Immunotherapy for the Treatment of Squamous Cell Carcinoma: Potential Benefits and Challenges
Abstract
:1. Introduction
2. The Etiology of SCC
2.1. External-Factors-Associated Genetic Mutation
2.2. Internal-Factors-Associated Genetic Mutation
3. Available Treatments for SCC
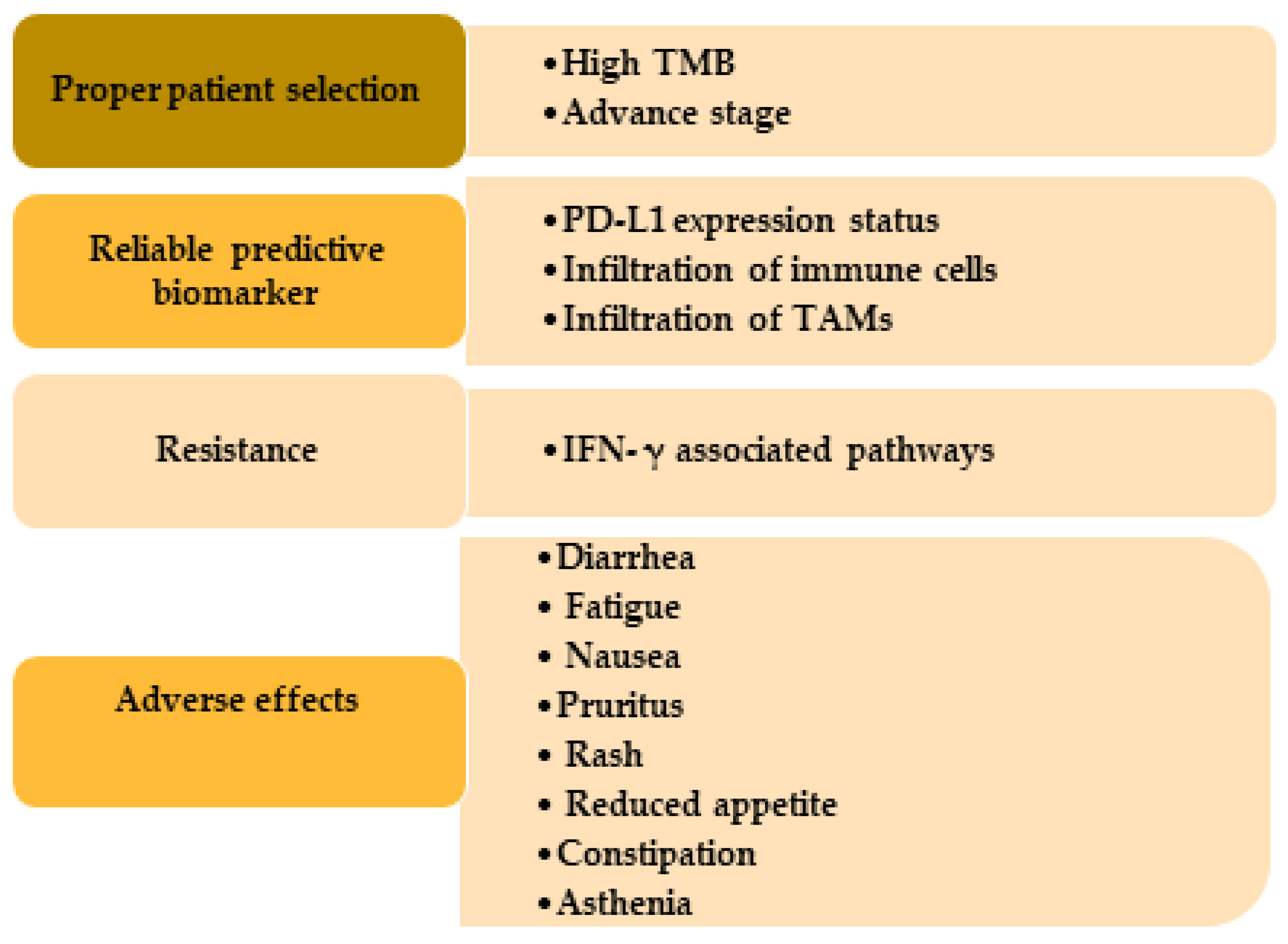
3.1. Factors That Determine the Potential Benefits of Immunotherapy in SCC Treatment
3.2. Potential Challenges of Immunotherapy in SCC Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NMSCs | nonmelanoma skin cancers |
| SCC | squamous cell carcinoma |
| UVR | ultraviolet radiation |
| BCC | basal cell carcinoma |
| FDA | Food and Drug Administration |
| CSCC | cutaneous squamous cell carcinoma |
| PUVA | PSORALEN (P) and ultraviolet light UVA |
| AK | actinic keratosis |
| BRAF | B type rapidly accelerated fibrosarcoma kinase |
| JAK | Janus kinase |
| PDE-5 | phosphodiesterase type 5 |
| OTRs | organ transplant recipients |
| TERT | telomerase reverse transcriptase |
| MAPK | mitogen-activated protein kinase |
| ROS | reactive oxygen species |
| FOXM1 | forkhead box M1 |
| COX2 | cyclooxygenase 2 |
| MMPs | matrix metalloproteinases |
| HPV | human papillomavirus |
| CDKN2A | cyclin-dependent kinase inhibitor 2A |
| FAT1 | FAT atypical cadherin 1 |
| CPDs | cyclobutane pyrimidine dimers |
| pRB | retinoblastoma phosphorylation |
| HNSCC | head and neck SCC |
| OSCC | oral SCC |
| 5-FU | 5-fluorouracil |
| EGFRis | epidermal growth factor receptor inhibitors |
| CTLA-4 | cytotoxic T-lymphocyte-associated antigen 4 |
| PD-1 | programmed cell death 1 |
| PD-L1 | programmed cell death-ligand 1 |
| TME | tumor microenvironment |
| TAMs | tumor-associated macrophages |
| TMB | Tumor mutational burden |
| NGS | Next-generation sequencing |
| TILs | tumor-infiltrating lymphocytes |
| FGF | fibroblast growth factor |
| EGF | epidermal growth factor |
| TGF-β | Transforming growth factor β |
| VEGF | vascular endothelial growth factor |
| CXCLs | C-X-C motif chemokine ligands |
| IFN-γ | interferon-gamma |
| ADAR1 | adenosine deaminase acting on RNA 1 |
| YAP | yes-associated protein |
| irAEs | immune-related adverse events |
References
- Leiter, U.; Eigentler, T.; Garbe, C. Epidemiology of skin cancer. Adv. Exp. Med. Biol. 2014, 810, 120–140. [Google Scholar] [CrossRef]
- Gloster, H.M., Jr.; Neal, K. Skin cancer in skin of color. J. Am. Acad. Dermatol. 2006, 55, 741–760. [Google Scholar] [CrossRef]
- Norval, M.; Wright, C.Y. The Epidemiology of Cutaneous Melanoma in the White and Black African Population Groups in South Africa. In Cutaneous Melanoma: Etiology and Therapy; Ward, W.H., Farma, J.M., Eds.; Codon Publications: Brisbane, QLD, Australia, 2017. [Google Scholar] [CrossRef]
- Fania, L.; Didona, D.; Di Pietro, F.R.; Verkhovskaia, S.; Morese, R.; Paolino, G.; Donati, M.; Ricci, F.; Coco, V.; Ricci, F.; et al. Cutaneous Squamous Cell Carcinoma: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2021, 9, 171. [Google Scholar] [CrossRef]
- Umar, S.A.; Tasduq, S.A. Ozone Layer Depletion and Emerging Public Health Concerns—An Update on Epidemiological Perspective of the Ambivalent Effects of Ultraviolet Radiation Exposure. Front. Oncol 2022, 12, 866733. [Google Scholar] [CrossRef]
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef]
- Xu, Y.G.; Aylward, J.L.; Swanson, A.M.; Spiegelman, V.S.; Vanness, E.R.; Teng, J.M.; Snow, S.N.; Wood, G.S. Nonmelanoma Skin Cancers: Basal Cell and Squamous Cell Carcinomas; Elsevier Inc.: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Ciazynska, M.; Kaminska-Winciorek, G.; Lange, D.; Lewandowski, B.; Reich, A.; Slawinska, M.; Pabianek, M.; Szczepaniak, K.; Hankiewicz, A.; Ulanska, M.; et al. The incidence and clinical analysis of non-melanoma skin cancer. Sci. Rep. 2021, 11, 4337. [Google Scholar] [CrossRef]
- Lukowiak, T.M.; Aizman, L.; Perz, A.; Miller, C.J.; Sobanko, J.F.; Shin, T.M.; Giordano, C.N.; Higgins, H.W., 2nd; Etzkorn, J.R. Association of Age, Sex, Race, and Geographic Region With Variation of the Ratio of Basal Cell to Cutaneous Squamous Cell Carcinomas in the United States. JAMA Dermatol. 2020, 156, 1192–1198. [Google Scholar] [CrossRef]
- Marinkovich, M.P. Tumour microenvironment: Laminin 332 in squamous-cell carcinoma. Nat. Rev. Cancer 2007, 7, 370–380. [Google Scholar] [CrossRef]
- Clayman, G.L.; Lee, J.J.; Holsinger, F.C.; Zhou, X.; Duvic, M.; El-Naggar, A.K.; Prieto, V.G.; Altamirano, E.; Tucker, S.L.; Strom, S.S.; et al. Mortality risk from squamous cell skin cancer. J. Clin. Oncol. 2005, 23, 759–765. [Google Scholar] [CrossRef]
- Tripathi, R.; Knusel, K.D.; Ezaldein, H.H.; Bordeaux, J.S.; Scott, J.F. Characteristics of Patients Hospitalized for Cutaneous Squamous Cell Carcinoma. Dermatol. Surg. 2020, 46, 742–746. [Google Scholar] [CrossRef]
