Abstract
Pregnancy complications such as maternal hyperglycemia increase perinatal mortality and morbidity, but risks are higher in males than in females. We hypothesized that fetal sex-dependent differences in placental palmitic-acid (PA) and oleic-acid (OA) metabolism influence such risks. Placental explants (n = 22) were incubated with isotope-labeled fatty acids (13C-PA or 13C-OA) for 24 or 48 h and the production of forty-seven 13C-PA lipids and thirty-seven 13C-OA lipids quantified by LCMS. Linear regression was used to investigate associations between maternal glycemia, BMI and fetal sex with 13C lipids, and between 13C lipids and birthweight centile. Placental explants from females showed greater incorporation of 13C-OA and 13C-PA into almost all lipids compared to males. Fetal sex also influenced relationships with maternal glycemia, with many 13C-OA and 13C-PA acylcarnitines, 13C-PA-diacylglycerols and 13C-PA phospholipids positively associated with glycemia in females but not in males. In contrast, several 13C-OA triacylglycerols and 13C-OA phospholipids were negatively associated with glycemia in males but not in females. Birthweight centile in females was positively associated with six 13C-PA and three 13C-OA lipids (mainly acylcarnitines) and was negatively associated with eight 13C-OA lipids, while males showed few associations. Fetal sex thus influences placental lipid metabolism and could be a key modulator of the impact of maternal metabolic health on perinatal outcomes, potentially contributing toward sex-specific adaptions in which females prioritize survival.
1. Introduction
Perinatal mortality and morbidity are higher in males than in females, but mechanisms underlying such sex differences remain inadequately understood. Males are generally born heavier than females, while females have greater fat mass and are more likely to survive suboptimal in utero conditions such as uteroplacental insufficiency and maternal hyperglycemia [,,,]. It has been suggested that female fetuses are more adaptable to in utero adversity, while male fetuses prioritize a growth strategy at the expense of survival []. Understanding the specific pathways involved in these sex-dependent differences could lead to novel strategies to improve offspring outcomes. It is postulated that sex-dependent differences in placental function and metabolism may partly account for these differences.
1.1. Palmitic Acid and Oleic Acid in Pregnancy
Palmitic acid (PA, 16:0) and oleic acid (OA, 18:1) constitute 60% of fatty acids in lipids present in the maternal plasma by the end of pregnancy, and in both placenta and the fetus, these fatty acids act as fuel and as building blocks for the synthesis of structural and signaling lipids [,,]. Both PA and OA are derived from diet and endogenous synthesis; thus, the fetus relies on both transplacental transfer and endogenous fetal–placental synthesis []. Therefore, sex-dependent variations in these processes may impact fetal growth and development [].
Excess PA is often negatively depicted for its potential involvement in the pathophysiology of adult chronic diseases such as cardiovascular disorders and diabetes, and excess intracellular un-esterified PA may induce lipotoxic effects [,], including in the placenta []. However, PA is also an essential fuel source, is a constituent of many signaling lipid molecules, is needed for the regulation of enzymatic activity though palmitoylation [], and is involved in the production of pro-inflammatory cytokines [], which are important in regulating pregnancy health and parturition. Conversely, OA is often positively depicted, showing no pro-inflammatory effects and appearing to counter many damaging effects of excess PA [,,]. For example, OA appears to protect trophoblasts from palmitate-induced death in vitro, possibly by inducing PA to be safely compartmentalized within lipid droplets [,,]. OA also increases placental amino acid transport and phosphorylation of ERK, mTOR, S6 kinase 1, and rpS6 [,] to regulate placental function including fetal nutritional supply.
1.2. The Influence of Maternal Glycemia, BMI and Fetal Sex on Maternal and Placental Lipids
Higher maternal glycemia and BMI are associated with increased maternal plasma lipids, which may increase the risk of fetal macrosomia [,] despite regulation of transplacental lipid transfer by placental lipid transport and metabolism []. Independently of fatty acid supply, maternal glycemia and BMI may also separately influence placental lipid composition to affect pregnancy outcomes in a sex-dependent manner. Maternal obesity is associated with increased placental uptake of radio-labeled OA in females but decreased uptake in males []. However, most placental lipidomic studies to date have not considered sex, making it difficult to explore the extent to which sex-dependent differences in placental lipid metabolism might be implicated in pathophysiological processes.
1.3. Aims and Hypotheses
In this study, we aimed to measure the incorporation of 13C-PA and 13C-OA into lipids in cultured placental villus explants. We hypothesized that there are sex-dependent differences specifically in the placental processing of PA and OA, independent of maternal fatty acid supply and fetal utilization. We also hypothesized that maternal glycemia and BMI associate with placental PA and OA metabolism in a sex-dependent manner and that such sex-dependent alterations are linked with birthweight.
2. Results
2.1. Placental Production of Fresh 13C-PA- and 13C-OA-Containing Lipids Quantified in Explant Tissue and in Conditioned Media
Stable isotope-labeled 13C-PA and 13C-OA were taken up and metabolized by placental explants into stable isotope-labeled lipids as well as being funneled into other metabolic processes such as elongation (the addition of carbons to a fatty acid chain) or B-oxidation (the removal of carbons from fatty acid chains for use as fuel) (graphical abstract and Figure 1). Quantifying the amount of each individual 13C-PA or 13C-OA metabolite in placental explants by LCMS thus enabled the metabolic capacity of different placentas to be compared.
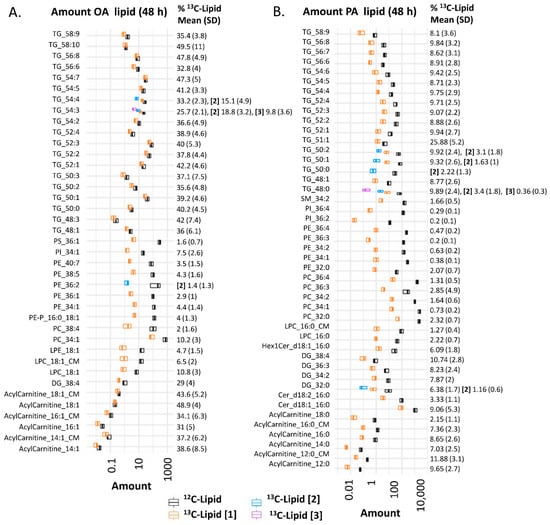
Figure 1.
Amount of lipid in placental explants (n = 22) incubated in 100 µM 13C-OA (A), or 100 µM 13C-PA (B) for 48 h. Boxplots (median and interquartile range) show the amount (pmol lipid/mg of dry explant) of 13C fatty acid-labeled lipids (orange: mono-labeled [1], light blue: di-labeled, purple: tri-labeled [3]) and endogenous 12C lipids (black) in placental explants and in conditioned media (CM). x-axis is log10 scaled. Numbers to the right show percentage of each lipid labeled with 13C out of the total of that specific lipid (100*Amount 13C lipid/total amount of 13C + 12C of corresponding lipid) alongside standard deviation (SD). Bold square brackets show percentage of each particular lipid di- [2] or tri- [3] labeled with 13C-PA or 13C-OA. Abbreviations; DG: diacylglycerol, Cer: ceramide, Hex: hexose, LPC: lysophosphatidylcholine, LPE: lysophosphatidylethanolamine, OA: oleic acid, PA: palmitic acid, PC: phosphatidylcholine, PE-P: phosphatidylethanolamine-plasmalogen, TG: triacylglycerols.
13C-PA and 13C-OA were incorporated into freshly produced stable-isotope-labeled glycerolipids (triacylglycerol (TG), diacylglycerol (DG)) and phospholipids (phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylethanolamine-plasmalogen (PE-P), phosphatidylinositol (PI), lysophosphatidylcholine (LPC), lysophosphatidylethanolamine (LPE)) (Figure 1 and graphical abstract). 13C-PA was also incorporated into sphingolipids including ceramides (Cer) and sphingomyelins (SM). Both 13C-PA and 13C-OA were metabolized into acylcarnitines and acylcarnitine beta oxidation products, and 13C-PA was elongated into a 13C16 labeled acylcarnitine 18:0. Incorporation of one stable isotope labeled fatty acid was most common, but several di- or tri-labeled lipids were also detected. 13C-PA- and 13C-OA-labeled lysophospholipids and acylcarnitines were also found in the conditioned explant media at both 24 and 48 h. Since other labeled lipids were not found in media, even those abundant in placental explants, it seems likely that these lipids are specifically secreted/exported by the placental explants.
The proportion of phospholipids that contained a labeled 13C (amount of 13C lipid/amount of 13C lipid plus matching endogenous 12C lipid) was much lower (12% for 13C-OA phospholipids as a class, <3% for 13C-PA phospholipids as a class) than the proportion of 13C- TGs, DGs and acylcarnitines (30–50% for 13C-OA lipids, 7–26% for 13C-PA lipids). This suggests that the turnover and replacement of glycerolipids and acylcarnitines is much faster than phospholipids, consistent with previous findings [].
2.2. Explants from Female Placentas Incorporate More 13C Lipids than Those from Male Placentas
Placental explants from females contained more of almost all 13C-OA lipids at 24 and 48 h, and 13C-PA lipids at 24 h of incubation (Figure 2A,B). That this sex difference was statistically significant for most lipids in most lipid classes suggests that sex-dependent events have a large impact on upstream processes such as fatty acid uptake or activation early during culture, leading to increased synthesis of diverse lipid classes. That an upstream process is involved is also supported by our finding that sex differences in the 13C-PA lipids are more apparent at 24 h than 48 h, since uptake and other related processes will become less rate limiting over time.
