Recent Research Progress of Ionic Liquid Dissolving Silks for Biomedicine and Tissue Engineering Applications
Abstract
:1. Introduction
2. Preparation Progress of SF Materials
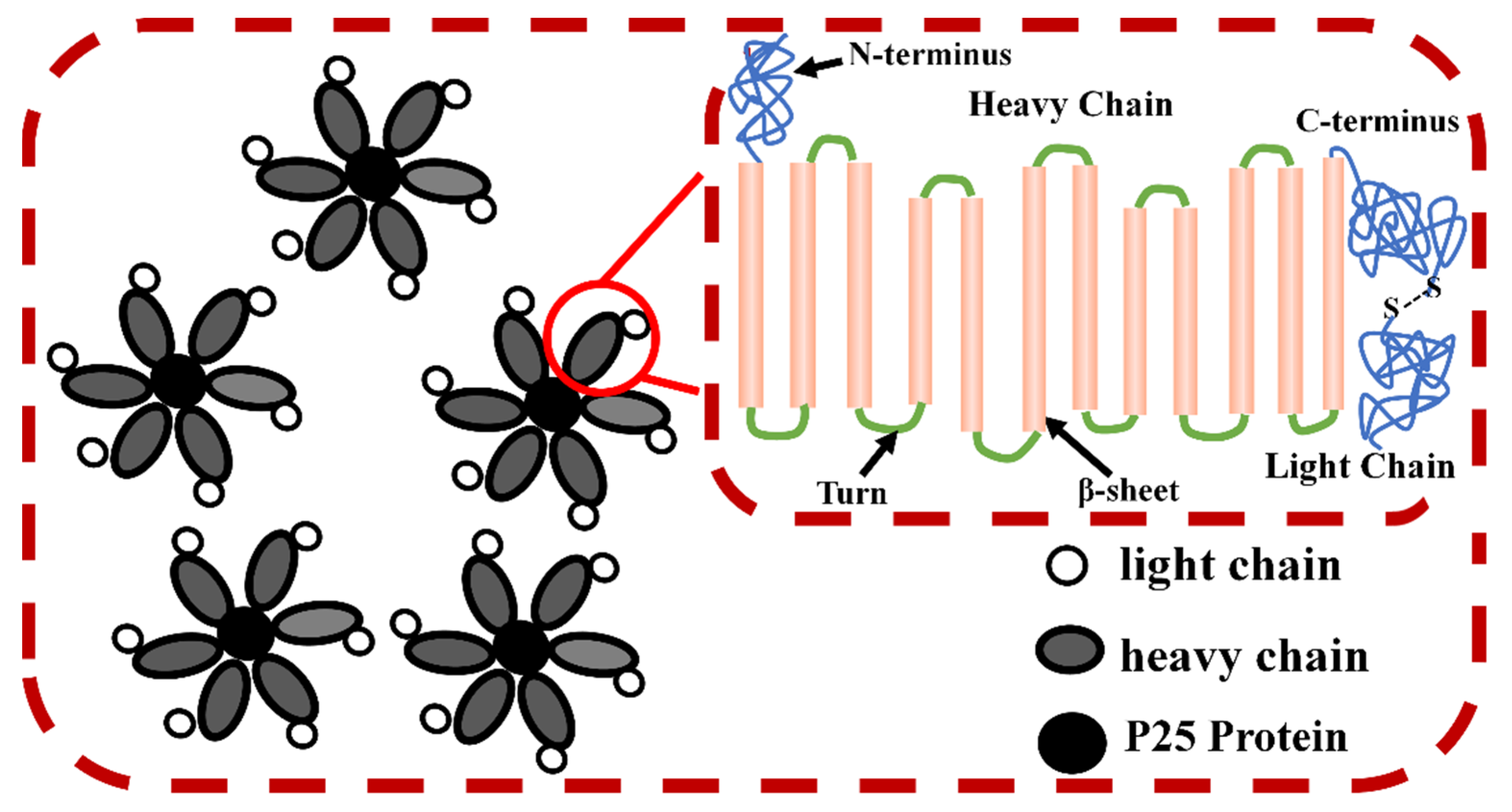
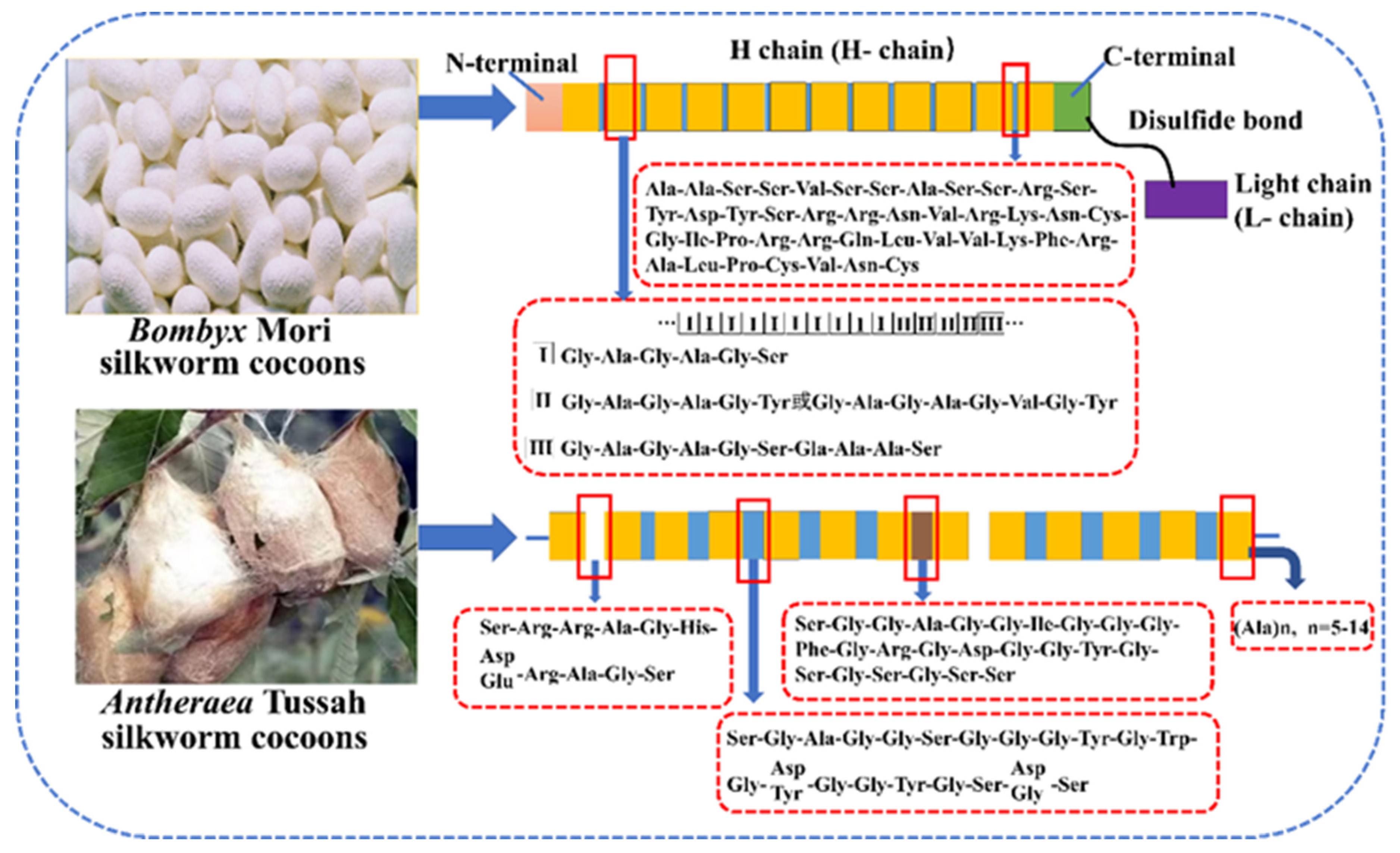
2.1. Composition, Structure and Properties of SF
2.2. Traditional Preparation Methods of SF Regeneration Solution
2.2.1. Lithium Bromide (LiBr) Dissolution Method
2.2.2. Lithium Thiocyanate (LiSCN) Dissolution Method
2.2.3. Calcium Chloride Ethanol Aqueous Solution (CaCl2-C2H5OH-H2O) Dissolution Method
2.2.4. Calcium Nitrate Methanol Aqueous Solution (Ca(NO3)2-CH3OH-H2O) Dissolution Method
2.2.5. Calcium Chloride Formic Acid Dissolution Method
2.3. IL Preparation Methods for SF Regeneration Solution
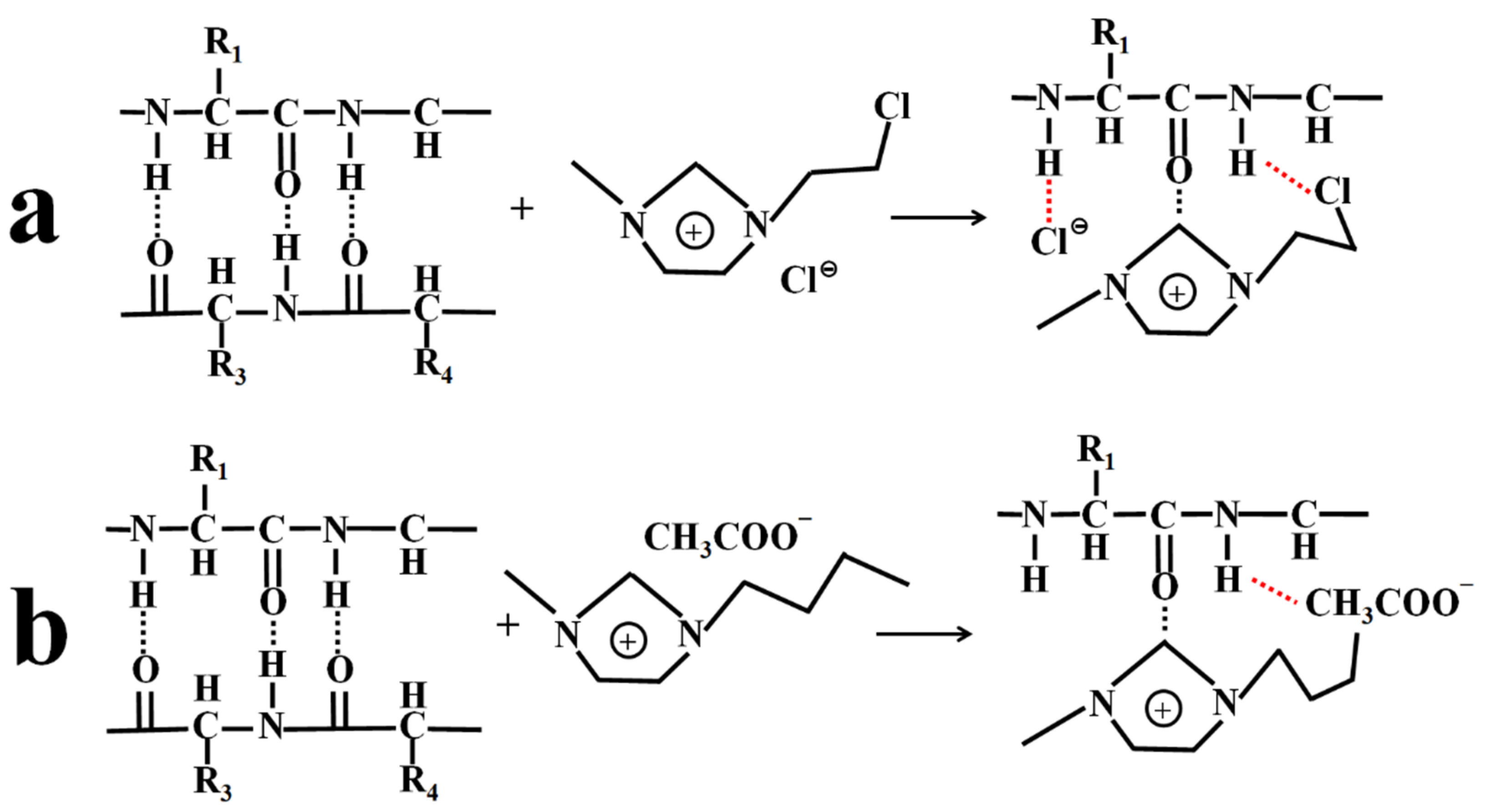
2.4. Mechanism of Dissolving SF via IL and the Influencing Factors
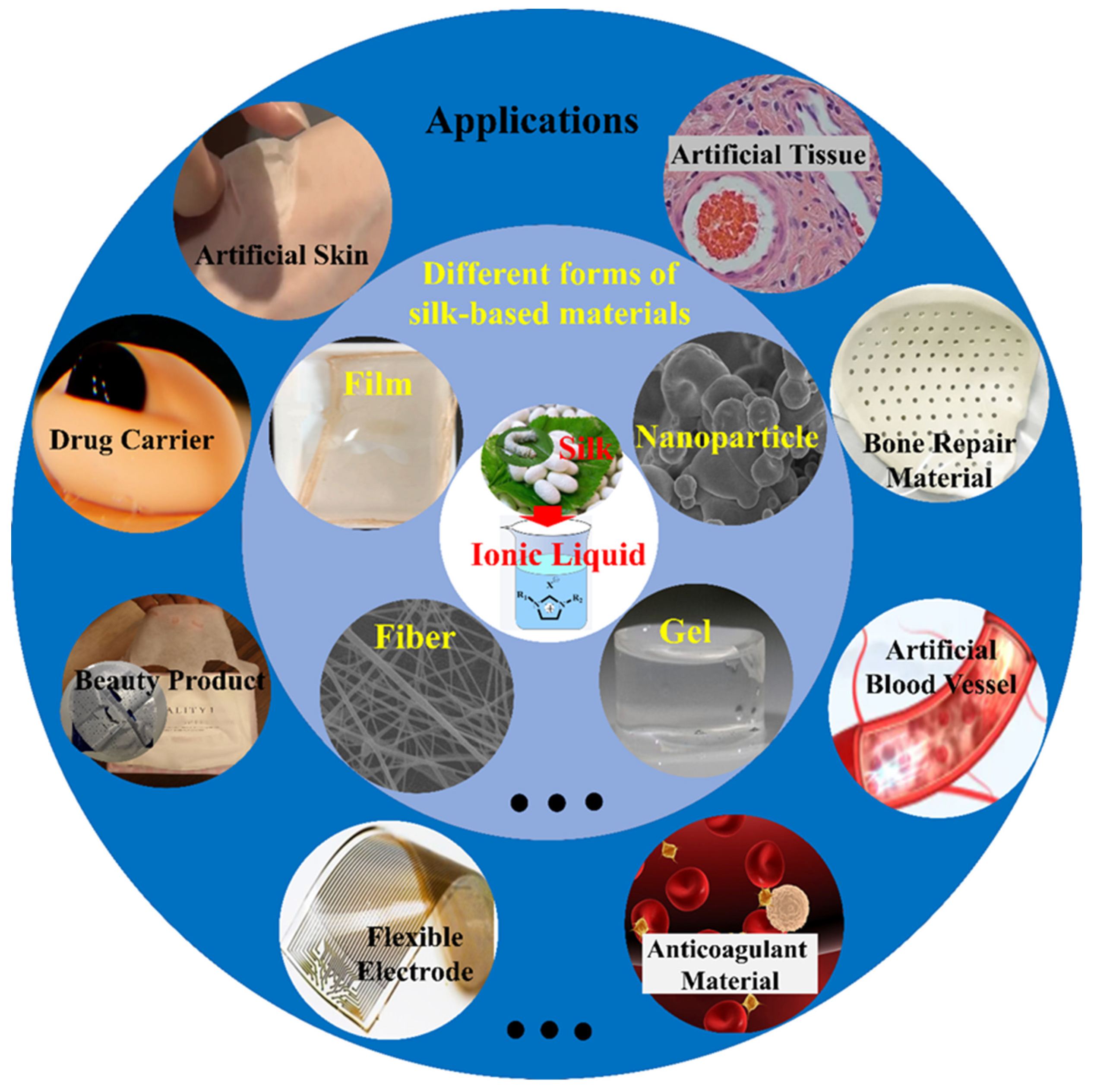
3. SF-Based Composites Prepared Using ILs for Biomedicine and Tissue Engineering Applications
3.1. SF/Natural Biopolymer Blends and Composite Materials
3.2. SF/Synthetic Polymer Composite Materials
3.3. SF/Inorganic Composite Materials
4. Conclusions and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luderer, G.; Madeddu, S.; Merfort, L.; Ueckerdt, F.; Pehl, M.; Pietzcker, R.; Rottoli, M.; Schreyer, F.; Bauer, N.; Baumstark, L.; et al. Impact of declining renewable energy costs on electrification in low-emission scenarios. Nat. Energy 2021, 7, 32–42. [Google Scholar] [CrossRef]
- Li, T.; Chen, C.J.; Brozena, A.H.; Hu, L.U.; Xu, L.X.; Driemeier, C.; Dai, J.Q.; Rojas, O.J.; Isogai, A.; Wagberg, L. Developing fibrillated cellulose as a sustainable technological material. Nature 2021, 590, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Sarute, U.; Hathaik, M. A critical review on cellulose: From fundamental to an approach on sensor technology. Renew. Sustain. Energy Rev. 2021, 57, 247–280. [Google Scholar]
- Berglund, J.; Mikkelsen, D.; Flanagan, B.M.; Dhital, S.; Gaunitz, S.; Henriksson, G.; Lindstrom, M.E.; Gidley, M.J.; Vilaplana, F. Wood hemicelluloses exert distinct biomechanical contributions to cellulose fibrillar networks. Nat. Commun. 2020, 11, 4692. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Neyrinck, A.M.; Zhang, Z.X.; Seethaler, B.; Nazare, J.A.; Sánchez, C.R.; Roumain, M.; Muccioli, G.G.; Bindels, L.B.; Cani, P.D.; et al. Metabolite profiling reveals the interaction of chitin-glucan with the gut microbiota. Gut Microbes 2020, 12, 1810530. [Google Scholar] [CrossRef] [PubMed]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef] [Green Version]
- Szlachta, M.; Neitola, R.; Peräniemi, S.; Vepsäläinen, J. Effective separation of uranium from mine process effluents using chitosan as a recyclable natural adsorbent. Sep. Purif. Technol. 2020, 253, 117493. [Google Scholar] [CrossRef]
- Silva, S.S.; Rodrigues, L.C.; Fernandes, E.M.; Gomes, J.M.; Vilas-Boas, Â.; Pirraco, R.P.; Reis, R.L. Approach on chitosan/virgin coconut oil-based emulsion matrices as a platform to design superabsorbent materials. Carbohydr. Polym. 2020, 249, 116839. [Google Scholar] [CrossRef]
- Knidri, H.E.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef]
- Costa, F.; Silva, R.; Boccaccini, A.R. 7-Fibrous protein-based biomaterials (silk, keratin, elastin, and resilin proteins) for tissue regeneration and repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Woodhead Publishing: Sawston, UK, 2018; pp. 175–204. [Google Scholar]
- Perrone, G.S.; Leisk, G.G.; Lo, T.J.; Moreau, J.E.; Haas, D.S.; Papenburg, B.J.; Golden, E.B.; Partlow, B.P.; Fox, S.E.; Ibrahim, A.M.S.; et al. The use of silk-based devices for fracture fixation. Nat. Commun. 2014, 5, 3385. [Google Scholar] [CrossRef] [Green Version]
- Shao, Z.Z.; Vollrath, F. Surprising strength of silkworm silk. Nature 2002, 418, 741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ling, C.; Zhang, A.L.; Liu, H.Y.; Jiang, Y.; Li, X.L.; Sheng, R.W.; Yao, Q.Q.; Chen, J.L. An all-silk-derived functional nanosphere matrix for sequential biomolecule delivery and in situ osteochondral regeneration. Bioact. Mater. 2020, 5, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Lee, G.J.; Leem, J.W.; Choi, S.; Kim, Y.L.; Song, Y.M. Revisiting silk: A lens-free optical physical unclonable function. Nat. Commun. 2022, 13, 247. [Google Scholar] [CrossRef] [PubMed]
