In Vitro Cytotoxicity of D18 and Y6 as Potential Organic Photovoltaic Materials for Retinal Prostheses
Abstract
:1. Introduction
2. Results
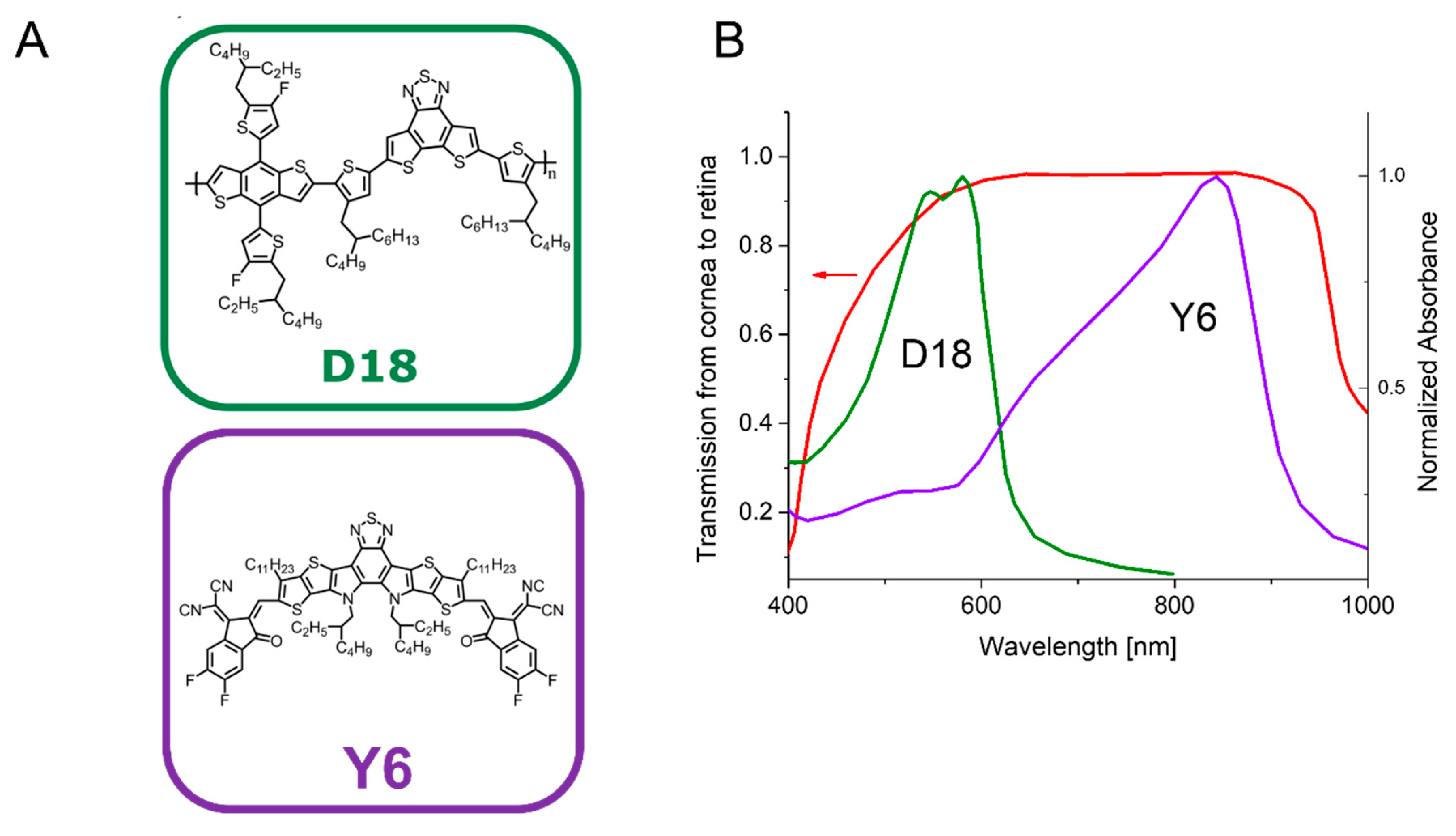
2.1. Selection of the Organic Semiconductor Materials
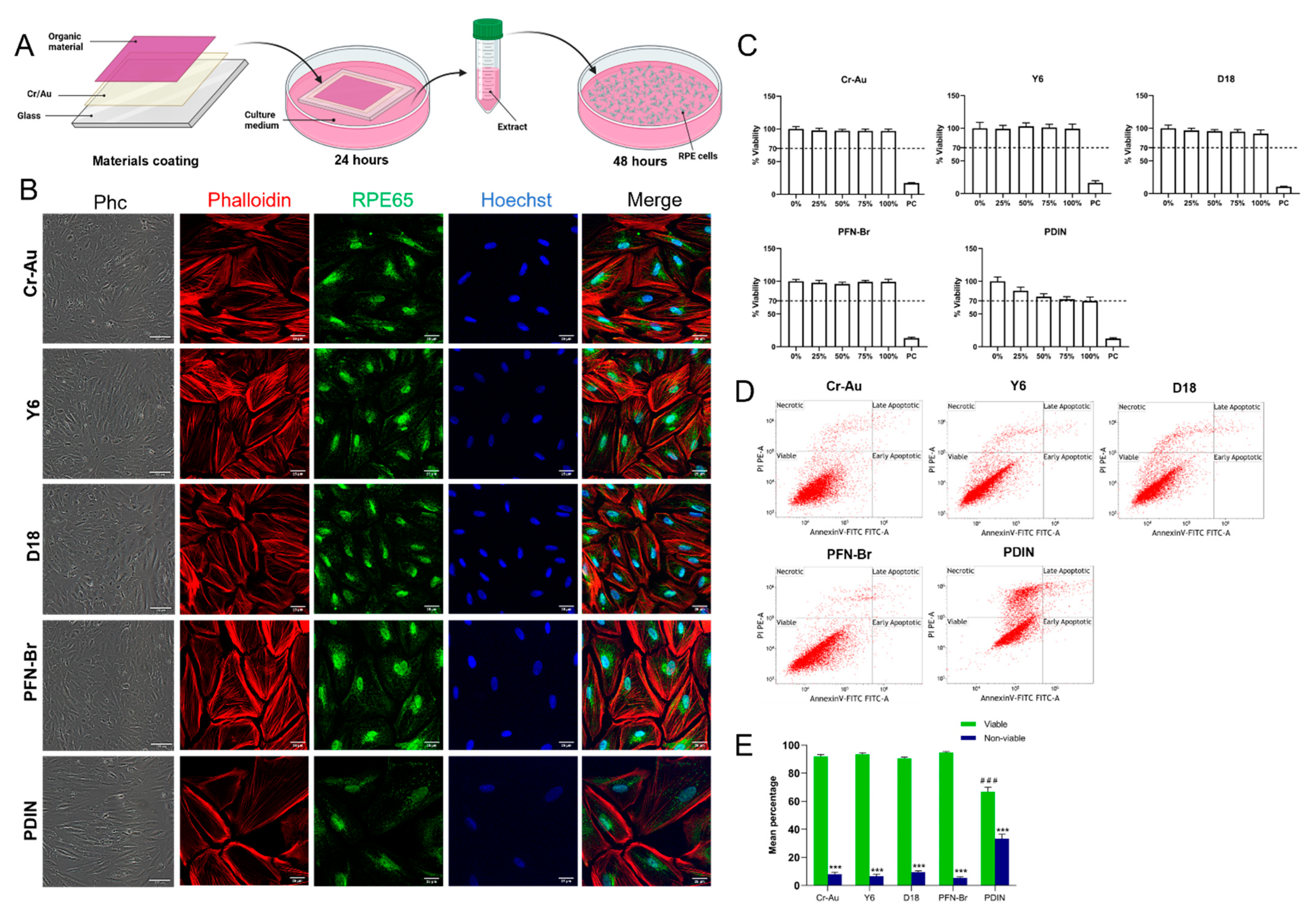
2.2. Cytotoxicity of the Materials’ Extract
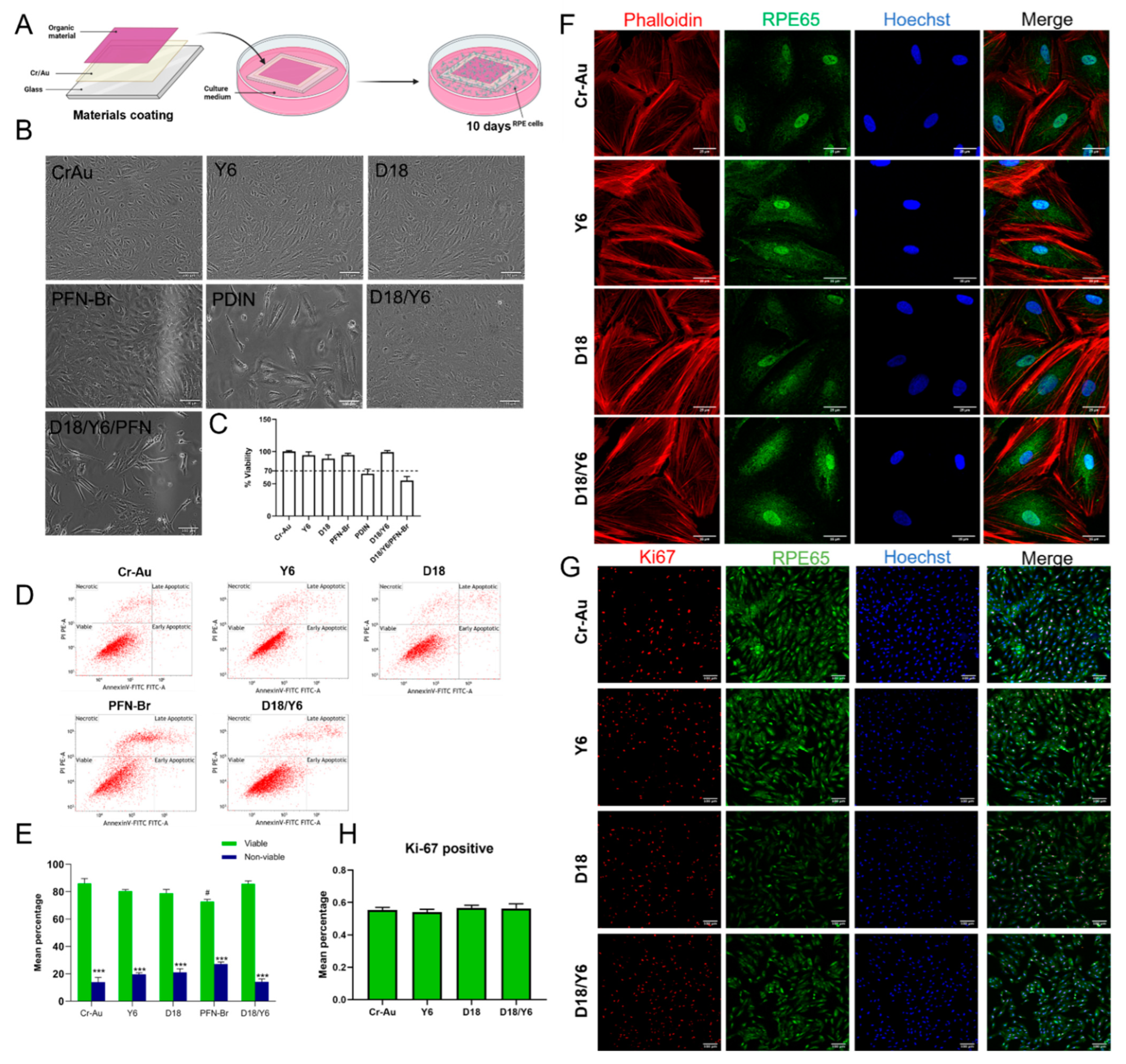
2.3. Cytotoxicity of the Materials by Direct Contact
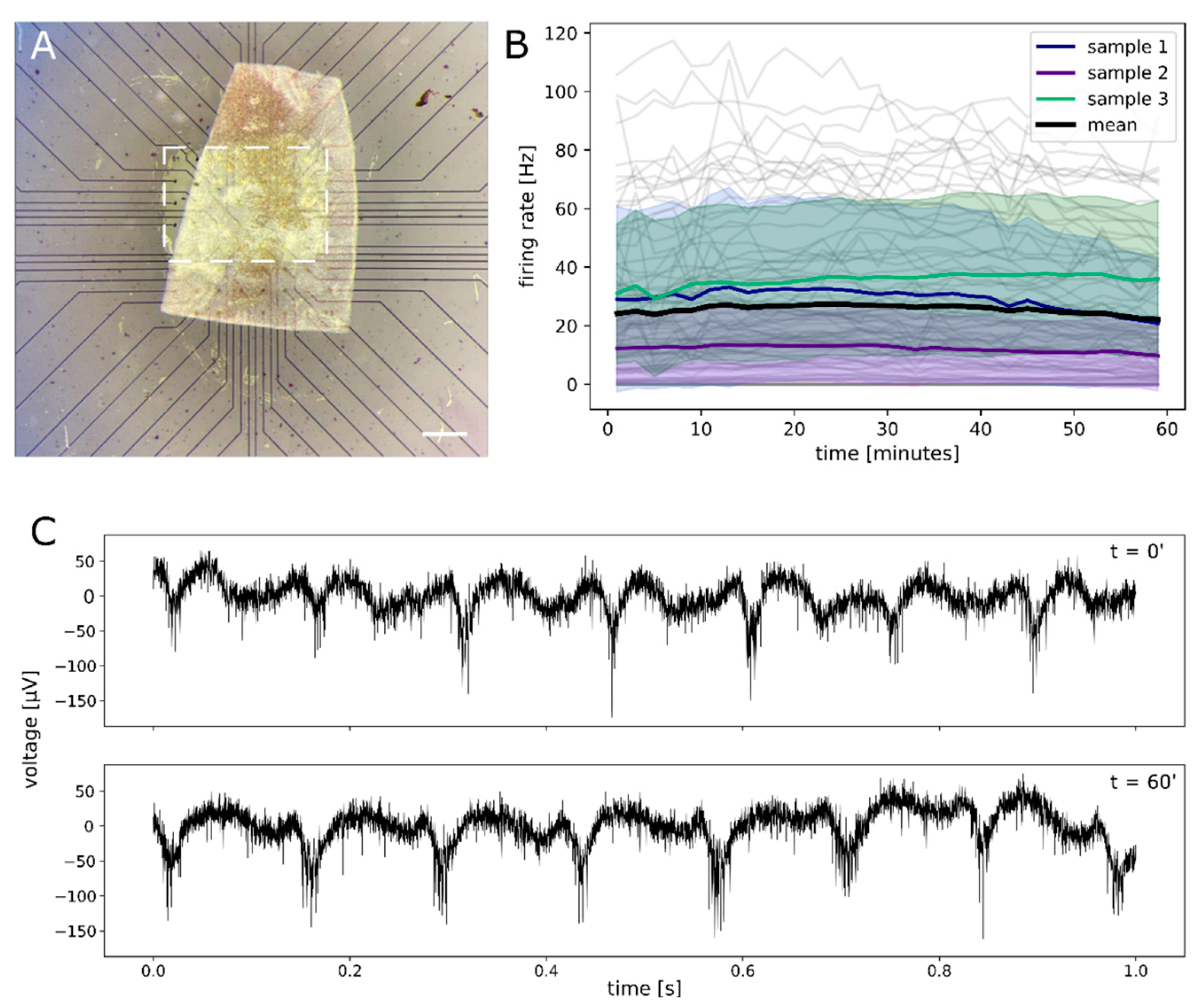
2.4. Functional Assessment of Retinal Explant
3. Discussion
4. Materials and Methods
4.1. Coating of the Organic Materials
4.2. Absorbance Measurement
4.3. Human RPE Cell Culture
4.4. MTT Cytotoxicity Assay
4.5. Apoptosis Analysis by Flow Cytometry
4.6. Immunofluorescence
4.7. Electrophysiological Recordings
4.8. Stastical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verbakel, S.K.; van Huet, R.A.C.; Boon, C.J.F.; den Hollander, A.I.; Collin, R.W.J.; Klaver, C.C.W.; Hoyng, C.B.; Roepman, R.; Klevering, B.J. Non-syndromic retinitis pigmentosa. Prog. Retin. Eye Res. 2018, 66, 157–186. [Google Scholar] [CrossRef]
- Sahel, J.A.; Marazova, K.; Audo, I. Clinical characteristics and current therapies for inherited retinal degenerations. Cold Spring Harb. Perspect. Med. 2014, 5, a017111. [Google Scholar] [CrossRef]