- American Cancer Society. Key Statistics for Basal and Squamous Cell Skin Cancers. Available online: https://www.cancer.org/cancer/basal-and-squamous-cell-skin-cancer/about/key-statistics.html#:~:text=Death%20from%20basal%20and%20squamous,been%20dropping%20in%20recent%20years (accessed on 12 June 2022).
- Rosenblatt, L.; Marks, R. Deaths due to squamous cell carcinoma in Australia: Is there a case for a public health intervention? Australas. J. Dermatol. 1996, 37, 26–29. [Google Scholar] [CrossRef]
- Tokez, S.; Wakkee, M.; Louwman, M.; Noels, E.; Nijsten, T.; Hollestein, L. Assessment of Cutaneous Squamous Cell Carcinoma (cSCC) In situ Incidence and the Risk of Developing Invasive cSCC in Patients With Prior cSCC In situ vs the General Population in the Netherlands, 1989–2017. JAMA Dermatol. 2020, 156, 973–981. [Google Scholar] [CrossRef]
- Waldman, A.; Schmults, C. Cutaneous Squamous Cell Carcinoma. Hematol. Oncol. Clin. N. Am. 2019, 33, 1–12. [Google Scholar] [CrossRef]
- Yan, W.; Wistuba, I.I.; Emmert-Buck, M.R.; Erickson, H.S. Squamous Cell Carcinoma—Similarities and Differences among Anatomical Sites. Am. J. Cancer Res. 2011, 1, 275–300. [Google Scholar]
- Alberti, A.; Bossi, P. Immunotherapy for Cutaneous Squamous Cell Carcinoma: Results and Perspectives. Front. Oncol. 2021, 11, 727027. [Google Scholar] [CrossRef]
- Kallini, J.R.; Hamed, N.; Khachemoune, A. Squamous cell carcinoma of the skin: Epidemiology, classification, management, and novel trends. Int. J. Dermatol. 2015, 54, 130–140. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Petersen, E.; Patel, R.; Migden, M.R. Cemiplimab-rwlc as first and only treatment for advanced cutaneous squamous cell carcinoma. Expert Rev. Clin. Pharmacol. 2019, 12, 947–951. [Google Scholar] [CrossRef]
- Gasparoto, T.H.; de Oliveira, C.E.; de Freitas, L.T.; Pinheiro, C.R.; Ramos, R.N.; da Silva, A.L.; Garlet, G.P.; da Silva, J.S.; Campanelli, A.P. Inflammatory events during murine squamous cell carcinoma development. J. Inflamm. 2012, 9, 46. [Google Scholar] [CrossRef] [Green Version]
- Hanai, N.; Shimizu, Y.; Kariya, S.; Yasumatsu, R.; Yokota, T.; Fujii, T.; Tsukahara, K.; Yoshida, M.; Hanyu, K.; Ueda, T.; et al. Effectiveness and safety of nivolumab in patients with head and neck cancer in Japanese real-world clinical practice: A multicenter retrospective clinical study. Int. J. Clin. Oncol. 2021, 26, 494–506. [Google Scholar] [CrossRef]
- Dotto, G.P.; Rustgi, A.K. Squamous Cell Cancers: A Unified Perspective on Biology and Genetics. Cancer Cell 2016, 29, 622–637. [Google Scholar] [CrossRef] [Green Version]
- Tunio, M.A.; Al Asiri, M.; Fagih, M.; Akasha, R. Primary squamous cell carcinoma of thyroid: A case report and review of literature. Head Neck Oncol. 2012, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Grabowski, J.; Saltzstein, S.L.; Sadler, G.; Blair, S. Squamous cell carcinoma of the breast: A review of 177 cases. Am. Surg. 2009, 75, 914–917. [Google Scholar]
- Malik, R.D.; Dakwar, G.; Hardee, M.E.; Sanfilippo, N.J.; Rosenkrantz, A.B.; Taneja, S.S. Squamous cell carcinoma of the prostate. Rev. Urol. 2011, 13, 56–60. [Google Scholar]
- Sevencan, N.O.; Cakmakliogullari, E.K.; Ozkan, A.E.; Kayhan, B. An unusual location of squamous cell carcinoma and a rare cutaneous infection caused by serratia marcescens on the tumoral tissue: A case report. Medicine 2018, 97, e12596. [Google Scholar] [CrossRef]
- Moan, J.; Grigalavicius, M.; Baturaite, Z.; Dahlback, A.; Juzeniene, A. The relationship between UV exposure and incidence of skin cancer. Photodermatol. Photoimmunol. Photomed. 2015, 31, 26–35. [Google Scholar] [CrossRef]
- Bernstein, S.C.; Lim, K.K.; Brodland, D.G.; Heidelberg, K.A. The many faces of squamous cell carcinoma. Dermatol. Surg. 1996, 22, 243–254. [Google Scholar] [CrossRef]
- Jaju, P.D.; Ransohoff, K.J.; Tang, J.Y.; Sarin, K.Y. Familial skin cancer syndromes: Increased risk of nonmelanotic skin cancers and extracutaneous tumors. J. Am. Acad. Dermatol. 2016, 74, 437–451. [Google Scholar] [CrossRef]
- Axibal, E.L.; Brown, M.R. Squamous Cell Carcinoma; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Johnson, N.M.; Prickett, K.A.; Phillips, M.A. Systemic medications linked to an increased risk for skin malignancy. Cutis 2019, 104, E32–E36. [Google Scholar]
- Schmidt, S.A.; Schmidt, M.; Mehnert, F.; Lemeshow, S.; Sorensen, H.T. Use of antihypertensive drugs and risk of skin cancer. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1545–1554. [Google Scholar] [CrossRef]
- Lichter, M.D.; Karagas, M.R.; Mott, L.A.; Spencer, S.K.; Stukel, T.A.; Greenberg, E.R. Therapeutic ionizing radiation and the incidence of basal cell carcinoma and squamous cell carcinoma. The New Hampshire Skin Cancer Study Group. Arch. Dermatol. 2000, 136, 1007–1011. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, R.P.; Bajdik, C.D.; Fincham, S.; Hill, G.B.; Keefe, A.R.; Coldman, A.; McLean, D.I. Chemical exposures, medical history, and risk of squamous and basal cell carcinoma of the skin. Cancer Epidemiol. Biomark. Prev. 1996, 5, 419–424. [Google Scholar]
