Clickable Biomaterials for Modulating Neuroinflammation
Abstract
:1. Introduction
2. Effectors, Stimuli, and Outcomes of Neuroinflammation
2.1. Cell Types and Phenotypes in Neuroinflammation
2.2. Neural Pathologies and the Role of Glial Cells
2.3. Role of Peripheral Immune Cells
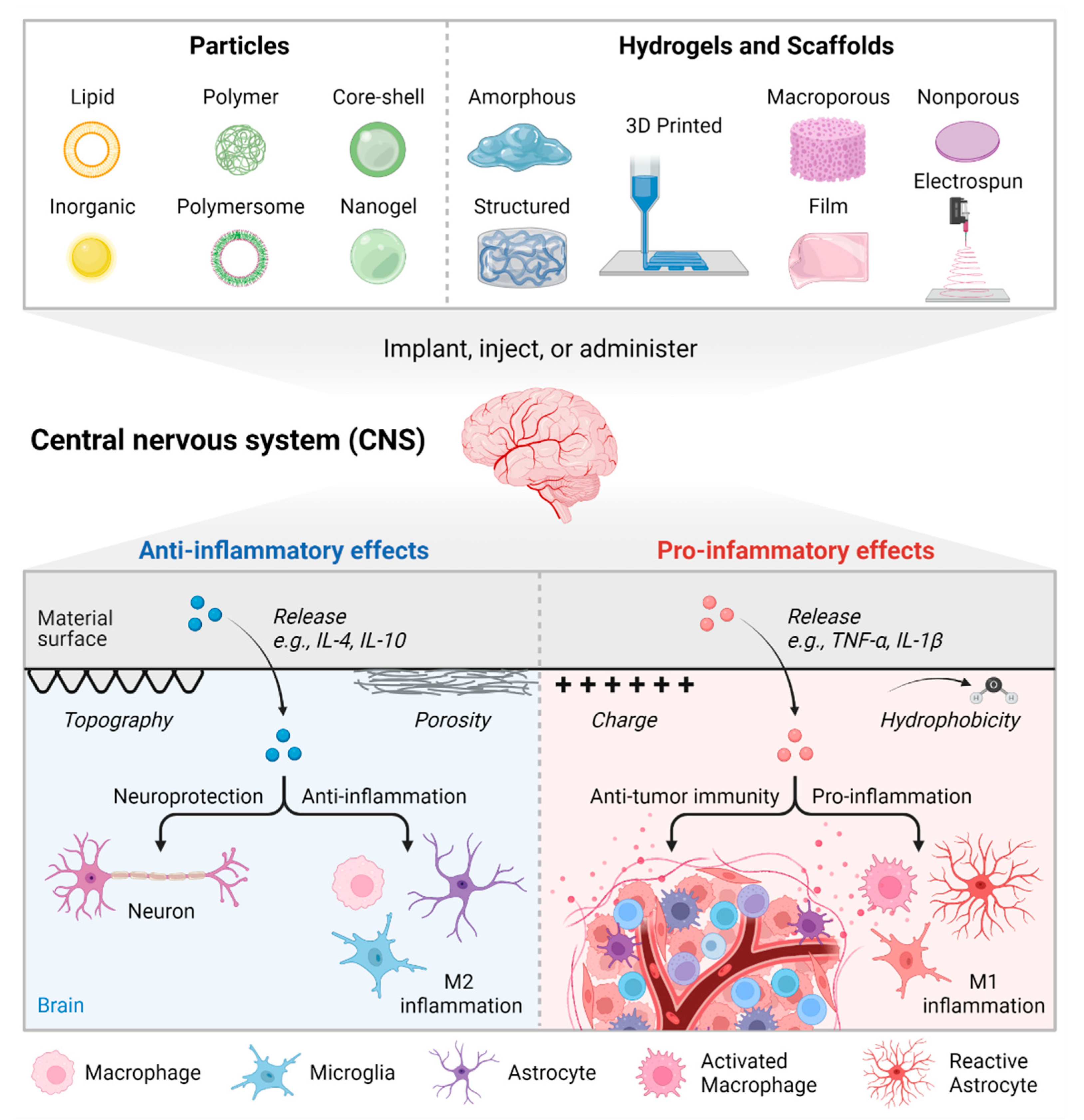
3. Biomaterial Strategies to Modulate Immune Cell Phenotypes
3.1. Particulate Biomaterials
3.2. Solid Scaffolds and Hydrogels
3.3. Materials for Glial Cell Modulation
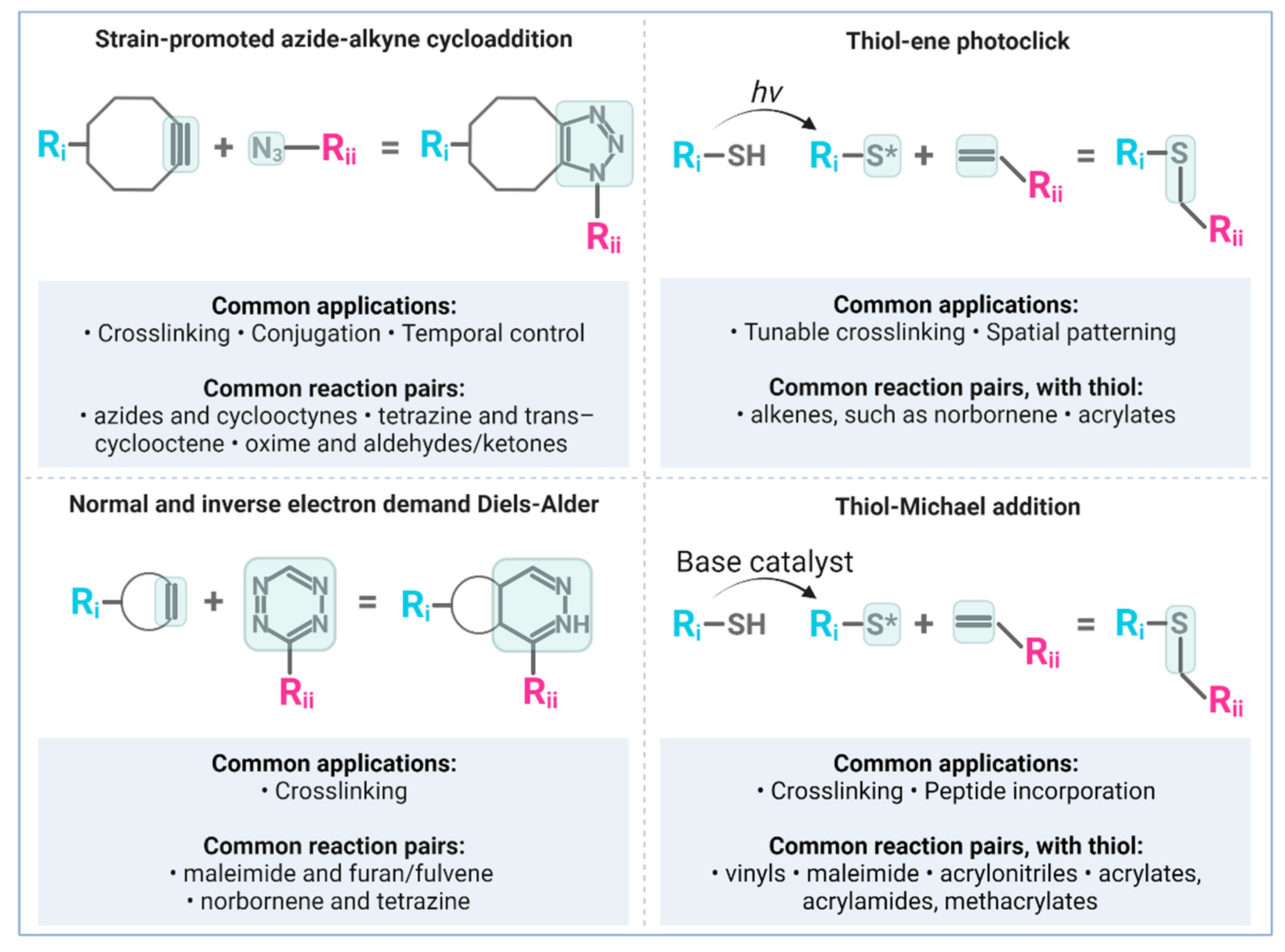
4. Bioorthogonal ‘Click’ Chemistry and Neural Biomaterials Applications
4.1. Copper-Free Strain-Promoted Azide-Alkyne Cycloaddition (SPAAC)
4.2. Diels-Alder and Inverse Electron-Demand Diels-Alder
4.3. Photo-Click Thiol-Ene Reactions
4.4. Thiol-Michael Addition
4.5. Click Applications for Immunomodulation
5. Click-Based Materials for Neuroimmune Modulation
5.1. Biomaterial Scaffolds and Hydrogels
5.2. Therapeutic Particles
5.3. Hydrogel Coatings
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ransohoff, R.M.; Schafer, D.; Vincent, A.; Blachère, N.E.; Bar-Or, A. Neuroinflammation: Ways in Which the Immune System Affects the Brain. Neurotherapeutics 2015, 12, 896–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl. S2), 136–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dyken, P.; Lacoste, B. Impact of Metabolic Syndrome on Neuroinflammation and the Blood–Brain Barrier. Front. Neurosci. 2018, 12, 930. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.A.; Banks, W.A. Age-Associated Changes in the Immune System and Blood–Brain Barrier Functions. Int. J. Mol. Sci. 2019, 20, 1632. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.Q.; Zhou, J.W. Neuroinflammation in the central nervous system: Symphony of glial cells. Glia 2019, 67, 1017–1035. [Google Scholar] [CrossRef] [PubMed]
- Locy, H.; de Mey, S.; de Mey, W.; De Ridder, M.; Thielemans, K.; Maenhout, S.K. Immunomodulation of the Tumor Microenvironment: Turn Foe Into Friend. Front. Immunol. 2018, 9, 2909. [Google Scholar] [CrossRef]
- Osipov, A.; Saung, M.T.; Zheng, L.; Murphy, A.G. Small molecule immunomodulation: The tumor microenvironment and overcoming immune escape. J. Immunother. Cancer 2019, 7, 224. [Google Scholar] [CrossRef] [Green Version]
- Varadé, J.; Magadán, S.; González-Fernández, Á. Human immunology and immunotherapy: Main achievements and challenges. Cell. Mol. Immunol. 2020, 18, 805–828. [Google Scholar] [CrossRef]
- Dellacherie, M.O.; Seo, B.R.; Mooney, D.J. Macroscale biomaterials strategies for local immunomodulation. Nat. Rev. Mater. 2019, 4, 379–397. [Google Scholar] [CrossRef]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef]
- Adu-Berchie, K.; Mooney, D.J. Biomaterials as local niches for immunomodulation. Acc. Chem. Res. 2020, 53, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, D.P.; Águas, A.P.; Barbosa, M.A.; Pelegrín, P.; Barbosa, J.N. The inflammasome in host response to biomaterials: Bridging inflammation and tissue regeneration. Acta Biomater. 2019, 83, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Anseth, K.S.; Klok, H.A. Click Chemistry in Biomaterials, Nanomedicine, and Drug Delivery. Biomacromolecules 2016, 17, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, X.; Han, Y.; Sun, H.Y.; Hilborn, J.; Shi, L. Click chemistry-based biopolymeric hydrogels for regenerative medicine. Biomed. Mater. 2021, 16, 022003. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.E.; Lemmel, S.A.; Yu, X.; Zhou, Q.A. Bioorthogonal Chemistry and Its Applications. Bioconjug. Chem. 2021, 32, 2457–2479. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhang, L.; Yang, L.; Zhu, F.; Ding, M.; Lin, F.; Wang, Z.; Li, Y. “Click” chemistry in polymeric scaffolds: Bioactive materials for tissue engineering. J. Control. Release 2018, 273, 160–179. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, A.L.; Chevalier, M.T.; Russo, L.; Pandit, A. Sweet tailoring of glyco-modulatory extracellular matrix-inspired biomaterials to target neuroinflammation. Cell Rep. Phys. Sci. 2021, 2, 100321. [Google Scholar] [CrossRef]
- Bellver-Landete, V.; Bretheau, F.; Mailhot, B.; Vallières, N.; Lessard, M.; Janelle, M.E.; Vernoux, N.; Tremblay, M.È.; Fuehrmann, T.; Shoichet, M.S.; et al. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat. Commun. 2019, 10, 518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, H.; Zhao, Y.; Hu, D.; Wang, S.; Yu, T.; Zhang, L. Depletion of microglia exacerbates injury and impairs function recovery after spinal cord injury in mice. Cell Death Dis. 2020, 11, 528. [Google Scholar] [CrossRef] [PubMed]
- Poulen, G.; Aloy, E.; Bringuier, C.M.; Mestre-Francés, N.; Artus, E.V.F.; Cardoso, M.; Perez, J.C.; Goze-Bac, C.; Boukhaddaoui, H.; Lonjon, N.; et al. Inhibiting microglia proliferation after spinal cord injury improves recovery in mice and nonhuman primates. Theranostics 2021, 11, 8640–8659. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, G.; Khakh, B.S.; Deming, T.J.; Sofroniew, M.V. Astrocyte scar formation aids CNS axon regeneration. Nature 2016, 532, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matejuk, A.; Vandenbark, A.A.; Offner, H. Cross-Talk of the CNS With Immune Cells and Functions in Health and Disease. Front. Neurol. 2021, 12, 750. [Google Scholar] [CrossRef]
- Vandenbark, A.A.; Offner, H.; Matejuk, S.; Matejuk, A. Microglia and astrocyte involvement in neurodegeneration and brain cancer. J. Neuroinflamm. 2021, 18, 298. [Google Scholar] [CrossRef]