- Aliramaji, S.; Zamanian, A.; Mozafari, M. Super-paramagnetic responsive silk fibroin/chitosan/magnetite scaffolds with tunable pore structures for bone tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Tanaka, K.; Arisaka, F.; Kimura, S.; Ohtomo, K.; Mizuno, S. Silk fibroin of Bombyx mori is secreted, assembling a high molecular mass elementary unit consisting of H-chain, L-chain, and P25, with a 6:6:1 molar ratio. J. Exp. Biol. 2000, 275, 40517–40528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, C.Y.; Lewis, R.V. Evidence from flagelliform silk cDNA for the structural basis of elasticity and modular nature of spider silks. J. Mol. Biol. 1998, 275, 773–784. [Google Scholar] [CrossRef]
- Sun, M.G.; Luo, Y.C.; Teng, T.; Guaiquil, V.; Zhou, Q.; McGinn, L.; Nazzal, O.; Walsh, M.; Lee, J.; Rosenblatt, M.I. Silk Film Stiffness Modulates Corneal Epithelial Cell Mechanosignaling. Macromol. Chem. Phys. 2021, 222, 2100013. [Google Scholar] [CrossRef]
- Neubauer, V.J.; Trossmann, V.T.; Jacobi, S.; Dobl, A.; Scheibel, T. Recombinant Spider Silk Gels Derived from Aqueous–Organic Solvents as Depots for Drugs. Angew. Chem. Int. Ed. 2021, 60, 11847–11851. [Google Scholar] [CrossRef]
- Drachuk, I.; Harbaugh, S.; Chavez, J.L.; Kelley-Loughnane, N. Improving the Activity of DNA-Encoded Sensing Elements through Confinement in Silk Microcapsules. ACS Appl. Mater. Interfaces 2020, 12, 48329–48339. [Google Scholar] [CrossRef]
- Zhang, J.; Allardyce, B.J.; Rajkhowa, R.; Kalita, S.; Dilley, R.J.; Wang, X.; Xin, L. Silk particles, microfibres and nanofibres: A comparative study of their functions in 3D printing hydrogel scaffolds. Mater. Sci. Eng. C 2019, 103, 109784. [Google Scholar] [CrossRef]
- Yang, F.T.; Cai, B.W.; Heng, H.; Gu, H.L.; Wang, F. Preparation, structure and properties of soy protein and silk fibroin composite nanofiber membrane by jet spinning. Sci. Sin. Chim. 2022, 52, 709–720. [Google Scholar] [CrossRef]
- Liu, Q.C.; Wang, F.; Gu, Z.G.; Ma, Q.Y.; Hu, X. Exploring the Structural Transformation Mechanism of Chinese and Thailand Silk Fibroin Fibers and Formic-Acid Fabricated Silk Films. Int. J. Mol. Sci. 2018, 19, 3309. [Google Scholar] [CrossRef] [Green Version]
- Shi, P.; Goh, J. Self-assembled silk fibroin particles: Tunable size and appearance. Powder Technol. 2012, 215, 85–90. [Google Scholar] [CrossRef]
- Li, M.; Ai, M.Y.; Yang, Y.X.; Yao, X.Y.; Zhou, Z.M.; Wang, H.; Wang, H.Y.; Li, C.; Xu, K. Silk-coated dexamethasone non-spherical microcrystals for local drug delivery to inner ear. Eur. J. Pharm. Sci. 2020, 150, 105336. [Google Scholar] [CrossRef]
- He, X.; Liu, X.Z.; Yang, J.; Du, H.B.; Chai, N.W.; Sha, Z.; Geng, M.R.; Zhou, X.J.; He, C.L. Tannic acid-reinforced methacrylated chitosan/methacrylated silk fibroin hydrogels with multifunctionality for accelerating wound healing. Carbohydr. Polym. 2020, 247, 116689. [Google Scholar] [CrossRef]
- Biswal, B.; Dan, A.K.; Sengupta, A.; Das, M.; Bindhani, B.K.; Das, D.; Parhi, P.K. Extraction of Silk Fibroin with Several Sericin Removal Processes and its Importance in Tissue Engineering: A Review. J. Polym. Environ. 2022, 30, 2222–2253. [Google Scholar] [CrossRef]
- Liu, J.Y.; Fang, Q.; Lin, H.; Yu, X.F.; Zheng, H.; Wan, Y. Alginate-poloxamer/silk fibroin hydrogels with covalently and physically cross-linked networks for cartilage tissue engineering. Carbohydr. Polym. 2020, 247, 116593. [Google Scholar] [CrossRef] [PubMed]
- Strassburg, S.; Zainuddin, S.; Scheibel, T. The Power of Silk Technology for Energy Applications. Adv. Energy Mater. 2021, 11, 2100519. [Google Scholar] [CrossRef]
- Mita, K.; Ichimura, S.; James, T.C. Highly repetitive structure and its organization of the silk fibroin gene. J. Mol. Evol. 1994, 38, 583–592. [Google Scholar] [CrossRef]
