bondi, Markus Drechsler, Walter Pula, Giuseppe Valacchi, and Elisabetta Esposito. 2022. "Dimethyl Fumarate-Loaded Transethosomes: A Formulative Study and Preliminary Ex Vivo and In Vivo Evaluation" International Journal of Molecular Sciences 23, no. 15: 8756. https://doi.org/10.3390/ijms23158756
APA StyleFerrara, F., Benedusi, M., Cervellati, F., Sguizzato, M., Montesi, L., Bondi, A., Drechsler, M., Pula, W., Valacchi, G., & Esposito, E. (2022). Dimethyl Fumarate-Loaded Transethosomes: A Formulative Study and Preliminary Ex Vivo and In Vivo Evaluation. International Journal of Molecular Sciences, 23(15), 8756. https://doi.org/10.3390/ijms23158756










