Delivery of Nucleotide Sugars to the Mammalian Golgi: A Very Well (un)Explained Story
Abstract
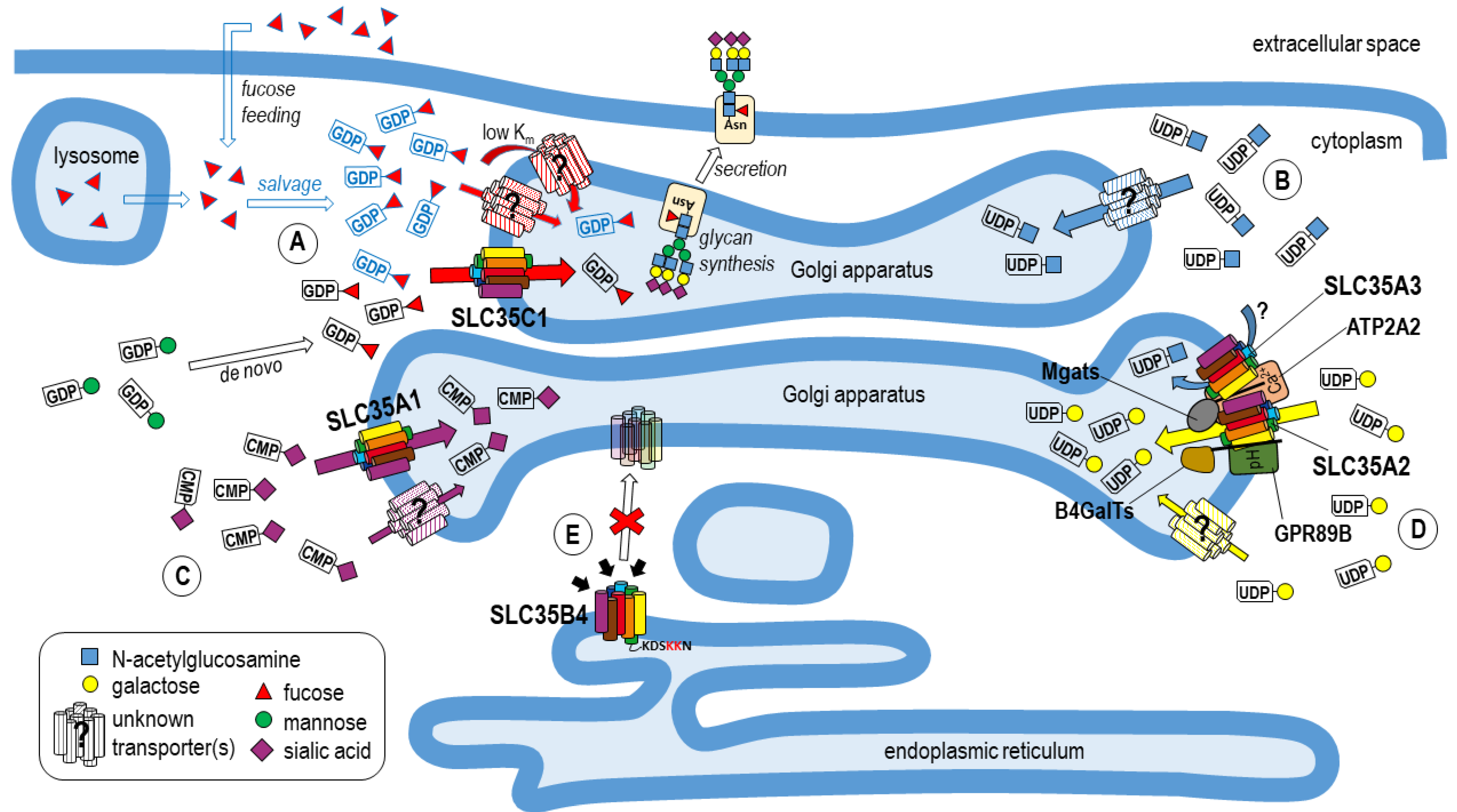
1. Introduction to Glycosylation
2. Methodological Approaches to the Nucleotide Sugar Transport
2.1. Glycosylation-Deficient Mutants
2.2. Functional Genetic Complementation
2.3. Microsome Penetration Assays
2.4. Heterologous Expression
2.5. Reconstitution into Liposomes
3. Nucleotide Sugar Transporters
3.1. Current State of Structural Studies on NSTs
3.2. Subcellular Localization of NSTs
3.3. Substrate Specificity of NSTs
3.4. Oligomerization of NSTs
3.5. Antiport Mechanism of NS Translocation
3.6. Pathologies Related with Defective NSTs
4. Studies of Nucleotide Sugar Transporters in Mammalian Cells
4.1. UDP-Galactose Supply
4.2. UDP-N-Acetylglucosamine Supply
4.3. CMP-Sialic Acid Supply
4.4. GDP-Fucose Supply
4.5. UDP-Xylose Supply
4.6. Mechanism of Transport of Nucleotide Sugars
4.7. Homo- and Heterologous Complexes of NSTs
4.8. Miscellaneous Controversial Data
5. Closing Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hart, G.W. Glycosylation. Curr. Opin. Cell Biol. 1992, 4, 1017–1023. [Google Scholar] [CrossRef]
- Paulson, J.C.; Colley, K.J. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J. Biol. Chem. 1989, 264, 17615–17618. [Google Scholar] [CrossRef]
- Hart, G.W. Dynamic O-linked glycosylation of nuclear and cytoskeletal proteins. Annu. Rev. Biochem. 1997, 66, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Kornfeld, R.; Kornfeld, S. Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 1985, 54, 631–664. [Google Scholar] [CrossRef] [PubMed]
- Strous, G.J. Initial glycosylation of proteins with acetylgalactosaminylserine linkages. Proc. Natl. Acad. Sci. USA 1979, 76, 2694–2698. [Google Scholar] [CrossRef]
- Abeijon, C.; Hirschberg, C.B. Hirschberg, Subcellular site of synthesis of the N-acetylgalactosamine (alpha 1-0) serine (or threonine) linkage in rat liver. J. Biol. Chem. 1987, 262, 4153–4159. [Google Scholar] [CrossRef]
- Ichikawa, S.; Ozawa, K.; Hirabayashi, Y. Molecular cloning and characterization of the mouse ceramide glucosyltransferase gene. Biochem. Biophys. Res. Commun. 1998, 253, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Sprong, H.; Kruithof, B.; Leijendekker, R.; Slot, J.W.; van Meer, G.; van der Sluijs, P. UDP-Galactose:Ceramide galactosyltransferase is a class I integral membrane protein of the endoplasmic reticulum. J. Biol. Chem. 1998, 273, 25880–25888. [Google Scholar] [CrossRef]
- Breton, C.; Mucha, J.; Jeanneau, C. Structural and functional features of glycosyltransferases. Biochimie 2001, 83, 713–718. [Google Scholar] [CrossRef]
- Luo, Y.; Haltiwanger, R.S. O-fucosylation of notch occurs in the endoplasmic reticulum. J. Biol. Chem. 2005, 280, 11289–11294. [Google Scholar] [CrossRef]
- Larsen, I.S.B.; Narimatsu, Y.; Joshi, H.J.; Siukstaite, L.; Harrison, O.J.; Brasch, J.; Goodman, K.M.; Hansen, L.; Shapiro, L.; Honig, B.; et al. Discovery of an O-mannosylation pathway selectively serving cadherins and protocadherins. Proc. Natl. Acad. Sci. USA 2017, 114, 11163–11168. [Google Scholar] [CrossRef] [PubMed]
- Coates, S.W.; Gurney, T., Jr.; Sommers, L.W.; Yeh, M.; Hirschberg, C.B. Subcellular localization of sugar nucleotide synthetases. J. Biol. Chem. 1980, 255, 9225–9229. [Google Scholar] [CrossRef]
- Münster, A.-K.; Eckhardt, M.; Potvin, B.; Mühlenhoff, M.; Stanley, P.; Gerardy-Schahn, R. Mammalian cytidine 5′-monophosphate N-acetylneuraminic acid synthetase: A nuclear protein with evolutionarily conserved structural motifs. Proc. Natl. Acad. Sci. USA 1998, 95, 9140–9145. [Google Scholar] [CrossRef] [PubMed]
- Stanley, P.; Caillibot, V.; Siminovitch, L. Selection and characterization of eight phenotypically distinct lines of lectin-resistant Chinese hamster ovary cell. Cell 1975, 6, 121–128. [Google Scholar] [CrossRef]
- Stanley, P.; Siminovitch, L. Complementation between mutants of CHO cells resistant to a variety of plant lectins. Somat. Cell Genet. 1977, 3, 391–405. [Google Scholar] [CrossRef]
- Stanley, P. Selection of specific wheat germ agglutinin-resistant (WgaR) phenotypes from Chinese hamster ovary cell populations containing numerous lecR genotypes. Mol. Cell. Biol. 1981, 1, 687–696. [Google Scholar]
- Stanley, P. Lectin-resistant CHO cells: Selection of new mutant phenotypes. Somat. Cell Genet. 1983, 9, 593–608. [Google Scholar] [CrossRef]
- Briles, E.B.; Li, E.; Kornfeld, S. Isolation of wheat germ agglutinin-resistant clones of Chinese hamster ovary cells deficient in membrane sialic acid and galactose. J. Biol. Chem. 1977, 252, 1107–1116. [Google Scholar] [CrossRef]
- Smith, W.L.; Nakajima, T.; Ballou, C.E. Biosynthesis of yeast mannan. Isolation of Kluyveromyces lactis mannan mutants and a study of the incorporation of N-acetyl-D-glucosamine into the polysaccharide side chains. J. Biol. Chem. 1975, 250, 3426–3435. [Google Scholar] [CrossRef]
- Douglas, R.H.; Ballou, C.E. Purification of an alpha-N-acetylglucosaminyltransferase from the yeast Kluyveromyces lactis and a study of mutants defective in this enzyme activity. Biochemistry 1982, 21, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Abeijon, C.; Mandon, E.C.; Robbins, P.W.; Hirschberg, C.B. A mutant yeast deficient in Golgi transport of uridine diphosphate N-acetylglucosamine. J. Biol. Chem. 1996, 271, 8851–8854. [Google Scholar] [CrossRef] [PubMed]
- Takegawa, K.; Tanaka, N.; Tabuchi, M.; Iwahara, S. Isolation and characterization of a glycosylation mutant from Schizosaccharomyces pombe. Biosci. Biotechnol. Biochem. 1996, 60, 1156–1159. [Google Scholar]