- Kaplan, D.L.; Adams, W.W.; Farmer, B.; Viney, C. Silk: Biology, structure, properties and genetics. In Silk Polymers: Materials Science and Biotechnology; Kaplan, D.L., Adams, W.W., Farmer, B., Viney, C., Eds.; ACS Symposium Series; Birkhäuser: Boston, MA, USA, 1994; Volume 544, pp. 2–16. [Google Scholar]
- Reizabal, A.; Costa, C.M.; Saiz, P.G.; Gonzalez, B.; Pérez-Álvarez, L.; Luis, R.F.D.; Garcia, A.; Vilela, J.L.V.; Méndez, S.L. Processing Strategies to Obtain Highly Porous Silk Fibroin Structures with Tailored Microstructure and Molecular Characteristics and Their Applicability in Water Remediation. J. Hazard. Mater. 2020, 403, 123675. [Google Scholar] [CrossRef] [PubMed]
- Moreira, I.P.; Esteves, C.; Palma, S.I.C.J.; Ramou, E.; Carvalho, A.L.M.; Roque, A.C.A. Synergy between silk fibroin and ionic liquids for active gas-sensing materials. Mater. Today Biol. 2022, 15, 100290. [Google Scholar] [CrossRef] [PubMed]
- El Seoud, O.A.; Keppeler, N.; Malek, N.I.; Galgano, P.D. Ionic Liquid-Based Surfactants: Recent Advances in Their Syntheses, Solution Properties, and Applications. Polymers 2021, 13, 1100. [Google Scholar] [CrossRef] [PubMed]
- Sayko, R.; Jacobs, M.; Dobrynin, A.V. Universality in Solution Properties of Polymers in Ionic Liquids. ACS Appl. Polym. Mater. 2022, 4, 1966–1973. [Google Scholar] [CrossRef]
- Koutsoukos, S.; Philippi, F.; Rauber, D.; Pugh, D.; Kay, C.W.M.; Welton, T. Effect of the cation structure on the properties of homobaric imidazolium ionic liquids. Phys. Chem. Chem. Phys. 2022, 24, 6453. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.D.; Seddon, K.R. Ionic liquids—Solvents of the future. Science 2003, 302, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Wang, J.J.; Lu, X.M.; Zhou, Q. Structures and Interactions of Ionic Liquids. Struct. Bond. 2014, 151, 1–197. [Google Scholar]
- Fox, D.; Fylstra, P.; Hanley, M.; Henderson, W.A.; Trulove, P.C.; Bellayer, S.; Gilman, J.; Long, D.H.C. The Preparation and Characterization of Bombyx Mori Silk Nanocomposites Using Ionic Liquids. ECS Trans. 2007, 3, 11–20. [Google Scholar] [CrossRef]
- Lujerdean, C.; Baci, G.M.; Cucu, A.A.; Dezmirean, D.S. The Contribution of Silk Fibroin in Biomedical Engineering. Insects 2022, 13, 286. [Google Scholar] [CrossRef] [PubMed]
- Kostag, M.; Jedvert, K.; Seoud, O.A.E. Engineering of sustainable biomaterial composites from cellulose and silk fibroin: Fundamentals and applications. Int. J. Biol. Macromol. 2020, 167, 687–718. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.Z.; Confalonieri, F.; Medina, N.; Zivanovic, Y.; Esnault, C.; Yang, T.; Jacquet, M.; Janin, J.; Duguet, M.; Perasso, R.; et al. Fine organization of Bombyx mori fibroin heavy chain gene. Nucleic Acids Res. 2000, 28, 2413–2419. [Google Scholar] [CrossRef] [Green Version]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef]
- Vollrath, F.; Porter, D. Silks as ancient models for modern polymers. Polymer 2009, 50, 5623–5632. [Google Scholar] [CrossRef] [Green Version]
- Ming, J.F.; Zuo, B.Q. Silk I Structure Formation Through Silk Fibroin Self-Assembly. J. Appl. Polym. Sci. 2012, 125, 2148–2154. [Google Scholar] [CrossRef]
- Wang, F.; Wolf, N.; Rocks, E.M.; Vuong, T.; Hu, X. Comparative studies of regenerated water-based Mori, Thai, Eri, Muga and Tussah silk fibroin films. J. Therm. Anal. Calorim. 2015, 122, 1069–1076. [Google Scholar] [CrossRef]
- Takahashi, Y.; Gehoh, M.; Yuzuriha, K. Structure refinement and diffuse streak scattering of silk (Bombyx mori). Int. J. Biol. Macromol. 1999, 24, 127–138. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Shao, Z.Z. Sol-Gel Transition of Regenerated Silk Fibroins in Ionic Liquid/Water Mixtures. ACS Biomater. Sci. Eng. 2016, 2, 12–18. [Google Scholar] [CrossRef]
- Marsh, R.E.; Corey, R.B.; Pauling, L. An investigation of the structure of silk fibroin. Biochim. Biophys. Acta. 1955, 16, 1–34. [Google Scholar] [CrossRef]
- Jin, H.J.; Kaplan, D.L. Mechanism of silk processing in insects and spiders. Nature 2003, 424, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.C.; Kundu, B.; Talukdar, S.; Bano, S.; Nayak, S.; Kundu, J.; Mandal, B.B.; Bhardwaj, N.; Botlagunta, M.; Dash, B.C.; et al. Nonmulberry silk biopolymers. Biopolymers 2012, 97, 455–467. [Google Scholar] [CrossRef]