- Nikakhlagh, S.; Saki, N.; Shoar, M.H.; Sartipipor, A.; Saki, S. Incidence of etiologic factors in squamous cell carcinoma of head and neck in ahvaz. Iran. J. Otorhinolaryngol. 2012, 24, 85–90. [Google Scholar]
- Stern, R.S.; Study, P.F.-U. The risk of squamous cell and basal cell cancer associated with psoralen and ultraviolet A therapy: A 30-year prospective study. J. Am. Acad. Dermatol. 2012, 66, 553–562. [Google Scholar] [CrossRef]
- Gallagher, R.P.; Hill, G.B.; Bajdik, C.D.; Coldman, A.J.; Fincham, S.; McLean, D.I.; Threlfall, W.J. Sunlight exposure, pigmentation factors, and risk of nonmelanocytic skin cancer. II. Squamous cell carcinoma. Arch. Dermatol. 1995, 131, 164–169. [Google Scholar]
- Trent, J.T.; Kirsner, R.S. Wounds and malignancy. Adv. Skin Wound Care 2003, 16, 31–34. [Google Scholar] [CrossRef]
- Karagas, M.R.; Stukel, T.A.; Greenberg, E.R.; Baron, J.A.; Mott, L.A.; Stern, R.S. Risk of subsequent basal cell carcinoma and squamous cell carcinoma of the skin among patients with prior skin cancer. Skin Cancer Prevention Study Group. JAMA 1992, 267, 3305–3310. [Google Scholar]
- Williams, K.; Mansh, M.; Chin-Hong, P.; Singer, J.; Arron, S.T. Voriconazole-associated cutaneous malignancy: A literature review on photocarcinogenesis in organ transplant recipients. Clin. Infect. Dis. 2014, 58, 997–1002. [Google Scholar] [CrossRef] [Green Version]
- Diepgen, T.L.; Mahler, V. The epidemiology of skin cancer. Br. J. Dermatol. 2002, 146 (Suppl. 61), 1–6. [Google Scholar] [CrossRef]
- Kim, Y.; He, Y.Y. Ultraviolet radiation-induced non-melanoma skin cancer: Regulation of DNA damage repair and inflammation. Genes Dis. 2014, 1, 188–198. [Google Scholar] [CrossRef] [Green Version]
- Lephart, E.D. Equol’s Anti-Aging Effects Protect against Environmental Assaults by Increasing Skin Antioxidant Defense and ECM Proteins While Decreasing Oxidative Stress and Inflammation. Cosmetics 2018, 5, 16. [Google Scholar]
- O’Connor, C.; Courtney, C.; Murphy, M. Shedding light on the myths of ultraviolet radiation in the COVID-19 pandemic. Clin. Exp. Dermatol. 2021, 46, 187–188. [Google Scholar] [CrossRef]
- Strzałka, W.; Zgłobicki, P.; Kowalska, E.; Bażant, A.; Dziga, D.; Banaś, A.K. The Dark Side of UV-Induced DNA Lesion Repair. Genes 2020, 11, 1450. [Google Scholar]
- Kozmin, S.; Slezak, G.; Reynaud-Angelin, A.; Elie, C.; de Rycke, Y.; Boiteux, S.; Sage, E. UVA radiation is highly mutagenic in cells that are unable to repair 7,8-dihydro-8-oxoguanine in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2005, 102, 13538–13543. [Google Scholar] [CrossRef] [Green Version]
- Mesa-Arango, A.C.; Flórez-Muñoz, S.V.; Sanclemente, G. Mechanisms of skin aging. Iatreia 2017, 30, 160–170. [Google Scholar]
- Goto, N.; Bazar, G.; Kovacs, Z.; Kunisada, M.; Morita, H.; Kizaki, S.; Sugiyama, H.; Tsenkova, R.; Nishigori, C. Detection of UV-induced cyclobutane pyrimidine dimers by near-infrared spectroscopy and aquaphotomics. Sci. Rep. 2015, 5, 11808. [Google Scholar] [CrossRef] [Green Version]
- Brash, D.E.; Rudolph, J.A.; Simon, J.A.; Lin, A.; McKenna, G.J.; Baden, H.P.; Halperin, A.J.; Ponten, J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 1991, 88, 10124–10128. [Google Scholar] [CrossRef] [Green Version]
- Brash, D.E.; Ziegler, A.; Jonason, A.S.; Simon, J.A.; Kunala, S.; Leffell, D.J. Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. J. Investig. Dermatol. Symp. Proc. 1996, 1, 136–142. [Google Scholar]
- Li, G.; Tron, V.; Ho, V. Induction of squamous cell carcinoma in p53-deficient mice after ultraviolet irradiation. J. Investig. Dermatol. 1998, 110, 72–75. [Google Scholar] [CrossRef] [Green Version]
- Melnikova, V.O.; Pacifico, A.; Chimenti, S.; Peris, K.; Ananthaswamy, H.N. Fate of UVB-induced p53 mutations in SKH-hr1 mouse skin after discontinuation of irradiation: Relationship to skin cancer development. Oncogene 2005, 24, 7055–7063. [Google Scholar] [CrossRef] [Green Version]
- Chasov, V.; Mirgayazova, R.; Zmievskaya, E.; Khadiullina, R.; Valiullina, A.; Stephenson Clarke, J.; Rizvanov, A.; Baud, M.G.J.; Bulatov, E. Key Players in the Mutant p53 Team: Small Molecules, Gene Editing, Immunotherapy. Front. Oncol. 2020, 10, 1460. [Google Scholar] [CrossRef]
- Smith, M.L.; Fornace, A.J., Jr. p53-mediated protective responses to UV irradiation. Proc. Natl. Acad. Sci. USA 1997, 94, 12255–12257. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, A.; Jonason, A.S.; Leffell, D.J.; Simon, J.A.; Sharma, H.W.; Kimmelman, J.; Remington, L.; Jacks, T.; Brash, D.E. Sunburn and p53 in the onset of skin cancer. Nature 1994, 372, 773–776. [Google Scholar] [CrossRef]
- Inman, G.J.; Wang, J.; Nagano, A.; Alexandrov, L.B.; Purdie, K.J.; Taylor, R.G.; Sherwood, V.; Thomson, J.; Hogan, S.; Spender, L.C.; et al. The genomic landscape of cutaneous SCC reveals drivers and a novel azathioprine associated mutational signature. Nat. Commun. 2018, 9, 3667. [Google Scholar] [CrossRef]
