Selective Deposition of Mo2C-Containing Coatings on {100} Facets of Synthetic Diamond Crystals
Abstract
:1. Introduction
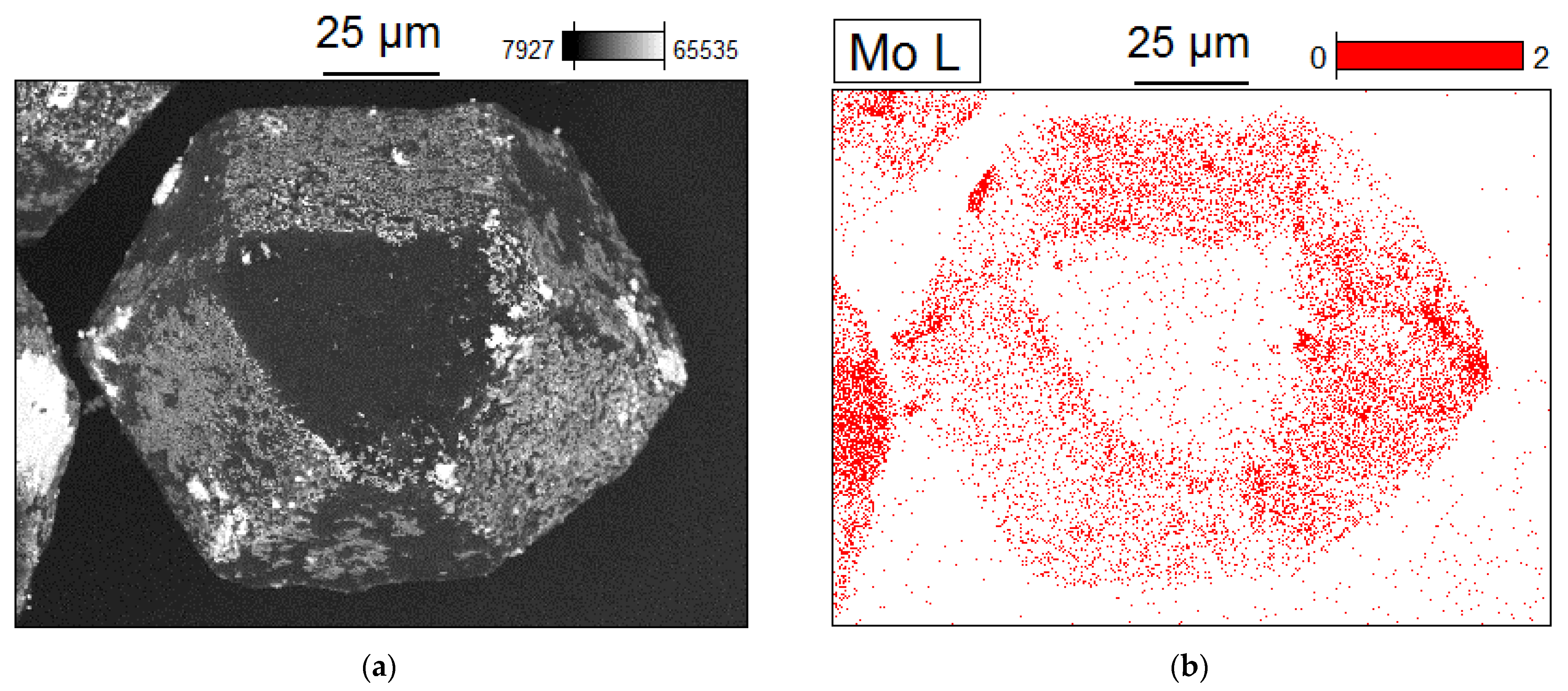
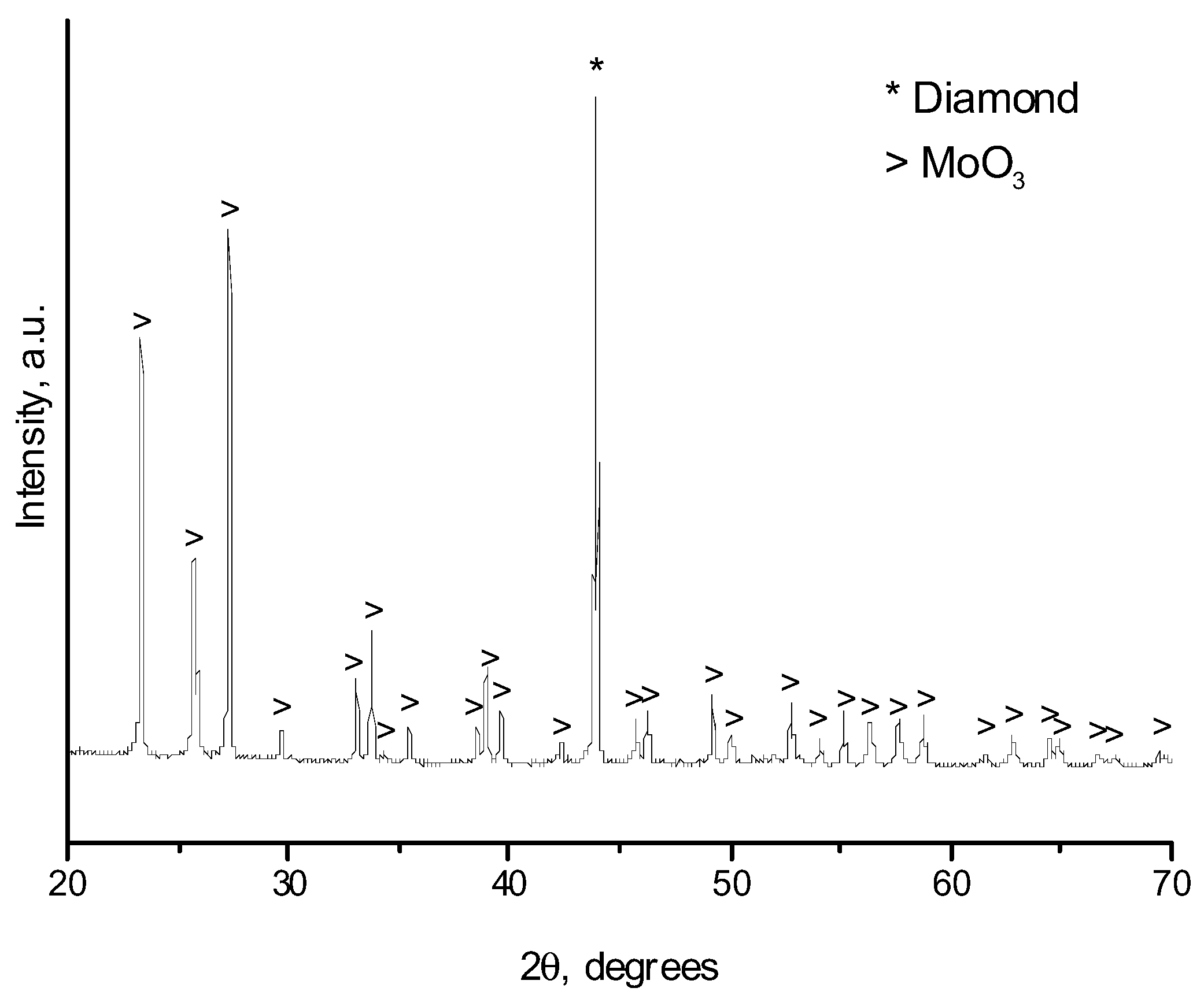
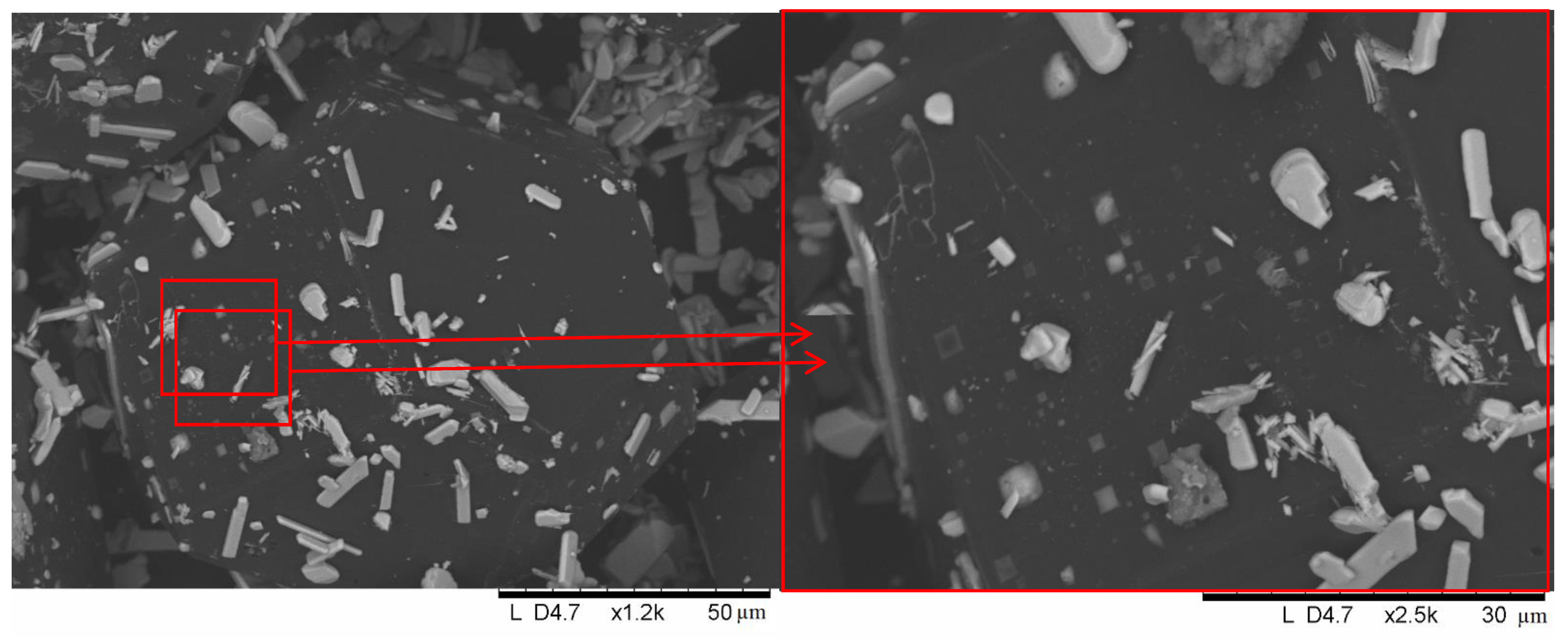
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dai, S.; Li, J.; Lu, N. Research progress of diamond/copper composites with high thermal conductivity. Diam. Relat. Mater. 2020, 108, 107993. [Google Scholar] [CrossRef]
- Zhao, D.; Zha, S.; Liu, D. Influence of sputtering and electroless plating of Cr/Cu dual-layer structure on thermal conductivity of diamond/copper composites. Diam. Relat. Mater. 2021, 115, 108296. [Google Scholar] [CrossRef]
- Jia, J.; Bai, S.; Xiong, D.; Xiao, J.; Yan, T. Enhanced thermal conductivity in diamond/copper composites with tungsten coatings on diamond particles prepared by magnetron sputtering method. Mater. Chem. Phys. 2020, 252, 123422. [Google Scholar] [CrossRef]