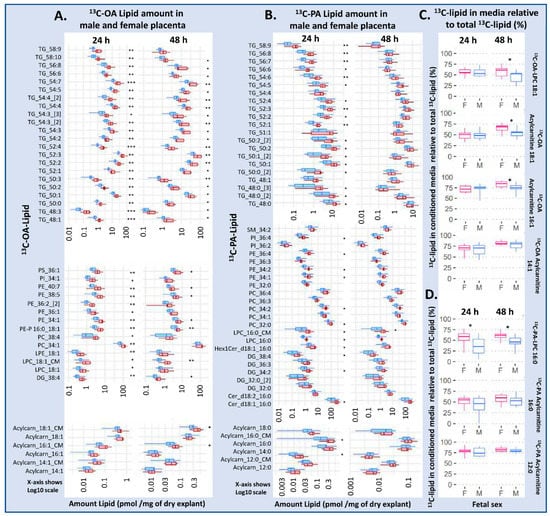
Figure 2.
Differences in amount of lipid in placental explants from males (blue) and females (red) treated with 100 µM 13C-OA (A), or 100 µM 13C-PA (B) for 48 h. (C,D) The percentage of labeled lipid found in media relative to the total amount of the specific 13C lipid in the media and explant, in males and females. Boxes and whiskers represent median, interquartile range and minimum and maximum. X axis is log10 scaled. Statistically significant differences between sexes following Benjamini–Hochberg correction for multiple testing * p < 0.05, ** p < 0.01, *** p < 0.001. Abbreviations, Cer: ceramide, DG: diacylglycerol, Hex1Cer: hexosylceramide, LPC: lysophosphatidylcholine, LPE: lysophosphatidylethanolamine, OA: oleic acid, PA: palmitic acid, PC: phosphatidylcholine, PE-P: phosphatidylethanolamine-plasmalogen, TG: triacylglycerols. F: female, M: male.
Conditioned media incubated with 13C-OA and explants from female placentas contained more 13C-OA LPC at 24 and 48 h, and more acylcarnitine 18:1 and 16:1 at 48 h compared with male cases. Meanwhile conditioned media from 13C-OA incubations had more 13C-PA LPC at 24 and 48 h, and more 13C-PA-acylcarnitine 16:0 at 24 h in female than in male cases. Further, the proportion of newly synthesized 13C lipids found in conditioned media compared to explants (amount in media/amount in media + amount in explant) was also higher in female cases, particularly for 13C-PA LPC, 13C-OA LPC, 13C-OA acyl carnitine 18:1 and 13C-OA acyl carnitine 16:1 (Figure 2C,D), suggesting that increased export occurs on top of the increased synthesis in females. Sex differences in lipid amount in placental explants and conditioned media remained similar after adjusting for maternal BMI or glycemia.
2.3. Placental Production of 13C Lipids Associated with Maternal Characteristics
We then examined relationships between amount of 13C lipid and maternal glycemia (fasting and 2 h post-load in a mid-gestation oral glucose tolerance test (OGTT)) or maternal BMI (Figure 3 and Figure 4). Of these measures, maternal 2 h glycemia (the time-point at which glycemia is most commonly elevated and fulfilling the criteria for gestational diabetes in our population [,]) showed the greatest number of associations with the amount of placental 13C-OA and 13C-PA lipids.
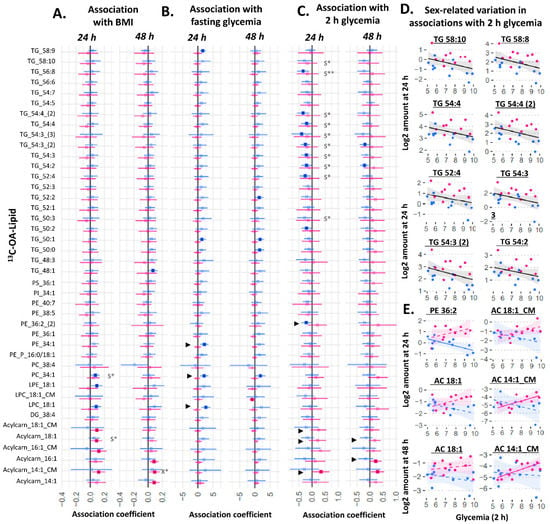
Figure 3.
Sex-stratified associations between the amount of 13C-OA lipid (outcome) and maternal glycemia or maternal BMI (predictor). X* represents p < 0.05 in linear regression with males and females combined (not shown). S* represents p < 0.05, while S** represents p < 0.01 in linear regression adjusted for sex (not shown). ► represents significant p < 0.05 for the interaction between sex and maternal glycemia on lipid amount. Linear regression was then run separately for males and females. Forest plots (A–C) show association coefficient and 95% confidence intervals for males (blue) and females (red) with significance (p < 0.05) shown by dark-colored filled squares. Unadjusted scatter plots illustrating examples of the relationship between 2 h glycemia and amount of lipid for (D) lipids where males and females show similar relationships and for (E) lipids where males and females show different relationships. The Benjamini–Hochberg method was used to correct for multiple testing. Abbreviations: Acylcarn and AC: acylcarnitine, DG: diacylglycerol, LPC: lysophosphatidylcholine, LPE: lysophosphatidylethanolamine, OA: oleic acid, PA: palmitic acid, PC: phosphatidylcholine, PE-P: phosphatidylethanolamine-plasmalogen, TG: triacylglycerols.
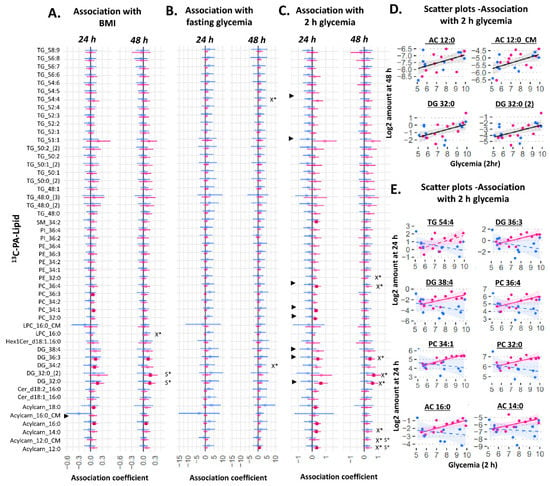
Figure 4.
Sex-stratified associations between the amount of 13C-PA lipid (outcome) and maternal glycemia or maternal BMI (predictor). X* represents p < 0.05 in linear regression with males and females combined (not shown). S* represents p < 0.05 in linear regression adjusted for sex (not shown). ► represents significant p < 0.05 for the interaction between sex and maternal glycemia on amount of lipid. Linear regression was then run separately for males and females. Forest plots (A–C) show association coefficient and 95% confidence intervals for males (blue) and females (red) with significance (p < 0.05) shown by dark-colored filled squares. Unadjusted scatter plots illustrating the relationship between 2 h glycemia and amount of lipid for (D) lipids where males and females show similar relationships and for (E) lipids where males and females show different relationships. The Benjamini–Hochberg method was used to correct for multiple testing. Abbreviations: Acylcarn and AC: Acylcarnitine, DG: diacylglycerol, Cer: ceramide, Hex: hexose, LPC: lysophosphatidylcholine, LPE: lysophosphatidylethanolamine, OA: oleic acid, PA: palmitic acid, PC: phosphatidylcholine, PE-P: phosphatidylethanolamine-plasmalogen, TG: triacylglycerols.
Among 13C-OA lipids, amounts of 13C-OA-TGs at 24 h were generally negatively associated with 2 h glycemia, which was significant for ten of them after adjusting for fetal sex (the combined analyses with males and females not shown, but lipids involved are indicated by *S in Figure 3C,D). In relation to glycemia, there were sex differences in its association with some phospholipids and acylcarnitines, including acylcarnitines found in conditioned media (significant sex*fasting-glycemia or sex*2 h-glycemia interactions are indicated by “►” in Figure 3). In sex-stratified analyses, female cases generally displayed a positive association for multiple 13C-OA-acylcarnitines with 2 h glycemia, while in males, more negative trends for TGs and the phospholipid 13C-OA-PE 36:2 were seen with increasing 2 h glycemia (Figure 3C), and positive associations for phospholipids (PE 34:1, PC 34:1, LPC 18:1) were observed with fasting glycemia (Figure 3B). Increasing divergence between males and females in the amount of these lipids were observed with increasing 2 h glycemia (Figure 3E). Overall, maternal BMI was positively associated with only 13C-OA acylcarnitine 18:1 and 13C-OA PC 34:1 after adjusting for sex, with no interactions observed between sex and BMI on 13C-OA lipids.
Among 13C-PA lipids, there was a general increase in the amount of 13C-PA-DGs (DG 32:0 (mono-labeled and di-labeled), and DG 36:3) and 13C-PA phospholipids (significant for the two most abundant phospholipids 13C-PA-PE 32:0 and PE 36:4) in association with increasing maternal 2 h glycemia at 48 h of culture (X* in Figure 4C,D). Further, there was a positive association with several 13C-PA derived acylcarnitines (13C-labeled-acylcarnitine 14:0 (explant) and 12:0 (explant and media) derived from beta-oxidized 13C-PA). Fasting glycemia also showed a general trend of positive associations with 13C-PA lipids, which were significant for DG 34:2 and TG 54:4 at 48 h. These associations remained similar after adjusting for fetal sex. However, significant interactions between sex and 2 h glycemia were observed for several 13C-PA lipids at 24 h. Sex-stratified regression models showed generally positive associations between 13C-PA lipids and 2 h glycemia in female, but not in male cases (Figure 4C,E). In females, significantly positive associations with 2 h glycemia were seen with the amount of four 13C-PA-derived acylcarnitines, two 13C-PA DGs, three 13C-PA phospholipids and one 13C-PA sphingolipid. Similar to 13C-OA lipids, these sex differences also showed increasing divergence with increasing 2 h glycemia (See selected scatter plots in Figure 4E). Maternal BMI was positively associated with only 13C-PA DG 32:0 (both mono and di-labeled) after adjusting for sex. Even though there were no significant interactions between sex and BMI, increases in the amount of 13C-PA DGs and 13C-PA-acylcarnitine with increasing BMI were more apparent in females.