- Silva, S.S.; Kundu, B.; Lu, S.; Reis, R.L.; Kundu, S.C. Chinese Oak Tasar Silkworm Antheraea pernyi Silk Proteins: Current Strategies and Future Perspectives for Biomedical Applications. Macromol. Biosci. 2019, 19, 1800252. [Google Scholar] [CrossRef]
- Fang, Q.; Chen, D.L.; Yang, Z.; Li, M. In vitro and in vivo research on using Antheraea pernyi silk fibroin as tissue engineering tendon scaffolds. Mater. Sci. Eng. 2009, 29, 1527–1534. [Google Scholar] [CrossRef]
- Minoura, N.; Aiba, S.I.; Higuchi, M.; Gotoh, Y.; Tsukada, M.; Imai, Y. Attachment and growth of fibroblast cells on silk fibroin. Biochem. Biophys. Res. Commun. 1995, 208, 511. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.Z.; Confalonieri, F.; Jacquet, M.; Perasso, R.; Li, Z.G.; Janin, J. Silk fibroin: Structural implications of a remarkable amino acid sequence. Proteins 2001, 44, 119–122. [Google Scholar] [CrossRef]
- Cho, H.J.; Ki, C.S.; Oh, H.; Lee, K.H.; Um, I.C. Molecular weight distribution and solution properties of silk fibroins with different dissolution conditions. Int. J. Biol. Macromol. 2012, 51, 336–341. [Google Scholar] [CrossRef]
- Wu, Z.W.; Feng, X.X.; Zhu, H.L.; Liu, N.; Sun, B.; Wu, B.W.; Chen, J.Y. Effects of different solvent systems on molecular mass of silk fibroin and properties of the regenerated silk fibroin membranes. Canye Kexue 2010, 36, 707–712. [Google Scholar]
- Chen, X.; Knight, D.P.; Shao, Z.Z.; Vollrath, F. Regenerated Bombyx silk solutions studied with rheometry and FTIR. Polymer 2001, 42, 9969–9974. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zhang, Y.Q.; Wei, Z.G. Dissolution and processing of silk fibroin for materials science. Crit. Rev. Biotechnol. 2021, 41, 406–424. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Zhang, Y.Q.; Wei, Z.G. Excess acetone extraction in silk protein solution greatly accelerates the regeneration progress of silk fibroin for desalting and purification. Int. J. Biol. Macromol. 2020, 146, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.D.; Golomb, M.C.; Georgakoudi, I.; Kaplan, D.L.; Omenetto, F.G. Bioactive Silk Protein Biomaterial Systems for Optical Devices. Biomacromolecules 2008, 9, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xiong, Y.; Yu, S.; Liu, S.W.; Liu, F.S.; Xie, C.X. Facile preparation for robust and freestanding silk fibroin films in a 1-butyl-3-methyl imidazolium acetate ionic liquid system. J. Appl. Polym. Sci. 2015, 132, 42822. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, Q.; Ming, J.; Dou, H.; Liu, Z.; Zuo, B.Q.; Qin, M.D.; Li, F.; Kaplan, D.L.; Zhang, X.G. Silk dissolution and regeneration at the nanofibril scale. J. Mater. Chem. B 2014, 2, 3879–3885. [Google Scholar] [CrossRef]
- Li, M.Z.; Tao, W.; Lu, S.Z.; Kuga, S. Compliant film of regenerated Antheraea pernyi silk fibroin by chemical crosslinking. Int. J. Biol. Macromol. 2003, 32, 159–163. [Google Scholar] [PubMed]
- Tao, W.; Li, M.Z.; Zhao, C.X. Structure and properties of regenerated Antheraea pemyi silk fibroin in aqueous solution. Int. J. Biol. Macromol. 2007, 40, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Noishiki, Y.; Nishiyama, Y.; Wada, M.; Kuga, S.; Magoshi, J. Mechanical Properties of Silk Fibroin–Microcrystalline Cellulose Composite Films. J. Appl. Polym. Sci. 2002, 86, 3425–3429. [Google Scholar]
- Um, I.C.; Kweon, H.; Park, Y.H.; Hudson, S. Structural characteristics and properties of the regenerated silk fibroin prepared from formic acid. Int. J. Biol. Macromol. 2001, 29, 91–97. [Google Scholar] [CrossRef]
- Kawahara, Y.; Furukawa, K.; Yamamoto, T. Self-Expansion Behavior of Silk Fibroin Film. Macromol. Mater. Eng. 2010, 291, 458–462. [Google Scholar] [CrossRef]
- Li, M.; Wu, Z.Y.; Zhang, C.S.; Lu, S.Z.; Yan, H.J.; Huang, D.; Ye, H.L. Study on porous silk fibroin materials. II. Preparation and characteristics of spongy porous silk fibroin materials. J. Appl. Polym. Sci. 2001, 79, 2192–2199. [Google Scholar] [CrossRef]
- Selmin, F.; Gennari, C.G.; Minghetti, P.; Marotta, L.A.; Viviani, B.; Vagdama, P.; Montanari, L.; Cilurzo, F. Enhanced hydration stability of Bombyx mori silk fibroin/PEG 600 composite scaffolds for tissue engineering. Polym. Adv. Technol. 2014, 25, 532–538. [Google Scholar] [CrossRef]