- Brown, V.L.; Harwood, C.A.; Crook, T.; Cronin, J.G.; Kelsell, D.P.; Proby, C.M. p16INK4a and p14ARF tumor suppressor genes are commonly inactivated in cutaneous squamous cell carcinoma. J. Investig. Dermatol. 2004, 122, 1284–1292. [Google Scholar] [CrossRef] [Green Version]
- Soufir, N.; Moles, J.P.; Vilmer, C.; Moch, C.; Verola, O.; Rivet, J.; Tesniere, A.; Dubertret, L.; Basset-Seguin, N. P16 UV mutations in human skin epithelial tumors. Oncogene 1999, 18, 5477–5481. [Google Scholar] [CrossRef] [Green Version]
- Conscience, I.; Jovenin, N.; Coissard, C.; Lorenzato, M.; Durlach, A.; Grange, F.; Birembaut, P.; Clavel, C.; Bernard, P. P16 is overexpressed in cutaneous carcinomas located on sun-exposed areas. Eur. J. Dermatol. 2006, 16, 518–522. [Google Scholar]
- Hodges, A.; Smoller, B.R. Immunohistochemical comparison of p16 expression in actinic keratoses and squamous cell carcinomas of the skin. Mod. Pathol. 2002, 15, 1121–1125. [Google Scholar] [CrossRef]
- Bito, T.; Ueda, M.; Ahmed, N.U.; Nagano, T.; Ichihashi, M. Cyclin D and retinoblastoma gene product expression in actinic keratosis and cutaneous squamous cell carcinoma in relation to p53 expression. J. Cutan. Pathol. 1995, 22, 427–434. [Google Scholar] [CrossRef]
- Populo, H.; Boaventura, P.; Vinagre, J.; Batista, R.; Mendes, A.; Caldas, R.; Pardal, J.; Azevedo, F.; Honavar, M.; Guimaraes, I.; et al. TERT promoter mutations in skin cancer: The effects of sun exposure and X-irradiation. J. Investig. Dermatol. 2014, 134, 2251–2257. [Google Scholar] [CrossRef] [Green Version]
- Scott, G.A.; Laughlin, T.S.; Rothberg, P.G. Mutations of the TERT promoter are common in basal cell carcinoma and squamous cell carcinoma. Mod. Pathol. 2014, 27, 516–523. [Google Scholar] [CrossRef] [Green Version]
- Beyersmann, D.; Hartwig, A. Carcinogenic metal compounds: Recent insight into molecular and cellular mechanisms. Arch. Toxicol. 2008, 82, 493–512. [Google Scholar] [CrossRef]
- Yu, H.S.; Liao, W.T.; Chai, C.Y. Arsenic carcinogenesis in the skin. J. Biomed. Sci. 2006, 13, 657–666. [Google Scholar] [CrossRef] [Green Version]
- Matsui, M.; Nishigori, C.; Toyokuni, S.; Takada, J.; Akaboshi, M.; Ishikawa, M.; Imamura, S.; Miyachi, Y. The role of oxidative DNA damage in human arsenic carcinogenesis: Detection of 8-hydroxy-2′-deoxyguanosine in arsenic-related Bowen’s disease. J. Investig. Dermatol. 1999, 113, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Chanda, S.; Dasgupta, U.B.; Guhamazumder, D.; Gupta, M.; Chaudhuri, U.; Lahiri, S.; Das, S.; Ghosh, N.; Chatterjee, D. DNA hypermethylation of promoter of gene p53 and p16 in arsenic-exposed people with and without malignancy. Toxicol. Sci. 2006, 89, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.W.; Lee, C.H.; Yu, H.S. Arsenic-Induced Carcinogenesis and Immune Dysregulation. Int. J. Environ. Res. Public Health 2019, 16, 2746. [Google Scholar] [CrossRef] [Green Version]
- Davidson, T.; Kluz, T.; Burns, F.; Rossman, T.; Zhang, Q.; Uddin, A.; Nadas, A.; Costa, M. Exposure to chromium (VI) in the drinking water increases susceptibility to UV-induced skin tumors in hairless mice. Toxicol. Appl. Pharmacol. 2004, 196, 431–437. [Google Scholar] [CrossRef]
- Leonardi-Bee, J.; Ellison, T.; Bath-Hextall, F. Smoking and the risk of nonmelanoma skin cancer: Systematic review and meta-analysis. Arch. Dermatol. 2012, 148, 939–946. [Google Scholar] [CrossRef]
- Stransky, N.; Egloff, A.M.; Tward, A.D.; Kostic, A.D.; Cibulskis, K.; Sivachenko, A.; Kryukov, G.V.; Lawrence, M.S.; Sougnez, C.; McKenna, A.; et al. The mutational landscape of head and neck squamous cell carcinoma. Science 2011, 333, 1157–1160. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, N.; Frederick, M.J.; Pickering, C.R.; Bettegowda, C.; Chang, K.; Li, R.J.; Fakhry, C.; Xie, T.X.; Zhang, J.; Wang, J.; et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science 2011, 333, 1154–1157. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Wu, J.; Wang, J.; Huang, R. Tobacco and oral squamous cell carcinoma: A review of carcinogenic pathways. Tob. Induc. Dis. 2019, 17, 29. [Google Scholar] [CrossRef]
- Nataraj, A.J.; Wolf, P.; Cerroni, L.; Ananthaswamy, H.N. p53 mutation in squamous cell carcinomas from psoriasis patients treated with psoralen + UVA (PUVA). J. Investig. Dermatol. 1997, 109, 238–243. [Google Scholar] [CrossRef] [Green Version]
- King, A.J.; Arnone, M.R.; Bleam, M.R.; Moss, K.G.; Yang, J.; Fedorowicz, K.E.; Smitheman, K.N.; Erhardt, J.A.; Hughes-Earle, A.; Kane-Carson, L.S.; et al. Dabrafenib; preclinical characterization, increased efficacy when combined with trametinib, while BRAF/MEK tool combination reduced skin lesions. PLoS ONE 2013, 8, e67583. [Google Scholar] [CrossRef] [Green Version]
- Poulikakos, P.I.; Zhang, C.; Bollag, G.; Shokat, K.M.; Rosen, N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 2010, 464, 427–430. [Google Scholar] [CrossRef] [Green Version]