- Vázquez-Domínguez, I.; Garanto, A.; Collin, R.W.J. Molecular Therapies for Inherited Retinal Diseases-Current Standing, Opportunities and Challenges. Genes 2019, 10, 654. [Google Scholar] [CrossRef] [Green Version]
- Bellapianta, A.; Cetkovic, A.; Bolz, M.; Salti, A. Retinal Organoids and Retinal Prostheses: An Overview. Int. J. Mol. Sci. 2022, 23, 2922. [Google Scholar] [CrossRef]
- Ayton, L.N.; Barnes, N.; Dagnelie, G.; Fujikado, T.; Goetz, G.; Hornig, R.; Jones, B.W.; Muqit, M.M.K.; Rathbun, D.L.; Stingl, K.; et al. An update on retinal prostheses. Clin. Neurophysiol. 2020, 131, 1383–1398. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.J. Retinal prostheses: Where to from here? Clin. Experiment. Ophthalmol. 2021, 49, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Hallum, L.E.; Dakin, S.C. Retinal Implantation of Electronic Vision Prostheses to Treat Retinitis Pigmentosa: A Systematic Review. Transl. Vis. Sci. Technol. 2021, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, A.K.; Behrend, M.R.; Kuroda, M.; Humayun, M.S.; Weiland, J.D. An in vitro model of a retinal prosthesis. IEEE Trans. Biomed. Eng. 2008, 55, 1744–1753. [Google Scholar] [CrossRef] [Green Version]
- Palanker, D.; Le Mer, Y.; Mohand-Said, S.; Muqit, M.; Sahel, J.A. Photovoltaic Restoration of Central Vision in Atrophic Age-Related Macular Degeneration. Ophthalmology 2020, 127, 1097–1104. [Google Scholar] [CrossRef]