- Li, H.; Wang, C.; Wu, L.; Chen, M.; Wu, C.; Wang, N.; Li, Z.; Tang, L.; Pang, Q. Optimization of process parameters, microstructure, and thermal conductivity properties of Ti-coated diamond/copper composites prepared by spark plasma sintering. J. Mater. Sci. Mater. Electron. 2021, 32, 9115–9125. [Google Scholar] [CrossRef]
- Zhu, C.; Cui, C.; Wua, X.; Zhang, B.; Yang, D.; Zhao, H.; Zheng, Z. Study on surface modification of diamond particles and thermal conductivity properties of their reinforced metal-based (Cu or Mg) composites. Diam. Relat. Mater. 2020, 108, 107998. [Google Scholar] [CrossRef]
- Ukhina, A.V.; Dudina, D.V.; Samoshkin, D.A.; Galashov, E.N.; Skovorodin, I.N.; Bokhonov, B.B. Effect of the Surface Modification of Synthetic Diamond with Nickel or Tungsten on the Properties of Copper–Diamond Composites. Inorg. Mater. 2018, 54, 426–433. [Google Scholar] [CrossRef]
- Chu, K.; Liu, Z.; Jia, C.; Chen, H.; Liang, X.; Gao, W.; Tian, W.; Guo, H. Thermal conductivity of SPS consolidated Cu/diamond composites with Cr-coated diamond particles. J. Alloy. Compd. 2010, 490, 453–458. [Google Scholar] [CrossRef]
- Ciupiński, Ł.; Kruszewski, M.J.; Grzonka, J.; Chmielewski, M.; Zielińsk, R.; Moszczyńska, D.; Michalski, A. Design of of interfacial Cr3C2 carbide layer via optimization of sintering parameters used to fabricate copper/diamond composites for thermal management applications. Mater. Des. 2017, 120, 170–185. [Google Scholar] [CrossRef]
- Okada, T.; Fukuoka, K.; Arata, Y.; Yonezawa, S.; Kiyokawa, H.; Takashima, M. Tungsten carbide coating on diamond particles in molten mixture of Na2CO3 and NaCl. Diam. Relat. Mater. 2015, 52, 11–17. [Google Scholar] [CrossRef]
- Kang, Q.; He, X.; Ren, S.; Zhang, L.; Wu, M.; Liu, T.; Liu, Q.; Guo, C.; Qu., X. Preparation of high thermal conductivity copper-diamond composites using molibdenum carbidecoated diamond particles. J. Mater. Sci. 2013, 48, 6133–6140. [Google Scholar] [CrossRef]