- Tabuchi, M.; Tanaka, N.; Iwahara, S.; Takegawa, K. The Schizosaccharomyces pombe gms1+ gene encodes an UDP-galactose transporter homologue required for protein galactosylation. Biochem. Biophys. Res. Commun. 1997, 232, 121–125. [Google Scholar] [CrossRef]
- Ballou, L.; Hitzeman, R.A.; Lewis, M.S.; Ballou, C.E. Vanadate-resistant yeast mutants are defective in protein glycosylation. Proc. Natl. Acad. Sci. USA 1991, 88, 3209–3212. [Google Scholar] [CrossRef]
- Dean, N.; Zhang, Y.B.; Poster, J.B. The VRG4 gene is required for GDP-mannose transport into the lumen of the Golgi in the yeast, Saccharomyces cerevisiae. J. Biol. Chem. 1997, 272, 31908–31914. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Hattori, S.; Kawakita, M. Isolation and characterization of mouse FM3A cell mutants which are devoid of Newcastle disease virus receptors. J. Virol. 1989, 63, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Meiss, H.K.; Green, R.F.; Rodriguez-Boulan, E.J. Lectin-resistant mutants of polarized epithelial cells. Mol. Cell. Biol. 1982, 2, 1287–1294. [Google Scholar]
- Brändli, A.W.; Hansson, G.C.; Rodriguez-Boulan, E.; Simons, K. A polarized epithelial cell mutant deficient in translocation of UDP-galactose into the Golgi complex. J. Biol. Chem. 1988, 263, 16283–16290. [Google Scholar] [CrossRef]
- Descoteaux, A.; Luo, Y.; Turco, S.J.; Beverley, S.M. A specialized pathway affecting virulence glycoconjugates of Leishmania. Science 1995, 269, 1869–1872. [Google Scholar] [CrossRef]
- Abeijon, C.; Robbins, P.W.; Hirschberg, C.B. Hirschberg, Molecular cloning of the Golgi apparatus uridine diphosphate-N-acetylglucosamine transporter from Kluyveromyces lactis. Proc. Natl. Acad. Sci. USA 1996, 93, 5963–5968. [Google Scholar] [CrossRef]
- Eckhardt, M.; Mühlenhoff, M.; Bethe, A.; Gerardy-Schahn, R. Expression cloning of the Golgi CMP-sialic acid transporter. Proc. Natl. Acad. Sci. USA 1996, 93, 7572–7576. [Google Scholar] [CrossRef]
- Ishida, N.; Miura, N.; Yoshioka, S.; Kawakita, M. Molecular cloning and characterization of a novel isoform of the human UDP-galactose transporter, and of related complementary DNAs belonging to the nucleotide-sugar transporter gene family. J. Biochem. 1996, 120, 1074–1078. [Google Scholar] [CrossRef]
- Miura, N.; Ishida, N.; Hoshino, M.; Yamauchi, M.; Hara, T.; Ayusawa, D.; Kawakita, M. Human UDP-galactose translocator: Molecular cloning of a complementary DNA that complements the genetic defect of a mutant cell line deficient in UDP-galactose translocator. J. Biochem. 1996, 120, 236–241. [Google Scholar] [CrossRef]
- Guillen, E.; Abeijon, C.; Hirschberg, C.B. Mammalian Golgi apparatus UDP-N-acetylglucosamine transporter: Molecular cloning by phenotypic correction of a yeast mutant. Proc. Natl. Acad. Sci. USA 1998, 95, 7888–7892. [Google Scholar] [CrossRef] [PubMed]
- Segawa, H.; Ishida, N.; Takegawa, K.; Kawakita, M. Schizosaccharomyces pombe UDP-galactose transporter: Identification of its functional form through cDNA cloning and expression in mammalian cells. FEBS Lett. 1999, 451, 295–298. [Google Scholar] [CrossRef]
- Ishida, N.; Ito, M.; Yoshioka, S.; Sun-Wada, G.-H.; Kawakita, M. Functional expression of human golgi CMP-sialic acid transporter in the Golgi complex of a transporter-deficient Chinese hamster ovary cell mutant. J. Biochem. 1998, 124, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Lühn, K.; Wild, M.K.; Eckhardt, M.; Gerardy-Schahn, R.; Vestweber, D. The gene defective in leukocyte adhesion deficiency II encodes a putative GDP-fucose transporter. Nat. Genet. 2001, 28, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Lühn, K.; Laskowska, A.; Pielage, J.; Klämbt, C.; Ipe, U.; Vestweber, D.; Wild, M.K. Identification and molecular cloning of a functional GDP-fucose transporter in Drosophila melanogaster. Exp. Cell Res. 2004, 301, 242–250. [Google Scholar] [CrossRef]
- Segawa, H.; Kawakita, M.; Ishida, N. Human and Drosophila UDP-galactose transporters transport UDP-N-acetylgalactosamine in addition to UDP-galactose. Eur. J. Biochem. 2002, 269, 128–138. [Google Scholar] [CrossRef]
- Nishikawa, A.; Mendez, B.; Jigami, Y.; Dean, N. Identification of a Candida glabrata homologue of the S. cerevisiae VRG4 gene, encoding the Golgi GDP-mannose transporter. Yeast 2002, 19, 691–698. [Google Scholar] [CrossRef]
- Martinez-Duncker, I.; Dupré, T.; Piller, V.; Piller, F.; Candelier, J.J.; Trichet, C.; Tchernia, G.; Oriol, R.; Mollicone, R. Genetic complementation reveals a novel human congenital disorder of glycosylation of type II, due to inactivation of the Golgi CMP-sialic acid transporter. Blood 2005, 105, 2671–2676. [Google Scholar] [CrossRef]
- Bakker, H.; Routier, F.; Oelmann, S.; Jordi, W.; Lommen, A.; Gerardy-Schahn, R.; Bosch, D. Molecular cloning of two Arabidopsis UDP-galactose transporters by complementation of a deficient Chinese hamster ovary cell line. Glycobiology 2005, 15, 193–201. [Google Scholar] [CrossRef]
- Kuhn, N.J.; White, A. Evidence for specific transport of uridine diphosphate galactose across the Golgi membrane of rat mammary gland. Biochem. J. 1976, 154, 243–244. [Google Scholar] [CrossRef]
- Carey, D.J.; Sommers, L.W.; Hirschberg, C.B. CMP-N-acetylneuraminic acid: Isolation from and penetration into mouse liver microsomes. Cell 1980, 19, 597–605. [Google Scholar] [CrossRef]
- Sommers, L.W.; Hirschberg, C.B. Transport of sugar nucleotides into rat liver Golgi. A new Golgi marker activity. J. Biol. Chem. 1982, 257, 10811–10817. [Google Scholar] [CrossRef]
- Schwarz, J.K.; Capasso, J.M.; Hirschberg, C.B. Translocation of adenosine 3′-phosphate 5′-phosphosulfate into rat liver Golgi vesicles. J. Biol. Chem. 1984, 259, 3554–3559. [Google Scholar] [CrossRef]