- Fujikado, T.; Kamei, M.; Sakaguchi, H.; Kanda, H.; Endo, T.; Hirota, M.; Morimoto, T.; Nishida, K.; Kishima, H.; Terasawa, Y.; et al. One-Year Outcome of 49-Channel Suprachoroidal-Transretinal Stimulation Prosthesis in Patients with Advanced Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6147–6157. [Google Scholar] [CrossRef] [Green Version]
- Zrenner, E.; Bartz-Schmidt, K.U.; Benav, H.; Besch, D.; Bruckmann, A.; Gabel, V.P.; Gekeler, F.; Greppmaier, U.; Harscher, A.; Kibbel, S.; et al. Subretinal electronic chips allow blind patients to read letters and combine them to words. Proc. Biol. Sci. 2011, 278, 1489–1497. [Google Scholar] [CrossRef]
- Lorach, H.; Goetz, G.; Smith, R.; Lei, X.; Mandel, Y.; Kamins, T.; Mathieson, K.; Huie, P.; Harris, J.; Sher, A.; et al. Photovoltaic restoration of sight with high visual acuity. Nat. Med. 2015, 21, 476–482. [Google Scholar] [CrossRef] [Green Version]
- Jole, M.; Leccardi, I.A.; Aurelia, N.; Chenais, L.; Ferlauto, L.; Kawecki, M.; Geneviève Zollinger, E.; Ghezzi, D. Photovoltaic organic interface for neuronal stimulation in the near-infrared. Commun. Mater. 2020, 1, 21. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Q.; Wang, H.; Wang, M. Multiscale engineering of functional organic polymer interfaces for neuronal stimulation and recording. Mater. Chem. Front. 2020, 4, 3444–3471. [Google Scholar] [CrossRef]