- Ma, S.; Zhao, N.; Shi, C.; Liu, E.; He, C.; He, F.; Ma, L. Mo2C coatings on diamond: Different effects on thermal conductivity of diamond/Al and diamond/Cu composites. Appl. Surf. Sci. 2017, 402, 372–383. [Google Scholar] [CrossRef]
- Liu, R.; Luo, G.; Li, Y.; Zhang, J.; Shen, Q.; Zhang, L. Microstructure and thermal properties of diamond/copper composites with Mo2C in-situ nano-coating. Surf. Coat. Technol. 2019, 360, 376–381. [Google Scholar] [CrossRef]
- Galashov, E.N.; Yusuf, A.A.; Mandrik, E.M. Cu/synthetic and impact-diamond composite heat-conducting substrates. J. Phys. Conf. Ser. 2016, 690, 012043. [Google Scholar] [CrossRef]
- Li, H.; Wang, C.; Ding, W.; Wu, L.; Wang, J.; Wei, T.; Hu, J.; Wu, C.; Chen, M.; Zhang, H.; et al. Microstructure evolution of diamond with molybdenum coating and thermal conductivity of diamond/copper composites fabricated by spark plasma sintering. J. Mater. Sci. Mater. Electron. 2022, 33, 15369–15384. [Google Scholar] [CrossRef]
- Chang, R.; Zang, J.; Wang, Y.; Yu, Y.; Lu, J.; Xu, X. Study of Ti-coated diamond grits prepared by Spark Plasma Coating. Diam. Relat. Mater. 2017, 77, 72–78. [Google Scholar] [CrossRef]
- Ukhina, A.V.; Dudina, D.V.; Bokhonov, B.B.; Savintseva, D.V.; Samoshkin, D.A.; Stankus, S.V. Morphological features and phase composition of W-containing coatings formed on diamond via its interaction with WO3. Diam. Relat. Mater. 2022, 123, 108876. [Google Scholar] [CrossRef]
- Fu, B. Surface Energy of Diamond Cubic Crystals and Anisotropy Analysis Revealed by Empirical Electron Surface Models. Adv. Mater. 2019, 8, 61–69. [Google Scholar] [CrossRef]
- De La Pierre, M.; Bruno, M.; Manfredotti, C.; Nestola, F.; Prencipe, M.; Manfredotti, C. The (100), (111) and (110) surfaces of diamond: An ab initio B3LYP study. Mol. Phys. 2014, 112, 1030–1103. [Google Scholar] [CrossRef]