- Li, Y.; Ritzel, R.M.; Khan, N.; Cao, T.; He, J.; Lei, Z.; Matyas, J.J.; Sabirzhanov, B.; Liu, S.; Li, H.; et al. Delayed microglial depletion after spinal cord injury reduces chronic inflammation and neurodegeneration in the brain and improves neurological recovery in male mice. Theranostics 2020, 10, 11376. [Google Scholar] [CrossRef] [PubMed]
- Banjara, M.; Ghosh, C. Sterile Neuroinflammation and Strategies for Therapeutic Intervention. Int. J. Inflam. 2017, 2017, 8385961. [Google Scholar] [CrossRef] [Green Version]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef]
- Marchetti, L.; Engelhardt, B. Immune cell trafficking across the blood-brain barrier in the absence and presence of neuroinflammation. Vasc. Biol. 2020, 2, H1. [Google Scholar] [CrossRef] [Green Version]
- Skaper, S.D.; Facci, L.; Zusso, M.; Giusti, P. An Inflammation-Centric View of Neurological Disease: Beyond the Neuron. Front. Cell. Neurosci. 2018, 12, 72. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Tyzack, G.E.; Hall, C.E.; Sibley, C.R.; Cymes, T.; Forostyak, S.; Carlino, G.; Meyer, I.F.; Schiavo, G.; Zhang, S.C.; Gibbons, G.M.; et al. A neuroprotective astrocyte state is induced by neuronal signal EphB1 but fails in ALS models. Nat. Commun. 2017, 8, 1164. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.B.; Song, L.J.; Wang, Q.; Kumar, G.; Yan, Y.Q.; Ma, C.G. Astrocytes: A double-edged sword in neurodegenerative diseases. Neural Regen. Res. 2021, 16, 1702–1710. [Google Scholar] [PubMed]
- Liu, L.R.; Liu, J.C.; Bao, J.S.; Bai, Q.Q.; Wang, G.Q. Interaction of Microglia and Astrocytes in the Neurovascular Unit. Front. Immunol. 2020, 11, 1024. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef] [Green Version]
- Jurga, A.M.; Paleczna, M.; Kadluczka, J.; Kuter, K.Z. Beyond the GFAP-Astrocyte Protein Markers in the Brain. Biomolecules 2021, 11, 1361. [Google Scholar] [CrossRef] [PubMed]
- Guttenplan, K.A.; Weigel, M.K.; Prakash, P.; Wijewardhane, P.R.; Hasel, P.; Rufen-Blanchette, U.; Münch, A.E.; Blum, J.A.; Fine, J.; Neal, M.C.; et al. Neurotoxic reactive astrocytes induce cell death via saturated lipids. Nature 2021, 599, 102–107. [Google Scholar] [CrossRef]
- Matejuk, A.; Ransohoff, R.M. Crosstalk Between Astrocytes and Microglia: An Overview. Front. Immunol. 2020, 11, 1416. [Google Scholar] [CrossRef]
- Clarke, L.E.; Liddelow, S.A.; Chakraborty, C.; Münch, A.E.; Heiman, M.; Barres, B.A. Normal aging induces A1-like astrocyte reactivity. Proc. Natl. Acad. Sci. USA 2018, 115, E1896–E1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niraula, A.; Sheridan, J.F.; Godbout, J.P. Microglia Priming with Aging and Stress. Neuropsychopharmacology 2017, 42, 318–333. [Google Scholar] [CrossRef] [Green Version]
- Marschallinger, J.; Iram, T.; Zardeneta, M.; Lee, S.E.; Lehallier, B.; Haney, M.S.; Pluvinage, J.V.; Mathur, V.; Hahn, O.; Morgens, D.W.; et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci. 2020, 23, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Holm, T.H.; Draeby, D.; Owens, T. Microglia are required for astroglial toll-like receptor 4 response and for optimal TLR2 and TLR3 response. Glia 2012, 60, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef] [PubMed]
- Kam, T.I.; Hinkle, J.T.; Dawson, T.M.; Dawson, V.L. Microglia and Astrocyte Dysfunction in Parkinson’s Disease. Neurobiol. Dis. 2020, 144, 105028. [Google Scholar] [CrossRef] [PubMed]
- Ponath, G.; Park, C.; Pitt, D. The Role of Astrocytes in Multiple Sclerosis. Front. Immunol. 2018, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, S.; Basilico, B.; Marrone, M.C.; Ragozzino, D. Microglia-neuron crosstalk: Signaling mechanism and control of synaptic transmission. Semin. Cell Dev. Biol. 2019, 94, 138–151. [Google Scholar] [CrossRef]
- Cardoso, V.; Chesné, J.; Ribeiro, H.; Garcia-Cassani, B.; Carvalho, T.; Bouchery, T.; Shah, K.; Barbosa-Morais, N.L.; Harris, N.; Veiga-Fernandes, H. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 2017, 549, 277–281. [Google Scholar] [CrossRef] [Green Version]
- Rostami, J.; Fotaki, G.; Sirois, J.; Mzezewa, R.; Bergström, J.; Essand, M.; Healy, L.; Erlandsson, A. Astrocytes have the capacity to act as antigen-presenting cells in the Parkinson’s disease brain. J. Neuroinflamm. 2020, 17, 119. [Google Scholar] [CrossRef]
- Damisah, E.C.; Hill, R.A.; Rai, A.; Chen, F.; Rothlin, C.V.; Ghosh, S.; Grutzendler, J. Astrocytes and microglia play orchestrated roles and respect phagocytic territories during neuronal corpse removal in vivo. Sci. Adv. 2020, 6, eaba3239. [Google Scholar] [CrossRef]
- Kigerl, K.A.; Gensel, J.C.; Ankeny, D.P.; Alexander, J.K.; Donnelly, D.J.; Popovich, P.G. Identification of Two Distinct Macrophage Subsets with Divergent Effects Causing either Neurotoxicity or Regeneration in the Injured Mouse Spinal Cord. J. Neurosci. 2009, 29, 13435–13444. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.J.; Zhu, H.; Zhang, N.; Shen, L.; Wang, R.; Zhou, J.S.; Hu, J.G.; Lü, H.Z. Temporal kinetics of macrophage polarization in the injured rat spinal cord. J. Neurosci. Res. 2015, 93, 1526–1533. [Google Scholar] [CrossRef]
- Hesketh, M.; Sahin, K.B.; West, Z.E.; Murray, R.Z. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int. J. Mol. Sci. 2017, 18, 1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.; Dai, Y.J.; Chen, G.; Cui, S. Sen Dissecting the Dual Role of the Glial Scar and Scar-Forming Astrocytes in Spinal Cord Injury. Front. Cell. Neurosci. 2020, 14, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Liu, X.; Chen, Z. Glial Scar—A Promising Target for Improving Outcomes After CNS Injury. J. Mol. Neurosci. 2020, 70, 340–352. [Google Scholar] [CrossRef]