- Heeger, A.J.; Sariciftci, N.S.; Namdas, E.B. Semiconducting and Metallic Polymers; Oxford University Press: Oxford, UK, 2011; Volume 21, pp. 391–393. [Google Scholar]
- Rand, D.; Jakešová, M.; Lubin, G.; Vėbraitė, I.; David-Pur, M.; Đerek, V.; Cramer, T.; Sariciftci, N.S.; Hanein, Y.; Głowacki, E.D. Direct Electrical Neurostimulation with Organic Pigment Photocapacitors. Adv. Mater. 2018, 30, 1707292. [Google Scholar] [CrossRef] [Green Version]
- Irimia-Vladu, M.; Glowacki, D.E.; Sariciftci, S.N.; Bauer, S. Green Materials for Electronics; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 1–53. [Google Scholar] [CrossRef]
- Vagni, P.; Airaghi Leccardi, M.J.I.; Vila, C.-H.; Zollinger, E.G.; Sherafatipour, G.; Wolfensberger, T.J.; Ghezzi, D. POLYRETINA restores light responses in vivo in blind Göttingen minipigs. Nat. Commun. 2022, 13, 3678. [Google Scholar] [CrossRef]
- Samba, R.; Herrmann, T.; Zeck, G. PEDOT–CNT coated electrodes stimulate retinal neurons at low voltage amplitudes and low charge densities. J. Neural Eng. 2015, 12, 016014. [Google Scholar] [CrossRef]
- Antognazza, M.R.; Di Paolo, M.; Ghezzi, D.; Mete, M.; Di Marco, S.; Maya-Vetencourt, J.F.; Maccarone, R.; Desii, A.; Di Fonzo, F.; Bramini, M.; et al. Characterization of a Polymer-Based, Fully Organic Prosthesis for Implantation into the Subretinal Space of the Rat. Adv. Healthc. Mater. 2016, 5, 2271–2282. [Google Scholar] [CrossRef]