- Bokhonov, B.B.; Kato, H. Selective growth of silver particles on the facets of synthetic diamon. CrystEngComm 2016, 18, 7430–7434. [Google Scholar] [CrossRef]
- Halicioglu, T. Calculation of surface energies for low index planes of diamond. Surf. Sci. Lett. 1991, 259, 714–718. [Google Scholar] [CrossRef]
- Vidyuk, T.M.; Dudina, D.V.; Korchagin, M.A.; Gavrilov, A.I.; Skripkina, T.S.; Ukhina, A.V.; Anisimov, A.G.; Bokhonov, B.B. Melting at the inter-particle contacts during Spark Plasma Sintering: Direct microstructural evidence and relation to particle morphology. Vacuum 2020, 181, 109566. [Google Scholar] [CrossRef]
- Vidyuk, T.M.; Dudina, D.V. Electric current-assisted joining of similar/dissimilar materials. Join. Process. Dissimilar Adv. Mater. 2022, 151–176. [Google Scholar] [CrossRef]

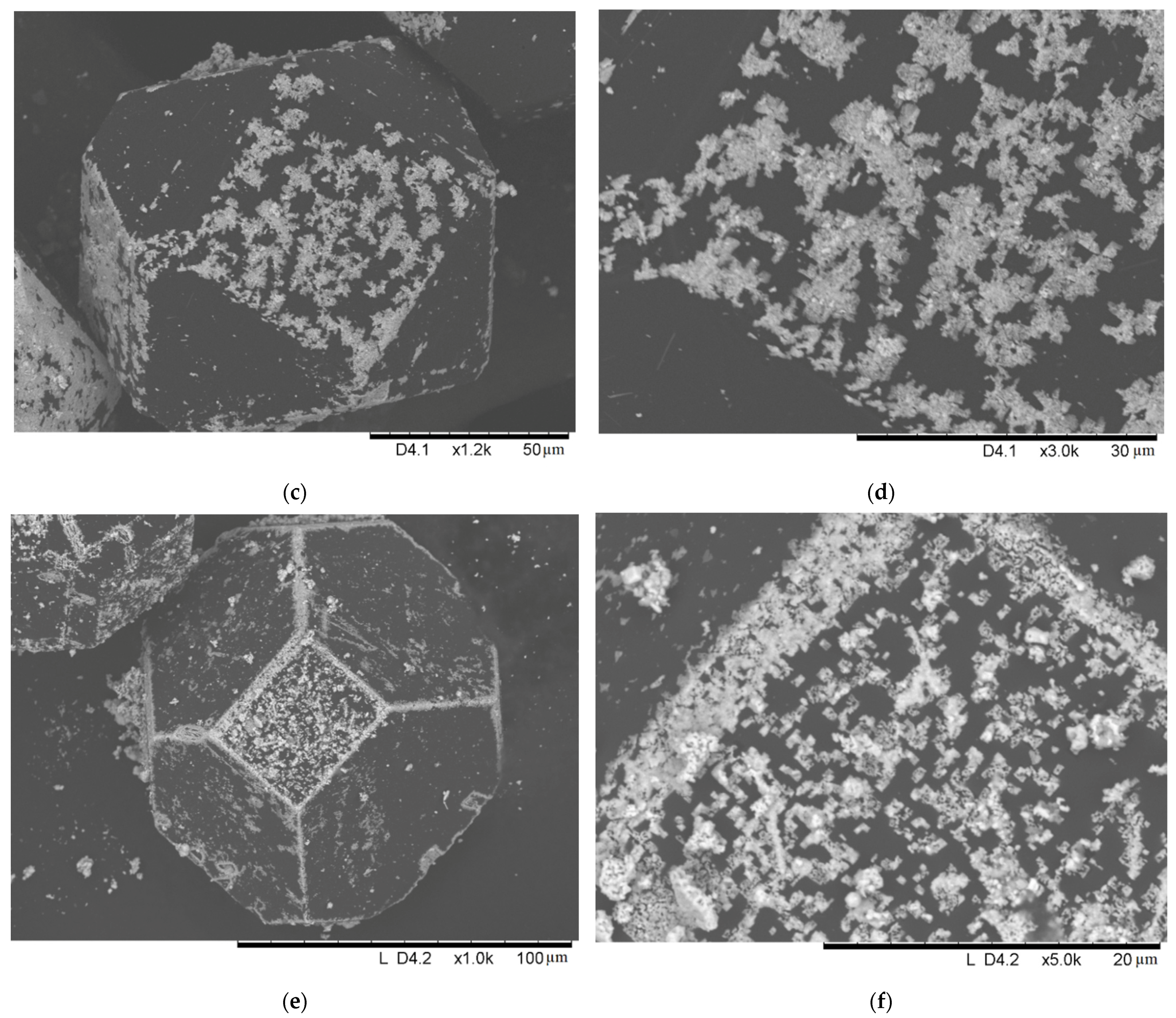