2.4. Birthweight Centile Is Associated with Placental 13C-OA Lipids and 13C-PA Lipids in Female Cases
We then investigated whether variations in the amount of explant or conditioned media 13C-OA or 13C-PA lipids could be associated with birthweight centile (standardized for gestational age using local reference). In a combined model including males and females, there was no association between any explant 13C-PA or 13C-OA lipids and birthweight centile at either 24 or 48 h. Adjusting for fetal sex, glycemia or BMI did not alter these results. However, two acylcarnitine species exported into the media at 48 h (13C-OA-acylcarnitine-14:1-CM and 13C-PA-acylcarnitine-12:0-CM) were positively associated with birthweight centile. Since significant interactions were found between sex and several 13C-OA or 13C-PA lipids on birthweight centile, sex-stratified analyses were conducted. Among female cases, generally negative associations were observed for 13C-OA-TGs at 24 h and positive associations for 13C-OA and 13C-PA acylcarnitines at 48 h. In contrast, few lipid species showed associations with birthweight in male cases.
Several other lipids also showed sex-divergent associations with birthweight (represented by ► in Figure 5 where there was significant interaction sex*amount of lipid on birthweight centile): 13C-OA-LPE showed a positive trend in males but negative in females; 13C-PA-SM 34:2, 13C-PA-DGs, and 13C-PA Cer-d18:2/16:0 showed negative trends in males but positive in females. This suggests that sex-dependent placental lipid metabolism may also play a part in explaining sex-dependent perinatal outcomes such as birthweight. Of note, the positive association coefficient between 13C-PA lipids and birthweight centile in female cases were particularly high for 13C16-PA acylcarnitine 16:0 (the major 13C-PA-derived acylcarnitine; estimate (95% CI) 53 (41–65) birthweight percentile units/(Log-2 pmol/mg)). This is equivalent to 40 birthweight percentile units per SD increase in 13C16-PA acylcarnitine 16:0.
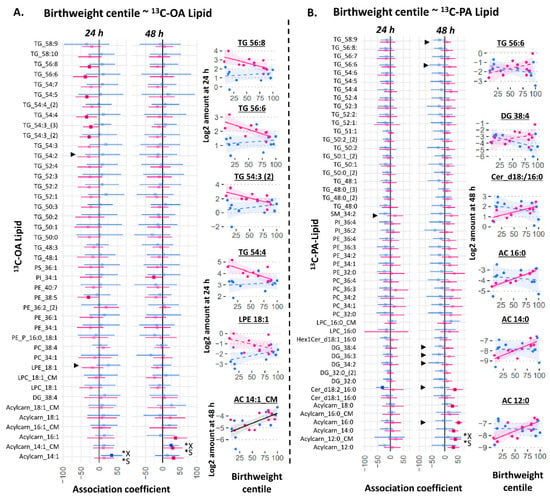
Figure 5.
Sex-stratified associations between birthweight centile (outcome) with the amount of placental 13C-OA lipid (A) or 13C-PA lipid (B) after 24 or 48 h of culture. Birthweight centile was standardized for gestational age by local references. X* represents p < 0.05 in linear regression with males and females combined (not shown). S* represents p < 0.05 in linear regression adjusted for sex (not shown). ► represents significant p < 0.05 for the interaction between sex and amount of lipid on birthweight centile. Linear regression was then run separately for males and females (shown in figure). Forest plots (A,B) show association coefficient and 95% confidence intervals for males (blue) and females (red) with significance (p < 0.05) shown by dark-colored filled squares. Unadjusted scatter plots illustrating the relationship between birthweight centile and amount of lipid. The Benjamini–Hochberg method was used to correct for multiple testing. Abbreviations: Acylcarn and AC: Acylcarnitine, DG: diacylglycerol, Cer: ceramide, Hex: hexose, LPC: lysophosphatidylcholine, LPE: lysophosphatidylethanolamine, OA: oleic acid, PA: palmitic acid, PC: phosphatidylcholine, PE-P: phosphatidylethanolamine-plasmalogen, TG: triacylglycerols.
Many of the 13C-OA and 13C-PA acylcarnitine species associated with birthweight centile in females were also positively associated with maternal glycemia (13C-OA-derived acylcarnitine 14:1-CM, 16:1 and 13C-PA-derived acylcarnitine 12:0, 14:0, 16:0) or BMI (13C-OA-derived acylcarnitine 14:1, 14:1-CM, 16:1 and 13C-PA-derived acylcarnitine 16:0). Thus, the increase in placental acylcarnitine synthesis appears to reflect key events by which higher maternal glycemia or BMI may lead to greater fetal growth.
2.5. The Relationship between mRNA Expression of Placental Fatty Acid Uptake Genes with Sex and Placental OA and PA Lipid Metabolism
Since most 13C-PA and 13C-OA lipids were higher in females than males, we hypothesized that these variations in placental lipid abundance could be due to sex differences in the expression of genes responsible for fatty acid uptake. However, when we investigated the placental mRNA abundance of fatty acid transport genes (SLC27A 1,2,3,4,6 and CD36), fatty acid binding proteins (FABP 3,4,5) or acyl CoA synthetases (ACSL 1,3,4,5,6) we found no significant difference in the relative expression levels in males compared with females (Figure 6).
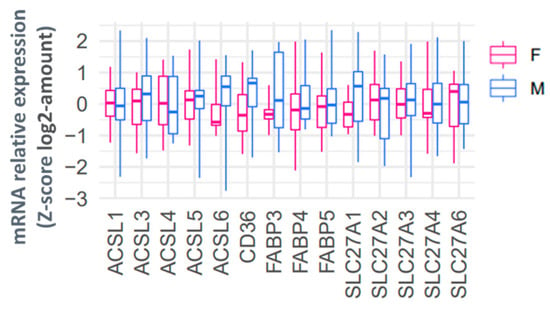
Figure 6.
Relative placental mRNA expression of genes involved in fatty acid uptake in females (red) and males (blue). mRNA abundance is normalized to the geometric mean of three housekeeping genes and then log2 transformed and converted to a z-score. Boxes show median and interquartile range while whiskers show minimum and maximum values.
However, our results suggest that the relative mRNA expression of these fatty acid uptake genes plays a significant role in regulating the fresh synthesis of specific placental lipids, with some sex differences. The relative mRNA expression of placental fatty acid uptake genes was generally positively associated with increased 13C-PA lipids at 48 h (Figure 7, *X), especially for ACSL3 (two DGs), ACSL4 (26 lipids), SLC27A2 (14 lipids), and SLC27A6 (three lipids). In sex-stratified analyses, the abundance of many 13C-PA lipids was positively associated with ACSL3, ACSL4, ACSL6, FABP5, SL27A1, SL27A2 and SL27A3 mRNA in females, while positive associations were only seen with ACSL4 mRNA in males. In contrast, mRNA expression of placental fatty acid uptake genes was generally not positively associated with 13C-OA lipids apart from SLC27A2 and 13C-OA TG 58:9 (Figure 8, *X). Instead, there were numerous significant negative associations seen for CD36 (PC 38:4), ACSL1 (four acylcarnitines), FABP3 (six lipids), SL27A1 (three lipids), SLC27A3 (PC 38:4). In sex-stratified analyses, ACSL3 mRNA was positively associated with 13C-OA acylcarnitines (three lipids) while SLC27A2 mRNA was positively associated with six 13C-OA TGs in females. Meanwhile in males, negative associations were observed between transcripts of FABP3, FABP4, and SLC27A2 with 13C-OA lipids.
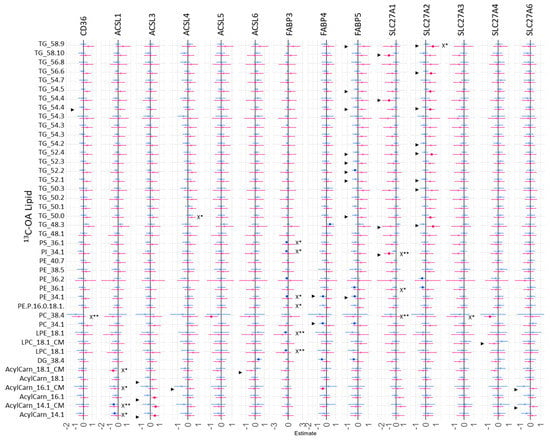
Figure 7.
Sex-stratified associations between the amount of 13C-OA lipids (outcome) with relative mRNA abundance after 48 h of culture. Lipid amount was log2 transformed, mRNA abundance was log-2 transformed and z-scored. Linear regression with males and females combined (not shown) with significance p < 0.05 is indicated by X*, whilst significance p < 0.01 is indicated by X**. Interaction between sex*relative mRNA abundance on lipid amount were then investigated with significant interactions p < 0.05 denoted by “►”. Linear regression was then run separately for males and females. Forest plots show association coefficient and 95% confidence intervals for males (blue) and females (red) with significance (p < 0.05 shown by dark-colored filled squares. The Benjamini–Hochberg method was used to correct for multiple testing.
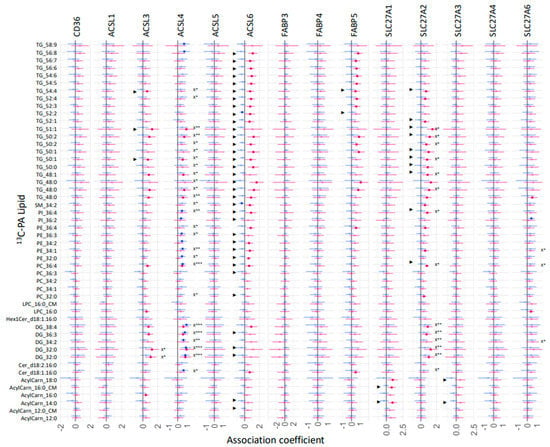
Figure 8.