- Hong, J.-W.; Yoon, C.; Jo, K.; Won, J.H.; Park, S. Recent advances in recording and modulation technologies for next-generation neural interfaces. Iscience 2021, 24, 103550. [Google Scholar] [CrossRef]
- Medagoda, D.I.; Ghezzi, D. Organic semiconductors for light-mediated neuromodulation. Commun. Mater. 2021, 2, 111. [Google Scholar] [CrossRef]
- Maya-Vetencourt, J.F.; Ghezzi, D.; Antognazza, M.R.; Colombo, E.; Mete, M.; Feyen, P.; Desii, A.; Buschiazzo, A.; Di Paolo, M.; Di Marco, S.; et al. A fully organic retinal prosthesis restores vision in a rat model of degenerative blindness. Nat. Mater. 2017, 16, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Ghezzi, D.; Antognazza, M.R.; Dal Maschio, M.; Lanzarini, E.; Benfenati, F.; Lanzani, G. A hybrid bioorganic interface for neuronal photoactivation. Nat. Commun. 2011, 2, 166. [Google Scholar] [CrossRef]
- Ferlauto, L.; Airaghi Leccardi, M.J.I.; Chenais, N.A.L.; Gilliéron, S.C.A.; Vagni, P.; Bevilacqua, M.; Wolfensberger, T.J.; Sivula, K.; Ghezzi, D. Design and validation of a foldable and photovoltaic wide-field epiretinal prosthesis. Nat. Commun. 2018, 9, 992. [Google Scholar] [CrossRef] [Green Version]