- Heidorn, S.J.; Milagre, C.; Whittaker, S.; Nourry, A.; Niculescu-Duvas, I.; Dhomen, N.; Hussain, J.; Reis-Filho, J.S.; Springer, C.J.; Pritchard, C.; et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 2010, 140, 209–221. [Google Scholar] [CrossRef]
- Zhu, G.A.; Sundram, U.; Chang, A.L. Two different scenarios of squamous cell carcinoma within advanced Basal cell carcinomas: Cases illustrating the importance of serial biopsy during vismodegib usage. JAMA Dermatol. 2014, 150, 970–973. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.V.; Chang, J.; Li, S.; Henry, A.S.; Wood, D.J.; Chang, A.L. Increased Risk of Cutaneous Squamous Cell Carcinoma After Vismodegib Therapy for Basal Cell Carcinoma. JAMA Dermatol. 2016, 152, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.Q.; Li, S.Q.; Li, S.; Kiamanesh, E.F.; Aasi, S.Z.; Kwong, B.Y.; Su Chang, A.L. A 10-year retrospective cohort study of ruxolitinib and association with nonmelanoma skin cancer in patients with polycythemia vera and myelofibrosis. J. Am. Acad. Dermatol. 2022, 86, 339–344. [Google Scholar] [CrossRef]
- March-Rodriguez, A.; Bellosillo, B.; Alvarez-Larran, A.; Besses, C.; Pujol, R.M.; Toll, A. Rapidly Growing and Aggressive Cutaneous Squamous Cell Carcinomas in a Patient Treated with Ruxolitinib. Ann. Dermatol. 2019, 31, 204–208. [Google Scholar] [CrossRef]
- Shin, D.; Lee, E.S.; Kim, J.; Guerra, L.; Naik, D.; Prida, X. Association Between the Use of Thiazide Diuretics and the Risk of Skin Cancers: A Meta-Analysis of Observational Studies. J. Clin. Med. Res. 2019, 11, 247–255. [Google Scholar] [CrossRef] [Green Version]
- D’Arcy, M.E.; Pfeiffer, R.M.; Rivera, D.R.; Hess, G.P.; Cahoon, E.K.; Arron, S.T.; Brownell, I.; Cowen, E.W.; Israni, A.K.; Triplette, M.A.; et al. Voriconazole and the Risk of Keratinocyte Carcinomas Among Lung Transplant Recipients in the United States. JAMA Dermatol. 2020, 156, 772–779. [Google Scholar] [CrossRef]
- Lanz, J.; Bouwes Bavinck, J.N.; Westhuis, M.; Quint, K.D.; Harwood, C.A.; Nasir, S.; Van-de-Velde, V.; Proby, C.M.; Ferrandiz, C.; Genders, R.E.; et al. Aggressive Squamous Cell Carcinoma in Organ Transplant Recipients. JAMA Dermatol. 2019, 155, 66–71. [Google Scholar] [CrossRef]
- Abikhair Burgo, M.; Roudiani, N.; Chen, J.; Santana, A.L.; Doudican, N.; Proby, C.; Felsen, D.; Carucci, J.A. Ruxolitinib inhibits cyclosporine-induced proliferation of cutaneous squamous cell carcinoma. JCI Insight 2018, 3, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Impola, U.; Jeskanen, L.; Ravanti, L.; Syrjanen, S.; Baldursson, B.; Kahari, V.M.; Saarialho-Kere, U. Expression of matrix metalloproteinase (MMP)-7 and MMP-13 and loss of MMP-19 and p16 are associated with malignant progression in chronic wounds. Br. J. Dermatol. 2005, 152, 720–726. [Google Scholar] [CrossRef]
- Filoni, A.; Cicco, G.; Cazzato, G.; Bosco, A.; Lospalluti, L.; Tucci, M.; Cimmino, A.; Foti, C.; Marzullo, A.; Bonamonte, D. Immune Disregulation in Cutaneous Squamous Cell Carcinoma of Patients with Recessive Dystrophic Epidermolysis Bullosa: A Single Pilot Study. Life 2022, 12, 213. [Google Scholar] [CrossRef]
- Condorelli, A.G.; Dellambra, E.; Logli, E.; Zambruno, G.; Castiglia, D. Epidermolysis Bullosa-Associated Squamous Cell Carcinoma: From Pathogenesis to Therapeutic Perspectives. Int. J. Mol. Sci. 2019, 20, 5707. [Google Scholar] [CrossRef] [Green Version]
- Weinberg, A.S.; Ogle, C.A.; Shim, E.K. Metastatic cutaneous squamous cell carcinoma: An update. Dermatol. Surg. 2007, 33, 885–899. [Google Scholar] [CrossRef]
- Feldman, S.R.; Fleischer, A.B., Jr. Progression of actinic keratosis to squamous cell carcinoma revisited: Clinical and treatment implications. Cutis 2011, 87, 201–207. [Google Scholar]
- Claveau, J.; Archambault, J.; Ernst, D.S.; Giacomantonio, C.; Limacher, J.J.; Murray, C.; Parent, F.; Zloty, D. Multidisciplinary management of locally advanced and metastatic cutaneous squamous cell carcinoma. Curr. Oncol. 2020, 27, e399–e407. [Google Scholar] [CrossRef]
- Rong, Y.; Zuo, L.; Shang, L.; Bazan, J.G. Radiotherapy treatment for nonmelanoma skin cancer. Expert Rev. Anticancer. Ther. 2015, 15, 765–776. [Google Scholar] [CrossRef]
- Cartei, G.; Cartei, F.; Interlandi, G.; Meneghini, G.; Jop, A.; Zingone, G.; Tabaro, G.; Mazzoleni, F. Oral 5-fluorouracil in squamous cell carcinoma of the skin in the aged. Am. J. Clin. Oncol. 2000, 23, 181–184. [Google Scholar] [CrossRef]
- Hashmi, A.A.; Hussain, Z.F.; Aijaz, S.; Irfan, M.; Khan, E.Y.; Naz, S.; Faridi, N.; Khan, A.; Edhi, M.M. Immunohistochemical expression of epidermal growth factor receptor (EGFR) in South Asian head and neck squamous cell carcinoma: Association with various risk factors and clinico-pathologic and prognostic parameters. World J. Surg. Oncol. 2018, 16, 118. [Google Scholar] [CrossRef]
