Design Principles of Hybrid Nanomaterials for Radiotherapy Enhanced by Photodynamic Therapy
Abstract
:1. Introduction
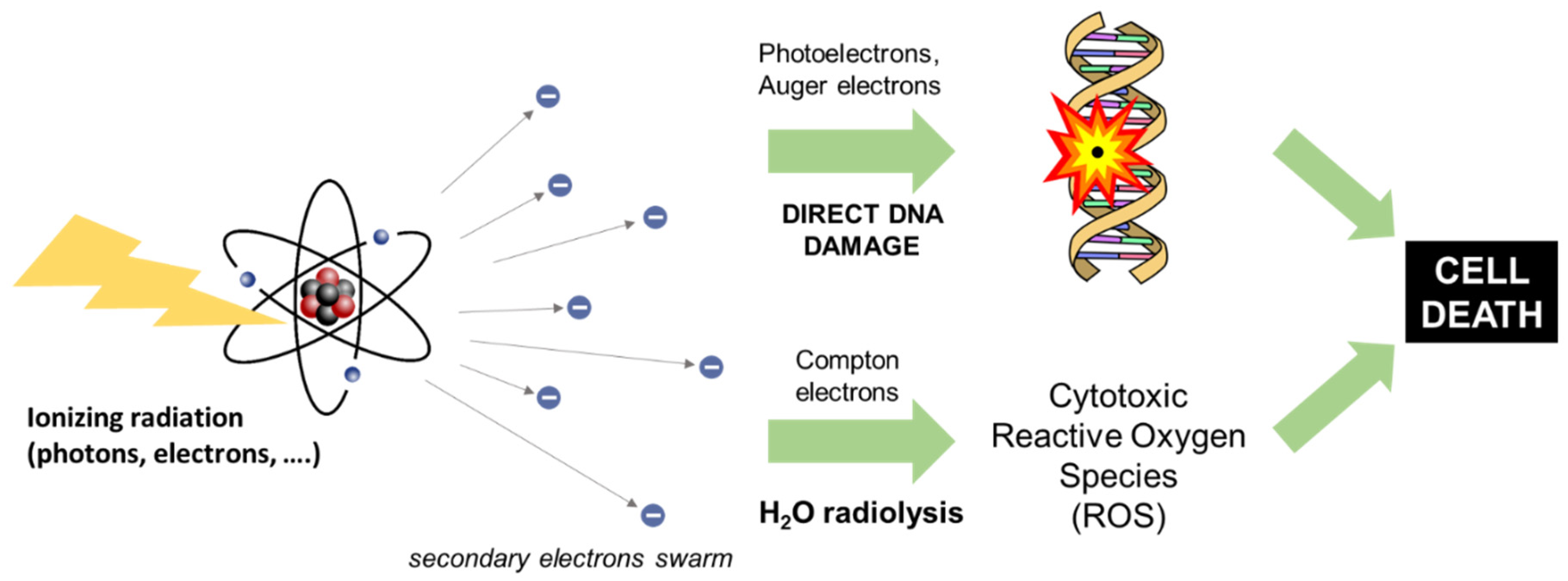
2. Principles of Radiotherapy
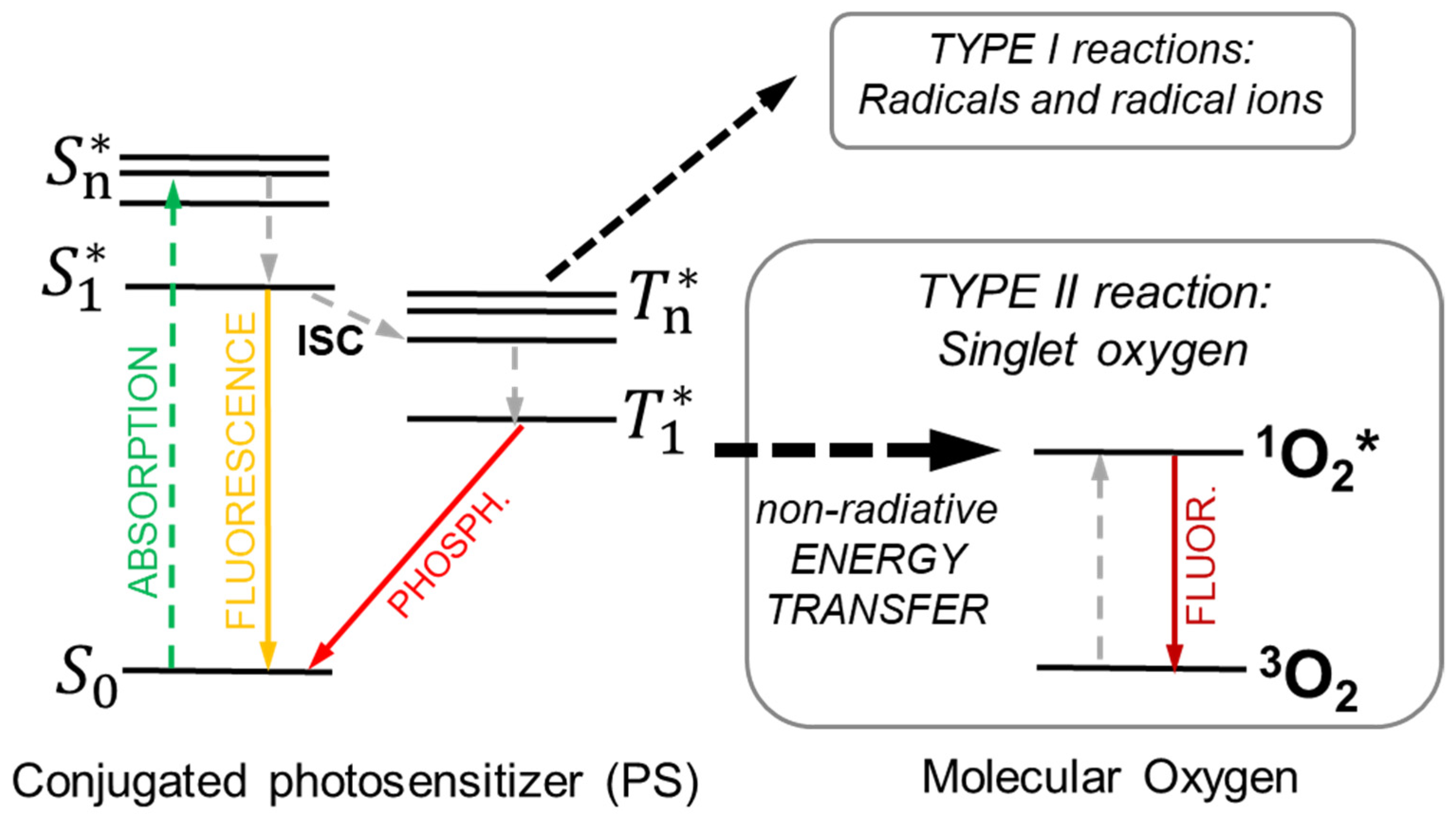
3. Principles of Photodynamic Therapy
4. Principles of Radiotherapy Enhanced by Photodynamic Therapy (X-PDT)
4.1. The Physics of X-PDT: The Radiosensitization Effect
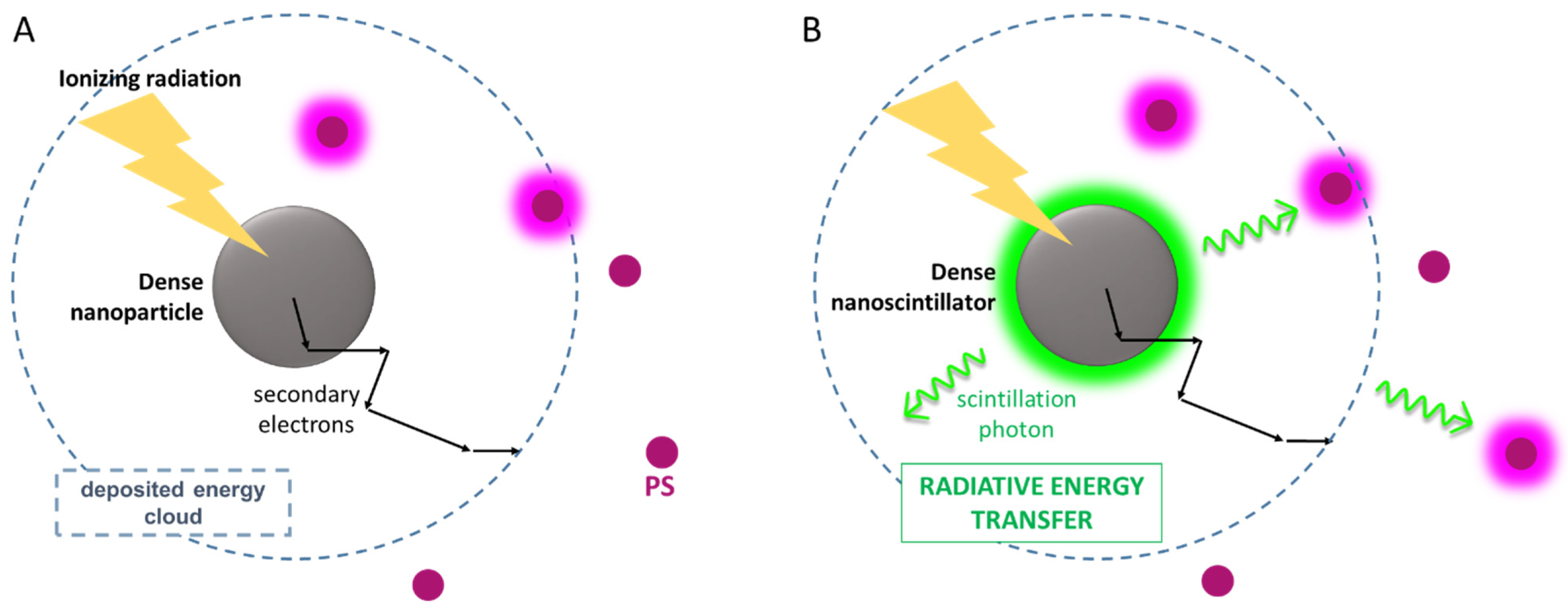
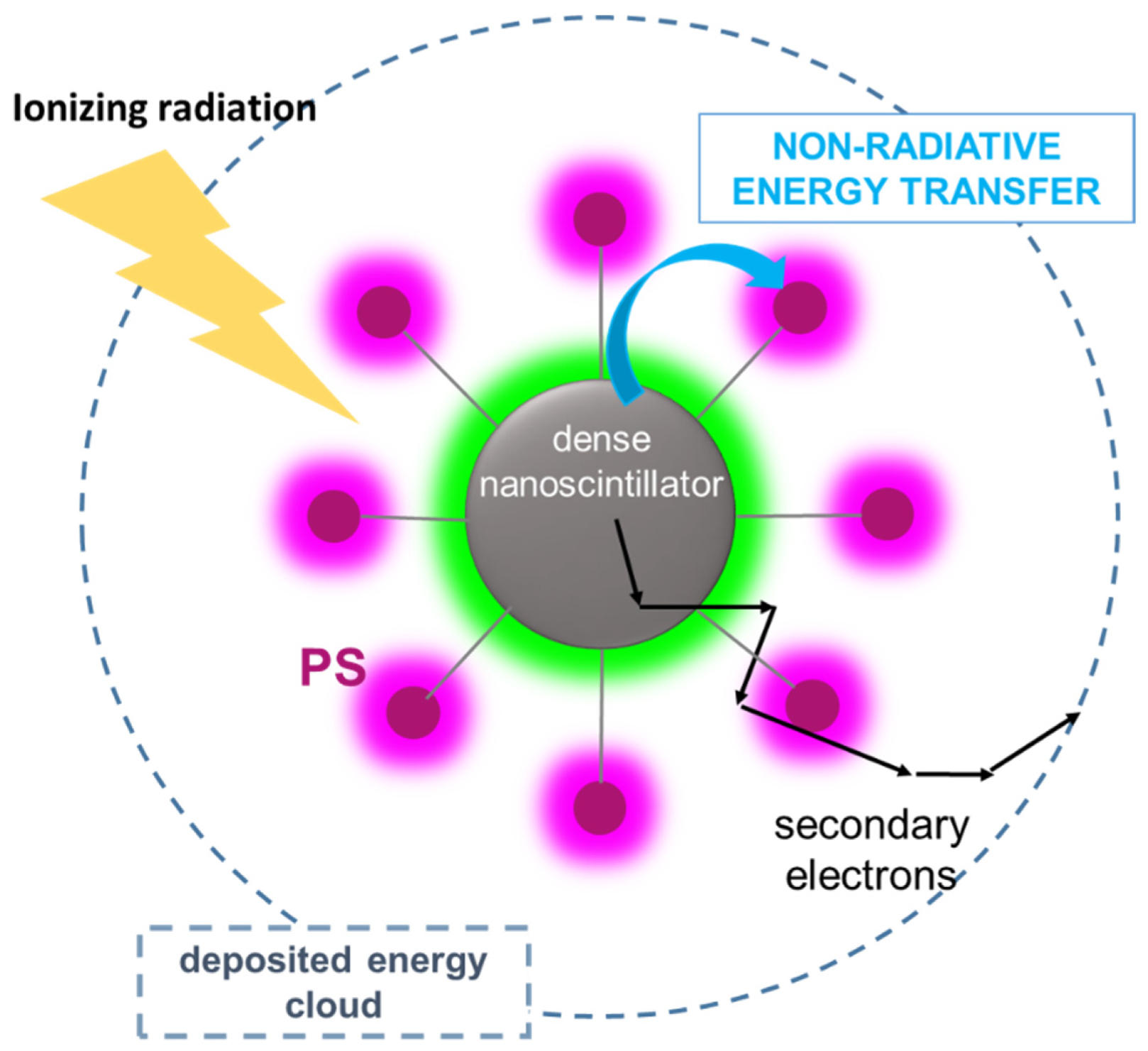
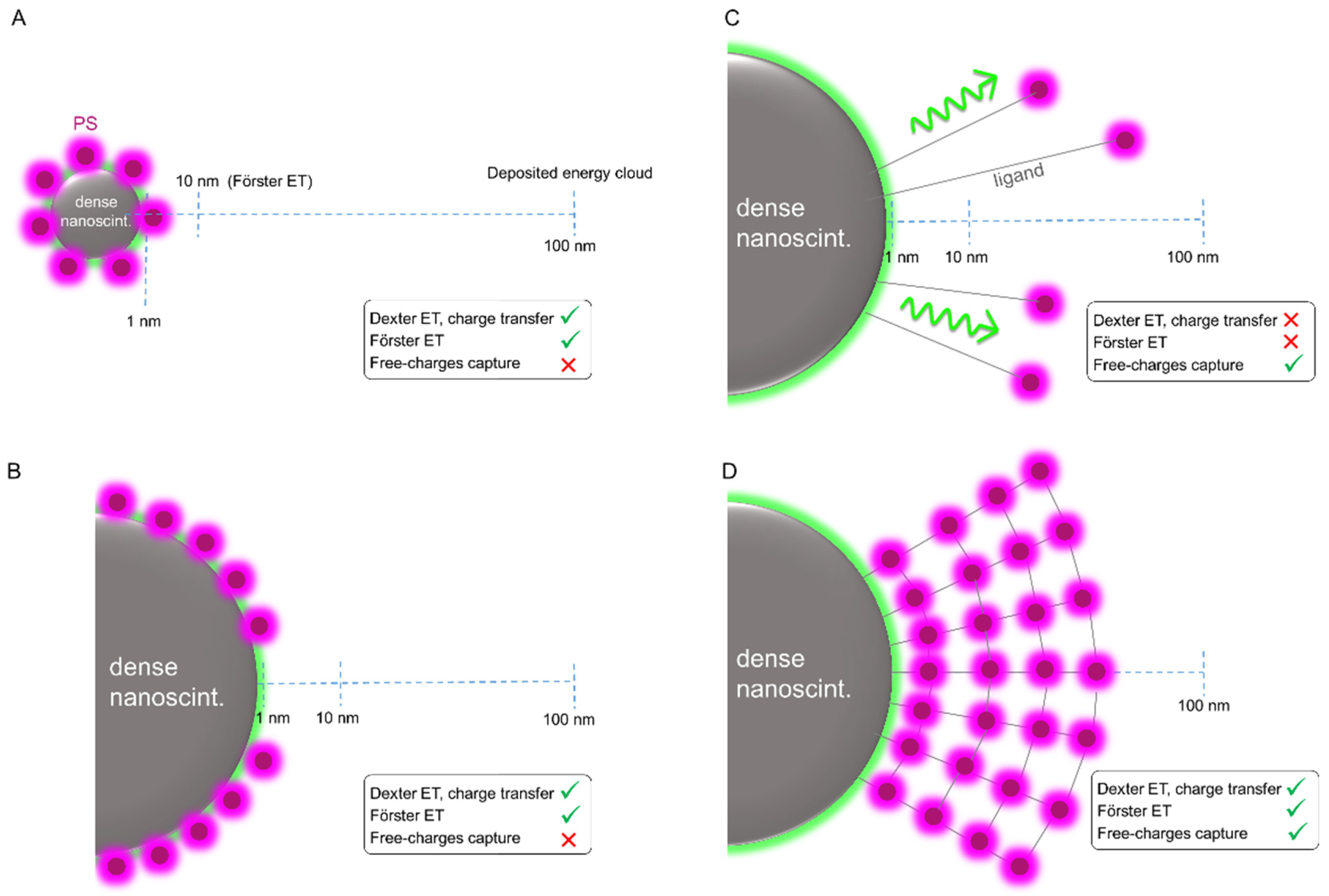
4.2. The Physics of X-PDT: Energy Transfer Mechanisms for PDT Activation
4.3. X-PDT Agents: Design and Optimization
5. Targeting and Surface Functionalization of X-PDT Systems
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, M.M. (Ed.) Basic Science of PET Imaging; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The role of radiotherapy in cancer treatment. Estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2005, 104, 1129–1137. [Google Scholar]
- Forastiere, A.A.; Goepfert, H.; Maor, M.; Pajak, T.F.; Weber, R.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; Chao, C.; et al. Concurrent Chemotherapy and Radiotherapy for Organ Preservation in Advanced Laryngeal Cancer. N. Engl. J. Med. 2003, 349, 2091–2098. [Google Scholar] [CrossRef] [Green Version]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Juarranz, Á.; Jaén, P.; Sanz-Rodríguez, F.; Cuevas, J.; González, S. Photodynamic therapy of cancer. Basic principles and applications. Clin. Transl. Oncol. 2008, 10, 148–154. [Google Scholar] [CrossRef]
- Cline, B.; Delahunty, I.; Xie, J. Nanoparticles to mediate X-ray-induced photodynamic therapy and Cherenkov radiation photodynamic therapy. WIREs Nanomed. Nanobiotechnol. 2018, 11, e1541. [Google Scholar] [CrossRef]
- Bistolfi, F. Red radioluminescence and radiochemiluminescence: Premises for a photodynamic tumour therapy with X-rays and haematoporphyrin derivatives. A working hypothesis. Panminerva Med. 2000, 42, 69–75. [Google Scholar]
- Larue, L.; Ben Mihoub, A.; Youssef, Z.; Colombeau, L.; Acherar, S.; André, J.C.; Arnoux, P.; Baros, F.; Vermandel, M.; Frochot, C. Using X-rays in photodynamic therapy: An overview. Photochem. Photobiol. Sci. 2018, 17, 1612–1650. [Google Scholar] [CrossRef]
- Deng, W.; McKelvey, K.J.; Guller, A.; Fayzullin, A.; Campbell, J.M.; Clement, S.; Habibalahi, A.; Wargocka, Z.; Liang, L.; Shen, C.; et al. Application of Mitochondrially Targeted Nanoconstructs to Neoadjuvant X-ray-Induced Photodynamic Therapy for Rectal Cancer. ACS Central Sci. 2020, 6, 715–726. [Google Scholar] [CrossRef]
- Clement, S.; Chen, W.; Deng, W.; Goldys, E.M. X-ray radiation-induced and targeted photodynamic therapy with folic acid-conjugated biodegradable nanoconstructs. Int. J. Nanomed. 2018, 13, 3553–3570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Zhang, J. Using Nanoparticles to Enable Simultaneous Radiation and Photodynamic Therapies for Cancer Treatment. J. Nanosci. Nanotechnol. 2006, 6, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Sun, M.; Wu, T.; Lu, Q.; Chen, B.; Huang, B. All-inorganic perovskite nanocrystals: Next-generation scintillation materials for high-resolution X-ray imaging. Nanoscale Adv. 2021, 4, 680–696. [Google Scholar] [CrossRef]
- Chen, H.; Wang, G.D.; Chuang, Y.-J.; Zhen, Z.; Chen, X.; Biddinger, P.; Hao, Z.; Liu, F.; Shen, B.; Pan, Z.; et al. Nanoscintillator-Mediated X-ray Inducible Photodynamic Therapy for In Vivo Cancer Treatment. Nano Lett. 2015, 15, 2249–2256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, G.; Ni, K.; Xu, R.; Lu, K.; Lin, Z.; Chan, C.; Lin, W. Nanoscale Metal-Organic Layers for Deeply Penetrating X-ray-Induced Photodynamic Therapy. Angew. Chem. Int. Ed. 2017, 56, 12102–12106. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Tang, W.; Lau, J.; Shen, Z.; Xie, J.; Shi, J.; Chen, X. Breaking the Depth Dependence by Nanotechnology-Enhanced X-Ray-Excited Deep Cancer Theranostics. Adv. Mater. 2019, 31, 1806381. [Google Scholar] [CrossRef]