- Perez, M.; Hirschberg, C.B. Topography of glycosylation reactions in the rough endoplasmic reticulum membrane. J. Biol. Chem. 1986, 261, 6822–6830. [Google Scholar] [CrossRef]
- Abeijon, C.; Orlean, P.; Robbins, P.W.; Hirschberg, C.B. Hirschberg, Topography of glycosylation in yeast: Characterization of GDPmannose transport and lumenal guanosine diphosphatase activities in Golgi-like vesicles. Proc. Natl. Acad. Sci. USA 1989, 86, 6935–6939. [Google Scholar] [CrossRef] [PubMed]
- Castro, O.; Chen, L.Y.; Parodi, A.J.; Abeijón, C. Uridine diphosphate-glucose transport into the endoplasmic reticulum of Saccharomyces cerevisiae: In vivo and in vitro evidence. Mol. Biol. Cell 1999, 10, 1019–1030. [Google Scholar] [CrossRef]
- Lübke, T.; von Figura, K.; Körner, C.; Marquardt, T. A new type of carbohydrate-deficient glycoprotein syndrome due to a decreased import of GDP-fucose into the golgi. J. Biol. Chem. 1999, 274, 25986–25989. [Google Scholar] [CrossRef]
- Waldman, B.C.; Rudnick, G. UDP-GlcNAc transport across the Golgi membrane: Electroneutral exchange for dianionic UMP. Biochemistry 1990, 29, 44–52. [Google Scholar] [CrossRef]
- Perez, M.; Hirschberg, C.B. Transport of sugar nucleotides into the lumen of vesicles derived from rat liver rough endoplasmic reticulum and Golgi apparatus. Methods Enzymol. 1987, 138, 709–715. [Google Scholar] [PubMed]
- Bossuyt, X.; Blanckaert, N. Functional characterization of carrier-mediated transport of uridine diphosphate N-acetylglucosamine across the endoplasmic reticulum membrane. Eur. J. Biochem. 1994, 223, 981–988. [Google Scholar] [CrossRef]
- Bossuyt, X.; Blanckaert, N. Carrier-mediated transport of intact UDP-glucuronic acid into the lumen of endoplasmic-reticulum-derived vesicles from rat liver. Biochem. J. 1994, 302 Pt 1, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Russell, D.G.; Beverley, S.M.; Turco, S.J. Golgi GDP-mannose uptake requires Leishmania LPG2. A member of a eukaryotic family of putative nucleotide-sugar transporters. J. Biol. Chem. 1997, 272, 3799–3805. [Google Scholar] [CrossRef]
- Berninsone, P.; Eckhardt, M.; Gerardy-Schahn, R.; Hirschberg, C.B. Functional expression of the murine Golgi CMP-sialic acid transporter in saccharomyces cerevisiae. J. Biol. Chem. 1997, 272, 12616–12619. [Google Scholar] [CrossRef]
- Tiralongo, J.; Ashikov, A.; Routier, F.; Eckhardt, M.; Bakker, H.; Gerardy-Schahn, R.; von Itzstein, M. Functional expression of the CMP-sialic acid transporter in Escherichia coli and its identification as a simple mobile carrier. Glycobiology 2005, 16, 73–81. [Google Scholar] [CrossRef]
- Sun-Wada, G.-H.; Yoshioka, S.; Ishida, N.; Kawakita, M. Functional expression of the human UDP-galactose transporters in the yeast Saccharomyces cerevisiae. J. Biochem. 1998, 123, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Yoshioka, S.; Chiba, Y.; Takeuchi, M.; Kawakita, M. Molecular cloning and functional expression of the human Golgi UDP-N-acetylglucosamine transporter. J. Biochem. 1999, 126, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, M.; Kawakita, M.; Ishida, N. Molecular characterization of human UDP-glucuronic acid/UDP-N-acetylgalactosamine transporter, a novel nucleotide sugar transporter with dual substrate specificity. FEBS Lett. 2001, 495, 87–93. [Google Scholar] [CrossRef]
- Suda, T.; Kamiyama, S.; Suzuki, M.; Kikuchi, N.; Nakayama, K.I.; Narimatsu, H.; Jigami, Y.; Aoki, T.; Nishihara, S. Molecular cloning and characterization of a human multisubstrate specific nucleotide-sugar transporter homologous to Drosophila fringe connection. J. Biol. Chem. 2004, 279, 26469–26474. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Kuba, T.; Aoki, K.; Miyatake, S.; Kawakita, M.; Sanai, Y. Identification and characterization of human Golgi nucleotide sugar transporter SLC35D2, a novel member of the SLC35 nucleotide sugar transporter family. Genomics 2005, 85, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Ashikov, A.; Routier, F.; Fuhlrott, J.; Helmus, Y.; Wild, M.; Gerardy-Schahn, R.; Bakker, H. The human solute carrier gene SLC35B4 encodes a bifunctional nucleotide sugar transporter with specificity for UDP-xylose and UDP-N-acetylglucosamine. J. Biol. Chem. 2005, 280, 27230–27235. [Google Scholar] [CrossRef]
- Berninsone, P.; Hwang, H.Y.; Zemtseva, I.; Horvitz, H.R.; Hirschberg, C.B. SQV-7, a protein involved in Caenorhabditis elegans epithelial invagination and early embryogenesis, transports UDP-glucuronic acid, UDP-N- acetylgalactosamine, and UDP-galactose. Proc. Natl. Acad. Sci. USA 2001, 98, 3738–3743. [Google Scholar] [CrossRef] [PubMed]
- Höflich, J.; Berninsone, P.; Göbel, C.; Gravato-Nobre, M.J.; Libby, B.J.; Darby, C.; Politz, S.M.; Hodgkin, J.; Hirschberg, C.B.; Baumeister, R. Loss of srf-3-encoded nucleotide sugar transporter activity in Caenorhabditis elegans alters surface antigenicity and prevents bacterial adherence. J. Biol. Chem. 2004, 279, 30440–30448. [Google Scholar] [CrossRef]
- Caffaro, C.E.; Hirschberg, C.B.; Berninsone, P.M. Independent and simultaneous translocation of two substrates by a nucleotide sugar transporter. Proc. Natl. Acad. Sci. USA 2006, 103, 16176–16181. [Google Scholar] [CrossRef] [PubMed]
- Caffaro, C.E.; Luhn, K.; Bakker, H.; Vestweber, D.; Samuelson, J.; Berninsone, P.; Hirschberg, C.B. A single Caenorhabditis elegans Golgi apparatus-type transporter of UDP-glucose, UDP-galactose, UDP-N-acetylglucosamine, and UDP-N-acetylgalactosamine. Biochemistry 2008, 47, 4337–4344. [Google Scholar] [CrossRef]