- Rischin, D.; Harrington, K.J.; Greil, R.; Soulieres, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Brana, I.; Neupane, P.; Bratland, A.; et al. Pembrolizumab alone or with chemotherapy for recurrent or metastatic head and neck squamous cell carcinoma: Health-related quality-of-life results from KEYNOTE-048. Oral. Oncol. 2022, 128, 105815. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef] [Green Version]
- Montaudie, H.; Viotti, J.; Combemale, P.; Dutriaux, C.; Dupin, N.; Robert, C.; Mortier, L.; Kaphan, R.; Duval-Modeste, A.B.; Dalle, S.; et al. Cetuximab is efficient and safe in patients with advanced cutaneous squamous cell carcinoma: A retrospective, multicentre study. Oncotarget 2020, 11, 378–385. [Google Scholar] [CrossRef] [Green Version]
- William, W.N., Jr.; Feng, L.; Ferrarotto, R.; Ginsberg, L.; Kies, M.; Lippman, S.; Glisson, B.; Kim, E.S. Gefitinib for patients with incurable cutaneous squamous cell carcinoma: A single-arm phase II clinical trial. J. Am. Acad. Dermatol. 2017, 77, 1110–1113.e2. [Google Scholar] [CrossRef]
- Hughes, B.G.M.; Munoz-Couselo, E.; Mortier, L.; Bratland, A.; Gutzmer, R.; Roshdy, O.; Gonzalez Mendoza, R.; Schachter, J.; Arance, A.; Grange, F.; et al. Pembrolizumab for locally advanced and recurrent/metastatic cutaneous squamous cell carcinoma (KEYNOTE-629 study): An open-label, nonrandomized, multicenter, phase II trial. Ann. Oncol. 2021, 32, 1276–1285. [Google Scholar] [CrossRef]
- Kato, K.; Cho, B.C.; Takahashi, M.; Okada, M.; Lin, C.Y.; Chin, K.; Kadowaki, S.; Ahn, M.J.; Hamamoto, Y.; Doki, Y.; et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 1506–1517. [Google Scholar] [CrossRef]
- Doki, Y.; Ajani, J.A.; Kato, K.; Xu, J.; Wyrwicz, L.; Motoyama, S.; Ogata, T.; Kawakami, H.; Hsu, C.H.; Adenis, A.; et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N. Engl. J. Med. 2022, 386, 449–462. [Google Scholar] [CrossRef]
- Hindson, J. Nivolumab plus chemotherapy or ipilimumab for advanced oesophageal squamous cell carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 216. [Google Scholar] [CrossRef]
- Soura, E.; Gagari, E.; Stratigos, A. Advanced cutaneous squamous cell carcinoma: How is it defined and what new therapeutic approaches are available? Curr. Opin. Oncol. 2019, 31, 461–468. [Google Scholar] [CrossRef]
- Cheng, G.; Dong, H.; Yang, C.; Liu, Y.; Wu, Y.; Zhu, L.; Tong, X.; Wang, S. A review on the advances and challenges of immunotherapy for head and neck cancer. Cancer Cell Int. 2021, 21, 406. [Google Scholar] [CrossRef]
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 341–351. [Google Scholar] [CrossRef] [Green Version]
- Guminski, A.D.; Lim, A.M.L.; Khushalani, N.I.; Schmults, C.D.; Hernandez-Aya, L.F.; Modi, B.; Dunn, L.; Hughes, B.G.M.; Chang, A.L.S.; Hauschild, A.; et al. Phase 2 study of cemiplimab, a human monoclonal anti-PD-1, in patients (pts) with metastatic cutaneous squamous cell carcinoma (mCSCC.; Group 1): 12-month follow-up. J. Clin. Oncol. 2019, 37, 9526. [Google Scholar] [CrossRef]
- Bhat, A.A.; Yousuf, P.; Wani, N.A.; Rizwan, A.; Chauhan, S.S.; Siddiqi, M.A.; Bedognetti, D.; El-Rifai, W.; Frenneaux, M.P.; Batra, S.K.; et al. Tumor microenvironment: An evil nexus promoting aggressive head and neck squamous cell carcinoma and avenue for targeted therapy. Signal. Transduct. Target. Ther. 2021, 6, 12. [Google Scholar] [CrossRef]
- Nisar, S.; Yousuf, P.; Masoodi, T.; Wani, N.A.; Hashem, S.; Singh, M.; Sageena, G.; Mishra, D.; Kumar, R.; Haris, M.; et al. Chemokine-Cytokine Networks in the Head and Neck Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 4584. [Google Scholar] [CrossRef]
- Peltanova, B.; Raudenska, M.; Masarik, M. Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: A systematic review. Mol. Cancer 2019, 18, 63. [Google Scholar] [CrossRef]
- Fu, Z.M.; Zhang, D.J.; Guo, Y.Y.; Han, S.; Guo, F.; Bai, J.; Wan, Y.N.; Guan, G.F.; Sun, K.W.; Yang, N. Expression of PD-L1 and CD4+ tumor-infiltrating lymphocytes predict survival in head and neck squamous cell carcinoma. Mol. Clin. Oncol. 2022, 16, 59. [Google Scholar] [CrossRef]
- Dos antos Pereira, J.; da Costa Miguel, M.C.; Guedes Queiroz, L.M.; da Silveira, E.J. Analysis of CD8+ and CD4+ cells in oral squamous cell carcinoma and their association with lymph node metastasis and histologic grade of malignancy. Appl. Immunohistochem. Mol. Morphol. 2014, 22, 200–205. [Google Scholar] [CrossRef]
- Freeman, A.; Bridge, J.A.; Maruthayanar, P.; Overgaard, N.H.; Jung, J.W.; Simpson, F.; Prow, T.W.; Soyer, H.P.; Frazer, I.H.; Freeman, M.; et al. Comparative immune phenotypic analysis of cutaneous Squamous Cell Carcinoma and Intraepidermal Carcinoma in immune-competent individuals: Proportional representation of CD8+ T-cells but not FoxP3+ Regulatory T-cells is associated with disease stage. PLoS ONE 2014, 9, e110928. [Google Scholar] [CrossRef]