- Bartus, K.; James, N.D.; Didangelos, A.; Bosch, K.D.; Verhaagen, J.; Yáñez-Muñoz, R.J.; Rogers, J.H.; Schneider, B.L.; Muir, E.M.; Bradbury, E.J. Large-Scale Chondroitin Sulfate Proteoglycan Digestion with Chondroitinase Gene Therapy Leads to Reduced Pathology and Modulates Macrophage Phenotype following Spinal Cord Contusion Injury. J. Neurosci. 2014, 34, 4822–4836. [Google Scholar] [CrossRef] [PubMed]
- Moeendarbary, E.; Weber, I.P.; Sheridan, G.K.; Koser, D.E.; Soleman, S.; Haenzi, B.; Bradbury, E.J.; Fawcett, J.; Franze, K. The soft mechanical signature of glial scars in the central nervous system. Nat. Commun. 2017, 8, 14787. [Google Scholar] [CrossRef]
- Kisucká, A.; Bimbová, K.; Bačová, M.; Gálik, J.; Lukáčová, N. Activation of Neuroprotective Microglia and Astrocytes at the Lesion Site and in the Adjacent Segments Is Crucial for Spontaneous Locomotor Recovery after Spinal Cord Injury. Cells 2021, 10, 1943. [Google Scholar] [CrossRef]
- Watanabe, S.; Alexander, M.; Misharin, A.V.; Budinger, G.R.S. The role of macrophages in the resolution of inflammation. J. Clin. Investig. 2019, 129, 2619–2628. [Google Scholar] [CrossRef] [Green Version]
- Sevenich, L. Brain-resident microglia and blood-borne macrophages orchestrate central nervous system inflammation in neurodegenerative disorders and brain cancer. Front. Immunol. 2018, 9, 697. [Google Scholar] [CrossRef]
- Francos-Quijorna, I.; Sánchez-Petidier, M.; Burnside, E.R.; Badea, S.R.; Torres-Espin, A.; Marshall, L.; De Winter, F.; Verhaagen, J.; Moreno-Manzano, V.; Bradbury, E.J.; et al. Chondroitin sulfate proteoglycans prevent immune cell phenotypic conversion and inflammation resolution via TLR4 in rodent models of spinal cord injury. Nat. Commun. 2022, 13, 2933. [Google Scholar] [CrossRef]
- Kopper, T.J.; Zhang, B.; Bailey, W.M.; Bethel, K.E.; Gensel, J.C. The effects of myelin on macrophage activation are phenotypic specific via cPLA2 in the context of spinal cord injury inflammation. Sci. Rep. 2021, 11, 6341. [Google Scholar] [CrossRef]
- Greenhalgh, A.D.; David, S.; Bennett, F.C. Immune cell regulation of glia during CNS injury and disease. Nat. Rev. Neurosci. 2020, 21, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, A.D.; Zarruk, J.G.; Healy, L.M.; Baskar Jesudasan, S.J.; Jhelum, P.; Salmon, C.K.; Formanek, A.; Russo, M.V.; Antel, J.P.; McGavern, D.B.; et al. Peripherally derived macrophages modulate microglial function to reduce inflammation after CNS injury. PLoS Biol. 2018, 16, e2005264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Decker, J.T.; Margul, D.J.; Smith, D.R.; Cummings, B.J.; Anderson, A.J.; Shea, L.D. Local Immunomodulation with Anti-inflammatory Cytokine-Encoding Lentivirus Enhances Functional Recovery after Spinal Cord Injury. Mol. Ther. 2018, 26, 1756–1770. [Google Scholar] [CrossRef] [Green Version]
- Tay, T.L.; Mai, D.; Dautzenberg, J.; Fernández-Klett, F.; Lin, G.; Sagar, S.; Datta, M.; Drougard, A.; Stempfl, T.; Ardura-Fabregat, A.; et al. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat. Neurosci. 2017, 20, 793–803. [Google Scholar] [CrossRef] [PubMed]
- González, H.; Pacheco, R. T-cell-mediated regulation of neuroinflammation involved in neurodegenerative diseases. J. Neuroinflamm. 2014, 11, 201. [Google Scholar] [CrossRef] [Green Version]
- Williams, G.P.; Schonhoff, A.M.; Jurkuvenaite, A.; Gallups, N.J.; Standaert, D.G.; Harms, A.S. CD4 T cells mediate brain inflammation and neurodegeneration in a mouse model of Parkinson’s disease. Brain 2021, 144, 2047–2059. [Google Scholar] [CrossRef]
- Rustenhoven, J.; Drieu, A.; Mamuladze, T.; de Lima, K.A.; Dykstra, T.; Wall, M.; Papadopoulos, Z.; Kanamori, M.; Salvador, A.F.; Baker, W.; et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell 2021, 184, 1000–1016.e27. [Google Scholar] [CrossRef]
- Louveau, A.; Herz, J.; Alme, M.N.; Salvador, A.F.; Dong, M.Q.; Viar, K.E.; Herod, S.G.; Knopp, J.; Setliff, J.C.; Lupi, A.L.; et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci. 2018, 21, 1380–1391. [Google Scholar] [CrossRef]
- Hu, X.; Deng, Q.; Ma, L.; Li, Q.; Chen, Y.; Liao, Y.; Zhou, F.; Zhang, C.; Shao, L.; Feng, J.; et al. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res. 2020, 30, 229–243. [Google Scholar] [CrossRef] [Green Version]
- Alves De Lima, K.; Rustenhoven, J.; Kipnis, J. Meningeal Immunity and Its Function in Maintenance of the Central Nervous System in Health and Disease. Annu. Rev. Immunol. 2020, 38, 597–620. [Google Scholar] [CrossRef]
- Da Mesquita, S.; Herz, J.; Wall, M.; Dykstra, T.; de Lima, K.A.; Norris, G.T.; Dabhi, N.; Kennedy, T.; Baker, W.; Kipnis, J. Aging-associated deficit in CCR7 is linked to worsened glymphatic function, cognition, neuroinflammation, and β-amyloid pathology. Sci. Adv. 2021, 7, 4601–4622. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Qin, C.; Chen, M.; Yu, H.H.; Chu, Y.H.; Chen, T.J.; Bosco, D.B.; Wu, L.J.; Bu, B.T.; Wang, W.; et al. Regulatory T cells protect against brain damage by alleviating inflammatory response in neuromyelitis optica spectrum disorder. J. Neuroinflamm. 2021, 18, 201. [Google Scholar] [CrossRef]
- Ooi, Y.C.; Tran, P.; Ung, N.; Thill, K.; Trang, A.; Fong, B.M.; Nagasawa, D.T.; Lim, M.; Yang, I. The role of regulatory T-cells in glioma immunology. Clin. Neurol. Neurosurg. 2014, 119, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Larochelle, C.; Wasser, B.; Jamann, H.; Löffel, J.T.; Cui, Q.L.; Tastet, O.; Schillner, M.; Luchtman, D.; Birkenstock, J.; Stroh, A.; et al. Pro-inflammatory T helper 17 directly harms oligodendrocytes in neuroinflammation. Proc. Natl. Acad. Sci. USA 2021, 118, e2025813118. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.L.; Kumar, G.; Agasing, A.; Ko, R.M.; Axtell, R.C. Role of TFH cells in promoting T Helper 17-induced neuroinflammation. Front. Immunol. 2018, 9, 382. [Google Scholar] [CrossRef] [Green Version]