- Maggioni, A.; Hadley, B.; Von Itzstein, M.; Tiralongo, J. Expression, solubilisation, and purification of a functional CMP-sialic acid transporter in Pichia pastoris. Protein Expr. Purif. 2014, 101, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Maggioni, A.; von Itzstein, M.; Gerardy-Schahn, R.; Tiralongo, J. Targeting the expression of functional murine CMP-sialic acid transporter to the E. coli inner membrane. Biochem. Biophys. Res. Commun. 2007, 362, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Förster-Fromme, K.; Schneider, S.; Sprenger, G.A.; Albermann, C. Functional expression of a human GDP-L-fucose transporter in Escherichia coli. Biotechnol. Lett. 2017, 39, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Milla, M.E.; Hirschberg, C.B. Reconstitution of Golgi vesicle CMP-sialic acid and adenosine 3′-phosphate 5′-phosphosulfate transport into proteoliposomes. Proc. Natl. Acad. Sci. USA 1989, 86, 1786–1790. [Google Scholar] [CrossRef]
- Milla, M.E.; Clairmont, C.A.; Hirschberg, C.B. Reconstitution into proteoliposomes and partial purification of the Golgi apparatus membrane UDP-galactose, UDP-xylose, and UDP-glucuronic acid transport activities. J. Biol. Chem. 1992, 267, 103–107. [Google Scholar] [CrossRef]
- Puglielli, L.; Mandon, E.C.; Rancour, D.; Menon, A.; Hirschberg, C.B. Identification and purification of the rat liver Golgi membrane UDP-N-acetylgalactosamine transporter. J. Biol. Chem. 1999, 274, 4474–4479. [Google Scholar] [CrossRef] [PubMed]
- Puglielli, L.; Hirschberg, C.B. Reconstitution, identification, and purification of the rat liver golgi membrane GDP-fucose transporter. J. Biol. Chem. 1999, 274, 35596–35600. [Google Scholar] [CrossRef]
- Segawa, H.; Soares, R.P.; Kawakita, M.; Beverley, S.M.; Turco, S.J. Reconstitution of GDP-mannose transport activity with purified Leishmania LPG2 protein in liposomes. J. Biol. Chem. 2005, 280, 2028–2035. [Google Scholar] [CrossRef]
- Martinez-Duncker, I.; Mollicone, R.; Codogno, P.; Oriol, R. The nucleotide-sugar transporter family: A phylogenetic approach. Biochimie 2003, 85, 245–260. [Google Scholar] [CrossRef]
- Eckhardt, M.; Gotza, B.; Gerardy-Schahn, R. Membrane topology of the mammalian CMP-sialic acid transporter. J. Biol. Chem. 1999, 274, 8779–8787. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.L.; Corey, R.; Stansfeld, P.J.; Newstead, S. Structural basis for substrate specificity and regulation of nucleotide sugar transporters in the lipid bilayer. Nat. Commun. 2019, 10, 4657. [Google Scholar] [CrossRef]
- Ahuja, S.; Whorton, M.R. Structural basis for mammalian nucleotide sugar transport. eLife 2019, 8, e45221. [Google Scholar] [CrossRef]
- Kabuß, R.; Ashikov, A.; Oelmann, S.; Gerardy-Schahn, R.; Bakker, H. Endoplasmic reticulum retention of the large splice variant of the UDP-galactose transporter is caused by a dilysine motif. Glycobiology 2005, 15, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.R.; Nilsson, T.; Peterson, P.A. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 1990, 9, 3153–3162. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, T.-L.L.; Vertel, B.M.; Colley, K.J. The CMP-sialic acid transporter is localized in the medial-trans Golgi and possesses two specific endoplasmic reticulum export motifs in its carboxyl-terminal cytoplasmic tail. J. Biol. Chem. 2006, 281, 31106–31118. [Google Scholar] [CrossRef] [PubMed]
- Nufer, O.; Guldbrandsen, S.; Degen, M.; Kappeler, F.; Paccaud, J.-P.; Tani, K.; Hauri, H.-P. Role of cytoplasmic C-terminal amino acids of membrane proteins in ER export. J. Cell Sci. 2002, 115 Pt 3, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-D.; Dean, N. Distinct protein domains of the yeast Golgi GDP-mannose transporter mediate oligomer assembly and export from the endoplasmic reticulum. J. Biol. Chem. 2000, 275, 17718–17727. [Google Scholar] [CrossRef]
- Yoshioka, S.; Sun-Wada, G.-H.; Ishida, N.; Kawakita, M. Expression of the human UDP-galactose transporter in the Golgi membranes of murine Had-1 cells that lack the endogenous transporter. J. Biochem. 1997, 122, 691–695. [Google Scholar] [CrossRef]
- Sprong, H.; Degroote, S.; Nilsson, T.; Kawakita, M.; Ishida, N.; van der Sluijs, P.; van Meer, G. Association of the Golgi UDP-galactose transporter with UDP-Galactose:Ceramide galactosyltransferase allows UDP-galactose import in the endoplasmic reticulum. Mol. Biol. Cell 2003, 14, 3482–3493. [Google Scholar] [CrossRef]
- Hirschberg, C.B.; Robbins, P.W.; Abeijon, C. Transporters of nucleotide sugars, ATP, and nucleotide sulfate in the endoplasmic reticulum and Golgi apparatus. Annu. Rev. Biochem. 1998, 67, 49–69. [Google Scholar] [CrossRef]
- Hong, K.; Ma, D.; Beverley, S.M.; Turco, S.J. The Leishmania GDP-mannose transporter is an autonomous, multi-specific, hexameric complex of LPG2 subunits. Biochemistry 2000, 39, 2013–2022. [Google Scholar] [CrossRef]
- Muraoka, M.; Miki, T.; Ishida, N.; Hara, T.; Kawakita, M. Variety of nucleotide sugar transporters with respect to the interaction with nucleoside mono- and diphosphates. J. Biol. Chem. 2007, 282, 24615–24622. [Google Scholar] [CrossRef] [PubMed]
- Selva, E.M.; Hong, K.; Baeg, G.-H.; Beverley, S.M.; Turco, S.J.; Perrimon, N.; Häcker, U. Dual role of the fringe connection gene in both heparan sulphate and fringe-dependent signalling events. Nat. Cell Biol. 2001, 3, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Taniguchi, M.; Muraoka, M.; Toyoda, H.; Sado, Y.; Kawakita, M.; Hayashi, S. UDP-sugar transporter implicated in glycosylation and processing of Notch. Nat. Cell Biol. 2001, 3, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Sosicka, P.; Bazan, B.; Maszczak-Seneczko, D.; Shauchuk, Y.; Olczak, T.; Olczak, M. SLC35A5 Protein-A Golgi Complex Member with Putative Nucleotide Sugar Transport Activity. Int. J. Mol. Sci. 2019, 20, 276. [Google Scholar] [CrossRef]