- Colombeau, L.; Acherar, S.; Baros, F.; Arnoux, P.; Gazzali, A.M.; Zaghdoudi, K.; Toussaint, M.; Vanderesse, R.; Frochot, C. Inorganic Nanoparticles for Photodynamic Therapy. In Light-Responsive Nanostructured Systems for Applications in Nanomedicine; Sortino, S., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 113–134. [Google Scholar]
- Ren, R.; Hao, X.-Y.; Li, H.-C.; Ke, M.-R.; Zheng, B.-Y.; Huang, J.-D. Progress in the development of nanosensitizers for X-ray-induced photodynamic therapy. Drug Discov. Today 2018, 23, 1791–1800. [Google Scholar] [CrossRef]
- Shrestha, S.; Wu, J.; Sah, B.; Vanasse, A.; Cooper, L.N.; Ma, L.; Li, G.; Zheng, H.; Chen, W.; Antosh, M.P. X-ray induced photodynamic therapy with copper-cysteamine nanoparticles in mice tumors. Proc. Natl. Acad. Sci. USA 2019, 116, 16823–16828. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Zhou, Z.; Pratx, G.; Chen, X.; Chen, H. Nanoscintillator-Mediated X-Ray Induced Photodynamic Therapy for Deep-Seated Tumors: From Concept to Biomedical Applications. Theranostics 2020, 10, 1296–1318. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Wang, G.D.; Nguyen, H.T.; Chen, H.; Cox, P.B.; Wang, L.; Nagata, K.; Hao, Z.; Wang, A.; Li, Z.; Xie, J. X-Ray Induced Photodynamic Therapy: A Combination of Radiotherapy and Photodynamic Therapy. Theranostics 2016, 6, 2295–2305. [Google Scholar] [CrossRef] [PubMed]
- Bulin, A.-L.; Broekgaarden, M.; Simeone, D.; Hasan, T. Low dose photodynamic therapy harmonizes with radiation therapy to induce beneficial effects on pancreatic heterocellular spheroids. Oncotarget 2019, 10, 2625–2643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulin, A.; Broekgaarden, M.; Chaput, F.; Baisamy, V.; Garrevoet, J.; Busser, B.; Brueckner, D.; Youssef, A.; Ravanat, J.; Dujardin, C.; et al. Radiation Dose-Enhancement Is a Potent Radiotherapeutic Effect of Rare-Earth Composite Nanoscintillators in Preclinical Models of Glioblastoma. Adv. Sci. 2020, 7, 2001675. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Shen, C.; Li, H.; Goldys, E.M.; Deng, W. X-ray induced photodynamic therapy (PDT) with a mitochondria-targeted liposome delivery system. J. Nanobiotechnol. 2020, 18, 87. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Wang, X.; Jiang, Z.; Yu, X.; Liu, X.; Mao, R.; Chen, X.; Li, W. Codoping Enhanced Radioluminescence of Nanoscintillators for X-ray-Activated Synergistic Cancer Therapy and Prognosis Using Metabolomics. ACS Nano 2019, 13, 10419–10433. [Google Scholar] [CrossRef]
- Rate, R.W.; Solin, L.J.; Turrisi, A.T. Palliative radiotherapy for metastatic malignant melanoma: Brain metastases, bone metastases, and spinal cord compression. Int. J. Radiat. Oncol. 1988, 15, 859–864. [Google Scholar] [CrossRef]
- Mott, J.; Daniel, J. Interactions of Electromagnetic Radiation and Subatomic Particles with Matter—Part 2. Clin. Oncol. 2021, 33, 455–460. [Google Scholar] [CrossRef]
- Lutz, W.; Winston, K.R.; Maleki, N. A system for stereotactic radiosurgery with a linear accelerator. Int. J. Radiat. Oncol. 1988, 14, 373–381. [Google Scholar] [CrossRef]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist; Lippincott Wilkins & Williams: Philadelphia, PA, USA, 2006. [Google Scholar]
- Kwatra, D.; Venugopal, A.; Anant, S. Nanoparticles in radiation therapy: A summary of various approaches to enhance radiosensitization in cancer. Transl. Cancer Res. 2013, 2, 330–342. [Google Scholar]
- Morrell, C.N. Reactive oxygen species: Finding the right balance. Circ. Res. 2008, 103, 571–572. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, D.; Stigbrand, T. Radiation-induced cell death mechanisms. Tumor Biol. 2010, 31, 363–372. [Google Scholar] [CrossRef]