Sex-stratified associations between the amount of 13C-PA lipids (outcome) with relative mRNA abundance after 48 h of culture. Lipid amount was log2 transformed, mRNA abundance was log-2 transformed and z-scored. Linear regression with males and females combined (not shown) with significance p < 0.05 is indicated by X*, whilst significance p < 0.01 or 0.001 is indicated by X** and X***. Interaction between sex and maternal glycemia on lipid amount were then investigated with significant interactions p < 0.05 denoted by “►”. Linear regression was then run separately for males and females. Forest plots show association coefficient and 95% confidence intervals for males (blue) and females (red) with significance (p < 0.05 shown by dark-colored filled circles. The Benjamini–Hochberg method was used to correct for multiple testing.
3. Discussion
3.1. Main Findings
The metabolism of the two most abundant fatty acids, OA and PA, by the placenta is strongly sex-dependent, and alters in response to variations in the in utero environment imposed by increasing maternal glycemia and BMI. In vitro, female placenta produced more 13C-OA- and 13C-PA-labeled lipids from every measured lipid class, suggesting that female placenta have generally greater capacity for fatty acid uptake and production. However, female placenta did not show increased mRNA expression of any of the tested placental fatty acid transporters or fatty acid CoA activation enzymes (a necessary step preceding lipid synthesis). Thus, increases in lipid production in females instead likely involve regulation at a post-transcriptional level such as increases in protein expression, activity or other yet-to-be-elucidated processes. Sex-dependent differences in OA and PA processing may lead to greater placental fat storage and fetal supply in females, consistent with findings of greater percentage body fat in female neonates than males []. It also supports the idea that females are more protected against sub-optimal nutritional supply such as in conditions of uteroplacental insufficiency, as well as in gestational diabetes and obesity, as reflected by lower perinatal morbidity and mortality compared with their male counterparts []. In females, but not males, we found positive associations between placental acylcarnitine metabolism and maternal glycemia, BMI, and birthweight. We postulate that regulation of placental beta-oxidation plays an important role in enabling female fetuses to better adapt to challenging in utero conditions, and they therefore display lower perinatal morbidity and mortality.
3.2. Sex Dependent Differences in Placental Lipid Metabolism
A previous ex vivo study had suggested that female placenta contained more endogenous un-esterified OA, OA-TGs, OA-acylcarnitines and more acylcarnitines of OA-beta-oxidation products than males []. Our in vitro findings of increased production of almost all OA and PA lipids in the placental explants of females compared to males suggest that such differences are a direct result of sex-dependent differences in placental lipid processing, rather than differences in maternal fatty acid supply or in fetal utilization. Such differences in placental lipid processing also likely explain the sex difference in potential lipid supply to the fetus, where venous cord blood (which contains nutritional supplies and signals picked up from the placenta for transport to the fetus) from females contains more un-esterified fatty acids (including saturated and mono-saturated fatty acids), phospholipids and glycerolipids than males [].
Many tissues demonstrate sex differences in fatty acid uptake, postulated to be due to differences in the expression and activity of genes involved in fatty acid uptake. For example, female mouse heart and rat liver show greater fatty acid uptake compared to males [,,,,,], and females show greater rat liver protein expression of FABP [,,] and greater CD36 mRNA expression in several human and rat tissues [,,]. Our findings suggest that in the placenta, sex differences in lipid abundance are not the result of simple increases in the mRNA expression of ACSL, FABP or SLC27 gene families. Instead, differences are likely due to augmentation in protein expression or activity of these genes, alterations in the operation of other sex-specific fatty acid transport mechanisms and other downstream lipid metabolic processes, or differences in the regulatory importance of fatty acid uptake compared to other lipid synthetic or catabolic processes. This is evidenced by the positive association between 13C-PA lipids with ACSL3, ACSL6, FABP5 and SLC27A2, and 13C-OA lipids with ACSL3 and SLC27A2 in females, but a negative association between 13C-OA lipids with FABP3 and FABP4 in males.
Our own PA results and many other studies suggest that FABP enhances transport of saturated fatty acids into cells, and this is thought to be because FABPs facilitate the transfer of fatty acids across aqueous cellular regions []. However, FABP can also assist in fatty acid export [,] as well as in binding to and thus regulating the activity of signaling molecules such as hormones, lipids and eicosanoids [,]. Our findings of negative associations between 13C-OA lipids with FABP3 and FABP4 in males may suggest that the aforementioned non-fatty-acid-uptake processes may play an important role. This would be consistent with previous reports that increasing the expression of FABP2 (I-FABP) in H141 cells decreased 3H-oleic acid uptake and its incorporations into phospholipids [], suggesting net efflux, while FABP4 null mice show both increased OA uptake and increased OA efflux [].
3.3. The Influence of Maternal Glycemia and BMI on Placental OA and PA Lipid Production
In the human in vivo study where 13C-PA was administered alongside other 13C-fatty acids, GDM was associated with increased total placental 13C-PA lipid [], consistent with our finding of generally positive associations between the amount of placental 13C-PA lipids with 2 h glycemia. The levels of endogenous placental non-esterified palmitic acid are also increased in GDM compared to controls []. Obesity reportedly had no influence on total 13C-OA placental lipid production in vitro [] and GDM did not associate with any change in 13C-OA placental lipids in vivo [], consistent with our own results showing no association between BMI or glycemia and 13C-OA placental lipids when sex was not considered.
While most existing literature does not report how glycemia might influence placental lipids in males and females separately, there are a few reports on sex differences in the influence of maternal BMI on placental OA lipids. One study reported a positive association between obesity and placental uptake of radio-labeled OA in females but a negative association in males []; placental lipid production was not quantified. Although we found that BMI was positively associated with 13C-OA and 13C-PA acylcarnitines in females, it seems unlikely that a general increase in fatty acid uptake would only increase acylcarnitines and not other lipid classes. Furthermore, we found that 13C-OA LPC, LPE and PC 34:1 positively associated with BMI in male cases, suggesting that the sex-dependent effects also demonstrate lipid-class specificity and predominantly occur in activities downstream from the fatty acid uptake process.
Greater serum LPC species have previously been reported to play a more important role in decreasing insulin sensitivity in adult males compared to adult females (2). Fatty acids meanwhile are bound to carnitine to form acyl-carnitines to facilitate their transport across the inner mitochondrial membranes, enabling beta oxidation and the utilization of fatty acids for energy []. Beta oxidation, similar to other alternative fuel pathways including glycolysis, polyol and pentose-phosphate pathways, ketogenesis and ketone oxidation, is important in the placenta, particularly as it is vulnerable to hypoxia exposure (e.g., with reduced maternal blood flow to the placental bed in situations such as regular uterine contractions in parturition, circulatory diversions to other maternal organs, maternal aortocaval compression) or to situations where the TCA cycle cannot easily be completed [,,,,]. Acylcarnitines also act as signaling molecules and as sources of activated fatty acid for lipid remodeling and protein palmitoylation [,,]. Thus, our findings of increased levels of acylcarnitines with increasing maternal 2 h glycemia and BMI in females, but not males, may represent a sex-specific adaptive strategy that promotes survival of female offspring in the face of in utero adversity. Increased beta oxidation could also reduce placental free-unesterified PA, which has lipotoxic effects in trophoblasts [,]. Increased beta oxidation could provide an alternative fuel source for the fetus if glucose utilization is impaired as observed with insulin resistance, hyperglycemia and obesity in adults [,,].
Our findings of increased OA-acylcarnitines in explants with increasing BMI in females contrast with the reduced endogenous OA-related acyl-carnitines (18:1, 16:1, 14:1) and free carnitine found in placental microvillous membrane (MVM) isolates [] from obese white Caucasian, non-GDM women carrying female compared to male offspring. Aside from the obvious experimental and population differences, this may reflect reduced carnitine availability in obesity rather than altered placental OA metabolism.
Female-specific associations of 13C-PA DGs with glycemia (2 h) and BMI and between 13C-OA LPC and LPE with maternal BMI may also suggest that alterations in these important lipid signaling molecules [,,,,] may also be part of a sex-specific adaptive strategy. Placental DGs are known to induce the release of human chorionic gonadotropin and placental lactogen [,,] and stimulate the production of progesterone [].
3.4. Placental Lipids and Birthweight
Our findings of sex-dependent associations between newly synthesized placental PA and OA lipids and birthweight centile suggest the involvement of placental lipid metabolism in the sex-specific regulation of fetal growth (Figure 9). Associations with birthweight were mainly observed in females, with positive associations seen for OA and PA acylcarnitines and a PA ceramide, and negative associations for OA phospholipids and OA-TGs.
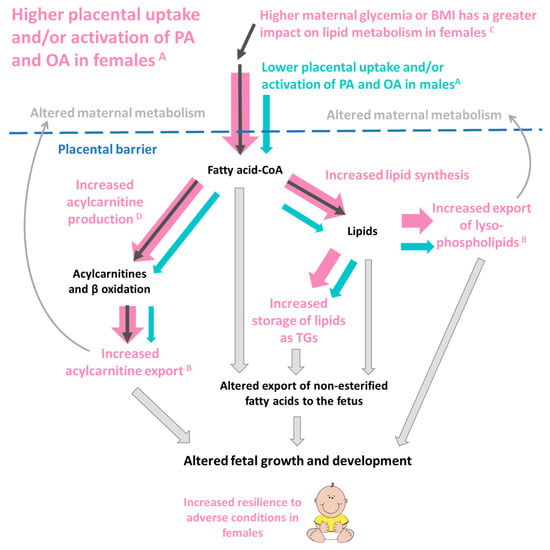
Figure 9.