- Ishikawa, H.O.; Ayukawa, T.; Nakayama, M.; Higashi, S.; Kamiyama, S.; Nishihara, S.; Aoki, K.; Ishida, N.; Sanai, Y.; Matsuno, K. Two pathways for importing GDP-fucose into the endoplasmic reticulum lumen function redundantly in the O-fucosylation of Notch in Drosophila. J. Biol. Chem. 2010, 285, 4122–4129. [Google Scholar] [CrossRef] [PubMed]
- Caffaro, C.E.; Hirschberg, C.B.; Berninsone, P.M. Functional redundancy between two Caenorhabditis elegans nucleotide sugar transporters with a novel transport mechanism. J. Biol. Chem. 2007, 282, 27970–27975. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Hashimoto, H.; Yoda, K. Molecular characterization of Vig4/Vrg4 GDP-mannose transporter of the yeast Saccharomyces cerevisiae. FEBS Lett. 1999, 458, 309–312. [Google Scholar] [CrossRef]
- Olczak, M.; Guillen, E. Characterization of a mutation and an alternative splicing of UDP-galactose transporter in MDCK-RCAr cell line. Biochim. Biophys. Acta 2006, 1763, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Maszczak-Seneczko, D.; Sosicka, P.; Majkowski, M.; Olczak, T.; Olczak, M. UDP-N-acetylglucosamine transporter and UDP-galactose transporter form heterologous complexes in the Golgi membrane. FEBS Lett. 2012, 586, 4082–4087. [Google Scholar] [CrossRef]
- Szulc, B.; Zadorozhna, Y.; Olczak, M.; Wiertelak, W.; Maszczak-Seneczko, D. Novel Insights into Selected Disease-Causing Mutations within the SLC35A1 Gene Encoding the CMP-Sialic Acid Transporter. Int. J. Mol. Sci. 2020, 22, 304. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, M.; Gerardy-Schahn, R. Molecular cloning of the hamster CMP-sialic acid transporter. Eur. J. Biochem. 1997, 248, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, N.J.; White, A. The role of nucleoside diphosphatase in a uridine nucleotide cycle associated with lactose synthesis in rat mammary-gland Golgi apparatus. Biochem. J. 1977, 168, 423–433. [Google Scholar] [CrossRef]
- Brandan, E.; Fleischer, B. Orientation and role of nucleosidediphosphatase and 5′-nucleotidase in Golgi vesicles from rat liver. Biochemistry 1982, 21, 4640–4645. [Google Scholar] [CrossRef] [PubMed]
- Capasso, J.M.; Hirschberg, C.B. Effect of nucleotides on translocation of sugar nucleotides and adenosine 3′-phosphate 5′-phosphosulfate into Golgi apparatus vesicles. Biochim. Biophys. Acta 1984, 777, 133–139. [Google Scholar] [CrossRef]
- Lopez-Avalos, M.D.; Uccelletti, D.; Abeijon, C.; Hirschberg, C.B. The UDPase activity of the Kluyveromyces lactis Golgi GDPase has a role in uridine nucleotide sugar transport into Golgi vesicles. Glycobiology 2001, 11, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Herrero, A.B.; Uccelletti, D.; Hirschberg, C.B.; Dominguez, A.; Abeijon, C. The Golgi GDPase of the fungal pathogen Candida albicans affects morphogenesis, glycosylation, and cell wall properties. Eukaryot. Cell 2002, 1, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Khatra, B.S.; Herries, D.G.; Brew, K. Some kinetic properties of human-milk galactosyl transferase. Eur. J. Biochem. 1974, 44, 537–560. [Google Scholar] [CrossRef]
- Nji, E.; Gulati, A.; Qureshi, A.A.; Coincon, M.; Drew, D. Structural basis for the delivery of activated sialic acid into Golgi for sialyation. Nat. Struct. Mol. Biol. 2019, 26, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Abeijon, C.; Yanagisawa, K.; Mandon, E.C.; Häusler, A.; Moremen, K.; Hirschberg, C.B.; Robbins, P.W. Guanosine diphosphatase is required for protein and sphingolipid glycosylation in the Golgi lumen of Saccharomyces cerevisiae. J. Cell Biol. 1993, 122, 307–323. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, C.; Caramelo, J.J.; Parodi, A.J. Absence of nucleoside diphosphatase activities in the yeast secretory pathway does not abolish nucleotide sugar-dependent protein glycosylation. J. Biol. Chem. 2005, 280, 40417–40427. [Google Scholar] [CrossRef]
- Bossuyt, X.; Blanckaert, N. Mechanism of stimulation of microsomal UDP-glucuronosyltransferase by UDP-N-acetylglucosamine. Biochem. J. 1995, 305 Pt 1, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, X.; Blanckaert, N. Differential regulation of UDP-GlcUA transport in endoplasmic reticulum and in Golgi membranes. J. Hepatol. 2001, 34, 210–214. [Google Scholar] [CrossRef]
- Lübke, T.; Marquardt, T.; Etzioni, A.; Hartmann, E.; Von Figura, K.; Körner, C. Complementation cloning identifies CDG-IIc, a new type of congenital disorders of glycosylation, as a GDP-fucose transporter deficiency. Nat. Genet. 2001, 28, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Etzioni, A.; Frydman, M.; Pollack, S.; Avidor, I.; Phillips, M.L.; Paulson, J.C.; Gershoni-Baruch, R. Brief report: Recurrent severe infections caused by a novel leukocyte adhesion deficiency. N. Engl. J. Med. 1992, 327, 1789–1792. [Google Scholar] [CrossRef]
- Sturla, L.; Puglielli, L.; Tonetti, M.; Berninsone, P.; Hirschberg, C.B.; de Flora, A.; Etzioni, A. Impairment of the Golgi GDP-L-fucose transport and unresponsiveness to fucose replacement therapy in LAD II patients. Pediatr. Res. 2001, 49, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Sturla, L.; Rampal, R.; Haltiwanger, R.S.; Fruscione, F.; Etzioni, A.; Tonetti, M. Differential terminal fucosylation of N-linked glycans versus protein O-fucosylation in leukocyte adhesion deficiency type II (CDG IIc). J. Biol. Chem. 2003, 278, 26727–26733. [Google Scholar] [CrossRef] [PubMed]
- Sturla, L.; Fruscione, F.; Noda, K.; Miyoshi, E.; Taniguchi, N.; Contini, P.; Tonetti, M. Core fucosylation of N-linked glycans in leukocyte adhesion deficiency/congenital disorder of glycosylation IIc fibroblasts. Glycobiology 2005, 15, 924–934. [Google Scholar] [CrossRef][Green Version]