- Ghezzi, D.; Antognazza, M.R.; MacCarone, R.; Bellani, S.; Lanzarini, E.; Martino, N.; Mete, M.; Pertile, G.; Bisti, S.; Lanzani, G.; et al. A polymer optoelectronic interface restores light sensitivity in blind rat retinas. Nat. Photonics 2013, 7, 400–406. [Google Scholar] [CrossRef] [Green Version]
- Hofinger, J.; Putz, C.; Mayr, F.; Gugujonovic, K.; Wielend, D.; Scharber, M.C. Understanding the low voltage losses in high-performance non-fullerene acceptor-based organic solar cells. Mater. Adv. 2021, 2, 4291–4302. [Google Scholar] [CrossRef]
- ÖNORM EN ISO 10993-5 Part 5; Tests for In Vitro Cytotoxicity. Austrian Standards Institue: Wien, Austria, 2009.
- Napoli, D.; Biagioni, M.; Billeri, F.; Di Marco, B.; Orsini, N.; Novelli, E.; Strettoi, E. Retinal pigment epithelium remodeling in mouse models of retinitis pigmentosa. Int. J. Mol. Sci. 2021, 22, 5381. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, Y.; Jin, K.; Qin, J.; Xu, J.; Li, W.; Xiong, J.; Liu, J.; Xiao, Z.; Sun, K.; et al. 18% Efficiency organic solar cells. Sci. Bull. 2020, 65, 272–275. [Google Scholar] [CrossRef] [Green Version]
- ISO-ISO 10993-12; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. International Organization for Standardization: Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/75769.html (accessed on 21 July 2022).