- Weber, F.; Byrne, S.N.; Le, S.; Brown, D.A.; Breit, S.N.; Scolyer, R.A.; Halliday, G.M. Transforming growth factor-beta1 immobilises dendritic cells within skin tumours and facilitates tumour escape from the immune system. Cancer Immunol. Immunother. 2005, 54, 898–906. [Google Scholar] [CrossRef]
- Dodagatta-Marri, E.; Meyer, D.S.; Reeves, M.Q.; Paniagua, R.; To, M.D.; Binnewies, M.; Broz, M.L.; Mori, H.; Wu, D.; Adoumie, M.; et al. alpha-PD-1 therapy elevates Treg/Th balance and increases tumor cell pSmad3 that are both targeted by alpha-TGFbeta antibody to promote durable rejection and immunity in squamous cell carcinomas. J. Immunother Cancer 2019, 7, 62. [Google Scholar] [CrossRef]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.P.; Wang, R.; Liu, Q.D.; Xu, X.W.; Wei, W.; Huang, X.T.; Peng, X.M.; Liu, Z.G. Combination of Tumor Mutational Burden and Specific Gene Mutations Stratifies Outcome to Immunotherapy Across Recurrent and Metastatic Head and Neck Squamous Cell Carcinoma. Front. Genet. 2021, 12, 756506. [Google Scholar] [CrossRef]
- Mata, D.A.; Williams, E.A.; Sokol, E.; Oxnard, G.R.; Fleischmann, Z.; Tse, J.Y.; Decker, B. Prevalence of UV Mutational Signatures Among Cutaneous Primary Tumors. JAMA Netw. Open 2022, 5, e223833. [Google Scholar] [CrossRef]
- Kalogirou, E.M.; Tosios, K.I.; Christopoulos, P.F. The Role of Macrophages in Oral Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 611115. [Google Scholar] [CrossRef]
- Essa, A.A.; Yamazaki, M.; Maruyama, S.; Abe, T.; Babkair, H.; Raghib, A.M.; Megahed, E.M.; Cheng, J.; Saku, T. Tumour-associated macrophages are recruited and differentiated in the neoplastic stroma of oral squamous cell carcinoma. Pathology 2016, 48, 219–227. [Google Scholar] [CrossRef]
- Pettersen, J.S.; Fuentes-Duculan, J.; Suarez-Farinas, M.; Pierson, K.C.; Pitts-Kiefer, A.; Fan, L.; Belkin, D.A.; Wang, C.Q.; Bhuvanendran, S.; Johnson-Huang, L.M.; et al. Tumor-associated macrophages in the cutaneous SCC microenvironment are heterogeneously activated. J. Investig. Dermatol. 2011, 131, 1322–1330. [Google Scholar] [CrossRef]
- Roh, M.R.; Zheng, Z.; Kim, H.S.; Kwon, J.E.; Jeung, H.C.; Rha, S.Y.; Chung, K.Y. Differential expression patterns of MMPs and their role in the invasion of epithelial premalignant tumors and invasive cutaneous squamous cell carcinoma. Exp. Mol. Pathol. 2012, 92, 236–242. [Google Scholar] [CrossRef]
- Rong, L.; Liu, Y.; Hui, Z.; Zhao, Z.; Zhang, Y.; Wang, B.; Yuan, Y.; Li, W.; Guo, L.; Ying, J.; et al. PD-L1 expression and its clinicopathological correlation in advanced esophageal squamous cell carcinoma in a Chinese population. Diagn. Pathol. 2019, 14, 6. [Google Scholar] [CrossRef]
- Derynck, R.; Turley, S.J.; Akhurst, R.J. TGFbeta biology in cancer progression and immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 9–34. [Google Scholar] [CrossRef]
- Kondo, Y.; Suzuki, S.; Takahara, T.; Ono, S.; Goto, M.; Miyabe, S.; Sugita, Y.; Ogawa, T.; Ito, H.; Satou, A.; et al. Improving function of cytotoxic T-lymphocytes by transforming growth factor-beta inhibitor in oral squamous cell carcinoma. Cancer Sci. 2021, 112, 4037–4049. [Google Scholar] [CrossRef]
- Son, H.K.; Kim, D.; Lim, Y.; Kim, J.; Park, I. A novel TGF-beta receptor II mutation (I227T/N236D) promotes aggressive phenotype of oral squamous cell carcinoma via enhanced EGFR signaling. BMC Cancer 2020, 20, 1163. [Google Scholar] [CrossRef]
- Xie, J.; Huang, L.; Lu, Y.G.; Zheng, D.L. Roles of the Wnt Signaling Pathway in Head and Neck Squamous Cell Carcinoma. Front. Mol. Biosci. 2020, 7, 590912. [Google Scholar] [CrossRef]
- Goodman, A.M.; Kato, S.; Chattopadhyay, R.; Okamura, R.; Saunders, I.M.; Montesion, M.; Frampton, G.M.; Miller, V.A.; Daniels, G.A.; Kurzrock, R. Phenotypic and Genomic Determinants of Immunotherapy Response Associated with Squamousness. Cancer Immunol. Res. 2019, 7, 866–873. [Google Scholar] [CrossRef] [Green Version]
- Burcher, K.M.; Lantz, J.W.; Gavrila, E.; Abreu, A.; Burcher, J.T.; Faucheux, A.T.; Xie, A.; Jackson, C.; Song, A.H.; Hughes, R.T.; et al. Relationship between Tumor Mutational Burden, PD-L1, Patient Characteristics, and Response to Immune Checkpoint Inhibitors in Head and Neck Squamous Cell Carcinoma. Cancers 2021, 13, 5733. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Liu, J.; Li, W.; Li, S.; Han, X. Lactoferricin B reverses cisplatin resistance in head and neck squamous cell carcinoma cells through targeting PD-L1. Cancer Med. 2018, 7, 3178–3187. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Bell, R.B.; Bifulco, C.B.; Burtness, B.; Gillison, M.L.; Harrington, K.J.; Le, Q.T.; Lee, N.Y.; Leidner, R.; Lewis, R.L.; et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J. Immunother. Cancer 2019, 7, 184. [Google Scholar] [CrossRef] [Green Version]