- Marquardt, T.; Brune, T.; Lühn, K.; Zimmer, K.P.; Körner, C.; Fabritz, L.; van der Werft, N.; Vormoor, J.; Freeze, H.H.; Louwen, F.; et al. Leukocyte adhesion deficiency II syndrome, a generalized defect in fucose metabolism. J. Pediatr. 1999, 134, 681–688. [Google Scholar] [CrossRef]
- Marquardt, T.; Lühn, K.; Srikrishna, G.; Freeze, H.H.; Harms, E.; Vestweber, D. Correction of leukocyte adhesion deficiency type II with oral fucose. Blood 1999, 94, 3976–3985. [Google Scholar] [CrossRef]
- Etzioni, A.; Tonetti, M. Fucose supplementation in leukocyte adhesion deficiency type II. Blood 2000, 95, 3641–3643. [Google Scholar] [CrossRef]
- Hidalgo, A.; Ma, S.; Peired, A.J.; Weiss, L.A.; Cunningham-Rundles, C.; Frenette, P.S. Insights into leukocyte adhesion deficiency type 2 from a novel mutation in the GDP-fucose transporter gene. Blood 2003, 101, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Willig, T.-N.; Breton-Gorius, J.; Elbim, C.; Mignotte, V.; Kaplan, C.; Mollicone, R.; Pasquier, C.; Filipe, A.; Miélot, F.; Cartron, J.-P.; et al. Macrothrombocytopenia with abnormal demarcation membranes in megakaryocytes and neutropenia with a complete lack of sialyl-Lewis-X antigen in leukocytes—A new syndrome? Blood 2001, 97, 826–828. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.; Ashikov, A.; Guillard, M.; Robben, J.H.; Schmidt, S.; Heuvel, B.V.D.; De Brouwer, A.P.; Gerardy-Schahn, R.; Deen, P.M.; Wevers, R.A.; et al. Intellectual disability and bleeding diathesis due to deficient CMP—Sialic acid transport. Neurology 2013, 81, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Ng, B.G.; Asteggiano, C.G.; Kircher, M.; Buckingham, K.J.; Raymond, K.; Nickerson, D.A.; Shendure, J.; Bamshad, M.J.; University of Washington Center for Mendelian Genomics; Ensslen, M.; et al. Freeze, Encephalopathy caused by novel mutations in the CMP-sialic acid transporter, SLC35A1. Am. J. Med. Genet. A 2017, 173, 2906–2911. [Google Scholar] [CrossRef] [PubMed]
- Kauskot, A.; Pascreau, T.; Adam, F.; Bruneel, A.; Reperant, C.; Lourenco-Rodrigues, M.-D.; Rosa, J.-P.; Petermann, R.; Maurey, H.; Auditeau, C.; et al. A mutation in the gene coding for the sialic acid transporter SLC35A1 is required for platelet life span but not proplatelet formation. Haematologica 2018, 103, e613–e617. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, B.; Horn, P.; Panitz, F.; Bendixen, E.; Petersen, A.H.; Holm, L.E.; Nielsen, V.H.; Agerholm, J.S.; Arnbjerg, J.; Bendixen, C. A missense mutation in the bovine SLC35A3 gene, encoding a UDP-N-acetylglucosamine transporter, causes complex vertebral malformation. Genome Res. 2006, 16, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Marini, C.; Hardies, K.; Pisano, T.; May, P.; Weckhuysen, S.; Cellini, E.; Suls, A.; Mei, D.; Balling, R.; Jonghe, P.D.; et al. Recessive mutations in SLC35A3 cause early onset epileptic encephalopathy with skeletal defects. Am. J. Med. Genet. A 2017, 173, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Edvardson, S.; Ashikov, A.; Jalas, C.; Sturiale, L.; Shaag, A.; Fedick, A.; Treff, N.R.; Garozzo, D.; Gerardy-Schahn, R.; Elpeleg, O. Mutations in SLC35A3 cause autism spectrum disorder, epilepsy and arthrogryposis. J. Med. Genet. 2013, 50, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, S.; Furuichi, T.; Nishimura, G.; Shibata, S.; Yanagishita, M.; Rimoin, D.L.; Superti-Furga, A.; Nikkels, P.G.; Ogawa, M.; Katsuyama, K.; et al. Nucleotide-sugar transporter SLC35D1 is critical to chondroitin sulfate synthesis in cartilage and skeletal development in mouse and human. Nat. Med. 2007, 13, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Furuichi, T.; Kayserili, H.; Hiraoka, S.; Nishimura, G.; Ohashi, H.; Alanay, Y.; Lerena, J.; Aslanger, A.D.; Koseki, H.; Cohn, D.H.; et al. Identification of loss-of-function mutations of SLC35D1 in patients with Schneckenbecken dysplasia, but not with other severe spondylodysplastic dysplasias group diseases. J. Med. Genet. 2009, 46, 562–568. [Google Scholar] [CrossRef]
- Kodera, H.; Nakamura, K.; Osaka, H.; Maegaki, Y.; Haginoya, K.; Mizumoto, S.; Kato, M.; Okamoto, N.; Iai, M.; Kondo, Y.; et al. De novo mutations in SLC35A2 encoding a UDP-galactose transporter cause early-onset epileptic encephalopathy. Hum. Mutat. 2013, 34, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- Ng, B.G.; Buckingham, K.J.; Raymond, K.; Kircher, M.; Turner, E.H.; He, M.; Smith, J.D.; Eroshkin, A.; Szybowska, M.; Losfeld, M.E.; et al. Freeze, Mosaicism of the UDP-galactose transporter SLC35A2 causes a congenital disorder of glycosylation. Am. J. Hum. Genet. 2013, 92, 632–636. [Google Scholar] [CrossRef]
- Dörre, K.; Olczak, M.; Wada, Y.; Sosicka, P.; Grüneberg, M.; Reunert, J.; Kurlemann, G.; Fiedler, B.; Biskup, S.; Hörtnagel, K.; et al. A new case of UDP-galactose transporter deficiency (SLC35A2-CDG): Molecular basis, clinical phenotype, and therapeutic approach. J. Inherit. Metab. Dis. 2015, 38, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Yates, T.M.; Suri, M.; Desurkar, A.; Lesca, G.; Wallgren-Pettersson, C.; Hammer, T.B.; Raghavan, A.; Poulat, A.L.; Møller, R.S.; Thuresson, A.C.; et al. SLC35A2-related congenital disorder of glycosylation: Defining the phenotype. Eur. J. Paediatr. Neurol. 2008, 22, 1095–1102. [Google Scholar] [CrossRef]
- Ng, B.G.; Sosicka, P.; Agadi, S.; Almannai, M.; Bacino, C.A.; Barone, R.; Botto, L.D.; Burton, J.E.; Carlston, C.; Chung, B.H.; et al. SLC35A2-CDG: Functional characterization, expanded molecular, clinical, and biochemical phenotypes of 30 unreported Individuals. Hum. Mutat. 2019, 40, 908–925. [Google Scholar] [CrossRef] [PubMed]
- Abuduxikuer, K.; Wang, J.-S. Four New Cases of SLC35A2-CDG With Novel Mutations and Clinical Features. Front. Genet. 2021, 12, 658786. [Google Scholar] [CrossRef]