- Wasser, B.; Luchtman, D.; Löffel, J.; Robohm, K.; Birkner, K.; Stroh, A.; Vogelaar, C.F.; Zipp, F.; Bittner, S. CNS-localized myeloid cells capture living invading T cells during neuroinflammation. J. Exp. Med. 2020, 217, e20190812. [Google Scholar] [CrossRef] [Green Version]
- Margul, D.J.; Park, J.; Boehler, R.M.; Smith, D.R.; Johnson, M.A.; McCreedy, D.A.; He, T.; Ataliwala, A.; Kukushliev, T.V.; Liang, J.; et al. Reducing neuroinflammation by delivery of IL-10 encoding lentivirus from multiple-channel bridges. Bioeng. Transl. Med. 2016, 1, 136–148. [Google Scholar] [CrossRef]
- Pierson, E.R.; Wagner, C.A.; Goverman, J.M. The contribution of neutrophils to CNS autoimmunity. Clin. Immunol. 2018, 189, 23–28. [Google Scholar] [CrossRef]
- Manda-Handzlik, A.; Demkow, U. The Brain Entangled: The Contribution of Neutrophil Extracellular Traps to the Diseases of the Central Nervous System. Cells 2019, 8, 1477. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.K.; Nair, A.; Gupta, M. Mast cells in neurodegenerative disease. Front. Cell. Neurosci. 2019, 13, 171. [Google Scholar] [CrossRef]
- Ahn, J.J.; Abu-Rub, M.; Miller, R.H. B Cells in Neuroinflammation: New Perspectives and Mechanistic Insights. Cells 2021, 10, 1605. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed]
- Iwai, Y.; Hamanishi, J.; Chamoto, K.; Honjo, T. Cancer immunotherapies targeting the PD-1 signaling pathway. J. Biomed. Sci. 2017, 24, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, J.; Giovannoni, G.; Hunter, S.F. Sphingosine 1-phosphate Receptor Modulator Therapy for Multiple Sclerosis: Differential Downstream Receptor Signalling and Clinical Profile Effects. Drugs 2021, 81, 207. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.F.; Bowen, J.D.; Reder, A.T. The Direct Effects of Fingolimod in the Central Nervous System: Implications for Relapsing Multiple Sclerosis. CNS Drugs 2016, 30, 135–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermersch, P.; Radue, E.-W.; Putzki, N.; Ritter, S.; Merschhemke, M.; Freedman, M.S. A comparison of multiple sclerosis disease activity after discontinuation of fingolimod and placebo. Mult. Scler. J.—Exp. Transl. Clin. 2017, 3, 2055217317730096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subei, A.M.; Cohen, J.A. Sphingosine 1-Phosphate Receptor Modulators in Multiple Sclerosis. CNS Drugs 2015, 29, 565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassi, S.; Mauri, L.; Prioni, S.; Cabitta, L.; Sonnino, S.; Prinetti, A.; Giussani, P. Sphingosine 1-Phosphate Receptors and Metabolic Enzymes as Druggable Targets for Brain Diseases. Front. Pharmacol. 2019, 10, 807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.Q.; Xiao, J.; Chen, M.; Zhou, L.Q.; Shang, K.; Qin, C.; Tian, D.S. Sphingosine-1-Phosphate Signaling in Ischemic Stroke: From Bench to Bedside and Beyond. Front. Cell. Neurosci. 2021, 15, 781098. [Google Scholar] [CrossRef]
- Kelly, S.H.; Shores, L.S.; Votaw, N.L.; Collier, J.H. Biomaterial strategies for generating therapeutic immune responses. Adv. Drug Deliv. Rev. 2017, 114, 3–18. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Mooney, D.J. Biomaterials and Emerging Anticancer Therapeutics: Engineering the Microenvironment. Nat. Rev. Cancer 2016, 16, 56. [Google Scholar] [CrossRef] [Green Version]
- Atukorale, P.U.; Raghunathan, S.P.; Raguveer, V.; Moon, T.J.; Zheng, C.; Bielecki, P.A.; Wiese, M.L.; Goldberg, A.L.; Covarrubias, G.; Hoimes, C.J.; et al. Nanoparticle encapsulation of synergistic immune agonists enables systemic co-delivery to tumor sites and interferon β-driven anti-tumor immunity. Cancer Res. 2019, 79, 5394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stabler, C.L.; Li, Y.; Stewart, J.M.; Keselowsky, B.G. Engineering immunomodulatory biomaterials for type 1 diabetes. Nat. Rev. Mater. 2019, 4, 429. [Google Scholar] [CrossRef]
- Abdulghani, S.; Mitchell, G.R. Biomaterials for In Situ Tissue Regeneration: A Review. Biomolecules 2019, 9, 750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadtler, K.; Estrellas, K.; Allen, B.W.; Wolf, M.T.; Fan, H.; Tam, A.J.; Patel, C.H.; Luber, B.S.; Wang, H.; Wagner, K.R.; et al. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science 2016, 352, 366–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andorko, J.I.; Jewell, C.M. Designing biomaterials with immunomodulatory properties for tissue engineering and regenerative medicine. Bioeng. Transl. Med. 2017, 2, 139–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maclean, F.L.; Horne, M.K.; Williams, R.J.; Nisbet, D.R. Review: Biomaterial systems to resolve brain inflammation after traumatic injury. APL Bioeng. 2018, 2, 021502. [Google Scholar] [CrossRef]
- Tsui, C.; Koss, K.; Churchward, M.A.; Todd, K.G. Biomaterials and glia: Progress on designs to modulate neuroinflammation. Acta Biomater. 2019, 83, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Letko Khait, N.; Ho, E.; Shoichet, M.S.; Letko Khait, N.; Ho, E.; Shoichet, M.S. Wielding the Double-Edged Sword of Inflammation: Building Biomaterial-Based Strategies for Immunomodulation in Ischemic Stroke Treatment. Adv. Funct. Mater. 2021, 31, 2010674. [Google Scholar] [CrossRef]