- Demaria, S.; Golden, E.B.; Formenti, S.C. Role of Local Radiation Therapy in Cancer Immunotherapy. JAMA Oncol. 2015, 1, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, X.; Zhai, S. The Rational Design and Biological Mechanisms of Nanoradiosensitizers. Nanomaterials 2020, 10, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhaegen, F.; Seuntjens, J. Monte Carlo modelling of external radiotherapy photon beams. Phys. Med. Biol. 2003, 48, R107–R164. [Google Scholar] [CrossRef]
- Mitin, T.; Zietman, A.L. Promise and Pitfalls of Heavy-Particle Therapy. J. Clin. Oncol. 2014, 32, 2855–2863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadeghi, M.; Enferadi, M.; Shirazi, A. External and internal radiation therapy: Past and future directions. J. Cancer Res. Ther. 2010, 6, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Ting, G.; Chang, C.-H.; Wang, H.-E.; Lee, T.-W. Nanotargeted Radionuclides for Cancer Nuclear Imaging and Internal Radiotherapy. J. Biomed. Biotechnol. 2010, 2010, 953537. [Google Scholar] [CrossRef] [Green Version]
- Dash, A.; Knapp, F.F.R.; Pillai, A.M.R. Targeted Radionuclide Therapy—An Overview. Curr. Radiopharm. 2013, 6, 152–180. [Google Scholar] [CrossRef]
- Skowronek, J. Current status of brachytherapy in cancer treatment—Short Overview. J. Contemp. Brachyther. 2017, 9, 581–589. [Google Scholar] [CrossRef]
- Mones, E.; Veronese, I.; Vedda, A.; Loi, G.; Fasoli, M.; Moretti, F.; Chiodini, N.; Cannillo, B.; Brambilla, M. Ce-doped optical fibre as radioluminescent dosimeter in radiotherapy. Radiat. Meas. 2008, 43, 888–892. [Google Scholar] [CrossRef]
- Hachadorian, R.L.; Bruza, P.; Jermyn, M.; Gladstone, D.J.; Pogue, B.W.; Jarvis, L.A. Imaging radiation dose in breast radiotherapy by X-ray CT calibration of Cherenkov light. Nat. Commun. 2020, 11, 2298–2299. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.; Healy, B.; Holloway, L.; Kuncic, Z.; Thwaites, D.; Baldock, C. Advances in kilovoltage x-ray beam dosimetry. Phys. Med. Biol. 2014, 59, R183–R231. [Google Scholar] [CrossRef] [PubMed]
- Dionysiou, D.D.; Stamatakos, G.S.; Gintides, D.; Uzunoglu, N.; Kyriaki, K. Critical Parameters Determining Standard Radiotherapy Treatment Outcome for Glioblastoma Multiforme: A Computer Simulation. Open Biomed. Eng. J. 2008, 2, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Voyant, C.; Julian, D.; Roustit, R.; Biffi, K.; Lantieri, C. Biological effects and equivalent doses in radiotherapy: A software solution. Rep. Pract. Oncol. Radiother. 2014, 19, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Lippitz, B.; Lindquist, C.; Paddick, I.; Peterson, D.; O’Neill, K.; Beaney, R. Stereotactic radiosurgery in the treatment of brain metastases: The current evidence. Cancer Treat. Rev. 2014, 40, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-H.; Choi, J.-W.; Kong, D.-S.; Seol, H.J.; Nam, D.-H.; Ryu, J.-W.; Kim, S.-T.; Suh, Y.-L.; Lee, J.-I. Histopathology and surgical outcome of symptomatic treatment-related changes after gamma knife radiosurgery in patients with brain metastases. Sci. Rep. 2022, 12, 3013. [Google Scholar] [CrossRef]
- Verellen, D.; De Ridder, M.; Linthout, N.; Tournel, K.; Soete, G.; Storme, G. Innovations in image-guided radiotherapy. Nat. Cancer 2007, 7, 949–960. [Google Scholar] [CrossRef]
- Xu, D.; Li, G.; Li, H.; Jia, F.J.M. Comparison of IMRT versus 3D-CRT in the treatment of esophagus cancer: A systematic review and meta-analysis. Medicine 2017, 96, e7685. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.W.; Dougherty, T.J. How Does Photodynamic Therapy Work? Photochem. Photobiol. 1992, 55, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Wagnieres, G.A.; Star, W.M.; Wilson, B.C. In Vivo Fluorescence Spectroscopy and Imaging for Oncological Applications. Photochem. Photobiol. 1998, 68, 603–632. [Google Scholar] [CrossRef]
- Foote, C.S. Definition of Type I And Type II Photosensitized Oxidation. Photochem. Photobiol. 1991, 54, 659. [Google Scholar] [CrossRef] [PubMed]