A postulation of how the sex-dependent placental metabolism of palmitic acid (PA) and oleic acid (OA) may influence offspring outcomes. A Female placenta produce more OA and PA labeled lipids from every measured lipid class, suggesting a greater capacity for fatty acid uptake and activation. B Female placenta also export a higher proportion of lysophospholipids and acylcarnitines compared to males. C In females, but not males, increases in maternal glycemia or BMI are associated with increases in placental PA and OA acylcarnitines and increases in PA lipids generally. In females, increased PA and OA acylcarnitines D are associated with increased birthweight. Thus, increased placental fatty acid uptake and incorporation in females (represented by the fatter pink arrows) could influence placental lipid reservoirs, local placental lipid activity, maternal–fetal lipid transfer and placental signaling to both fetus and mother. Placental lipid processing could influence maternal and fetal metabolism through both direct and indirect lipid-mediated pathways and hence impact fetal development.
Ceramide d18:2_16:0 is one of most abundant PA lipids in the placenta and was positively associated with birthweight in females but was negatively associated in males. A previous study had found increased ceramide in placenta from normal-weight fetuses compared to those with intra-uterine growth restriction (IUGR), consistent with our current findings in females []. Ceramides likely affect fetal growth by acting as signaling molecules, decreasing the expression of nutrient transporters [,,], and regulating placental inflammation, insulin signaling, AKT activation, and amino acid transport [,,,,,,]. Negative associations of birthweight centile with OA-TGs in female cases, meanwhile, might indicate reduced sequestration of OA into TG storage, making it more available for transfer to the fetus, especially since cord plasma OA has previously been linked with increased birthweight [].
Umbilical cord blood acylcarnitines are associated with being small or large for gestational age relative to appropriately grown controls [,,,]; however, there have been no studies of the role of placental acylcarnitines. We found that conditioned media showed increased 12:0 and 14:1 acylcarnitines in association with birthweight in females. Placentally synthesized acylcarnitines are known to be transferred to the fetus [,,] as metabolic fuel as a source of carnitine and as signaling molecules potentially regulating insulin action and adiposity [,,]. We speculate that in growth restriction, placental export of acylcarnitines acts mainly as an alternative fuel source to promote fetal survival, but with maternal hyperglycemia and obesity, increased placental acylcarnitines may also act as signals to limit excessive growth and hence perinatal morbidity and mortality.
3.5. Strengths and Limitations
Our targeted LCMS method allowed us to separately interrogate the metabolism of almost 100 different stable isotope-labeled lipids, enabling us to explore the importance of many different lipidomic pathways. Our study was able to quantify not only 13C-PA and 13C-OA acylcarnitine but also the acylcarnitine derivatives of 13C-PA and 13C-OA after beta oxidation and elongation, enabling us to trace PA and OA through several cycles of beta-oxidation, which was not possible in previous studies. Quantification of 13C16-labeled acylcarnitine 18:0 further enabled us to trace the elongated PA into stearic acid (18:0), the first documented evidence of placental PA elongation despite the placenta being known to express several elongases []. We were also able to quantify the export of 13C-acylcarnitines and lysophospholipids into conditioned media. However, placental explant-type experiments cannot indicate whether this represents export to the mother or to the fetus. Stable isotope-labeled placental explant experiments are difficult to perform on large numbers of placentas, limiting our ability to explore the effects of multiple clinical factors simultaneously or to explore mediation effects. As only two cases were treated with insulin, we were unable to assess how insulin might impact placental OA and PA lipid metabolism.
4. Materials and Methods
4.1. Placental Collection
Placenta were collected with written informed consent from 22 women recruited at the National University Hospital, Singapore. Placentas were collected from non-smoking mothers who delivered by elective Caesarean section after 37 weeks’ gestation and who underwent a three-time-point 75 g oral glucose tolerance test (OGTT) at mid-gestation. Cases were matched for first trimester BMI and GDM (WHO 2013 criteria) to ensure a balance of cases across the sexes (Table 1).

Table 1.
Clinical characteristics of study population.
Cases of known pre-existing diabetes mellitus or possible pre-existing diabetes diagnosed in pregnancy (defined by antenatal OGTT results of fasting glycemia ≥ 7.0 mmol/L or 2 h glycemia ≥ 11.1 mmol/L) were excluded. Researchers were blinded to the clinical characteristics of the women and neonates involved until the completion of all explant culture experiments. Of the women with GDM, nine were diet controlled and only two were insulin treated.
4.2. Placenta Collection and Placental Explant Culture
Villous placental explants were cultured as previously described []. Briefly, five pieces of villous placental tissue were biopsied from five different sites across the placenta. These biopsies were then cut into small explants (approximately 3 mm2) and added to each experimental well, with one explant coming from each different placental biopsy. All placental processing was finished within two hours of delivery. Explants were cultured in 12-well plates (NunclonTM Delta surface, Thermofisher, Waltham, MA, USA) in serum-free CMRL media (1.8 mL, GIBCO 1066-L Glutamine, Thermofisher, Waltham, MA, USA) containing 5 mM glucose, P5030, Biowest, Nuaillé, France) with 1.5% BSA (HI Clone fraction V, Culture grade, pH 7.00 lyophilized powder, Cytiva, Marlborough, MA, USA) and either 13C16 PA (100 µM) or 13C18 OA (100 µM). Stable isotope-labeled fatty acids (>99 atom % 13C, >99% (CP)) were purchased from Sigma Aldrich (Saint Louis, MO, USA). Triplicate wells were run for each condition. Levels of PA and OA in maternal blood are highly variable, and those available for placental uptake in vivo during pregnancy are unknown since these fatty acids also originate from esterified plasma lipids. We therefore chose to add 100 µM to represent levels around those of maternal blood and cord blood [,], a concentration that was high enough to enable quantification of a range of 13C-labeled placental lipids in both placental explants and conditioned media.
Explants were incubated at 37 °C in a humidified atmosphere of 5% CO2/air for 24 or 48 h. Explants were then harvested with triplicates combined in a pre-weighed Omnitube (Waltham, MA, USA). Explants were washed with PBS (1 mL) and excess PBS removed after centrifugation. Explant media from triplicate wells (800 µL from each) were combined into an Eppendorf tube, allowed to settle for 5 min then clear supernatant (1 mL) transferred into a fresh Eppendorf. Samples were stored at −80 °C until lipid extraction.
4.3. RNA Extraction, cDNA Synthesis and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA from frozen placental biopsies was extracted using phenol–chloroform method and further purified using the RNeasy Mini Kit (Qiagen, Hilden, Germany) following manufacturer’s instructions. RNA concentrations and quality were then determined by using a Nanodrop ND-1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Placental RNA was reverse transcribed to cDNA with Superscript III reverse transcriptase (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. To determine mRNA expression of lipid genes, RT-qPCR was performed with 5 ng cDNA in duplicate 10 µL reactions using TaqMan Fast Advanced Master Mix (Thermo Fisher Scientific, Waltham, MA, USA) on the Applied Biosystems 7500 Fast Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA) under the fast settings (95 °C for 20 s, followed by 45 cycles of 95 °C for 3 s and 60 °C for 30 s). Inventoried FAM-labeled TaqMan probes (Thermo Fisher Scientific, Waltham, MA, USA) were used for 3 housekeeping genes—CYC1 (cytochrome C1, Hs00357718_m1), SDHA (succinate dehydrogenase complex, subunit A, Hs00188166_m1) and TBP (TATA-box binding protein, Hs00427620_m1); 5 ACSL family genes—ACSL1 (Hs00242530_m1), ACSL3 (Hs00244853_m1), ACSL4 (Hs00244871_m1), ACSL5 (Hs01061754_m1) and ACSL6 (Hs00922295_m1); 3 FABP family genes—FABP3 (Hs00997360_m1), FABP4 (Hs01086177_m1) and FABP5 (Hs02339439_g1); 5 SLC27A family genes—SLC27A1 (Hs01587911_m1), SLC27A2 (Hs01113391_m1), SLC27A3 (Hs00225680_m1), SLC27A4 (Hs00192700_m1) and SLC27A6 (Hs00204034_m1) as well as CD36 (Hs00354519_m1). Relative expression of each gene was then calculated by the formula 2(−ΔCT) and normalized to the geometric mean of expression of the three control genes [].
4.4. Lipid Quantification by LCMS and Statistical Analysis
Lipids were extracted and analyzed by LCMS as described in Supplementary S1–S3. Placental lipid content was expressed as pmol lipid in explant/mg dry weight of explant, and lipid concentration in conditioned media was expressed as pmol lipid in media/mg dry weight of explant. For statistical analysis, lipid amounts were log2 transformed (to achieve an approximately normal distribution). Linear regression was run for each lipid (outcome: lipid amount at 24 or 48 h) with each variable of interest (predictor: fetal sex, maternal BMI or fasting/post-load 2 h glycemia). Linear regression models were run in R (Vienna, Austria) version "Kick Things" with ‘tidyverse’ Version: 1.3.1 and ‘mediation’ Version: 4.5.0 packages. Where indicated, multiple linear regressions were then performed with mutual adjustments for these variables. Interactions between sex and each maternal factor on lipid amount were observed for multiple lipids; hence, stratified analyses by sex were also conducted for each lipid. Birthweight centile (customized for gestational age using local standards) was also analyzed as an outcome against each lipid (predictor) with interactions observed between sex and lipid amount, leading us to perform sex-stratified linear regression. For all tests, the Benjamini–Hochberg method was used to correct for multiple testing of lipids and time-points to minimize false discovery with statistical significance set at a two-sided alpha level of p < 0.05.