- Menzler, J.; Channappa, L.; Zeck, G. Rhythmic Ganglion Cell Activity in Bleached and Blind Adult Mouse Retinas. PLoS ONE 2014, 9, e106047. [Google Scholar] [CrossRef] [Green Version]
- Goo, Y.S.; Ahn, K.N.; Song, Y.J.; Ahn, S.H.; Han, S.K.; Ryu, S.B.; Kim, K.H. Spontaneous Oscillatory Rhythm in Retinal Activities of Two Retinal Degeneration (rd1 and rd10) Mice. Korean J. Physiol. Pharmacol. 2011, 15, 415–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeck, G. Aberrant activity in degenerated retinas revealed by electrical imaging. Front. Cell. Neurosci. 2016, 10, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menzler, J.; Zeck, G. Network oscillations in rod-degenerated mouse retinas. J. Neurosci. 2011, 31, 2280–2291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, S.; Haselier, C.; Mataruga, A.; Thumann, G.; Walter, P.; Müller, F. Pharmacological Analysis of Intrinsic Neuronal Oscillations in rd10 Retina. PLoS ONE 2014, 9, e99075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, S.; Leng, X.; Li, Y.; Zheng, Z.; Zhang, X.; Zhang, Y.; Zhou, H.; Bi, S.; Leng, X.; Li, Y.; et al. Interfacial Modification in Organic and Perovskite Solar Cells. Adv. Mater. 2019, 31, 1805708. [Google Scholar] [CrossRef] [PubMed]
- Oosterhout, S.D.; Braunecker, W.A.; Owczarczyk, Z.R.; Ayzner, A.L.; Toney, M.F.; Olson, D.C.; Kopidakis, N. Molecular engineering to improve carrier lifetimes for organic photovoltaic devices with thick active layers. Org. Electron. 2017, 47, 57–65. [Google Scholar] [CrossRef]
- Liu, Z.R.; Rill, R.L. N,N′-Bis[3,3′-(dimethylamino)propylamine]-3,4,9,10-perylenetetracarboxylic Diimide, a Dicationic Perylene Dye for Rapid Precipitation and Quantitation of Trace Amounts of DNA. Anal. Biochem. 1996, 236, 139–145. [Google Scholar] [CrossRef]
- Abdelhameed, M.; Martir, D.R.; Chen, S.; Xu, W.Z.; Oyeneye, O.O.; Chakrabarti, S.; Zysman-Colman, E.; Charpentier, P.A. Tuning the Optical Properties of Silicon Quantum Dots via Surface Functionalization with Conjugated Aromatic Fluorophores. Sci. Rep. 2018, 8, 3050. [Google Scholar] [CrossRef]
- Castro-Gálvez, Z.; Garrido-Armas, M.; Palacios-Arreola, M.I.; Torres-Flores, U.; Rivera-Torruco, G.; Valle-Rios, R.; Amador-Muñoz, O.; Hernández-Hernández, A.; Arenas-Huertero, F. Cytotoxic and genotoxic effects of Benzo[ghi]perylene on the human bronchial cell line NL-20. Toxicol. Vitr. 2019, 61, 104645. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, Y.; Wan, X.; Li, C.; Zhang, X.; Wang, Y.; Ke, X.; Xiao, Z.; Ding, L.; Xia, R.; et al. Organic and solution-processed tandem solar cells with 17.3% efficiency. Science 2018, 361, 1094–1098. [Google Scholar] [CrossRef] [Green Version]
- Saltzman, W.M.; Kyriakides, T.R. Cell Interactions with Polymers. In Principles of Tissue Engineering, 4th ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 385–406. [Google Scholar] [CrossRef]
- Sytnyk, M.; Jakešová, M.; Litviňuková, M.; Mashkov, O.; Kriegner, D.; Stangl, J.; Nebesářová, J.; Fecher, F.W.; Schöfberger, W.; Sariciftci, N.S.; et al. Cellular interfaces with hydrogen-bonded organic semiconductor hierarchical nanocrystals. Nat. Commun. 2017, 8, 91. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, E.; Hou, L.; Zhang, F.; Andersson, M.R.; Inganäs, O. Mixed solvents for reproducible photovoltaic bulk heterojunctions. J. Photonics Energy 2011, 1, 011122. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, Y.; Song, R.; Peng, Z.; Xia, L.; Wu, M.; Xiong, K.; Wang, B.; Lin, Y.; Xu, X.; et al. Polymer solar cells spray coated with non-halogenated solvents. Sol. Energy Mater. Sol. Cells 2017, 161, 52–61. [Google Scholar] [CrossRef]