- Plaetzer, K.; Krammer, B.; Berlanda, J.; Berr, F.; Kiesslich, T. Photophysics and photochemistry of photodynamic therapy: Fundamental aspects. Lasers Med. Sci. 2009, 24, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Bacellar, I.O.L.; Tsubone, T.M.; Pavani, C.; Baptista, M.S. Photodynamic Efficiency: From Molecular Photochemistry to Cell Death. Int. J. Mol. Sci. 2015, 16, 20523–20559. [Google Scholar] [CrossRef] [Green Version]
- Sharman, W.M.; Allen, C.M.; van Lier, J.E. [35] Role of activated oxygen species in photodynamic therapy. Methods Enzymol. 2000, 319, 376–400. [Google Scholar] [CrossRef] [PubMed]
- Moan, J.; Berg, K. The Photodegradation of Porphyrins in Cells Can Be Used to Estimate the Lifetime of Singlet Oxygen. Photochem. Photobiol. 1991, 53, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Dysart, J.S.; Patterson, M.S. Characterization of Photofrin photobleaching for singlet oxygen dose estimation during photodynamic therapy of MLL cells in vitro. Phys. Med. Biol. 2005, 50, 2597–2616. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Al-Husein, B.; Abdalla, M.; Trepte, M.; DeRemer, D.L.; Somanath, P.R. Antiangiogenic Therapy for Cancer: An Update, Pharmacotherapy. J. Hum. Pharmacol. Drug Ther. 2012, 32, 1095–1111. [Google Scholar] [CrossRef] [Green Version]
- Star, W.M.; Marijnissen, H.P.; Berg-Blok, A.E.V.D.; Versteeg, A.J.; Franken, A.K.; Reinhold, H.S. Destruction of rat mammary tumor and normal tissue microcirculation by hematoporphyrin derivative photoradiation observed in vivo in sandwich observation chambers. Cancer Res. 1986, 46, 2532–2540. [Google Scholar]
- Foster, T.; Primavera, M.C.; Marder, V.J.; Hilf, R.; Sporn, A.L. Photosensitized release of von Willebrand factor from cultured human endothelial cells. Cancer Res. 1991, 51, 3261–3266. [Google Scholar]
- Nelson, J.S.; Liaw, L.-H.; Berns, M. Tumor Destruction in Photodynamic Therapy. Photochem. Photobiol. 1987, 46, 829–836. [Google Scholar] [CrossRef]
- Fingar, V.H.; Wieman, T.J.; Haydon, P.S. The effects of thrombocytopenia on vessel stasis and macromolecular leakage after photodynamic therapy using photofrin. Photochem. Photobiol. 1997, 66, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Fingar, V.H.; Kik, P.K.; Haydon, P.S.; Cerrito, P.B.; Tseng, M.; Abang, E.; Wieman, T.J. Analysis of acute vascular damage after photodynamic therapy using benzoporphyrin derivative (BPD). Br. J. Cancer 1999, 79, 1702–1708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantl, G.; Lattuada, D.; Nicolin, A.; Taroni, P.; Valentini, G.; Cubeddu, R. Antitumor immunity induced by photodynamic therapy with aluminum disulfonated phthalocyanines and laser light. Anti-Cancer Drugs 1994, 5, 443–447. [Google Scholar] [CrossRef]
- Korbelik, M.; Dougherty, G.J. Photodynamic therapy-mediated immune response against subcutaneous mouse tumors. Cancer Res. 1999, 59, 1941–1946. [Google Scholar]
- Schweitzer, V.G.; Visscher, D. Photodynamic therapy for treatment of AIDS-related oral Kaposi’s sarcoma. Otolaryngol.—Head Neck Surg. 1990, 102, 639–649. [Google Scholar] [CrossRef]
- Tardivo, J.P.; Giglio, A.D.; Paschoal, L.H.; Baptista, M.S. New Photodynamic Therapy Protocol to Treat AIDS-Related Kaposi’s Sarcoma. Photomed. Laser Surg. 2006, 24, 528–531. [Google Scholar] [CrossRef]
- Hebeda, K.M.; Huizing, M.T.; Brouwer, P.A.; van der Meulen, F.W.; Hulsebosch, H.J.; Reiss, P.; Oosting, J.; Veenhof, C.H.; Bakker, P.J. Photodynamic therapy in AIDS-related cutaneous Kaposi’s sarcoma. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. Off. Publ. Int. Retrovirol. Assoc. 1995, 10, 61–70. [Google Scholar]
- Moghissi, K.; Dixon, K.; Gibbins, S. A Surgical View of Photodynamic Therapy in Oncology: A Review. Surg. J. 2015, 1, e1–e15. [Google Scholar] [CrossRef] [Green Version]
- Crapanzano, R.; Secchi, V.; Villa, I. Co-Adjuvant Nanoparticles for Radiotherapy Treatments of Oncological Diseases. Appl. Sci. 2021, 11, 7073. [Google Scholar] [CrossRef]