- Hotaling, N.A.; Tang, L.; Irvine, D.J.; Babensee, J.E. Biomaterial strategies for immunomodulation. Annu. Rev. Biomed. Eng. 2015, 17, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Akiva, E.; Est Witte, S.; Meyer, R.A.; Rhodes, K.R.; Green, J.J. Polymeric micro- and nanoparticles for immune modulation. Biomater. Sci. 2018, 7, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Segura, T. Biomaterials-Mediated Regulation of Macrophage Cell Fate. Front. Bioeng. Biotechnol. 2020, 8, 609297. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, R.; Hernaez-Estrada, B.; Hernandez, R.M.; Santos-Vizcaino, E.; Spiller, K.L. Immunomodulatory Biomaterials for Tissue Repair. Chem. Rev. 2021, 121, 11305–11335. [Google Scholar] [CrossRef]
- Sharma, P.; Alakesh, A.; Jhunjhunwala, S. The consequences of particle uptake on immune cells. Trends Pharmacol. Sci. 2022, 43, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Darling, N.J.; Sideris, E.; Hamada, N.; Carmichael, S.T.; Segura, T. Injectable and Spatially Patterned Microporous Annealed Particle (MAP) Hydrogels for Tissue Repair Applications. Adv. Sci. 2018, 5, 1801046. [Google Scholar] [CrossRef]
- Soni, S.S.; Rodell, C.B. Polymeric materials for immune engineering: Molecular interaction to biomaterial design. Acta Biomater. 2021, 133, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Getts, D.R.; Shea, L.D.; Miller, S.D.; King, N.J.C. Harnessing nanoparticles for immune modulation. Trends Immunol. 2015, 36, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Malachowski, T.; Hassel, A. Engineering nanoparticles to overcome immunological barriers for enhanced drug delivery. Eng. Regen. 2020, 1, 35–50. [Google Scholar] [CrossRef]
- Singh, A.; Peppas, N.A. Hydrogels and scaffolds for immunomodulation. Adv. Mater. 2014, 26, 6530–6541. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, S.R.; Ayad, N.G.; Lee, J.K. Neuroinflammation Treatment via Targeted Delivery of Nanoparticles. Front. Cell. Neurosci. 2020, 14, 329. [Google Scholar] [CrossRef] [PubMed]
- Paris, J.L.; Baeza, A. Nano- and Microscale Drug Delivery Approaches for Therapeutic Immunomodulation. ChemNanoMat 2021, 7, 773–788. [Google Scholar] [CrossRef]
- O’Brien, E.M.; Spiller, K.L. Pro-inflammatory polarization primes Macrophages to transition into a distinct M2-like phenotype in response to IL-4. J. Leukoc. Biol. 2022, 111, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Wofford, K.L.; Cullen, D.K.; Spiller, K.L. Modulation of macrophage phenotype via phagocytosis of drug-loaded microparticles. J. Biomed. Mater. Res. Part A 2019, 107, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Walker, A.; Mitragotri, S. Role of Particle Size in Phagocytosis of Polymeric Microspheres. Pharm. Res. 2008, 25, 1815. [Google Scholar] [CrossRef] [Green Version]
- Garapaty, A.; Champion, J.A. Shape of ligand immobilized particles dominates and amplifies the macrophage cytokine response to ligands. PLoS ONE 2019, 14, e0217022. [Google Scholar]
- Lewis, J.S.; Stewart, J.M.; Marshall, G.P.; Carstens, M.R.; Zhang, Y.; Dolgova, N.V.; Xia, C.; Brusko, T.M.; Wasserfall, C.H.; Clare-Salzler, M.J.; et al. Dual-Sized Microparticle System for Generating Suppressive Dendritic Cells Prevents and Reverses Type 1 Diabetes in the Nonobese Diabetic Mouse Model. ACS Biomater. Sci. Eng. 2019, 5, 2631–2646. [Google Scholar] [CrossRef] [Green Version]
- Becicka, W.M.; Bielecki, P.A.; Lorkowski, M.E.; Moon, T.J.; Zhang, Y.; Atukorale, P.U.; Covarrubias, G.; Karathanasis, E. The effect of PEGylation on the efficacy and uptake of an immunostimulatory nanoparticle in the tumor immune microenvironment. Nanoscale Adv. 2021, 3, 4961–4972. [Google Scholar] [CrossRef] [PubMed]
- Klyachko, N.L.; Polak, R.; Haney, M.J.; Zhao, Y.; Gomes Neto, R.J.; Hill, M.C.; Kabanov, A.V.; Cohen, R.E.; Rubner, M.F.; Batrakova, E.V. Macrophages with cellular backpacks for targeted drug delivery to the brain. Biomaterials 2017, 140, 79. [Google Scholar] [CrossRef]
- Doshi, N.; Swiston, A.J.; Gilbert, J.B.; Alcaraz, M.L.; Cohen, R.E.; Rubner, M.F.; Mitragotri, S. Cell-Based Drug Delivery Devices Using Phagocytosis-Resistant Backpacks. Adv. Mater. 2011, 23, H105–H109. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Gilbert, J.B.; Kumar, S.; Gupta, V.; Cohen, R.E.; Rubner, M.F.; Mitragotri, S. Monocyte-mediated delivery of polymeric backpacks to inflamed tissues: A generalized strategy to deliver drugs to treat inflammation. J. Control. Release 2015, 199, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Frei, A.W.; Labrada, I.M.; Rong, Y.; Liang, J.P.; Samojlik, M.M.; Sun, C.; Barash, S.; Keselowsky, B.G.; Bayer, A.L.; et al. Immunosuppressive PLGA TGF-β1 Microparticles Induce Polyclonal and Antigen-Specific Regulatory T Cells for Local Immunomodulation of Allogeneic Islet Transplants. Front. Immunol. 2021, 12, 1484. [Google Scholar] [CrossRef]
- Allen, R.; Chizari, S.; Ma, J.A.; Raychaudhuri, S.; Lewis, J.S. Combinatorial, Microparticle-Based Delivery of Immune Modulators Reprograms the Dendritic Cell Phenotype and Promotes Remission of Collagen-Induced Arthritis in Mice. ACS Appl. Bio Mater. 2019, 2, 2388–2404. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Miao, L.; Gao, W.; Chen, Z.; McHugh, K.J.; Sun, Y.; Tochka, Z.; Tomasic, S.; Sadtler, K.; Hyacinthe, A.; et al. Engineered PLGA microparticles for long-term, pulsatile release of STING agonist for cancer immunotherapy. Sci. Transl. Med. 2020, 12, 6606. [Google Scholar] [CrossRef]
- Koerner, J.; Horvath, D.; Herrmann, V.L.; MacKerracher, A.; Gander, B.; Yagita, H.; Rohayem, J.; Groettrup, M. PLGA-particle vaccine carrying TLR3/RIG-I ligand Riboxxim synergizes with immune checkpoint blockade for effective anti-cancer immunotherapy. Nat. Commun. 2021, 12, 2935. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.P.; Lampe, K.J. Fabricating PLGA microparticles with high loads of the small molecule antioxidant N-acetylcysteine that rescue oligodendrocyte progenitor cells from oxidative stress. Biotechnol. Bioeng. 2018, 115, 246–256. [Google Scholar] [CrossRef]