- Maya-Vetencourt, J.F.; Di Marco, S.; Mete, M.; Di Paolo, M.; Ventrella, D.; Barone, F.; Elmi, A.; Manfredi, G.; Desii, A.; Sannita, W.G.; et al. Biocompatibility of a Conjugated Polymer Retinal Prosthesis in the Domestic Pig. Front. Bioeng. Biotechnol. 2020, 8, 1188. [Google Scholar] [CrossRef]
- Tong, Y.; Xiao, Z.; Du, X.; Zuo, C.; Li, Y.; Lv, M.; Yuan, Y.; Yi, C.; Hao, F.; Hua, Y.; et al. Progress of the key materials for organic solar cells. Sci. China Chem. 2020, 63, 758–765. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.L.; Lau, T.K.; Lu, X.; Zhu, C.; Peng, H.; Johnson, P.A.; et al. Single-Junction Organic Solar Cell with over 15% Efficiency Using Fused-Ring Acceptor with Electron-Deficient Core. Joule 2019, 3, 1140–1151. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, H.; Zhang, S.; Qin, Y.; Zhang, J.; Yang, L.; Li, W.; Wei, Z.; Gao, F.; Hou, J. Fluorination vs. chlorination: A case study on high performance organic photovoltaic materials. Sci. China Chem. 2018, 61, 1328–1337. [Google Scholar] [CrossRef]
- Fan, B.; Zhang, D.; Li, M.; Zhong, W.; Zeng, Z.; Ying, L.; Huang, F.; Cao, Y. Achieving over 16% efficiency for single-junction organic solar cells. Sci. China Chem. 2019, 62, 746–752. [Google Scholar] [CrossRef]
- Yan, T.; Song, W.; Huang, J.; Peng, R.; Huang, L.; Ge, Z.; Yan, T.T.; Song, W.; Huang, J.M.; Peng, R.X.; et al. 16.67% Rigid and 14.06% Flexible Organic Solar Cells Enabled by Ternary Heterojunction Strategy. Adv. Mater. 2019, 31, 1902210. [Google Scholar] [CrossRef]
- Quiroga, R.Q.; Nadasdy, Z.; Ben-Shaul, Y. Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Comput. 2004, 16, 1661–1687. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cetkovic, A.; Bellapianta, A.; Irimia-Vladu, M.; Hofinger, J.; Yumusak, C.; Corna, A.; Scharber, M.C.; Zeck, G.; Sariciftci, N.S.; Bolz, M.; et al. In Vitro Cytotoxicity of D18 and Y6 as Potential Organic Photovoltaic Materials for Retinal Prostheses. Int. J. Mol. Sci. 2022, 23, 8666. https://doi.org/10.3390/ijms23158666
Cetkovic A, Bellapianta A, Irimia-Vladu M, Hofinger J, Yumusak C, Corna A, Scharber MC, Zeck G, Sariciftci NS, Bolz M, et al. In Vitro Cytotoxicity of D18 and Y6 as Potential Organic Photovoltaic Materials for Retinal Prostheses. International Journal of Molecular Sciences. 2022; 23(15):8666. https://doi.org/10.3390/ijms23158666
Chicago/Turabian StyleCetkovic, Ana, Alessandro Bellapianta, Mihai Irimia-Vladu, Jakob Hofinger, Cigdem Yumusak, Andrea Corna, Markus Clark Scharber, Günther Zeck, Niyazi Serdar Sariciftci, Matthias Bolz, and et al. 2022. "In Vitro Cytotoxicity of D18 and Y6 as Potential Organic Photovoltaic Materials for Retinal Prostheses" International Journal of Molecular Sciences 23, no. 15: 8666. https://doi.org/10.3390/ijms23158666
APA StyleCetkovic, A., Bellapianta, A., Irimia-Vladu, M., Hofinger, J., Yumusak, C., Corna, A., Scharber, M. C., Zeck, G., Sariciftci, N. S., Bolz, M., & Salti, A. (2022). In Vitro Cytotoxicity of D18 and Y6 as Potential Organic Photovoltaic Materials for Retinal Prostheses. International Journal of Molecular Sciences, 23(15), 8666. https://doi.org/10.3390/ijms23158666