5. Conclusions
Our study suggests that placental lipid metabolism is dependent on fetal sex. Female placentas produce more PA and OA lipids, and the stage at which such sex-dependent regulation occurs appears to be downstream of the transcriptional regulation of placental fatty acid uptake genes. Furthermore, fetal sex influences the relationship between maternal glycemia and maternal BMI with placental PA and OA lipid metabolism, as well as between placental PA and OA lipid metabolism and birthweight, with associations mainly observed in females rather than in males. We postulate that sex differences in placental lipid processing likely contribute to sex differences in perinatal outcomes. The ability to adapt placental lipid processing to decrease vulnerability to adverse conditions in utero potentially contributes toward the overall lower perinatal morbidity and mortality risk in female offspring.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23158685/s1.
Author Contributions
Conceptualization, S.-Y.C., O.C.W., R.M.L., K.M.G. and A.C.-G.; Methodology, O.C.W., H.E.J.Y., V.K.B.C.-H., A.C.-G. and M.R.W.; Software, O.C.W., H.E.J.Y. and V.K.B.C.-H.; Validation, O.C.W., H.E.J.Y. and V.K.B.C.-H.; Formal Analysis, O.C.W., H.E.J.Y., V.K.B.C.-H. and S.-Y.C., Investigation, O.C.W., R.A.P., P.S., H.E.J.Y., T.K.L.M., N.S., V.K.B.C.-H. and S.-Y.C.; Resources, O.C.W., H.E.J.Y., T.K.L.M., R.A.P., P.S., N.S., A.K.B., A.C.-G., M.R.W. and S.-Y.C.; Data Curation, O.C.W., V.K.B.C.-H., P.S. and H.E.J.Y.; Writing—Original Draft Preparation, O.C.W., H.E.J.Y. and S.-Y.C., Writing—Review and Editing, O.C.W., H.E.J.Y., T.K.L.M., V.K.B.C.-H., R.A.P., P.S., N.S., A.C.-G., A.K.B., K.M.G., R.M.L., M.R.W. and S.-Y.C.; Visualization, O.C.W., V.K.B.C.-H., H.E.J.Y., R.M.L. and S.-Y.C.; Supervision, A.C.-G., A.K.B., K.M.G., R.M.L., M.R.W. and S.-Y.C.; Project Administration, O.C.W., H.E.J.Y., R.A.P., P.S., N.S., A.K.B. and S.-Y.C.; Funding Acquisition, A.C.-G., A.K.B., K.M.G., R.M.L., M.R.W. and S.-Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by a Clinician Scientist Award awarded to S.-Y.C. from the Singapore National Medical Research Council (NMRC/CSA-INV/0010/2016, MOH-CSAINV19nov-0002), by the National University of Singapore, National University Health System Singapore, and the Singapore Institute for Clinical Sciences A*STAR. The Singapore Lipidomics Incubator receives funding from the Life Sciences Institute, the National University of Singapore Yong Loo Lin School of Medicine, the National Research Foundation (grant number NRFI2015-05), and A*STAR (IAF-ICP I1901E0040). K.M.G. is supported by the UK Medical Research Council (MC_UU_12011/4), the National Institute for Health Research (NIHR Senior Investigator (NF-SI-0515-10042) and NIHR Southampton Biomedical Research Centre (IS-BRC-1215-20004)), the European Union (Erasmus+ Programme ImpENSA 598488-EPP-1-2018-1-DE-EPPKA2-CBHE-JP). Funders played no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. Ethical approval was obtained from the National Healthcare Group Domain Specific Review Board (2016/00183).
Informed Consent Statement
Informed written consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank Samantha Grace Loon Magadia, Celes Maria Catherine Dado, and Chen Zhenzhi in coordinating the recruitment of the women involved in this study, the staff of the National University Hospital who kindly assisted with placental collection, and the women who generously donated their placenta for research.
Conflicts of Interest
S.-Y.C. and K.M.G. are part of an academic consortium that has received research funding from Abbott Nutrition, Société Des Produits Nestlé S.A., Danone and BenevolentAI Bio Ltd. for work unrelated to this manuscript. S.-Y.C. and K.M.G. are co-inventors on patent filings by Nestlé S.A., which covers the use of inositol in human health applications, but which do not draw on the work in this manuscript. Godfrey has received reimbursement for speaking at conferences sponsored by companies selling nutritional products. Chan has received reimbursement and honoraria into her research funds from Nestlé S.A. for speaking at a conference. The other authors have no financial or personal conflict of interest to declare.
References
- Voldner, N.; Frey Frøslie, K.; Godang, K.; Bollerslev, J.; Henriksen, T. Determinants of birth weight in boys and girls. Hum. Ontogenet. Int. J. Interdiscip. Dev. Res. 2009, 3, 7–12. [Google Scholar] [CrossRef]
- Roland, M.C.P.; Friis, C.M.; Godang, K.; Bollerslev, J.; Haugen, G.; Henriksen, T. Maternal factors associated with fetal growth and birthweight are independent determinants of placental weight and exhibit differential effects by fetal sex. PLoS ONE 2014, 9, e87303. [Google Scholar] [CrossRef] [PubMed]
- Group, H.S.C.R. Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: Associations with maternal body mass index. BJOG Int. J. Obstetr. Gynaecol. 2010, 117, 575–584. [Google Scholar]
- Clifton, V.L. Review: Sex and the Human Placenta: Mediating Differential Strategies of Fetal Growth and Survival. Placenta 2010, 31, S33–S39. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.G.; Kajantie, E.; Osmond, C.; Thornburg, K.; Barker, D.J. Boys live dangerously in the womb. Am. J. Hum. Biol. 2010, 22, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Villa, P.M.; Laivuori, H.; Kajantie, E.; Kaaja, R. Free fatty acid profiles in preeclampsia. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 17–21. [Google Scholar] [CrossRef]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic acid: Physiological role, metabolism and nutritional implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef]
- Eastman, A.J.; Moore, R.E.; Townsend, S.D.; Gaddy, J.A.; Aronoff, D.M. The Influence of Obesity and Associated Fatty Acids on Placental Inflammation. Clin. Therap. 2021, 43, 265–278. [Google Scholar] [CrossRef]
- Herrera, E. Metabolic adaptations in pregnancy and their implications for the availability of substrates to the fetus. Eur. J. Clin. Nutr. 2000, 54, S47–S51. [Google Scholar] [CrossRef]
- Colvin, B.N.; Longtine, M.S.; Chen, B.; Costa, M.L.; Nelson, D.M. Oleate attenuates palmitate-induced endoplasmic reticulum stress and apoptosis in placental trophoblasts. Reproduction 2017, 153, 369–380. [Google Scholar] [CrossRef]
- Yang, X.; Haghiac, M.; Glazebrook, P.; Minium, J.; Catalano, P.M.; Hauguel-de Mouzon, S. Saturated fatty acids enhance TLR4 immune pathways in human trophoblasts. Hum. Reprod. 2015, 30, 2152–2159. [Google Scholar] [CrossRef] [PubMed]
- Lager, S.; Jansson, T.; Powell, T.L. Differential regulation of placental amino acid transport by saturated and unsaturated fatty acids. Am. J. Physiol. Cell Physiol. 2014, 307, C738–C744. [Google Scholar] [CrossRef] [PubMed]
- Ricchi, M.; Odoardi, M.R.; Carulli, L.; Anzivino, C.; Ballestri, S.; Pinetti, A.; Fantoni, L.I.; Marra, F.; Bertolotti, M.; Banni, S. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J. Gastroenterol. Hepatol. 2009, 24, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Pathmaperuma, A.; Mana, P.; Cheung, S.; Kugathas, K.; Josiah, A.; Koina, M.; Broomfield, A.; Delghingaro-Augusto, V.; Ellwood, D.; Dahlstrom, J. Fatty acids alter glycerolipid metabolism and induce lipid droplet formation, syncytialisation and cytokine production in human trophoblasts with minimal glucose effect or interaction. Placenta 2010, 31, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.; Ortega-Senovilla, H. Implications of Lipids in Neonatal Body Weight and Fat Mass in Gestational Diabetic Mothers and Non-Diabetic Controls. Curr. Diabetes Rep. 2018, 18, 7. [Google Scholar] [CrossRef]
- Lewis, R.M.; Wadsack, C.; Desoye, G. Placental fatty acid transfer. Curr. Opin. Clin. Nutr. Metabol. Care 2018, 21, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Brass, E.; Hanson, E.; O’Tierney-Ginn, P.F. Placental oleic acid uptake is lower in male offspring of obese women. Placenta 2013, 34, 503–509. [Google Scholar] [CrossRef]
- Watkins, O.C.; Islam, M.O.; Selvam, P.; Pillai, R.A.; Cazenave-Gassiot, A.; Bendt, A.K.; Karnani, N.; Godfrey, K.M.; Lewis, R.M.; Wenk, M.R.; et al. Metabolism of 13C-Labeled Fatty Acids in Term Human Placental Explants by Liquid Chromatography–Mass Spectrometry. Endocrinology 2019, 160, 1394–1408. [Google Scholar] [CrossRef]