- Oelmann, S.; Stanley, P.; Gerardy-Schahn, R. Point mutations identified in Lec8 Chinese hamster ovary glycosylation mutants that inactivate both the UDP-galactose and CMP-sialic acid transporters. J. Biol. Chem. 2001, 276, 26291–26300. [Google Scholar] [CrossRef] [PubMed]
- Toma, L.; Pinhal, M.A.; Dietrich, C.P.; Nader, H.; Hirschberg, C.B. Transport of UDP-galactose into the Golgi lumen regulates the biosynthesis of proteoglycans. J. Biol. Chem. 1996, 271, 3897–3901. [Google Scholar] [CrossRef]
- Maszczak-Seneczko, D.; Olczak, T.; Wunderlich, L.; Olczak, M. Comparative analysis of involvement of UGT1 and UGT2 splice variants of UDP-galactose transporter in glycosylation of macromolecules in MDCK and CHO cell lines. Glycoconj. J. 2011, 28, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Szulc, B.; Sosicka, P.; Maszczak-Seneczko, D.; Skurska, E.; Shauchuk, A.; Olczak, T.; Freeze, H.H.; Olczak, M. Biosynthesis of GlcNAc-rich N- and O-glycans in the Golgi apparatus does not require the nucleotide sugar transporter SLC35A3. J. Biol. Chem. 2020, 295, 16445–16463. [Google Scholar] [CrossRef] [PubMed]
- Maszczak-Seneczko, D.; Olczak, T.; Jakimowicz, P.; Olczak, M. Overexpression of UDP-GlcNAc transporter partially corrects galactosylation defect caused by UDP-Gal transporter mutation. FEBS Lett. 2011, 585, 3090–3094. [Google Scholar] [CrossRef]
- Olczak, M.; Maszczak-Seneczko, D.; Sosicka, P.; Jakimowicz, P.; Olczak, T. UDP-Gal/UDP-GlcNAc chimeric transporter complements mutation defect in mammalian cells deficient in UDP-Gal transporter. Biochem. Biophys. Res. Commun. 2013, 434, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Sosicka, P.; Jakimowicz, P.; Olczak, T.; Olczak, M. Short N-terminal region of UDP-galactose transporter (SLC35A2) is crucial for galactosylation of N-glycans. Biochem. Biophys. Res. Commun. 2014, 454, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Maszczak-Seneczko, D.; Sosicka, P.; Olczak, T.; Jakimowicz, P.; Majkowski, M.; Olczak, M. UDP-N-acetylglucosamine transporter (SLC35A3) regulates biosynthesis of highly branched N-glycans and keratan sulfate. J. Biol. Chem. 2013, 288, 21850–21860. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Honke, K.; Fukuda, M.; Narimatsu, H.; Yamaguchi, Y.; Angata, T. Handbook of Glycosyltransferases and Related genes, 2nd ed.; Springer: Tokyo, Japan, 2014. [Google Scholar]
- Song, W.; Isaji, T.; Nakano, M.; Liang, C.; Fukuda, T.; Gu, J. O-GlcNAcylation regulates β1,4-GlcNAc-branched N-glycan biosynthesis via the OGT/SLC35A3/GnT-IV axis. FASEB J. 2022, 36, e22149. [Google Scholar] [CrossRef] [PubMed]
- Bazan, B.; Wiktor, M.; Maszczak-Seneczko, D.; Olczak, T.; Kaczmarek, B.; Olczak, M. Lysine at position 329 within a C-terminal dilysine motif is crucial for the ER localization of human SLC35B4. PLoS ONE 2018, 13, e0207521. [Google Scholar] [CrossRef]
- Eckhardt, M.; Gotza, B.; Gerardy-Schahn, R. Mutants of the CMP-sialic acid transporter causing the Lec2 phenotype. J. Biol. Chem. 1998, 273, 20189–20195. [Google Scholar] [CrossRef]
- Deutscher, S.L.; Nuwayhid, N.; Stanley, P.; Briles, E.I.B.; Hirschberg, C.B. Translocation across Golgi vesicle membranes: A CHO glycosylation mutant deficient in CMP-sialic acid transport. Cell 1984, 39 Pt 1, 295–299. [Google Scholar] [CrossRef]
- Lim, S.F.; Lee, M.M.; Zhang, P.; Song, Z. The Golgi CMP-sialic acid transporter: A new CHO mutant provides functional insights. Glycobiology 2008, 18, 851–860. [Google Scholar] [CrossRef]
- Hellbusch, C.C.; Sperandio, M.; Frommhold, D.; Yakubenia, S.; Wild, M.K.; Popovici, D.; Vestweber, D.; Grone, H.J.; Von Figura, K.; Lubke, T.; et al. Golgi GDP-fucose transporter-deficient mice mimic congenital disorder of glycosylation IIc/leukocyte adhesion deficiency II. J. Biol. Chem. 2007, 282, 10762–10772. [Google Scholar] [CrossRef]
- Skurska, E.; Szulc, B.; Maszczak-Seneczko, D.; Wiktor, M.; Wiertelak, W.; Makowiecka, A.; Olczak, M. Incorporation of fucose into glycans independent of the GDP-fucose transporter SLC35C1 preferentially utilizes salvaged over de novo GDP-fucose. J. Biol. Chem. 2022, 298, 102206. [Google Scholar] [CrossRef]
- Hirschberg, C.B. Golgi nucleotide sugar transport and leukocyte adhesion deficiency II. J. Clin. Investig. 2001, 108, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, K.; Miyoshi, E. Fucosylation and gastrointestinal cancer. World J. Hepatol. 2010, 2, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Hou, X.; Shi, S.; Körner, C.; Stanley, P. Slc35c2 promotes Notch1 fucosylation and is required for optimal Notch signaling in mammalian cells. J. Biol. Chem. 2010, 285, 36245–36254. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Sleeman, J.E.; Coughtrie, M.W.H.; Burchell, B. Molecular and functional characterization of microsomal UDP-glucuronic acid uptake by members of the nucleotide sugar transporter (NST) family. Biochem. J. 2006, 400, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Maszczak-Seneczko, D.; Olczak, T.; Olczak, M. Subcellular localization of UDP-GlcNAc, UDP-Gal and SLC35B4 transporters. Acta Biochim. Pol. 2011, 58, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Capasso, J.M.; Hirschberg, C.B. Mechanisms of glycosylation and sulfation in the Golgi apparatus: Evidence for nucleotide sugar/nucleoside monophosphate and nucleotide sulfate/nucleoside monophosphate antiports in the Golgi apparatus membrane. Proc. Natl. Acad. Sci. USA 1984, 81, 7051–7055. [Google Scholar] [CrossRef]
- Berninsone, P.; Miret, J.J.; Hirschberg, C.B. The Golgi guanosine diphosphatase is required for transport of GDP-mannose into the lumen of Saccharomyces cerevisiae Golgi vesicles. J. Biol. Chem. 1994, 269, 207–211. [Google Scholar] [CrossRef]