- Kuncic, Z.; Lacombe, S. Biology, Nanoparticle radio-enhancement: Principles, progress and application to cancer treatment. Phys. Med. Biol. 2018, 63, 02TR01. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Yao, M.; Ma, L.; Hossu, M.; Han, X.; Juzenas, P.; Chen, W. X-ray-induced nanoparticle-based photodynamic therapy of cancer. Nanomedicine 2014, 9, 2339–2351. [Google Scholar] [CrossRef] [PubMed]
- Procházková, L.; Pelikanova, I.T.; Mihóková, E.; Dědic, R.; Čuba, V. Novel scintillating nanocomposite for X-ray induced photodynamic therapy. Radiat. Meas. 2018, 121, 13–17. [Google Scholar] [CrossRef]
- Gadzhimagomedova, Z.; Zolotukhin, P.; Kit, O.; Kirsanova, D.; Soldatov, A. Nanocomposites for X-Ray Photodynamic Therapy. Int. J. Mol. Sci. 2020, 21, 4004. [Google Scholar] [CrossRef]
- Villa, I.; Moretti, F.; Fasoli, M.; Rossi, A.; Hattendorf, B.; Dujardin, C.; Niederberger, M.; Vedda, A.; Lauria, A. The Bright X-Ray Stimulated Luminescence of HfO 2 Nanocrystals Activated by Ti Ions. Adv. Opt. Mater. 2020, 8, 1901348. [Google Scholar] [CrossRef]
- Zhang, X.; Wasson, M.C.; Shayan, M.; Berdichevsky, E.K.; Ricardo-Noordberg, J.; Singh, Z.; Papazyan, E.K.; Castro, A.J.; Marino, P.; Ajoyan, Z.; et al. A historical perspective on porphyrin-based metal–organic frameworks and their applications. Coord. Chem. Rev. 2021, 429, 213615. [Google Scholar] [CrossRef]
- Spinelli, A.E.; Boschi, F. Photodynamic Therapy Using Cerenkov and Radioluminescence Light. Front. Phys. 2021, 9, 637120. [Google Scholar] [CrossRef]
- Kotagiri, N.; Cooper, M.L.; Rettig, M.; Egbulefu, C.; Prior, J.; Cui, G.; Karmakar, P.; Zhou, M.; Yang, X.; Sudlow, G.; et al. Radionuclides transform chemotherapeutics into phototherapeutics for precise treatment of disseminated cancer. Nat. Commun. 2018, 9, 275. [Google Scholar] [CrossRef]
- Pratt, E.C.; Shaffer, T.M.; Zhang, Q.; Drain, C.M.; Grimm, J. Nanoparticles as multimodal photon transducers of ionizing radiation. Nat. Nanotechnol. 2018, 13, 418–426. [Google Scholar] [CrossRef]
- Glaser, A.K.; Zhang, R.; Andreozzi, J.M.; Gladstone, D.J.; Pogue, B.W. Cherenkov radiation fluence estimates in tissue for molecular imaging and therapy applications. Phys. Med. Biol. 2015, 60, 6701–6718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa, I.; Villa, C.; Crapanzano, R.; Secchi, V.; Tawfilas, M.; Trombetta, E.; Porretti, L.; Brambilla, A.; Campione, M.; Torrente, Y.; et al. Functionalized Scintillating Nanotubes for Simultaneous Radio- and Photodynamic Therapy of Cancer. ACS Appl. Mater. Interfaces 2021, 13, 12997–13008. [Google Scholar] [CrossRef] [PubMed]
- Sengar, P.; Juárez, P.; Verdugo-Meza, A.; Arellano, D.L.; Jain, A.; Chauhan, K.; Hirata, G.A.; Fournier, P.G.J. Development of a functionalized UV-emitting nanocomposite for the treatment of cancer using indirect photodynamic therapy. J. Nanobiotechnol. 2018, 16, 19. [Google Scholar] [CrossRef]
- Song, L.; Li, P.-P.; Yang, W.; Lin, X.-H.; Liang, H.; Chen, X.-F.; Liu, G.; Li, J.; Yang, H.-H. Low-Dose X-ray Activation of W(VI)-Doped Persistent Luminescence Nanoparticles for Deep-Tissue Photodynamic Therapy. Adv. Funct. Mater. 2018, 28, 1707496. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Zhu, W.; Yi, X.; Dong, Z.; Xu, X.; Chen, M.; Yang, K.; Lu, G.; Jiang, L.; et al. Nanoscale metal−organic frameworks for combined photodynamic & radiation therapy in cancer treatment. Biomaterials 2016, 97, 1–9. [Google Scholar] [CrossRef]
- Lu, K.; He, C.; Lin, W. Nanoscale Metal–Organic Framework for Highly Effective Photodynamic Therapy of Resistant Head and Neck Cancer. J. Am. Chem. Soc. 2014, 136, 16712–16715. [Google Scholar] [CrossRef] [Green Version]
- Rossi, F.; Bedogni, E.; Bigi, F.; Rimoldi, T.; Cristofolini, L.; Pinelli, S.; Alinovi, R.; Negri, M.; Dhanabalan, S.C.; Attolini, G.; et al. Porphyrin conjugated SiC/SiOx nanowires for X-ray-excited photodynamic therapy. Sci. Rep. 2015, 5, 7606. [Google Scholar] [CrossRef]