- Rama, M.; Vijayalakshmi, U. Influence of Silk fibroin on the preparation of nanofibrous scaffolds for the effective use in Osteoregenerative Applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102182. [Google Scholar] [CrossRef]
- Mathur, A.B.; Tonelli, A.; Rathke, T.; Hudson, S. The dissolution and characterization of Bombyx mori silk fibroin in calcium nitrate-methanol solution and the regeneration of films. Biopolymers 1997, 42, 61–74. [Google Scholar] [CrossRef]
- Huang, J.W.; Jin, Z.G.; Zuo, B.; Zhang, F.; Qin, J.Z.; Shen, Y.C.; Yao, M. Preparation and characterization of a novel Cassava silk film containing nanopore structure. Mater. Lett. 2020, 260, 126942. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, F.; Torculas, M.; Lofland, S.; Hu, X. Formic Acid Regenerated Mori, Tussah, Eri, Thai, and Muga Silk Materials: Mechanism of Self-Assembly. ACS Biomater. Sci. Eng. 2019, 5, 6361–6373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yang, R.C.; Zhang, P.; Qin, J.Z.; Fan, Z.H.; Zuo, B.Q. Water-Rinsed Nonmulberry Silk Film for Potential Tissue Engineering Applications. ACS Omega 2019, 4, 3114–3121. [Google Scholar] [CrossRef]
- Thimm, B.W.; Wüst, S.; Hofmann, S.; Hagenmüller, H.; Müller, R. Initial cell pre-cultivation can maximize ECM mineralization by human mesenchymal stem cells on silk fibroin scaffolds. Acta Biomater. 2011, 7, 2218–2228. [Google Scholar] [CrossRef]
- Wang, Y.; Rudym, D.D.; Walsh, A.; Abrahamsen, L.; Kim, H.J.; Kim, H.S.; Head, C.K.; Kaplan, D.L. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials 2008, 29, 3415–3428. [Google Scholar] [CrossRef] [Green Version]
- Phillips, D.M.; Drummy, L.F.; Conrady, D.G.; Fox, D.M.; Naik, R.R.; Stone, M.O.; Trulove, P.C.; Long, H.C.D.; Mantz, R.A. Dissolution and regeneration of Bombyx mori silk fibroin using ionic liquids. J. Am. Chem. Soc. 2004, 126, 14350–14351. [Google Scholar] [CrossRef]
- Dymek, C.J., Jr.; Grossie, D.A.; Fratini, A.V.; Adams, W.W. Evidence for the presence of hydrogen-bonded ion-ion interactions in the molten salt precursor, 1-methyl-3-ethylimidazolium chloride. J. Mol. Struct. 1989, 213, 25–34. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Y.H.; Chen, X.; Shao, Z.Z. Investigation of rheological properties and conformation of silk fibroin in the solution of AmimCl. Biomacromolecules 2012, 13, 1875–1881. [Google Scholar] [CrossRef]
- Wang, H.Y.; Wei, Z.G.; Zhang, Y.Q. Dissolution and regeneration of silk from silkworm Bombyx mori in ionic liquids and its application to medical biomaterials - ScienceDirect. Int. J. Biol. Macromol. 2020, 143, 594–601. [Google Scholar] [CrossRef]
- Xie, C.X.; Li, W.J.; Liang, Q.Q.; Yu, S.T.; Li, L. Fabrication of robust silk fibroin film by controlling the content of β-sheet via the synergism of Uv-light and ionic liquids. Appl. Surf. Sci. 2019, 492, 55–65. [Google Scholar] [CrossRef]
- Walker, A.A.; Holland, C.; Sutherland, T.D. More than one way to spin a crystallite: Multiple trajectories through liquid crystallinity to solid silk. Proc. R. Soc. B. 2015, 282, 0259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuster, M.G.; Carissimi, G.; Montalbán, M.G.; Víllora, G. Improving Anticancer Therapy with Naringenin-Loaded Silk Fibroin Nanoparticles. Nanomaterials 2020, 10, 718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, D.M.; Drummy, L.F.; Naik, R.R.; Long, H.C.D.; Fox, D.M.; Trulove, P.C.; Mantz, R.A. Regenerated silk fiber wet spinning from an ionic liquid solution. J. Mater. Chem. 2005, 15, 4206–4208. [Google Scholar] [CrossRef]
- Carissimi, G.; Baronio, C.M.; Montalbán, M.G.; Víllora, G.; Barth, A. On the Secondary Structure of Silk Fibroin Nanoparticles Obtained Using Ionic Liquids: An Infrared Spectroscopy Study. Polymers 2020, 12, 1294. [Google Scholar] [CrossRef]
- Blessing, B.; Trout, C.; Morales, A.; Rybacki, K.; Love, S.A.; Lamoureux, G.; Malley, S.M.O.; Hu, X.; Cruz, D.S.D.L. Morphology and ionic conductivity relationship in silk/cellulose biocomposites. Polym Int. 2019, 68, 1580–1590. [Google Scholar] [CrossRef]