- Cecchelli, R.; Cacan, R.; Verbert, A. Mechanism of UDP-sugar transport into intracellular vesicles. Occurrence of UDP-GlcNAc/UDP and UDP-Gal/UDP antiports. FEBS Lett. 1986, 208, 407–412. [Google Scholar] [CrossRef]
- Sosicka, P.; Maszczak-Seneczko, D.; Bazan, B.; Shauchuk, Y.; Kaczmarek, B.; Olczak, M. An insight into the orphan nucleotide sugar transporter SLC35A4. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 825–838. [Google Scholar] [CrossRef]
- Wiertelak, W.; Olczak, M.; Maszczak-Seneczko, D. Demonstration of Heterologous Complexes formed by Golgi-Resident Type III Membrane Proteins using Split Luciferase Complementation Assay. J. Vis. Exp. 2020, 163, e61669. [Google Scholar] [CrossRef]
- Khoder-Agha, F.; Sosicka, P.; Conde, M.E.; Hassinen, A.; Glumoff, T.; Olczak, M.; Kellokumpu, S. N-acetylglucosaminyltransferases and nucleotide sugar transporters form multi-enzyme-multi-transporter assemblies in golgi membranes in vivo. Cell. Mol. Life Sci. 2019, 76, 1821–1832. [Google Scholar] [CrossRef]
- Maszczak-Seneczko, D.; Sosicka, P.; Kaczmarek, B.; Majkowski, M.; Luzarowski, M.; Olczak, T.; Olczak, M. UDP-galactose (SLC35A2) and UDP-N-acetylglucosamine (SLC35A3) Transporters Form Glycosylation-related Complexes with Mannoside Acetylglucosaminyltransferases (Mgats). J. Biol. Chem. 2015, 290, 15475–15486. [Google Scholar] [CrossRef]
- Wiertelak, W.; Sosicka, P.; Olczak, M.; Maszczak-Seneczko, D. Analysis of homologous and heterologous interactions between UDP-galactose transporter and beta-1,4-galactosyltransferase 1 using NanoBiT. Anal. Biochem. 2020, 593, 113599. [Google Scholar] [CrossRef] [PubMed]
- Shauchuk, A.; Szulc, B.; Maszczak-Seneczko, D.; Wiertelak, W.; Skurska, E.; Olczak, M. N-glycosylation of the human β1,4-galactosyltransferase 4 is crucial for its activity and Golgi localization. Glycoconj. J. 2020, 37, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Wiktor, M.; Wiertelak, W.; Maszczak-Seneczko, D.; Balwierz, P.J.; Szulc, B.; Olczak, M. Identification of novel potential interaction partners of UDP-galactose (SLC35A2), UDP-N-acetylglucosamine (SLC35A3) and an orphan (SLC35A4) nucleotide sugar transporters. J. Proteom. 2021, 249, 104321. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.W.; Aebi, M.; Darvill, A.G.; Kinoshita, T.; Packer, N.H.; Prestegard, J.H.; et al. Essentials of Glycobiology, 4th ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2022. [Google Scholar]
- Moriarity, J.L.; Hurt, K.J.; Resnick, A.C.; Storm, P.B.; Laroy, W.; Schnaar, R.L.; Snyder, S.H. UDP-glucuronate decarboxylase, a key enzyme in proteoglycan synthesis: Cloning, characterization, and localization. J. Biol. Chem. 2002, 277, 16968–16975. [Google Scholar] [CrossRef] [PubMed]
- Hadley, B.; Maggioni, A.; Ashikov, A.; Day, C.J.; Haselhorst, T.; Tiralongo, J. Structure and function of nucleotide sugar transporters: Current progress. Comput. Struct. Biotechnol. J. 2014, 10, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Hadley, B.; Litfin, T.; Day, C.J.; Haselhorst, T.; Zhou, Y.; Tiralongo, J. Nucleotide Sugar Transporter SLC35 Family Structure and Function. Comput. Struct. Biotechnol. J. 2019, 17, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
| Nucleotide Sugar Full Name | Abbreviation |
|---|---|
| cytidine monophosphate sialic acid | CMP-Sia |
| guanosine diphosphate mannose | GDP-Man |
| guanosine diphosphate fucose | GDP-Fuc |
| uridine diphosphate galactose | UDP-Gal |
| uridine diphosphate glucose | UDP-Glc |
| uridine diphosphate glucuronic acid | UDP-GlcA |
| uridine diphosphate N-acetylglucosamine | UDP-GlcNAc |
| uridine diphosphate N-acetylgalactosamine | UDP-GalNAc |
| uridine diphosphate xylose | UDP-Xyl |
| NST Name | Molecular Features | Subcellular Localization | Substrate Specificity | Method of Discovery | Controversial Results/Observations |
|---|---|---|---|---|---|
| SLC35A1 | 36.8 kDa, 337 aa | GA | CMP-Sia | Gene cloning by complementation of the Lec2 mutant cell line [36] |
|
| SLC35A2 | 41.0 kDa, 393 aa (UGT1), 41.3 kDa, 396 aa (UGT2) | GA (UGT1), ER/GA (UGT2) | UDP-Gal, UDP-GalNAc | Gene cloning by complementation of the Had-1 mutant cell line [33] |
|
| SLC35A3 | 36.0 kDa, 325 aa | GA | UDP-GlcNAc | Gene cloning by complementation of the K. lactis mutant [34] |
|
| SLC35C1 | 39.8 kDa, 364 aa | GA | GDP-Fuc | Gene cloning by complementation of the cells derived from LADII patients [11,37] | |
| SLC35B4 | 37.4 kDa, 331 aa | GA/ER | UDP-Xyl, UDP-GlcNAc | Transport assay in a Saccharomyces cerevisiae heterologous system [63] |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maszczak-Seneczko, D.; Wiktor, M.; Skurska, E.; Wiertelak, W.; Olczak, M. Delivery of Nucleotide Sugars to the Mammalian Golgi: A Very Well (un)Explained Story. Int. J. Mol. Sci. 2022, 23, 8648. https://doi.org/10.3390/ijms23158648
Maszczak-Seneczko D, Wiktor M, Skurska E, Wiertelak W, Olczak M. Delivery of Nucleotide Sugars to the Mammalian Golgi: A Very Well (un)Explained Story. International Journal of Molecular Sciences. 2022; 23(15):8648. https://doi.org/10.3390/ijms23158648
Chicago/Turabian StyleMaszczak-Seneczko, Dorota, Maciej Wiktor, Edyta Skurska, Wojciech Wiertelak, and Mariusz Olczak. 2022. "Delivery of Nucleotide Sugars to the Mammalian Golgi: A Very Well (un)Explained Story" International Journal of Molecular Sciences 23, no. 15: 8648. https://doi.org/10.3390/ijms23158648
APA StyleMaszczak-Seneczko, D., Wiktor, M., Skurska, E., Wiertelak, W., & Olczak, M. (2022). Delivery of Nucleotide Sugars to the Mammalian Golgi: A Very Well (un)Explained Story. International Journal of Molecular Sciences, 23(15), 8648. https://doi.org/10.3390/ijms23158648