- Allen, R.P.; Bolandparvaz, A.; Ma, J.A.; Manickam, V.A.; Lewis, J.S. Latent, Immunosuppressive Nature of Poly(lactic-co-glycolic acid) Microparticles. ACS Biomater. Sci. Eng. 2018, 4, 900. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, A.; Girelli, M.; Arosio, D.; Polito, L.; Podini, P.; Martino, G.; Seneci, P.; Muzio, L.; Menegon, A. Intracisternal delivery of PEG-coated gold nanoparticles results in high brain penetrance and long-lasting stability. J. Nanobiotechnol. 2019, 17, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moyano, D.F.; Liu, Y.; Ayaz, F.; Hou, S.; Puangploy, P.; Duncan, B.; Osborne, B.A.; Rotello, V.M. Immunomodulatory effects of coated gold nanoparticles in LPS-stimulated in vitro and in vivo murine model systems. Chem 2016, 1, 320–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roach, K.A.; Stefaniak, A.B.; Roberts, J.R. Metal nanomaterials: Immune effects and implications of physicochemical properties on sensitization, elicitation, and exacerbation of allergic disease. J. Immunotoxicol. 2019, 16, 87–124. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Veerman, R.E.; Güçlüler Akpinar, G.; Eldh, M.; Gabrielsson, S. Immune Cell-Derived Extracellular Vesicles—Functions and Therapeutic Applications. Trends Mol. Med. 2019, 25, 382–394. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Ratajczak, J. Extracellular microvesicles/exosomes: Discovery, disbelief, acceptance, and the future? Leukemia 2020, 34, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Riazifar, M.; Mohammadi, M.R.; Pone, E.J.; Yeri, A.; Lasser, C.; Segaliny, A.I.; McIntyre, L.L.; Shelke, G.V.; Hutchins, E.; Hamamoto, A.; et al. Stem Cell-Derived Exosomes as Nanotherapeutics for Autoimmune and Neurodegenerative Disorders. ACS Nano 2019, 13, 6670–6688. [Google Scholar] [CrossRef] [PubMed]
- Gorabi, A.M.; Kiaie, N.; Barreto, G.E.; Read, M.I.; Tafti, H.A.; Sahebkar, A. The Therapeutic Potential of Mesenchymal Stem Cell–Derived Exosomes in Treatment of Neurodegenerative Diseases. Mol. Neurobiol. 2019, 56, 8157–8167. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Zhao, Y.; Banks, W.A.; Bullock, K.M.; Haney, M.; Batrakova, E.; Kabanov, A.V. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 2017, 142, 1–12. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, H.X.; He, C.P.; Fan, S.; Zhu, Y.L.; Qi, C.; Huang, N.P.; Xiao, Z.D.; Lu, Z.H.; Tannous, B.A.; et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef]
- Lin, Y.; Lu, Y.; Li, X. Biological characteristics of exosomes and genetically engineered exosomes for the targeted delivery of therapeutic agents. J. Drug Targetin 2019, 28, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Pan, J.J.; Li, Y.; Zhang, Z.; Yang, G.Y. Native and Bioengineered Exosomes for Ischemic Stroke Therapy. Front. Cell Dev. Biol. 2021, 9, 450. [Google Scholar] [CrossRef]
- You, D.G.; Lim, G.T.; Kwon, S.; Um, W.; Oh, B.H.; Song, S.H.; Lee, J.; Jo, D.G.; Cho, Y.W.; Park, J.H. Metabolically engineered stem cell–derived exosomes to regulate macrophage heterogeneity in rheumatoid arthritis. Sci. Adv. 2021, 7, eabe0083. [Google Scholar] [CrossRef]
- Has, C.; Sunthar, P. A comprehensive review on recent preparation techniques of liposomes. J. Liposome Res. 2020, 30, 336–365. [Google Scholar] [CrossRef]
- Brennan, M.; Layrolle, P.; Mooney, D.J. Biomaterials Functionalized with MSC Secreted Extracellular Vesicles and Soluble Factors for Tissue Regeneration. Adv. Funct. Mater. 2020, 30, 1909125. [Google Scholar] [CrossRef] [PubMed]
- Murali, V.P.; Holmes, C.A. Biomaterial-based extracellular vesicle delivery for therapeutic applications. Acta Biomater. 2021, 124, 88–107. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, R.; Cameron, A.R.; Kelly, D.J.; Kearney, C.J.; O’Brien, F.J. Biomaterial based modulation of macrophage polarization: A review and suggested design principles. Mater. Today 2015, 18, 313–325. [Google Scholar] [CrossRef]
- Veiseh, O.; Vegas, A.J. Domesticating the Foreign Body Response: Recent Advances and Applications. Adv. Drug Deliv. Rev. 2019, 144, 148. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Pullen, N.A.; Oskeritzian, C.A.; Ryan, J.J.; Bowlin, G.L. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials 2013, 34, 4439–4451. [Google Scholar] [CrossRef] [Green Version]
- Tylek, T.; Blum, C.; Hrynevich, A.; Schlegelmilch, K.; Schilling, T.; Dalton, P.D.; Groll, J. Precisely defined fiber scaffolds with 40 μm porosity induce elongation driven M2-like polarization of human macrophages. Biofabrication 2020, 12, 025007. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Dai, F.; Zhang, S.; Xu, F.; Xu, Z.; Liao, S.; Zeng, L.; Song, L.; Ai, F. Pore Size of 3D-Printed Polycaprolactone/Polyethylene Glycol/Hydroxyapatite Scaffolds Affects Bone Regeneration by Modulating Macrophage Polarization and the Foreign Body Response. ACS Appl. Mater. Interfaces 2022, 14, 20693–20707. [Google Scholar] [CrossRef]
- Sridharan, R.; Cavanagh, B.; Cameron, A.R.; Kelly, D.J.; O’Brien, F.J. Material stiffness influences the polarization state, function and migration mode of macrophages. Acta Biomater. 2019, 89, 47–59. [Google Scholar] [CrossRef]
- Sridharan, R.; Ryan, E.J.; Kearney, C.J.; Kelly, D.J.; O’Brien, F.J. Macrophage Polarization in Response to Collagen Scaffold Stiffness Is Dependent on Cross-Linking Agent Used to Modulate the Stiffness. ACS Biomater. Sci. Eng. 2019, 5, 544–552. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, J.; Wu, X.; Wu, Q.; Li, Y.; Zhou, Y.; Li, L.; Bu, H. Genipin crosslinking reduced the immunogenicity of xenogeneic decellularized porcine whole-liver matrices through regulation of immune cell proliferation and polarization. Sci. Rep. 2016, 6, 24779. [Google Scholar] [CrossRef]