- Aris, I.M.; Soh, S.E.; Tint, M.T.; Liang, S.; Chinnadurai, A.; Saw, S.M.; Rajadurai, V.S.; Kwek, K.; Meaney, M.J.; Godfrey, K.M. Effect of maternal glycemia on neonatal adiposity in a multiethnic Asian birth cohort. J. Clin. Endocrinol. Metab. 2014, 99, 240–247. [Google Scholar] [CrossRef]
- Chen, L.-W.; Soh, S.E.; Tint, M.-T.; Loy, S.L.; Yap, F.; Tan, K.H.; Lee, Y.S.; Shek, L.P.-C.; Godfrey, K.M.; Gluckman, P.D. Combined analysis of gestational diabetes and maternal weight status from pre-pregnancy through post-delivery in future development of type 2 diabetes. Sci. Rep. 2021, 11, 5021. [Google Scholar] [CrossRef]
- Catalano, P.M.; Kirwan, J.P. Maternal factors that determine neonatal size and body fat. Curr. Diabetes Rep. 2001, 1, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Powell, T.L.; Barner, K.; Madi, L.; Armstrong, M.; Manke, J.; Uhlson, C.; Jansson, T.; Ferchaud-Roucher, V. Sex-specific responses in placental fatty acid oxidation, esterification and transfer capacity to maternal obesity. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2021, 1866, 158861. [Google Scholar] [CrossRef] [PubMed]
- Mansell, T.; Vlahos, A.; Collier, F.; Ponsonby, A.-L.; Vuillermin, P.; Ellul, S.; Tang, M.L.K.; Burgner, D.; Saffery, R.; Vuillermin, P.; et al. The newborn metabolome: Associations with gestational diabetes, sex, gestation, birth mode, and birth weight. Pediatr. Res. 2021, 91, 1864–1873. [Google Scholar] [CrossRef]
- Goldenberg, J.R.; Wang, X.; Lewandowski, E.D. Acyl CoA synthetase-1 links facilitated long chain fatty acid uptake to intracellular metabolic trafficking differently in hearts of male versus female mice. J. Mol. Cell. Cardiol. 2016, 94, 26995156. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, D.; Zhou, S.; Kokkotou, E.; Berk, P.D. Sex differences in hepatic fatty acid uptake reflect a greater affinity of the transport system in females. Am. J. Physiol. Gastrointest. Liver Physiol. 1992, 263, G380–G385. [Google Scholar] [CrossRef]
- Ockner, R.K.; Burnett, D.A.; Lysenko, N.; Manning, J.A. Sex differences in long chain fatty acid utilization and fatty acid binding protein concentration in rat liver. J. Clin. Investig. 1979, 64, 172–181. [Google Scholar] [CrossRef]
- Kushlan, M.C.; Gollan, J.L.; Ma, W.L.; Ockner, R.K. Sex differences in hepatic uptake of long chain fatty acids in single-pass perfused rat liver. J. Lipid Res. 1981, 22, 431–436. [Google Scholar] [CrossRef]
- Ockner, R.K.; Lysenko, N.; Manning, J.A.; Monroe, S.E.; Burnett, D.A. Sex steroid modulation of fatty acid utilization and fatty acid binding protein concentration in rat liver. J. Clin. Investig. 1980, 65, 1013–1023. [Google Scholar] [CrossRef]
- Luxon, B.A.; Weisiger, R.A. Sex differences in intracellular fatty acid transport: Role of cytoplasmic binding proteins. Am. J. Physiol. Gastrointest. Liver Physiol. 1993, 265, G831–G841. [Google Scholar] [CrossRef]
- Stahlberg, N.; Rico-Bautista, E.; Fisher, R.M.; Wu, X.; Cheung, L.; Flores-Morales, A.; Tybring, G.; Norstedt, G.; Tollet-Egnell, P. Female-Predominant Expression of Fatty Acid Translocase/CD36 in Rat and Human Liver. Endocrinology 2004, 145, 1972–1979. [Google Scholar] [CrossRef]
- Xie, P.; Wang, X.-P.; Bu, Z.; Zou, X.-T. Differential expression of fatty acid transporters and fatty acid synthesis-related genes in crop tissues of male and female pigeons (Columba livia domestica) during incubation and chick rearing. Br. Poult. Sci. 2017, 58, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Yang, D.; Tang, B.; Yang, Y. Oleic acid induces smooth muscle foam cell formation and enhances atherosclerotic lesion development via CD36. Lipids Health Disease 2011, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Atshaves, B.P.; Storey, S.M.; Petrescu, A.; Greenberg, C.C.; Lyuksyutova, O.I.; Smith, R.; Schroeder, F. Expression of fatty acid binding proteins inhibits lipid accumulation and alters toxicity in L cell fibroblasts. Am. J. Physiol. Cell Physiol. 2002, 283, C688–C703. [Google Scholar] [CrossRef]
- Duttaroy, A.K. Transport of fatty acids across the human placenta: A review. Prog. Lipid Res. 2009, 48, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Baar, R.A.; Dingfelder, C.S.; Smith, L.A.; Bernlohr, D.A.; Wu, C.; Lange, A.J.; Parks, E.J. Investigation of in vivo fatty acid metabolism in AFABP/aP2−/− mice. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E187–E193. [Google Scholar] [CrossRef] [PubMed]
- Maatman, R.G.H.J.; van Moerkerk, H.T.B.; Nooren, I.M.A.; van Zoelen, E.J.J.; Veerkamp, J.H. Expression of human liver fatty acid-binding protein in Escherichia coli and comparative analysis of its binding characteristics with muscle fatty acid-binding protein. Biochim. Biophys. Acta BBA Lipids Lipid Metab. 1994, 1214, 8068722. [Google Scholar] [CrossRef]
- Thumser, A.E.; Voysey, J.E.; Wilton, D.C. The binding of lysophospholipids to rat liver fatty acid-binding protein and albumin. Biochem. J. 1994, 301, 801–806. [Google Scholar] [CrossRef]
- Prows, D.R.; Schroeder, F. Metallothionein-IIAPromoter Induction Alters Rat Intestinal Fatty Acid Binding Protein Expression, Fatty Acid Uptake, and Lipid Metabolism in Transfected L-Cells. Arch. Biochem. Biophys. 1997, 340, 135–143. [Google Scholar] [CrossRef]
- Coe, N.R.; Simpson, M.A.; Bernlohr, D.A. Targeted disruption of the adipocyte lipid-binding protein (aP2 protein) gene impairs fat cell lipolysis and increases cellular fatty acid levels. J. Lipid Res. 1999, 40, 967–972. [Google Scholar] [CrossRef]
- Larqué, E.; Pagán, A.; Prieto, M.T.; Blanco, J.E.; Gil-Sánchez, A.; Zornoza-Moreno, M.; Ruiz-Palacios, M.; Gázquez, A.; Demmelmair, H.; Parrilla, J.J. Placental fatty acid transfer: A key factor in fetal growth. Ann. Nutr. Metab. 2014, 64, 247–253. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, Z.; Guo, F.; Wang, H.; Long, W.; Wang, H.; Yu, B. Placental metabolic profiling in gestational diabetes mellitus: An important role of fatty acids. J. Clin. Lab. Anal. 2021, 35, e24096. [Google Scholar] [CrossRef] [PubMed]
- Gázquez, A.; Prieto-Sánchez, M.T.; Blanco-Carnero, J.E.; Ruíz-Palacios, M.; Nieto, A.; van Harskamp, D.; Oosterink, J.E.; Schierbeek, H.; van Goudoever, J.B.; Demmelmair, H.; et al. Altered materno-fetal transfer of 13C-polyunsaturated fatty acids in obese pregnant women. Clin. Nutr. 2020, 39, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Shenai, J.P.; Borum, P.R.; Mohan, P.; Donlevy, S.C. Carnitine status at birth of newborn infants of varying gestation. Pediatr. Res. 1983, 17, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, N.; Hagerman, D.; Villee, C. Stimulation of fatty acid synthesis by oestradiol in vitro. Biochem. J. 1960, 76, 297. [Google Scholar] [CrossRef]
- Shambaugh, G., 3rd; Koehler, R.; Freinkel, N. Fetal fuels II: Contributions of selected carbon fuels to oxidative metabolism in rat conceptus. Am. J. Physiol. Endocrinol. Metab. 1977, 233, E457. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E.; Murray, A.J. Oxygen and placental development; parallels and differences with tumour biology. Placenta 2017, 56, 14–18. [Google Scholar] [CrossRef]
- Oey, N.; Den Boer, M.; Ruiter, J.; Wanders, R.; Duran, M.; Waterham, H.; Boer, K.; van der Post, J.; Wijburg, F. High activity of fatty acid oxidation enzymes in human placenta: Implications for fetal-maternal disease. J. Inherit. Metab. Disease 2003, 26, 385–392. [Google Scholar] [CrossRef]
- Shekhawat, P.; Bennett, M.J.; Sadovsky, Y.; Nelson, D.M.; Rakheja, D.; Strauss, A.W. Human placenta metabolizes fatty acids: Implications for fetal fatty acid oxidation disorders and maternal liver diseases. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E1098–E1105. [Google Scholar] [CrossRef]
- Jones, L.L.; McDonald, D.A.; Borum, P.R. Acylcarnitines: Role in brain. Progr. Lipid Res. 2010, 49, 61–75. [Google Scholar] [CrossRef]
- Arduini, A.; Mancinelli, G.; Radatti, G.L.; Dottori, S.; Molajoni, F.; Ramsay, R.R. Role of carnitine and carnitine palmitoyltransferase as integral components of the pathway for membrane phospholipid fatty acid turnover in intact human erythrocytes. J. Biol. Chem. 1992, 267, 12673–12681. [Google Scholar] [CrossRef]