- Prat, A.; Navarro, A.; Pare, L.; Reguart, N.; Galvan, P.; Pascual, T.; Martinez, A.; Nuciforo, P.; Comerma, L.; Alos, L.; et al. Immune-Related Gene Expression Profiling After PD-1 Blockade in Non-Small Cell Lung Carcinoma, Head and Neck Squamous Cell Carcinoma, and Melanoma. Cancer Res. 2017, 77, 3540–3550. [Google Scholar] [CrossRef] [Green Version]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef]
- Lei, Q.; Wang, D.; Sun, K.; Wang, L.; Zhang, Y. Resistance Mechanisms of Anti-PD1/PDL1 Therapy in Solid Tumors. Front. Cell Dev. Biol. 2020, 8, 672. [Google Scholar] [CrossRef]
- Ishizuka, J.J.; Manguso, R.T.; Cheruiyot, C.K.; Bi, K.; Panda, A.; Iracheta-Vellve, A.; Miller, B.C.; Du, P.P.; Yates, K.B.; Dubrot, J.; et al. Loss of ADAR1 in tumours overcomes resistance to immune checkpoint blockade. Nature 2019, 565, 43–48. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Lin, W.; Shuai, P. ADAR1 overexpression is associated with cervical cancer progression and angiogenesis. Diagn. Pathol. 2017, 12, 12. [Google Scholar] [CrossRef] [Green Version]
- Qiao, J.J.; Chan, T.H.; Qin, Y.R.; Chen, L. ADAR1: A promising new biomarker for esophageal squamous cell carcinoma? Expert Rev. Anticancer Ther. 2014, 14, 865–868. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Peng, Z.; Qin, M.; Liu, Y.; Wang, J.; Zhang, C.; Lin, J.; Dong, T.; Wang, L.; Li, S.; et al. Interferon-gamma induces tumor resistance to anti-PD-1 immunotherapy by promoting YAP phase separation. Mol. Cell 2021, 81, 1216–1230.e1219. [Google Scholar] [CrossRef]
- Ge, L.; Smail, M.; Meng, W.; Shyr, Y.; Ye, F.; Fan, K.H.; Li, X.; Zhou, H.M.; Bhowmick, N.A. Yes-associated protein expression in head and neck squamous cell carcinoma nodal metastasis. PLoS ONE 2011, 6, e27529. [Google Scholar] [CrossRef] [Green Version]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
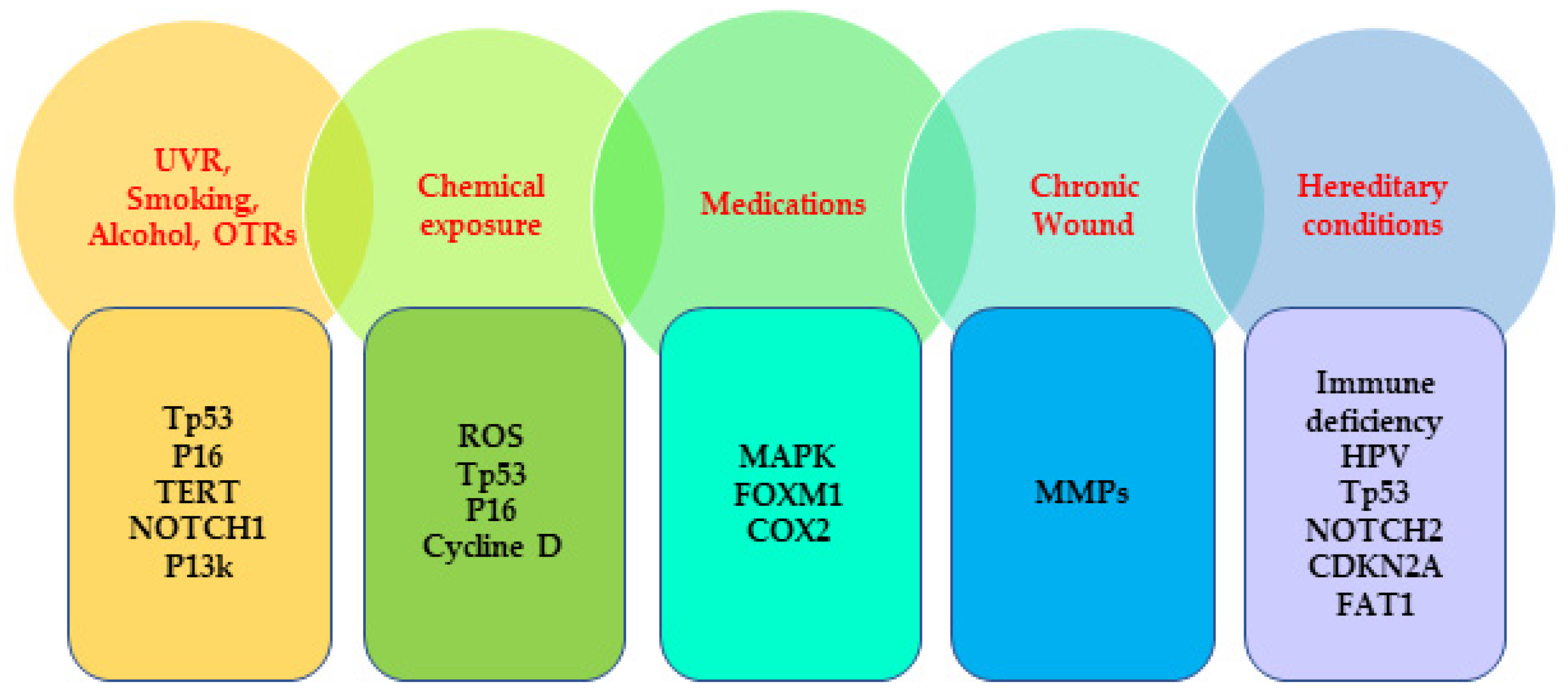

| Factor Type | Major Factors | Specific Factors |
|---|---|---|
| Extrinsic Factors | Ultraviolet radiation (UVR) | Ultraviolet (UV)B, UVA |
| Radiotherapy | ||
| Chemical exposure | nickel, arsenic, chromium, hydrocarbon, pesticides, herbicides, insecticides, fungicides, petroleum products, (gasoline and oil), grease, and diesel fumes | |
| Habitual factors | cigarette smoking, alcohol consumption, dietary factors (iron deficiency, malnutrition, oral hygiene), 8-methoxypsoralen (P) and UVA (PUVA) treatment | |
| Medications | BRAF inhibitors; vemurafenib, dabrafenib, sonic hedgehog-inhibiting agents; vismodegib; JAK inhibitors; ruxolitinib, PDE-5 inhibitors, antihypertensive drugs; diuretics, antifungal medication; voriconazole | |
| Intrinsic factors | Age | more than 60 |
| Sex | male | |
| Skin type | pale skin | |
| Precancerous lesion | actinic keratosis, Bowen’s disease | |
| History of immune suppression | post transplantation, cancer therapy | |
| Chronic medical conditions | organ transplant recipients, chronic wounds (chronic osteomyelitis, chronic venous ulcers) | |
| Viral infections | HIV/AIDS, human papillomavirus, Epstein–Barr virus, John Cunningham virus | |
| Hereditary conditions | (xeroderma pigmentosum, oculocutaneous albinism, epidermolysis bullosa, dyskeratosis congenita, Huriez syndrome, epidermodysplasia verruciformis, Rothmund–Thomson syndrome, Bloom syndrome, Werner syndrome, GATA2 deficiency, DOCK8 deficiency, Fanconi anemia | |
| Past history of NMSC |
| Antibody | Target | Study Type | Total Number of Patients (n) | Disease/Tumor Type | Overall Response Rate (ORR) | Reference |
|---|---|---|---|---|---|---|
| Cemiplimab | PD-1 | Phase-I, open-label, multicenter study | 26 | advanced cSCC | 50% | [106] |
| Phase-II, nonrandomized, global, | 59 | 47% | ||||
| Pembrolizumab | PD-1 | phase II, global, open-label, nonrandomized, | 159 | locally advanced or recurrent and/or metastatic cSCC | 50% | [100] |
| Nivolumab | PD-1 | Phase III, multicenter, randomized (1:1), active-controlled, open-label | 419 | unresectable advanced, recurrent or metastatic esophageal SCC (ESCC) | 19.3% | [101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansary, T.M.; Hossain, M.R.; Komine, M.; Ohtsuki, M. Immunotherapy for the Treatment of Squamous Cell Carcinoma: Potential Benefits and Challenges. Int. J. Mol. Sci. 2022, 23, 8530. https://doi.org/10.3390/ijms23158530
Ansary TM, Hossain MR, Komine M, Ohtsuki M. Immunotherapy for the Treatment of Squamous Cell Carcinoma: Potential Benefits and Challenges. International Journal of Molecular Sciences. 2022; 23(15):8530. https://doi.org/10.3390/ijms23158530
Chicago/Turabian StyleAnsary, Tuba M., MD Razib Hossain, Mayumi Komine, and Mamitaro Ohtsuki. 2022. "Immunotherapy for the Treatment of Squamous Cell Carcinoma: Potential Benefits and Challenges" International Journal of Molecular Sciences 23, no. 15: 8530. https://doi.org/10.3390/ijms23158530
APA StyleAnsary, T. M., Hossain, M. R., Komine, M., & Ohtsuki, M. (2022). Immunotherapy for the Treatment of Squamous Cell Carcinoma: Potential Benefits and Challenges. International Journal of Molecular Sciences, 23(15), 8530. https://doi.org/10.3390/ijms23158530