- Badylak, S.F.; Gilbert, T.W. Immune response to biologic scaffold materials. Semin. Immunol. 2008, 20, 109–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dziki, J.L.; Huleihel, L.; Scarritt, M.E.; Badylak, S.F. Extracellular Matrix Bioscaffolds as Immunomodulatory Biomaterials. Tissue Eng. Part A 2017, 23, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Rowley, A.T.; Nagalla, R.R.; Wang, S.W.; Liu, W.F. Extracellular matrix-based strategies for immunomodulatory biomaterials engineering. Adv. Healthc. Mater. 2019, 8, e1801578. [Google Scholar] [CrossRef] [PubMed]
- Sicari, B.M.; Peter Rubin, J.; Dearth, C.L.; Wolf, M.T.; Ambrosio, F.; Boninger, M.; Turner, N.J.; Weber, D.J.; Simpson, T.W.; Wyse, A.; et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci. Transl. Med. 2014, 6, 234ra58. [Google Scholar] [CrossRef] [Green Version]
- Keane, T.J.; Londono, R.; Turner, N.J.; Badylak, S.F. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 2012, 33, 1771–1781. [Google Scholar] [CrossRef]
- Meng, F.W.; Slivka, P.F.; Dearth, C.L.; Badylak, S.F. Solubilized extracellular matrix from brain and urinary bladder elicits distinct functional and phenotypic responses in macrophages. Biomaterials 2015, 46, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Huleihel, L.; Dziki, J.L.; Bartolacci, J.G.; Rausch, T.; Scarritt, M.E.; Cramer, M.C.; Vorobyov, T.; LoPresti, S.T.; Swineheart, I.T.; White, L.J.; et al. Macrophage phenotype in response to ECM bioscaffolds. Semin. Immunol. 2017, 29, 2. [Google Scholar] [CrossRef]
- Hussey, G.S.; Molina, C.P.; Cramer, M.C.; Tyurina, Y.Y.; Tyurin, V.A.; Lee, Y.C.; El-Mossier, S.O.; Murdock, M.H.; Timashev, P.S.; Kagan, V.E.; et al. Lipidomics and RNA sequencing reveal a novel subpopulation of nanovesicle within extracellular matrix biomaterials. Sci. Adv. 2020, 6, eaay4361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damian, C.; Ghuman, H.; Mauney, C.; Azar, R.; Reinartz, J.; Badylak, S.F.; Modo, M. Post-Stroke Timing of ECM Hydrogel Implantation Affects Biodegradation and Tissue Restoration. Int. J. Mol. Sci. 2021, 22, 11372. [Google Scholar] [CrossRef] [PubMed]
- Cornelison, R.C.; Gonzalez-Rothi, E.J.; Porvasnik, S.L.; Wellman, S.M.; Park, J.H.; Fuller, D.D.; Schmidt, C.E. Injectable hydrogels of optimized acellular nerve for injection in the injured spinal cord. Biomed. Mater. 2018, 13, 034110. [Google Scholar] [CrossRef] [PubMed]
- Ghuman, H.; Mauney, C.; Donnelly, J.; Massensini, A.R.; Badylak, S.F.; Modo, M. Biodegradation of ECM hydrogel promotes endogenous brain tissue restoration in a rat model of stroke. Acta Biomater. 2018, 80, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Yaldiz, B.; Saglam-Metiner, P.; Yesil-Celiktas, O. Decellularised extracellular matrix-based biomaterials for repair and regeneration of central nervous system. Expert Rev. Mol. Med. 2021, 23, e25. [Google Scholar] [CrossRef] [PubMed]
- OʼShea, T.M.; Wollenberg, A.L.; Kim, J.H.; Ao, Y.; Deming, T.J.; Sofroniew, M.V. Foreign body responses in mouse central nervous system mimic natural wound responses and alter biomaterial functions. Nat. Commun. 2020, 11, 6203. [Google Scholar] [CrossRef] [PubMed]
- Zuidema, J.M.; Gilbert, R.J.; Gottipati, M.K. Biomaterial Approaches to Modulate Reactive Astroglial Response. Cells. Tissues. Organs 2018, 205, 372. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.; Sakiyama-Elbert, S. Using Biomaterials to Promote Pro-Regenerative Glial Phenotypes After Nervous System Injuries. Biomed. Mater. 2018, 13, 024104. [Google Scholar] [CrossRef] [PubMed]
- Gottipati, M.K.; Zuidema, J.M.; Gilbert, R.J. Biomaterial strategies for creating in vitro astrocyte cultures resembling in vivo astrocyte morphologies and phenotypes. Curr. Opin. Biomed. Eng. 2020, 14, 67. [Google Scholar] [CrossRef]
- Gottipati, M.K.; D’Amato, A.R.; Ziemba, A.M.; Popovich, P.G.; Gilbert, R.J. TGFβ3 is Neuroprotective and Alleviates the Neurotoxic Response Induced by Aligned Poly-L-Lactic Acid Fibers on Naïve and Activated Primary Astrocytes. Acta Biomater. 2020, 117, 273. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, G.; Tian, J.; Qiu, J.; Jia, Y.; Feng, D.; Wei, Z.; Li, S.; Xu, F. Matrix stiffness changes affect astrocyte phenotype in an in vitro injury model. NPG Asia Mater. 2021, 13, 35. [Google Scholar] [CrossRef]
- Johnson, C.D.L.; Zuidema, J.M.; Kearns, K.R.; Maguire, A.B.; Desmond, G.P.; Thompson, D.M.; Gilbert, R.J. The Effect of Electrospun Fiber Diameter on Astrocyte-Mediated Neurite Guidance and Protection. ACS Appl. Bio Mater. 2019, 2, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Madl, C.M.; Heilshorn, S.C. Bioorthogonal Strategies for Engineering Extracellular Matrices. Adv. Funct. Mater. 2018, 28, 1706046. [Google Scholar] [CrossRef]
- Arkenberg, M.R.; Nguyen, H.D.; Lin, C.C. Recent advances in bio-orthogonal and dynamic crosslinking of biomimetic hydrogels. J. Mater. Chem. B 2020, 8, 7835–7855. [Google Scholar] [CrossRef] [PubMed]
- Nimmo, C.M.; Owen, S.C.; Shoichet, M.S. Diels-Alder Click cross-linked hyaluronic acid hydrogels for tissue engineering. Biomacromolecules 2011, 12, 824–830. [Google Scholar] [CrossRef] [Green Version]