- Arduini, A.; Denisova, N.; Virmani, A.; Avrova, N.; Federici, G.; Arrigoni-Martelli, E. Evidence for the Involvement of Carnitine-Dependent Long-Chain Acyltransferases in Neuronal Triglyceride and Phospholipid Fatty Acid Turnover. J. Neurochem. 1994, 62, 1530–1538. [Google Scholar] [CrossRef]
- Batchuluun, B.; Al Rijjal, D.; Prentice, K.J.; Eversley, J.A.; Burdett, E.; Mohan, H.; Bhattacharjee, A.; Gunderson, E.P.; Liu, Y.; Wheeler, M.B. Elevated Medium-Chain Acylcarnitines Are Associated With Gestational Diabetes Mellitus and Early Progression to Type 2 Diabetes and Induce Pancreatic β-Cell Dysfunction. Diabetes 2018, 67, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Schooneman, M.G.; Vaz, F.M.; Houten, S.M.; Soeters, M.R. Acylcarnitines: Reflecting or inflicting insulin resistance? Diabetes 2013, 62, 23258903. [Google Scholar] [CrossRef]
- Sánchez-Pintos, P.; de Castro, M.-J.; Roca, I.; Rite, S.; López, M.; Couce, M.-L. Similarities between acylcarnitine profiles in large for gestational age newborns and obesity. Sci. Rep. 2017, 7, 16267. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhu, W.; Chen, C.; Yan, B.; Zhu, L.; Chen, X.; Peng, C. The mechanisms of lysophosphatidylcholine in the development of diseases. Life Sci. 2020, 247, 117443. [Google Scholar] [CrossRef]
- Gokina, N.I.; Bonev, A.D.; Gokin, A.P.; Goloman, G. Role of impaired endothelial cell Ca2+ signaling in uteroplacental vascular dysfunction during diabetic rat pregnancy. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H935–H945. [Google Scholar] [CrossRef][Green Version]
- Tóth, M. Attenuation of diacylglycerol signal in the primordial human placenta: Role of phosphatidylcholine formation. Biochim. Biophys. Acta BBA Lipids Lipid Metab. 1993, 1210, 105–112. [Google Scholar] [CrossRef]
- Eichmann, T.O.; Lass, A. DAG tales: The multiple faces of diacylglycerol—Stereochemistry, metabolism, and signaling. Cell. Mol. Life Sci. 2015, 72, 3931–3952. [Google Scholar] [CrossRef] [PubMed]
- Drzazga, A.; Sowińska, A.; Koziołkiewicz, M. Lysophosphatidylcholine and lysophosphatidylinosiol—Novel promissing signaling molecules and their possible therapeutic activity. Acta Poloniae Pharm. 2014, 71, 887–899. [Google Scholar]
- Iwashita, M.; Watanabe, M.; Setoyama, T.; Mimuro, T.; Nakayamab, S.; Adachi, T.; Takeda, Y.; Sakamoto, S. Effects of diacylglycerol and gonadotropin-releasing hormone on human chorionic gonadotropin release by cultured trophoblast cells. Placenta 1992, 13, 213–221. [Google Scholar] [CrossRef]
- Harman, I.; Zeitler, P.; Ganong, B.; Bell, R.M.; Handwerger, S. Sn-1,2-Diacylglycerols and Phorbol Esters Stimulate the Synthesis and Release of Human Placental Lactogen from Placental Cells: A Role for Protein Kinase C*. Endocrinology 1986, 119, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Sane, A.; Harman, I.; Quarfordt, S.; Costello, A.; Handwergera, S. Characterization of placental lactogen release from perifused human trophoblast cells. Placenta 1988, 9, 129–138. [Google Scholar] [CrossRef]
- Kato, H.; Kato, M.; Kasugai, M.; Mizutani, S.; Ninagawa, T.; Tomoda, Y. Sn-1, 2-diacylglycerols and phorbol ester stimulate the production of progesterone from the human placenta. Eur. J. Endocrinol. 1989, 121, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, S.; Yinon, Y.; Xu, J.; Ermini, L.; Sallais, J.; Tagliaferro, A.; Todros, T.; Post, M.; Caniggia, I. Aberrant TGFβ signalling contributes to dysregulation of sphingolipid metabolism in intrauterine growth restriction. J. Clin. Endocrinol. Metab. 2015, 100, E986–E996. [Google Scholar] [CrossRef] [PubMed]
- Guenther, G.G.; Peralta, E.R.; Rosales, K.R.; Wong, S.Y.; Siskind, L.J.; Edinger, A.L. Ceramide starves cells to death by downregulating nutrient transporter proteins. Proc. Natl. Acad. Sci. USA 2008, 105, 17402–17407. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Griffiths, H.R. Ceramides reduce CD36 cell surface expression and oxidised LDL uptake by monocytes and macrophages. Arch. Biochem. Biophys. 2006, 450, 89–99. [Google Scholar] [CrossRef]
- Hyde, R.; Hajduch, E.; Powell, D.J.; Taylor, P.M.; Hundal, H.S. Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J. 2005, 19, 15611152. [Google Scholar] [CrossRef]
- Singh, A.T.; Dharmarajan, A.; Aye, I.L.M.H.; Keelan, J.A. Ceramide biosynthesis and metabolism in trophoblast syncytialization. Mol. Cell. Endocrinol. 2012, 362, 48–59. [Google Scholar] [CrossRef]
- Signorelli, P.; Avagliano, L.; Reforgiato, M.R.; Toppi, N.; Casas, J.; Fabriàs, G.; Marconi, A.M.; Ghidoni, R.; Caretti, A. De novo ceramide synthesis is involved in acute inflammation during labor. Biol. Chem. 2016, 397, 147–155. [Google Scholar] [CrossRef]
- Jansson, N.; Rosario, F.J.; Gaccioli, F.; Lager, S.; Jones, H.N.; Roos, S.; Jansson, T.; Powell, T.L. Activation of Placental mTOR Signaling and Amino Acid Transporters in Obese Women Giving Birth to Large Babies. J. Clin. Endocrinol. Metab. 2013, 98, 105–113. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Lin, S.-Y.; Lee, C.-N.; Wu, H.-T.; Kuo, C.-H.; Kuo, H.-C.; Chuang, C.-C.; Kuo, C.-H.; Chen, S.-C.; Fan, K.-C. Maternal Plasma Lipids During Pregnancy, Insulin-like Growth Factor-1 and Excess Foetal Growth. J. Clin. Endocrinol. Metab. 2021, 106, e3461–e3472. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Kawabata, T.; Kagawa, Y.; Shoji, K.; Kimura, F.; Miyazawa, T.; Tatsuta, N.; Arima, T.; Yaegashi, N.; Nakai, K. Associations of umbilical cord fatty acid profiles and desaturase enzyme indices with birth weight for gestational age in Japanese infants. Prostaglandins Leukot. Essent. Fat. Acids 2021, 165, 102233. [Google Scholar] [CrossRef] [PubMed]
- Giannacopoulou, C.; Evangeliou, A.; Matalliotakis, I.; Relakis, K.; Sbirakis, N.; Hatzidaki, E.; Koumandakis, E. Effects of gestation age and of birth weight in the concentration of carnitine in the umblical plasma. Clin. Exp. Obstet. Gynaecol. 1998, 25, 42–45. [Google Scholar]
- El-Wahed, M.A.; El-Farghali, O.; ElAbd, H.; El-Desouky, E.; Hassan, S. Metabolic derangements in IUGR neonates detected at birth using UPLC-MS. Egypt. J. Med. Hum. Genet. 2017, 18, 281–287. [Google Scholar] [CrossRef]
- Sánchez-Pintos, P.; Perez-Munuzuri, A.; Cocho, J.Á.; Fernández-Lorenzo, J.R.; Fraga, J.M.; Couce, M.L. Evaluation of carnitine deficit in very low birth weight preterm newborns small for their gestational age. J. Mater. Fetal Neonat. Med. 2016, 29, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Sommerfeld, E.; Penn, D.; Sodha, R.J.; Prögler, M.; Novak, M.; Schneider, H. Transfer and metabolism of carnitine and carnitine esters in the in vitro perfused human placenta. Pediatr. Res. 1985, 19, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.; Monkus, E.F.; Chung, D.; Buch, M. Carnitine in the perinatal metabolism of lipids I. Relationship between maternal and fetal plasma levels of carnitine and acylcarnitines. Pediatrics 1981, 67, 95–100. [Google Scholar] [CrossRef]
- Rodríguez-Cruz, M.; González, R.S.; Maldonado, J.; López-Alarcón, M.; Bernabe-García, M. The effect of gestational age on expression of genes involved in uptake, trafficking and synthesis of fatty acids in the rat placenta. Gene 2016, 591, 403–410. [Google Scholar] [CrossRef]
- Watkins, O.C.; Selvam, P.; Pillai, R.A.; Cracknell-Hazra, V.; Yong, H.E.J.; Sharma, N.; Cazenave-Gassiot, A.; Bendt, A.K.; Godfrey, K.M.; Lewis, R.M.; et al. Placental 13C-DHA metabolism and relationship with maternal BMI, glycemia and birthweight. Mol. Med. 2021, 27, 84. [Google Scholar] [CrossRef]
- Benassayag, C.; Mignot, T.; Haourigui, M.; Civel, C.; Hassid, J.; Carbonne, B.; Nunez, E.; Ferre, F. High polyunsaturated fatty acid, thromboxane A2, and alpha-fetoprotein concentrations at the human feto-maternal interface. J. Lipid Res. 1997, 38, 276–286. [Google Scholar] [CrossRef]
- Abdelmagid, S.A.; Clarke, S.E.; Nielsen, D.E.; Badawi, A.; El-Sohemy, A.; Mutch, D.M.; Ma, D.W. Comprehensive profiling of plasma fatty acid concentrations in young healthy Canadian adults. PLoS ONE 2015, 10, e0116195. [Google Scholar] [CrossRef] [PubMed]
- Pillai, R.A.; Islam, M.O.; Selvam, P.; Sharma, N.; Chu, A.H.; Watkins, O.C.; Godfrey, K.M.; Lewis, R.M.; Chan, S.Y. Placental Inositol Reduced in Gestational Diabetes as Glucose alters Inositol Transporters and IMPA1 enzyme expression. J. Clin. Endocrinol. Metab. 2020, 106, e875–e890. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).