- Chen, H.; Sun, X.; Wang, G.D.; Nagata, K.; Hao, Z.; Wang, A.; Li, Z.; Xie, J.; Shen, B. LiGa5O8:Cr-based theranostic nanoparticles for imaging-guided X-ray induced photodynamic therapy of deep-seated tumors. Mater. Horizons 2017, 4, 1092–1101. [Google Scholar] [CrossRef]
- Elmenoufy, A.H.; Tang, Y.; Hu, J.; Xu, H.; Yang, X. A novel deep photodynamic therapy modality combined with CT imaging established via X-ray stimulated silica-modified lanthanide scintillating nanoparticles. Chem. Commun. 2015, 51, 12247–12250. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Zou, X.; Hossu, M.; Chen, W. Synthesis of ZnS:Ag,Co water-soluble blue afterglow nanoparticles and application in photodynamic activation. Nanotechnology 2016, 27, 315602. [Google Scholar] [CrossRef]
- Clement, S.; Chen, W.; Anwer, A.G.; Goldys, E.M. Verteprofin conjugated to gold nanoparticles for fluorescent cellular bioimaging and X-ray mediated photodynamic therapy. Mikrochim. Acta 2017, 184, 1765–1771. [Google Scholar] [CrossRef]
- Carter, J.D.; Cheng, N.N.; Qu, Y.; Suarez, G.D.; Guo, T. Nanoscale Energy Deposition by X-ray Absorbing Nanostructures. J. Phys. Chem. B 2007, 111, 11622–11625. [Google Scholar] [CrossRef] [PubMed]
- Bulin, A.-L.; Vasil’Ev, A.; Belsky, A.; Amans, D.; Ledoux, G.; Dujardin, C. Modelling energy deposition in nanoscintillators to predict the efficiency of the X-ray-induced photodynamic effect. Nanoscale 2015, 7, 5744–5751. [Google Scholar] [CrossRef]
- Mattiello, S.; Mecca, S.; Ronchi, A.; Calascibetta, A.; Mattioli, G.; Pallini, F.; Meinardi, F.; Beverina, L.; Monguzzi, A. Diffusion-Free Intramolecular Triplet–Triplet Annihilation in Engineered Conjugated Chromophores for Sensitized Photon Upconversion. ACS Energy Lett. 2022, 7, 2435–2442. [Google Scholar] [CrossRef]
- Pogue, B.W.; Wilson, B.C. Optical and x-ray technology synergies enabling diagnostic and therapeutic applications in medicine. J. Biomed. Opt. 2018, 23, 121610. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Mahler, B.; Houel, J.; Kulzer, F.; Ledoux, G.; Vasil’Ev, A.; Dujardin, C. Perspectives for CdSe/CdS spherical quantum wells as rapid-response nano-scintillators. Nanoscale 2021, 13, 19578–19586. [Google Scholar] [CrossRef]
- Perego, J.; Villa, I.; Pedrini, A.; Padovani, E.C.; Crapanzano, R.; Vedda, A.; Dujardin, C.; Bezuidenhout, C.X.; Bracco, S.; Sozzani, P.E.; et al. Composite fast scintillators based on high-Z fluorescent metal–organic framework nanocrystals. Nat. Photonics 2021, 15, 393–400. [Google Scholar] [CrossRef]
- Liu, F.; Wu, R.; Wei, J.; Nie, W.; Mohite, A.D.; Brovelli, S.; Manna, L.; Li, H. Recent Progress in Halide Perovskite Radiation Detectors for Gamma-Ray Spectroscopy. ACS Energy Lett. 2022, 7, 1066–1085. [Google Scholar] [CrossRef]
- Wang, K.K.-H.; Finlay, J.C.; Busch, T.M.; Hahn, S.M.; Zhu, T.C. Explicit dosimetry for photodynamic therapy: Macroscopic singlet oxygen modeling. J. Biophotonics 2010, 3, 304–318. [Google Scholar] [CrossRef] [Green Version]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Plenum Press: New York, NY, USA, 2006. [Google Scholar]
- Montalti, M.; Credi, A.; Prodi, L.; Gandolfi, M.T. Handbook of Photochemistry; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Ni, K.; Lan, G.; Chan, C.; Quigley, B.; Lu, K.; Aung, T.; Guo, N.; La Riviere, P.; Weichselbaum, R.R.; Lin, W. Nanoscale metal-organic frameworks enhance radiotherapy to potentiate checkpoint blockade immunotherapy. Nat. Commun. 2018, 9, 2351. [Google Scholar] [CrossRef]
- Lu, K.; He, C.; Guo, N.; Chan, C.; Ni, K.; Lan, G.; Tang, H.; Pelizzari, C.; Fu, Y.-X.; Spiotto, M.T.; et al. Low-dose X-ray radiotherapy–radiodynamic therapy via nanoscale metal–organic frameworks enhances checkpoint blockade immunotherapy. Nat. Biomed. Eng. 2018, 2, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, A.; Capitani, C.; Pinchetti, V.; Gariano, G.; Zaffalon, M.L.; Meinardi, F.; Brovelli, S.; Monguzzi, A. High Photon Upconversion Efficiency with Hybrid Triplet Sensitizers by Ultrafast Hole-Routing in Electronic-Doped Nanocrystals. Adv. Mater. 2020, 32, 2002953. [Google Scholar] [CrossRef] [PubMed]