- Hadadi, A.; Whittaker, J.W.; Verrill, D.E.; Hu, X.; Larini, L.; Cruz, D.S.D.L. A Hierarchical Model to Understand the Processing of Polysaccharides/Protein-Based Films in Ionic Liquids. Biomacromolecules 2018, 19, 3970–3982. [Google Scholar] [CrossRef]
- Stanton, J.; Xue, Y.; Pandher, P.; Malek, L.; Brown, T.; Hu, X.; Salas-de la Cruz, D. Impact of Ionic Liquid Type on the Structure, Morphology and Properties of Silk-Cellulose Biocomposite Materials. Int. J. Biol. Macromol. 2018, 108, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Hardacre, C.; Holbrey, J.D.; Nieuwenhuyzen, M.; Youngs, T.G. Structure and solvation in ionic liquids. Acc. Chem. Res. 2007, 40, 1146–1155. [Google Scholar] [CrossRef]
- Vyas, S.K.; Shukla, S.R. Degumming of Tasar silk using imidazolium-based ionic liquids. J. Text. Inst. 2020, 111, 1364–1370. [Google Scholar] [CrossRef]
- Ren, H.P.; Sun, S.Q.; Li, L. Dissolution performance of ionic liquid for silk fibroin. Appl. Chem. Ind. 2014, 43, 1256–1262. [Google Scholar]
- Zhang, H.H.; Hu, C.G.; Shao, H.L. Dissolution of Silk Fibroin in lonic Liquids and the Structure of the Regenerated Silk Fiber. Polym. Mater. Sci. Eng. 2011, 27, 66–68. [Google Scholar]
- Susanin, A.I.; Sashina, E.S.; Novoselov, N.P.; Zakharov, V.V. Change of Silk Fibroin Molecular Mass During Dissolution in Ionic Liquids. Fibre Chem. 2020, 52, 208–213. [Google Scholar] [CrossRef]
- Wang, W.Q.; Liu, Y.Z.; Wang, S.Q.; Fu, X.M.; Zhao, T.C.; Chen, X.; Shao, Z.Z. Physically Cross-Linked Silk Fibroin-Based Tough Hydrogel Electrolyte with Exceptional Water Retention and Freezing Tolerance. ACS Appl. Mater. Interfaces 2020, 12, 25353–25362. [Google Scholar] [CrossRef]
- Pérez, A.A.L.; Montalbán, M.G.; Cervantes, S.D.A.; Cragnolini, F.; Cenis, J.L.; Víllora, G. Production of silk fibroin nanoparticles using ionic liquids and high-power ultrasounds. J. Appl. Polym. Sci. 2015, 132, 41702. [Google Scholar]
- Jiang, C.; Wang, X.; Gunawidjaja, R.; Lin, Y.H.; Gupta, M.K.; Kaplan, D.L.; Naik, R.R.; Tsukruk, V.V. Mechanical properties of robust ultrathin silk fibroin films. Adv. Funct. Mater. 2007, 17, 2229–2237. [Google Scholar] [CrossRef]
- Goujon, N.; Rajkhowa, R.; Wang, X.; Byrne, N. Effect of solvent on ionic liquid dissolved regenerated antheraea assamensis silk fibroin. J. Appl. Polym. Sci. 2013, 128, 4411–4416. [Google Scholar] [CrossRef]
- Fernandes, T.C.D.; Rodrigues, H.M.R.; Paz, F.A.A.; Sousa, J.F.M.; Valente, A.J.M.; Silva, M.M.; Bermudez, V.D.Z.; Pereira, R.F.P. Highly Conducting Bombyx mori Silk Fibroin-Based Electrolytes Incorporating Glycerol, Dimethyl Sulfoxide and [Bmim]PF6. J. Electrochem. Soc. 2020, 167, 070551. [Google Scholar] [CrossRef]
- Yao, M.; Su, D.H.; Wang, W.Q.; Chen, X.; Shao, Z.Z. Fabrication of air-stable and conductive silk fibroin gels. ACS Appl. Mater. Interfaces 2018, 10, 38466–38475. [Google Scholar] [CrossRef]
- Silva, S.S.; Oliveira, N.M.; Oliveira, M.B.; Soares da Costa, D.P.; Naskar, D.; Mano, J.F.; Kundu, S.C.; Reis, R.L. Fabrication and characterization of Eri silk fibers-based sponges for biomedical application. Acta Biomater. 2016, 32, 178–189. [Google Scholar] [CrossRef] [Green Version]
- DeFrates, K.; Markiewicz, T.; Callaway, K.; Xue, Y.; Stanton, J.; de la Cruz, D.S.; Hu, X. Structure-property relationships of Thai silk-microcrystalline cellulose biocomposite materials fabricated from ionic liquid. Int. J. Biol. Macromol. 2017, 104, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Stanton, J.; Xue, Y.; Waters, J.C.; Lewis, A.; Cowan, D.; Hu, X.; Cruz, D.S.D.L. Structure-property relationships of blended polysaccharide and protein biomaterials in ionic liquid. Cellulose 2017, 24, 1775–1789. [Google Scholar] [CrossRef]
- Shang, S.M.; Zhu, L.; Fan, J.T. Physical properties of silk fibroin/cellulose blend films regenerated from the hydrophilic ionic liquid. Carbohyd. Polym. 2011, 86, 462–468. [Google Scholar] [CrossRef]
- Tian, D.; Li, T.; Zhang, R.C.; Wu, Q.; Chen, T.H.; Sun, P.C.; Ramamoorthy, A. Conformations and Intermolecular Interactions in Cellulose/Silk Fibroin Blend Films: A Solid-State NMR Perspective. J. Phys. Chem. B. 2017, 121, 6108–6116. [Google Scholar] [CrossRef]