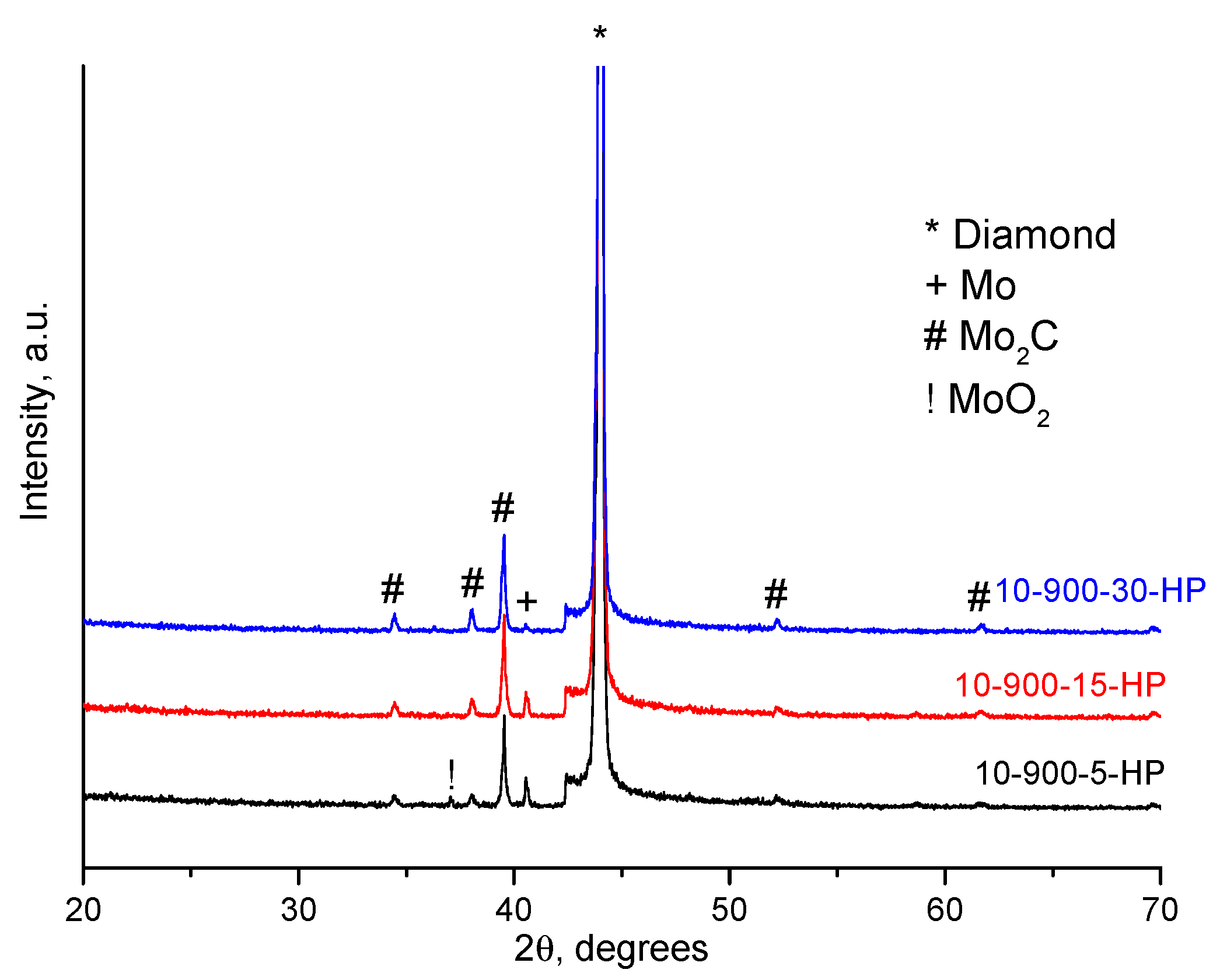
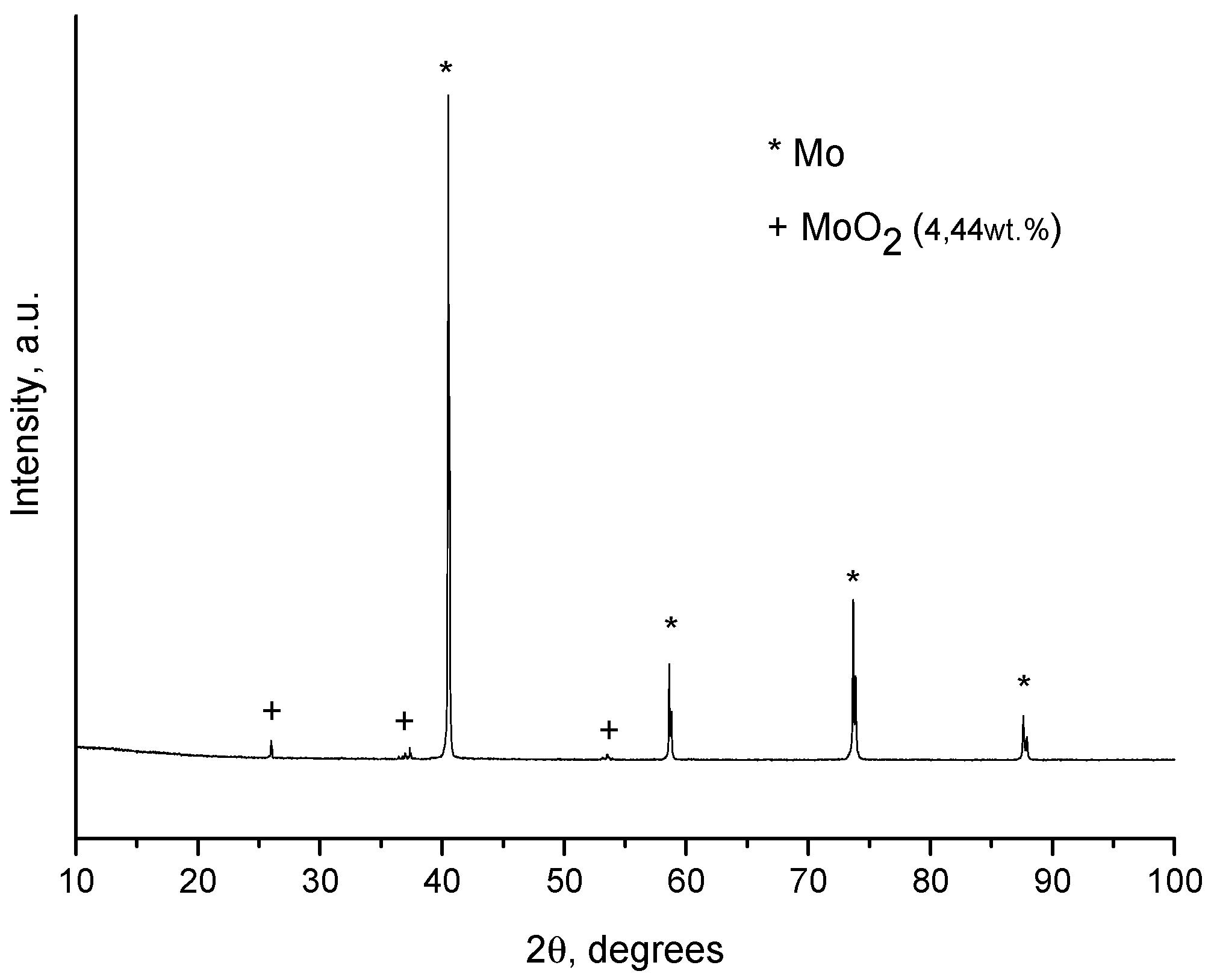
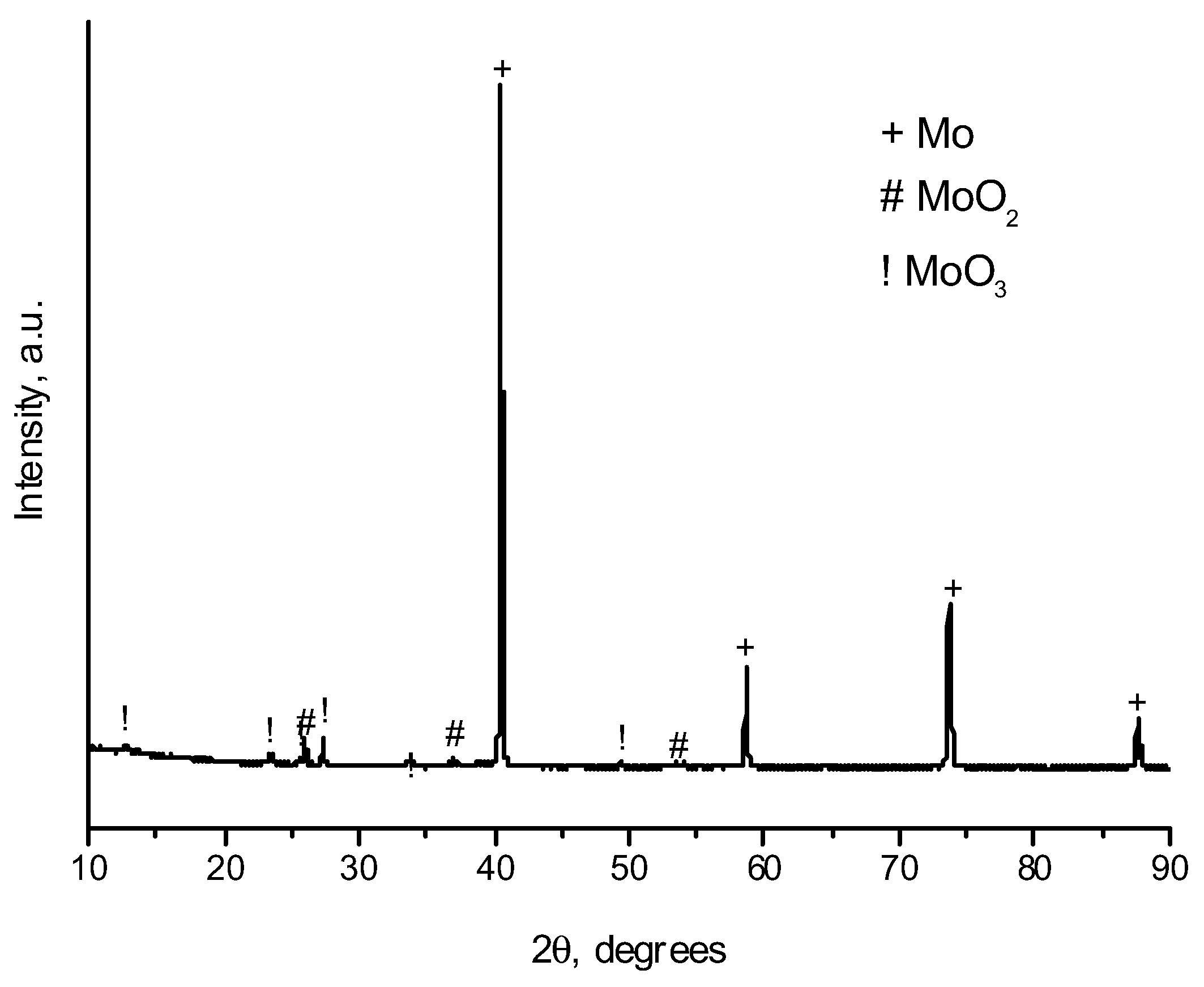
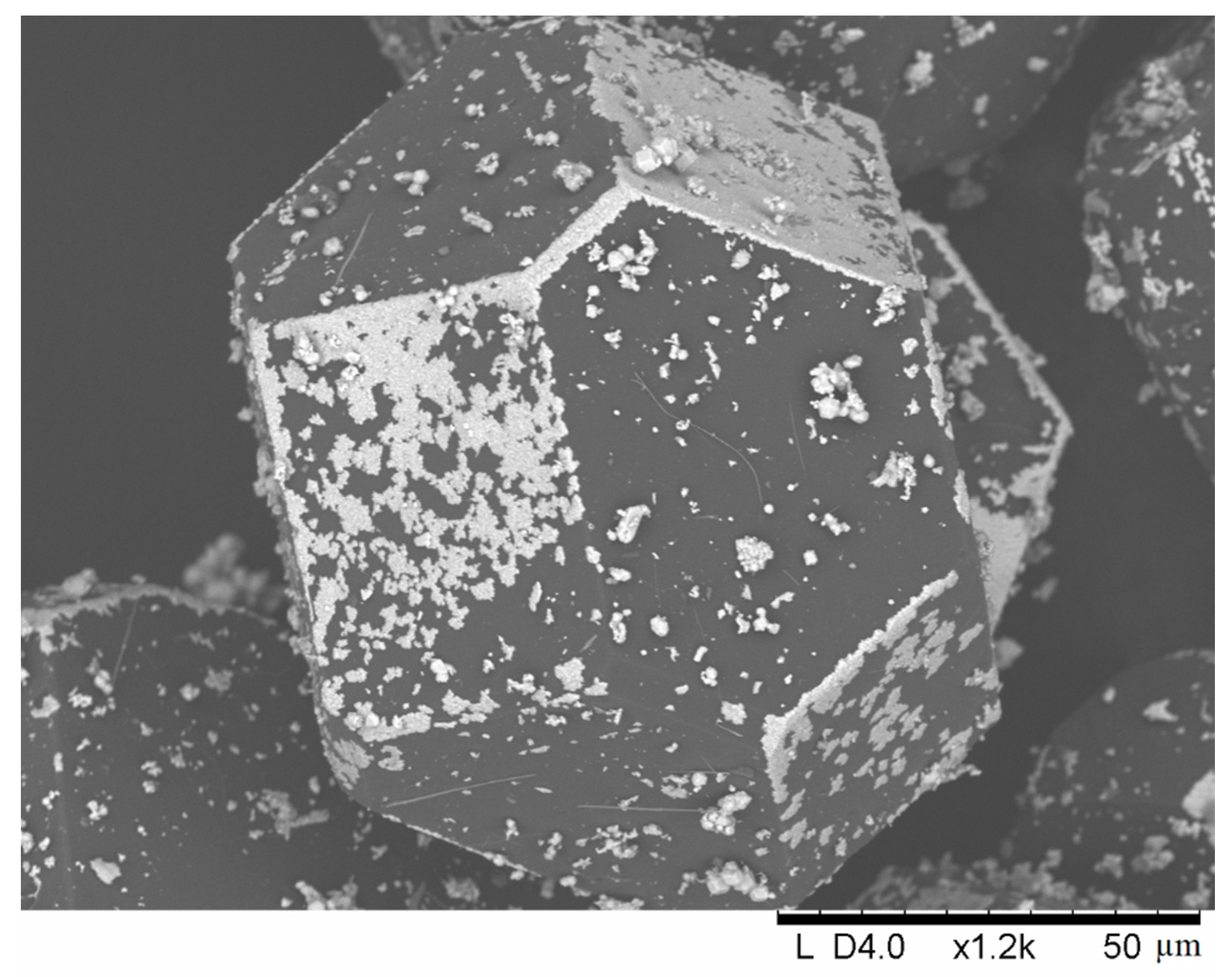
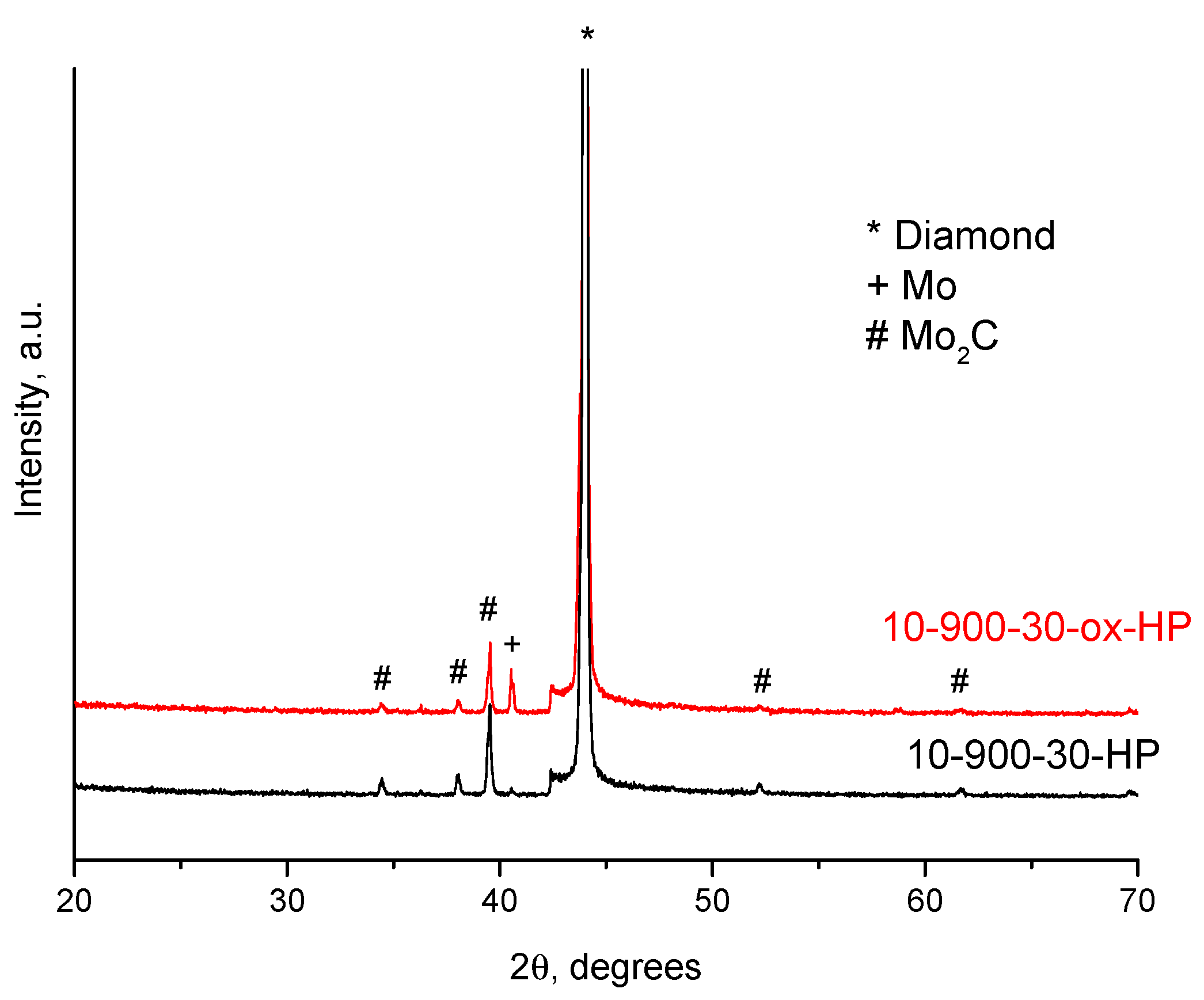
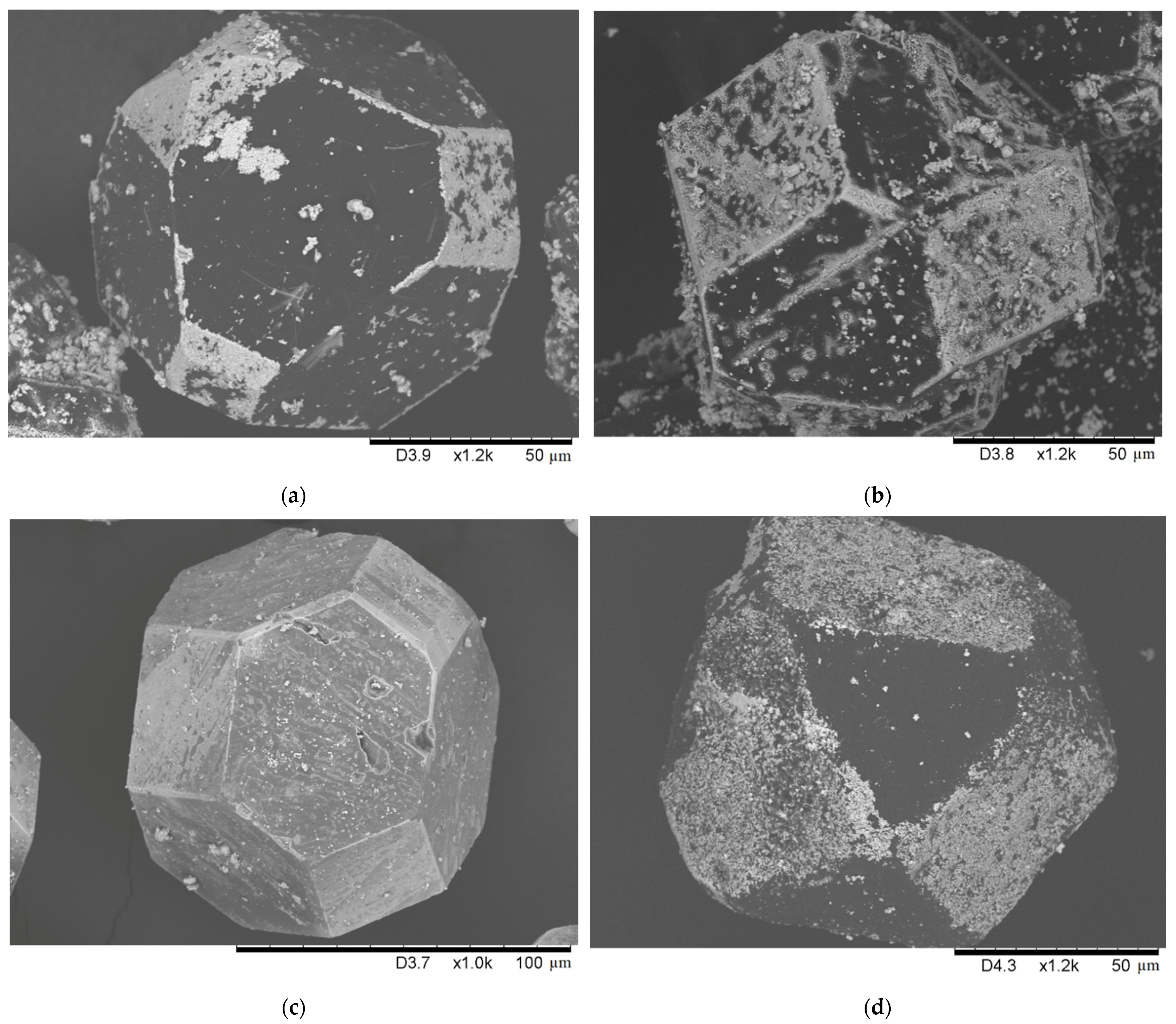
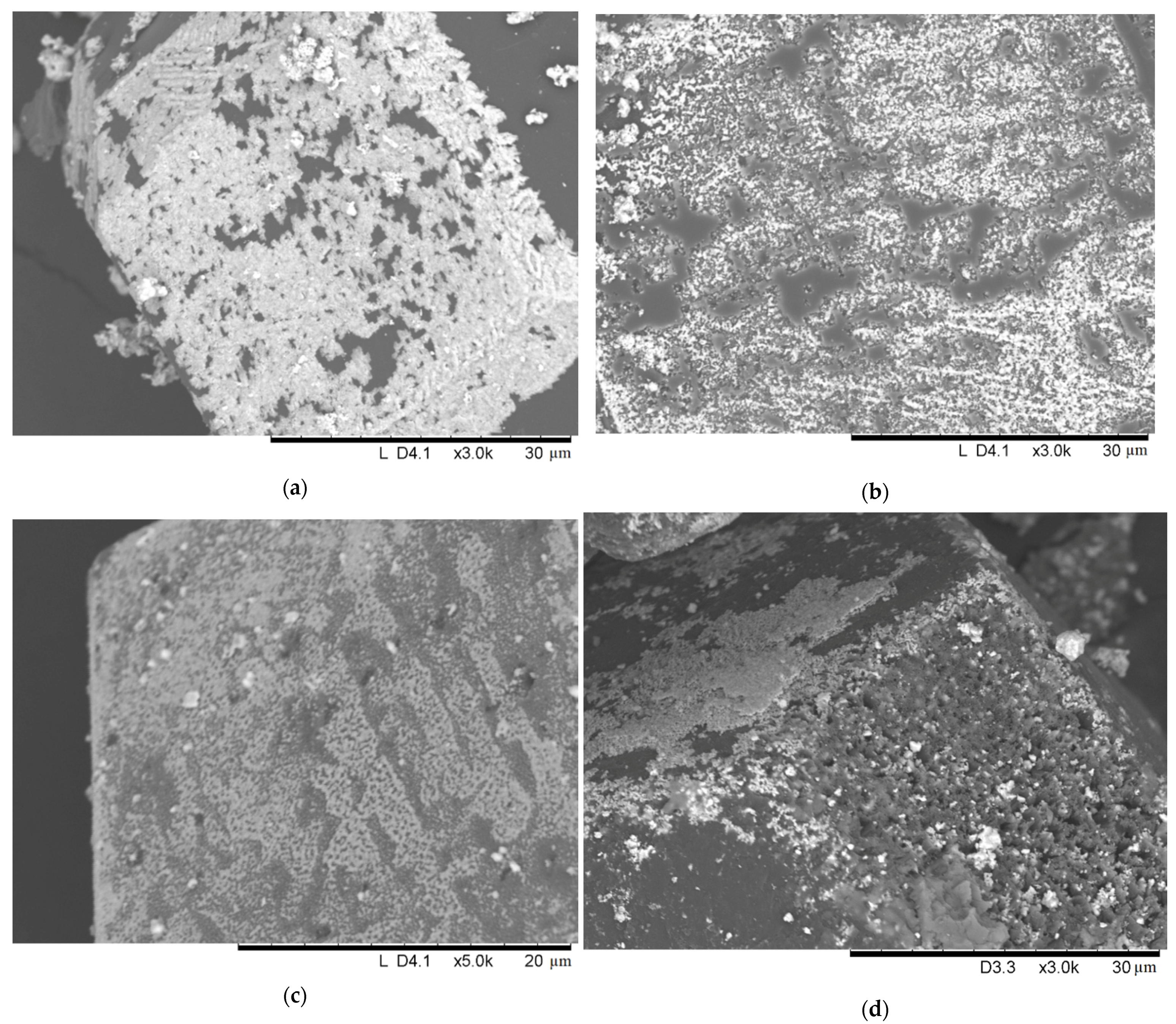

| Sample Notation | Mo Concentration in the Starting Mixture, Vol.% | Treatment Temperature, °C | Treatment Time, min | Treatment Method |
|---|---|---|---|---|
| 10-900-5-HP | 10 | 900 | 5 | HP |
| 10-900-15-HP | 10 | 900 | 15 | HP |
| 10-900-30-HP | 10 | 900 | 30 | HP |
| 10-1000-15-HP | 10 | 1000 | 15 | HP |
| 10-1000-30-HP | 10 | 1000 | 30 | HP |
| 50-1000-15-HP | 50 | 1000 | 15 | HP |
| 10-1000-15-SPS | 10 | 1000 | 15 | SPS |
| 10-900-30-ox-HP | 10 * | 900 | 30 | HP |
| 50-650-60-air | 50 | 650 | 60 | Annealing in air |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ukhina, A.V.; Bokhonov, B.B.; Dudina, D.V. Selective Deposition of Mo2C-Containing Coatings on {100} Facets of Synthetic Diamond Crystals. Int. J. Mol. Sci. 2022, 23, 8511. https://doi.org/10.3390/ijms23158511
Ukhina AV, Bokhonov BB, Dudina DV. Selective Deposition of Mo2C-Containing Coatings on {100} Facets of Synthetic Diamond Crystals. International Journal of Molecular Sciences. 2022; 23(15):8511. https://doi.org/10.3390/ijms23158511
Chicago/Turabian StyleUkhina, Arina V., Boris B. Bokhonov, and Dina V. Dudina. 2022. "Selective Deposition of Mo2C-Containing Coatings on {100} Facets of Synthetic Diamond Crystals" International Journal of Molecular Sciences 23, no. 15: 8511. https://doi.org/10.3390/ijms23158511
APA StyleUkhina, A. V., Bokhonov, B. B., & Dudina, D. V. (2022). Selective Deposition of Mo2C-Containing Coatings on {100} Facets of Synthetic Diamond Crystals. International Journal of Molecular Sciences, 23(15), 8511. https://doi.org/10.3390/ijms23158511