- Villa, I.; Gonzalez, B.S.; Orfano, M.; Cova, F.; Secchi, V.; Colombo, C.; Páterek, J.; Kučerková, R.; Babin, V.; Mauri, M.; et al. The Sensitization of Scintillation in Polymeric Composites Based on Fluorescent Nanocomplexes. Nanomaterials 2021, 11, 3387. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Richa; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular Mechanisms of Ultraviolet Radiation-Induced DNA Damage and Repair. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberman, A.; Mendez, N.; Trogler, W.C.; Kummel, A.C. Synthesis and surface functionalization of silica nanoparticles for nanomedicine. Surf. Sci. Rep. 2014, 69, 132–158. [Google Scholar] [CrossRef] [Green Version]
- Yi, Z.; Luo, Z.; Qin, X.; Chen, Q.; Liu, X. Lanthanide-Activated Nanoparticles: A Toolbox for Bioimaging, Therapeutics, and Neuromodulation. Accounts Chem. Res. 2020, 53, 2692–2704. [Google Scholar] [CrossRef]
- Hong, E.J.; Choi, D.G.; Shim, M.S. Targeted and effective photodynamic therapy for cancer using functionalized nanomaterials. Acta Pharm. Sin. B 2016, 6, 297–307. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; An, S.; Ward, R.; Yang, Y.; Guo, X.-X.; Li, W.; Xu, T.-R. G protein-coupled receptors as promising cancer targets. Cancer Lett. 2016, 376, 226–239. [Google Scholar] [CrossRef]
- Lu, Y.; Sega, E.; Leamon, C.P.; Low, P.S. Folate receptor-targeted immunotherapy of cancer: Mechanism and therapeutic potential. Adv. Drug Deliv. Rev. 2004, 56, 1161–1176. [Google Scholar] [CrossRef]
- Samani, R.K.; Tavakoli, M.B.; Maghsoudinia, F.; Motaghi, H.; Hejazi, S.H.; Mehrgardi, M.A. Trastuzumab and folic acid functionalized gold nanoclusters as a dual-targeted radiosensitizer for megavoltage radiation therapy of human breast cancer. Eur. J. Pharm. Sci. 2020, 153, 105487. [Google Scholar] [CrossRef]
- Pietersz, A.G.; Wang, X.; Yap, M.L.; Lim, B.; Peter, K. Therapeutic targeting in nanomedicine: The future lies in recombinant antibodies. Nanomedicine 2017, 12, 1873–1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Y.; Zhou, F.; Zhang, D.; Chen, Q.; Xing, D. A graphene oxide based smart drug delivery system for tumor mitochondria-targeting photodynamic therapy. Nanoscale 2016, 8, 3530–3538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roby, A.; Erdogan, S.; Torchilin, V.P. Enhanced in vivo antitumor efficacy of poorly soluble PDT agent, meso-tetraphenylporphine, in PEG-PE-based tumor-targeted immunomicelles. Cancer Biol. Ther. 2007, 6, 1136–1142. [Google Scholar] [CrossRef] [Green Version]
- Olivo, M.; Bhuvaneswari, R.; Lucky, S.S.; Dendukuri, N.; Thong, P.S.-P. Targeted Therapy of Cancer Using Photodynamic Therapy in Combination with Multi-faceted Anti-Tumor Modalities. Pharmaceuticals 2010, 3, 1507–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, L.; Liu, J.; Shi, J. Nuclear-Targeting Gold Nanorods for Extremely Low NIR Activated Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 15952–15961. [Google Scholar] [CrossRef]
- Pan, L.; He, Q.; Liu, J.; Chen, Y.; Ma, M.; Zhang, L.; Shi, J. Nuclear-Targeted Drug Delivery of TAT Peptide-Conjugated Monodisperse Mesoporous Silica Nanoparticles. J. Am. Chem. Soc. 2012, 134, 5722–5725. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, G.; Zhai, S.; Zhang, W.; Xing, D. Specific photoacoustic cavitation through nucleus targeted nanoparticles for high-efficiency tumor therapy. Nano Res. 2020, 13, 719–728. [Google Scholar] [CrossRef]
- Zuo, H.D.; Yao, W.W.; Chen, T.W.; Zhu, J.; Zhang, J.J.; Pu, Y.; Liu, G.; Zhang, X.M. The Effect of Superparamagnetic Iron Oxide with iRGD Peptide on the Labeling of Pancreatic Cancer Cells In Vitro: A Preliminary Study. BioMed Res. Int. 2014, 2014, 852352. [Google Scholar] [CrossRef] [Green Version]
- Benezra, M.; Penate-Medina, O.; Zanzonico, P.B.; Schaer, D.; Ow, H.; Burns, A.; DeStanchina, E.; Longo, V.; Herz, E.; Iyer, S.; et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J. Clin. Investig. 2011, 121, 2768–2780. [Google Scholar] [CrossRef] [Green Version]