- Blessing, B.; Trout, C.; Morales, A.; Rybacki, K.; Love, S.A.; Lamoureux, G.; Mally, S.M.; Hu, X.; Cruz, D.S.D.L. The Impact of Composition and Morphology on Ionic Conductivity of Silk/Cellulose Bio-Composites Fabricated from Ionic Liquid and Varying Percentages of Coagulation Agents. Int. J. Mol. Sci. 2020, 21, 4695. [Google Scholar] [CrossRef]
- Love, S.A.; Popov, E.; Rybacki, K.; Hu, X.; Cruz, D.S.D.L. Facile treatment to fine-tune cellulose crystals in cellulose-silk biocomposites through hydrogen peroxide. Int. J. Biol. Macromol. 2020, 147, 569–575. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Abdan, K.; Kaneko, T. A concise review on the physicochemical properties of biopolymer blends prepared in ionic liquids. Molecules 2021, 26, 216. [Google Scholar] [CrossRef]
- Gonçalves, C.; Gomes, J.M.; Maia, F.R.; Radhouani, H.; Silva, S.S.; Reis, R.L.; Oliveira, J.M. Fabrication of biocompatible porous SAIB/silk fibroin scaffolds using ionic liquids. Mater. Chem. Front. 2021, 5, 6582–6591. [Google Scholar] [CrossRef]
- Gomes, J.M.; Silva, S.S.; Fernandes, E.M.; Lobo, F.C.M.; Martín-Pastor, M.; Taboada, P.; Reis, R.L. Silk fibroin/cholinium gallate-based architectures as therapeutic tools. Acta Biomater. 2022, 147, 168–184. [Google Scholar] [CrossRef]
- Deng, Q.Q.; Wang, F.; Gough, C.R.; Hu, X. Tunable microphase-regulated silk fibroin/poly (lactic acid) biocomposite materials generated from ionic liquids. Int. J. Biol. Macromol. 2022, 197, 55–67. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhang, C.C.; Xu, W.L.; Liu, H.T.; Ouyang, C.X. Blend films of silk fibroin and water-insoluble polyurethane prepared from an ionic liquid. Mater. Lett. 2011, 65, 2489–2491. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Yang, H.W.; Tang, L.Y.; Li, C.L. Preparation and characterization of silk fibroin /polyvinyl alcohol composite membrane. J. Text. Res. 2018, 39, 12061294. [Google Scholar]
- Pereira, R.F.P.; Zehbe, K.; Günter, C.; Santos, T.D.; Nunes, S.C.; Paz, F.A.A.; Silva, M.M.; Granja, P.L.; Taubert, A.; Bermudez, V.D.Z. Ionic Liquid-Assisted Synthesis of Mesoporous Silk Fibroin/Silica Hybrids for Biomedical Applications. ACS Omega 2018, 3, 10811–10822. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Bai, L.; Zhang, L.L.; Sun, G.P.; Zhang, X.W.; Dong, S.J. A microporous silk carbon–ionic liquid composite for the electrochemical sensing of dopamine. Analyst 2016, 141, 2447–2453. [Google Scholar] [CrossRef] [PubMed]
- Rath, T.; Pramanik, N.; Kumar, S. High electrochemical performance flexible solid-state supercapacitor based on Co-doped reduced graphene oxide and silk fibroin composites. Energy 2017, 141, 1982–1988. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.C.; Cheng, Y.H.; Zhang, J.M.; Mi, Q.Y.; Yin, C.C.; Wu, J.; Zhang, J. Super-rapid and highly-efficient esterification of cellulose to achieve an accurate chromatographic analysis of its molecular weight. Carbohydr. Polym. 2022, 286, 119301. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Pinkert, A.; Marsh, K.N.; Pang, S.S.; Staiger, M.P. Ionic liquids and their interaction with cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar] [CrossRef]
- Yuan, X.M.; Cheng, G. From cellulose fibrils to single chains: Understanding cellulose dissolution in ionic liquids. Phys. Chem. Chem. Phys. 2015, 17, 31592–31607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, R.F.P.; Pereira, R.B.; Gonçalves, R.; Silva, M.P.; Costa, C.M.; Silva, M.M.; Bermudez, V.D.Z.; Méndez, S.L. Silk Fibroin Separators: A Step Toward Lithium-Ion Batteries with Enhanced Sustainability. ACS Appl. Mater. Interfaces 2018, 10, 5385–5394. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.Q.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.L.; Chen, L.M.; Jiang, Y.C.; Jia, Y.R.; Huang, Y.X.; Zhang, Y.S. Study on the Properties of the PVA/SF Blend Membranes. Adv. Mater. Res. 2013, 781–784, 546–549. [Google Scholar] [CrossRef]