| Nanoscintillator | PDT Agent | Targeting Strategy | Studies | Ref. |
|---|---|---|---|---|
| Ce(3+)-doped lanthanum(III) fluoride nanoparticles | protoporphyrin IX (PpIX) | poly(D,L-lactide-co-glycolide (PLGA) microspheres | in vitro | [76] |
| mesoporous silica (passive X-PDT) | Rose Bengal (RB) | - | in vitro in vivo | [27] |
| chrysotile nanotube | Erythrosine B, Rose Bengal, meso-tetra(4-sulfonatophenyl)porphyrin | mPEG2K-phosphate (PEO) | in vitro | [85] |
| Mesoporous silica coated NPs | protoporphyrin IX (PpIX) | folic acid (FA) | in vitro in vivo | [86] |
| ZnGa2O4:Cr | ZnPcS Phtalocyanine | - | in vitro in vivo | [87] |
| Hf-based Metal-organic Frameworks | TCPP (tetrakis (4-carboxyphenyl) porphyrin) | - | in vitro in vivo | [88] |
| Hf-based Metal-organic Frameworks | 5,15-di(p-benzoato)porphyrin (H2DBP) | - | in vitro in vivo | [89] |
| SiC/SiOx core/shell nanowires | tetracarboxyphenyl porphyrin | - | in vitro | [90] |
| LiGa5O8:Cr | 2,3-naphthalocyanine | mesoporous silica shells conjugated with cetuximab | in vitro in vivo | [91] |
| LaF3:Tb | Rose Bengal (RB) | - | in vivo | [92] |
| SrAl2O4:Eu2+ | merocyanine 540 (MC540) | coated with two layers of silica | in vitro in vivo | [15] |
| co-doped ZnS (ZnS:Ag,Co) NPs | protoporphyrin IX (PpIX) | - | in vitro | [93] |
| Au nanoparticles | verteporfin (VP) | SH-PEG or SH-PEG-NH2 | in vitro | [94] |
| (PLGA) polymeric nanoparticles | verteporfin (VP) | folic acid (FA) | in vitro | [12] |
| Type of NPs | Active Targeting | Application | Tests | Ref. |
|---|---|---|---|---|
| Mesoporous silica-coated NPs functionalized with protoporphyrin IX (PpIX) | Folic acid (FA) | X-PDT | In vitro; in vivo. | [86] |
| (PLGA) polymeric NPs incorporating verteporfin (VP) | Folic acid (FA) | X-PDT | In vitro | [12] |
| Gold nanoclusters | trastuzumab (Herceptin®) and/or folic acid (FA) | dual-targeted RT | In vitro | [115] |
| graphene oxide (NGO) functionalized with pyropheophorbide-a (PPa) and polyethylene-glycol (PEG) | integrin αvβ3 monoclonal antibody (mAb); | tumor mitochondria-targeted PDT | In vitro | [117] |
| meso-tetraphenylporphine (TPP) loaded polyethylene glycol-phosphatidyl ethanolamine (PEG-PE) micelles | monoclonal 2C5 antibody (mAb 2C5) | PDT | In vitro; in vivo. | [118] |
| gold nanorods | nuclear location signal peptides (GNRs) | photothermal therapy (PTT) | In vitro; in vivo | [120] |
| poly (N,N-dimethylacrylamide) (PDMA), and near-infrared (NIR) light absorbing agent (hCyR) NPs | TAT peptide | photoacoustic therapy (PA) | In vitro; in vivo | [122] |
| iron oxide NPs | iRGD peptide | Multimodal probe (diagnostic) | In vitro | [123] |
| Cy5 dye-encapsulating core-shell silica NPs | polyethylene glycol (PEG) chains; ανβ3 integrin–targeting cRGDY peptide ligands | Multimodal probe (diagnostic) | In vitro; in vivo | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Secchi, V.; Monguzzi, A.; Villa, I. Design Principles of Hybrid Nanomaterials for Radiotherapy Enhanced by Photodynamic Therapy. Int. J. Mol. Sci. 2022, 23, 8736. https://doi.org/10.3390/ijms23158736
Secchi V, Monguzzi A, Villa I. Design Principles of Hybrid Nanomaterials for Radiotherapy Enhanced by Photodynamic Therapy. International Journal of Molecular Sciences. 2022; 23(15):8736. https://doi.org/10.3390/ijms23158736
Chicago/Turabian StyleSecchi, Valeria, Angelo Monguzzi, and Irene Villa. 2022. "Design Principles of Hybrid Nanomaterials for Radiotherapy Enhanced by Photodynamic Therapy" International Journal of Molecular Sciences 23, no. 15: 8736. https://doi.org/10.3390/ijms23158736
APA StyleSecchi, V., Monguzzi, A., & Villa, I. (2022). Design Principles of Hybrid Nanomaterials for Radiotherapy Enhanced by Photodynamic Therapy. International Journal of Molecular Sciences, 23(15), 8736. https://doi.org/10.3390/ijms23158736