- Arcos, D.; Regí, M.V. Sol-gel silica-based biomaterials and bone tissue regeneration. Acta Biomater. 2010, 6, 2874–2888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ries, M.E.; Hine, P.J. Time-temperature superposition of the dissolution of silk fibers in the Ionic liquid 1-ethyl-3-methylimidazolium acetate. Biomacromolecules 2021, 22, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- El Seoud, O.A.; Kostag, M.; Possidonio, S.; Dignani, M.T.; Pires, P.A.R.; Lourenço, M.C. Dissolution of silk fibroin in mixtures of ionic liquids and dimethyl sulfoxide: On the relative importance of temperature and binary solvent composition. Polymers 2021, 14, 13. [Google Scholar] [CrossRef] [PubMed]

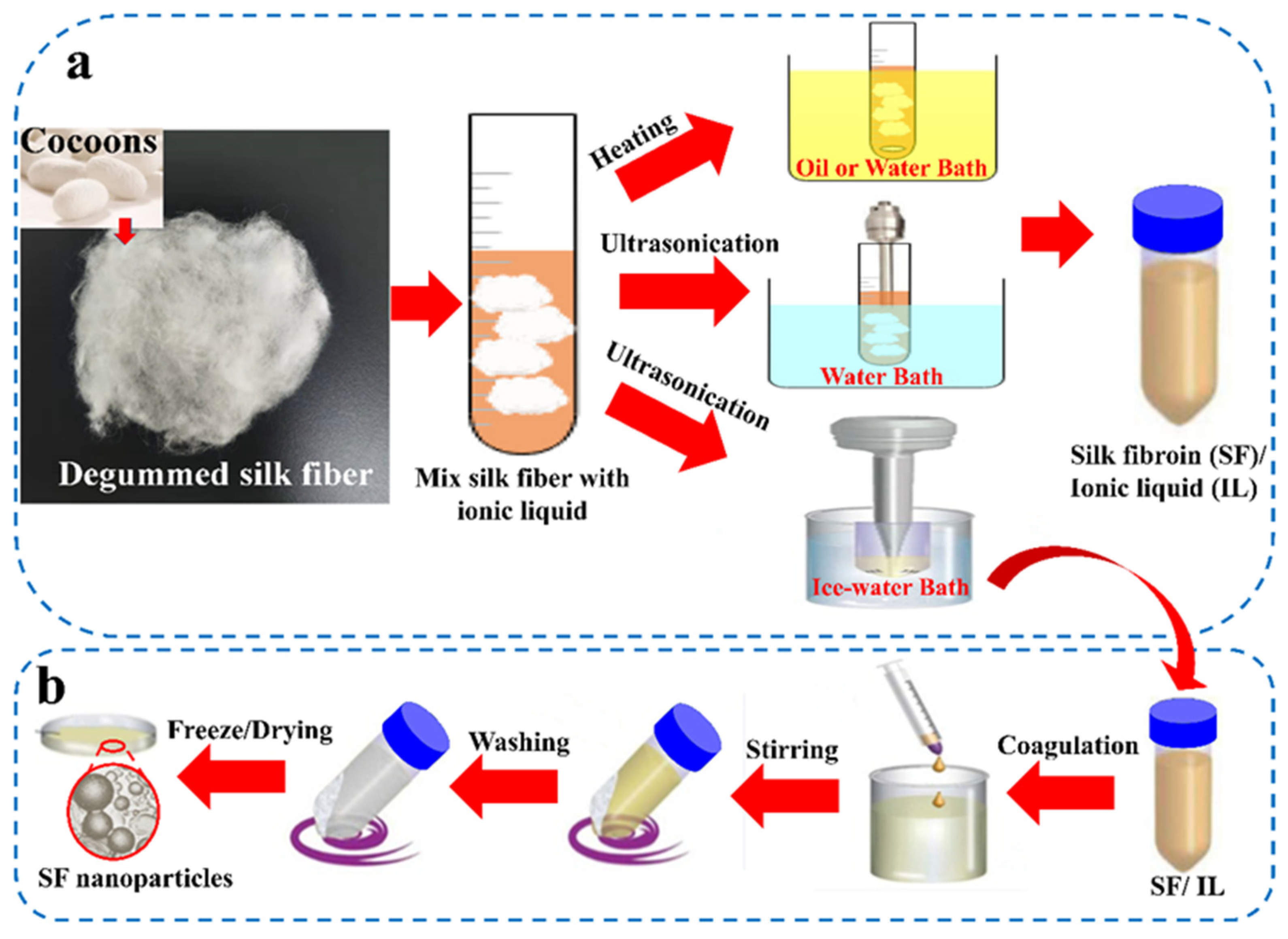
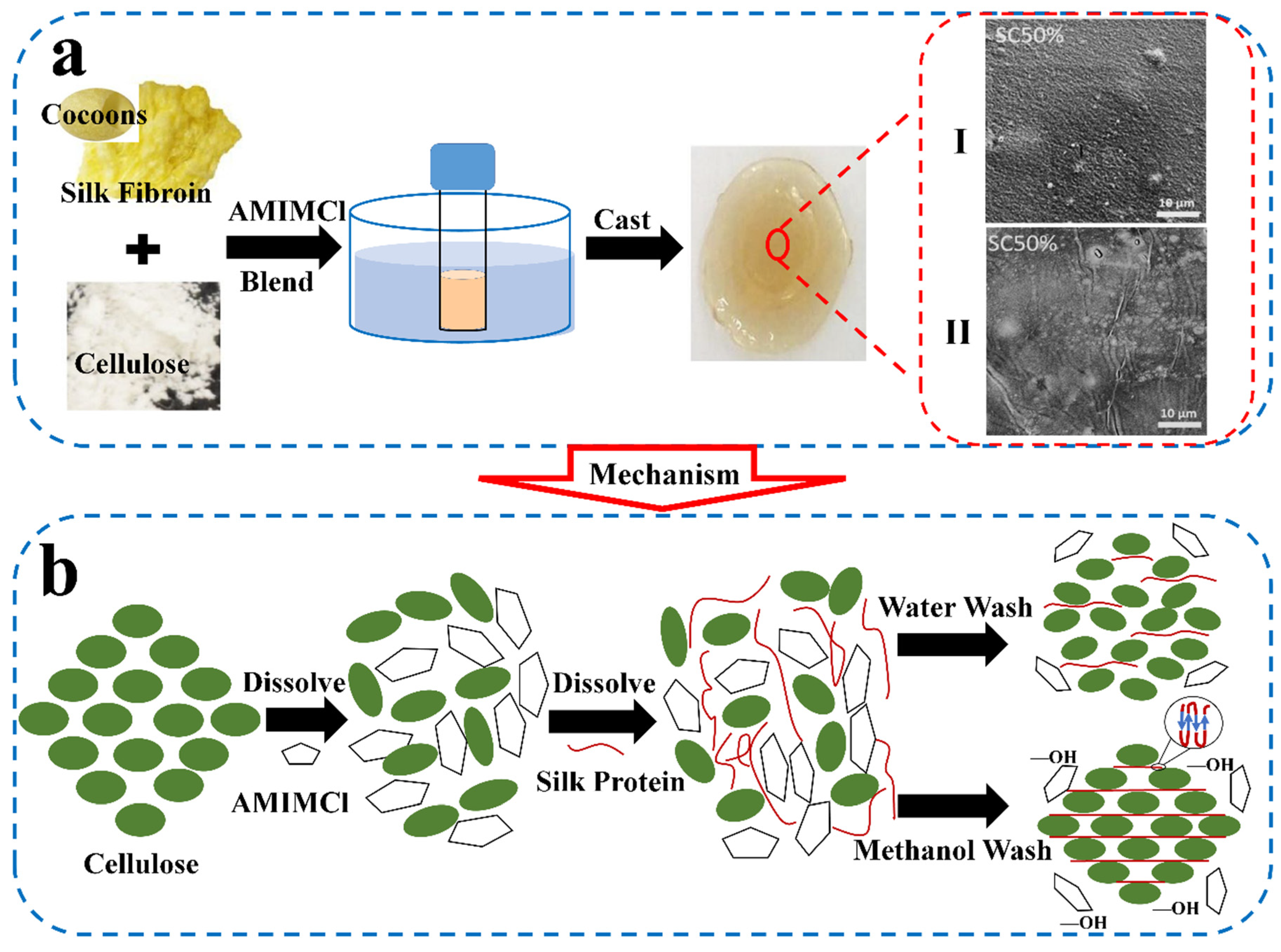
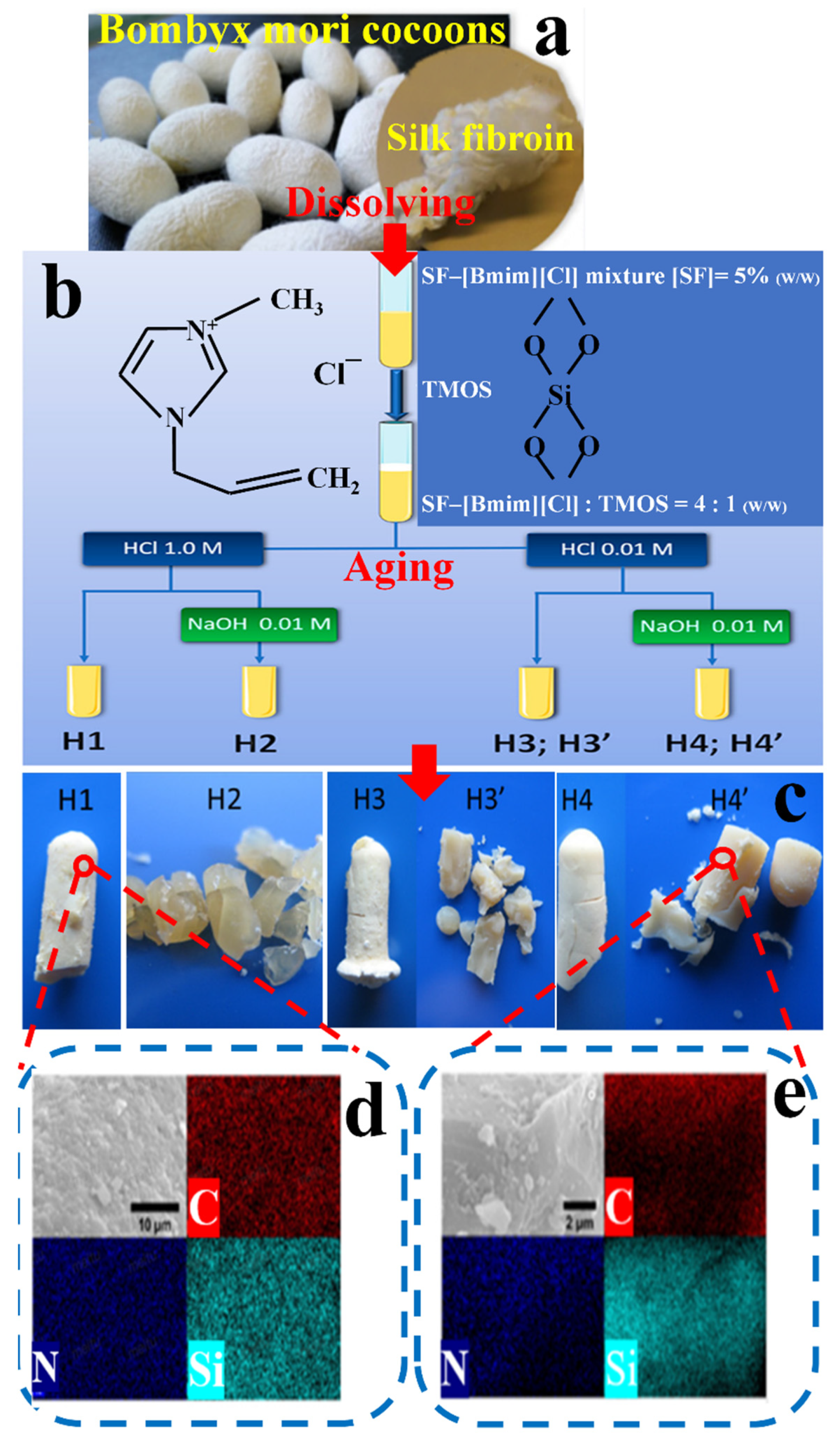
| Components | Solvent | Shape | Applications | References |
|---|---|---|---|---|
| SF | [EMIM]Ac Anp | Hydrogels | Biomedical materials | [49] |
| [EMIM]AC Bom | Gels | Conductive gel, biomedical materials | [49,101] | |
| [BMIM]AC Bom | Films | Tissue engineering materials | [63,82,83] | |
| [AMIM]Cl Bom | Regenerated silk Fiber | Tissue engineering materials | [81] | |
| [BMIM]Br Bom | Films | Tissue engineering materials | [83] | |
| [BMIM]Cl Bom | Films | Artificial skin coating | [82,97,98] | |
| [BMIM]Cl Ana [BMIM]Ac Ana | Films | Tissue engineering materials | [99] | |
| [BMIM]Ac Eri | Sponge | Cartilage-related biomedical materials | [102] | |
| SF-cellulose | [EMIM]Cl | Films | Electrode materials | [80] |
| [BMIM]Br [BMIM]MeSO3 | Films | Biomedical materials | [90] | |
| [AMIM]Cl | Films | Tissue engineering materials | [90,103,104] | |
| [AMIM]Cl | Films | Biomedical materials | [103] | |
| [BMIM]Cl | Films | Biomedical materials | [105,106] | |
| [EMIM]AC | Nanoparticles, Films | Coagulation materials, Electrode materials, Tissue engineering materials | [80] [87] [107,108] | |
| [BMIM]Cl | Fiber | biomedicine and tissue materials | [109] | |
| SF-carbon nanotubes | [EMIM]Cl | Films | Tissue engineering materials | [40] |
| SF-naringin | [EMIM]AC | Nanoparticles | Drug delivery materials | [85] |
| SF-chitin | [AMIM]Cl | Films | Biomedical materials | [90] |
| SF-KCl | [EMIM]AC | Gels | Flexible ionic conductive hydrogel | [96] |
| SF-Glycerol & Dimethyl sulfoxide | [BMIM]PF6 | Films | Electrolyte film, Energy materials | [100] |
| SF-sucrose acetate isobutyrate | [BMIM][Ac] | Scaffolds | Tissue engineering materials | [110] |
| SF-cholinium gallate | Bio-ILs | Sponge | Biomedical materials | [111] |
| SF-chitosan | [AMIM]Cl [BMIM]Ac | Films Hydrogels | Tissue engineering materials Biomedical materials | [90,109] |
| SF-polylactic acid | [BMIM]Cl | Films | Drug delivery materials | [112] |
| SF-polyurethane | [BMIM]Cl | Films | Coagulation material | [113] |
| SF- polyvinyl alcohol | [AMIM]Cl | Films | Tissue engineering materials | [114] |
| SF-silica | [BMIM]Cl | 3D scaffolds | Bone tissue materials | [82,115] |
| SF-carbon | [BMIM]PF6 | 3D scaffolds | Electrochemical sensor | [116] |
| SF-graphene oxide | [EMIM]BF4 | Films | Electrode material | [117] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heng, H.; Deng, Q.; Yang, Y.; Wang, F. Recent Research Progress of Ionic Liquid Dissolving Silks for Biomedicine and Tissue Engineering Applications. Int. J. Mol. Sci. 2022, 23, 8706. https://doi.org/10.3390/ijms23158706
Heng H, Deng Q, Yang Y, Wang F. Recent Research Progress of Ionic Liquid Dissolving Silks for Biomedicine and Tissue Engineering Applications. International Journal of Molecular Sciences. 2022; 23(15):8706. https://doi.org/10.3390/ijms23158706
Chicago/Turabian StyleHeng, Hang, Qianqian Deng, Yipeng Yang, and Fang Wang. 2022. "Recent Research Progress of Ionic Liquid Dissolving Silks for Biomedicine and Tissue Engineering Applications" International Journal of Molecular Sciences 23, no. 15: 8706. https://doi.org/10.3390/ijms23158706
APA StyleHeng, H., Deng, Q., Yang, Y., & Wang, F. (2022). Recent Research Progress of Ionic Liquid Dissolving Silks for Biomedicine and Tissue Engineering Applications. International Journal of Molecular Sciences, 23(15), 8706. https://doi.org/10.3390/ijms23158706