- Sawicki, L.A.; Kloxin, A.M. Design of thiol–ene photoclick hydrogels using facile techniques for cell culture applications. Biomater. Sci. 2014, 2, 1612–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, A.C.; Gutmann, M.; Lühmann, T.; Meinel, L. Bioorthogonal strategies for site-directed decoration of biomaterials with therapeutic proteins. J. Control. Release 2018, 273, 68–85. [Google Scholar] [CrossRef]
- Ren, X.; Evangelista-Leite, D.; Wu, T.; Rajab, K.T.; Moser, P.T.; Kitano, K.; Economopoulos, K.P.; Gorman, D.E.; Bloom, J.P.; Tan, J.J.; et al. Metabolic glycan labeling and chemoselective functionalization of native biomaterials. Biomaterials 2018, 182, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, M.; Bechold, J.; Seibel, J.; Meinel, L.; Lühmann, T. Metabolic Glycoengineering of Cell-Derived Matrices and Cell Surfaces: A Combination of Key Principles and Step-by-Step Procedures. ACS Biomater. Sci. Eng. 2019, 5, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Spicer, C.D.; Pashuck, E.T.; Stevens, M.M. Achieving Controlled Biomolecule-Biomaterial Conjugation. Chem. Rev. 2018, 118, 7702–7743. [Google Scholar] [CrossRef] [PubMed]
- Jivan, F.; Yegappan, R.; Pearce, H.; Carrow, J.K.; McShane, M.; Gaharwar, A.K.; Alge, D.L. Sequential Thiol-Ene and Tetrazine Click Reactions for the Polymerization and Functionalization of Hydrogel Microparticles. Biomacromolecules 2016, 17, 3516–3523. [Google Scholar] [CrossRef] [PubMed]
- Brudno, Y.; Pezone, M.J.; Snyder, T.K.; Uzun, O.; Moody, C.T.; Aizenberg, M.; Mooney, D.J. Replenishable drug depot to combat post-resection cancer recurrence. Biomaterials 2018, 178, 373–382. [Google Scholar] [CrossRef]
- Hui, E.; Sumey, J.L.; Caliari, S.R. Click-functionalized hydrogel design for mechanobiology investigations. Mol. Syst. Des. Eng. 2021, 6, 670–707. [Google Scholar] [CrossRef]
- Gutmann, M.; Braun, A.; Seibel, J.; Lühmann, T. Bioorthogonal Modification of Cell Derived Matrices by Metabolic Glycoengineering. ACS Biomater. Sci. Eng. 2018, 4, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Idiago-López, J.; Moreno-Antolín, E.; de la Fuente, J.M.; Fratila, R.M. Nanoparticles and bioorthogonal chemistry joining forces for improved biomedical applications. Nanoscale Adv. 2021, 3, 1261–1292. [Google Scholar] [CrossRef]
- Agard, N.J.; Prescher, J.A.; Bertozzi, C.R. A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 2004, 126, 15046–15047. [Google Scholar] [CrossRef] [PubMed]
- Knight, G.T.; Sha, J.; Ashton, R.S. Micropatterned, clickable culture substrates enable in situ spatiotemporal control of human PSC-derived neural tissue morphology. Chem. Commun. 2015, 51, 5238–5241. [Google Scholar] [CrossRef] [Green Version]
- Knight, G.T.; Lundin, B.F.; Iyer, N.; Ashton, L.M.T.; Sethares, W.A.; Willett, R.M.; Ashton, R.S. Engineering induction of singular neural rosette emergence within hPSC-derived tissues. eLife 2018, 7, e37549. [Google Scholar] [CrossRef]
- Adil, M.M.; Vazin, T.; Ananthanarayanan, B.; Rodrigues, G.M.C.; Rao, A.T.; Kulkarni, R.U.; Miller, E.W.; Kumar, S.; Schaffer, D.V. Engineered hydrogels increase the post-transplantation survival of encapsulated hESC-derived midbrain dopaminergic neurons. Biomaterials 2017, 136, 1–11. [Google Scholar] [CrossRef]
- Desai, R.M.; Koshy, S.T.; Hilderbrand, S.A.; Mooney, D.J.; Joshi, N.S. Versatile click alginate hydrogels crosslinked via tetrazine-norbornene chemistry. Biomaterials 2015, 50, 30–37. [Google Scholar] [CrossRef]
- Alge, D.L.; Azagarsamy, M.A.; Donohue, D.F.; Anseth, K.S. Synthetically tractable click hydrogels for three-dimensional cell culture formed using tetrazine-norbornene chemistry. Biomacromolecules 2013, 14, 949–953. [Google Scholar] [CrossRef]
- Hardy, J.G.; Lin, P.; Schmidt, C.E. Biodegradable hydrogels composed of oxime crosslinked poly(ethylene glycol), hyaluronic acid and collagen: A tunable platform for soft tissue engineering. J. Biomater. Sci. Polym. Ed. 2015, 26, 143–161. [Google Scholar] [CrossRef] [Green Version]
- Stöckmann, H.; Neves, A.A.; Stairs, S.; Brindle, K.M.; Leeper, F.J. Exploring isonitrile-based click chemistry for ligation with biomolecules. Org. Biomol. Chem. 2011, 9, 7303–7305. [Google Scholar] [CrossRef]
- Sletten, E.M.; Bertozzi, C.R. A bioorthogonal quadricyclane ligation. J. Am. Chem. Soc. 2011, 133, 17570–17573. [Google Scholar] [CrossRef] [PubMed]
- Madl, C.M.; Lesavage, B.L.; Dewi, R.E.; Dinh, C.B.; Stowers, R.S.; Khariton, M.; Lampe, K.J.; Nguyen, D.; Chaudhuri, O.; Enejder, A.; et al. Maintenance of neural progenitor cell stemness in 3D hydrogels requires matrix remodelling. Nat. Mater. 2017, 16, 1233–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.Y.; Lin, W.; Gao, J.; Shi, X.; Davaritouchaee, M.; Nielsen, A.E.; Mancini, R.J.; Wang, Z. PH-Responsive Nanoparticles Targeted to Lungs for Improved Therapy of Acute Lung Inflammation/Injury. ACS Appl. Mater. Interfaces 2019, 11, 16380–16390. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zheng, J.; Wang, H.; Becker, M.L.; Leipzig, N.D. Neural stem cell encapsulation and differentiation in strain promoted crosslinked polyethylene glycol-based hydrogels. J. Biomater. Appl. 2018, 32, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Gregoritza, M.; Brandl, F.P. The Diels-Alder reaction: A powerful tool for the design of drug delivery systems and biomaterials. Eur. J. Pharm. Biopharm. 2015, 97, 438–453. [Google Scholar] [CrossRef]
- Cadamuro, F.; Russo, L.; Nicotra, F. Biomedical Hydrogels Fabricated Using Diels-Alder Crosslinking. European J. Org. Chem. 2021, 2021, 374–382. [Google Scholar] [CrossRef]
- Wu, H.; Devaraj, N.K. Inverse Electron-Demand Diels-Alder Bioorthogonal Reactions. In Cycloadditions in Bioorthogonal Chemistry. Topics in Current Chemistry Collections; Vrabel, M., Carell, T., Eds.; Springer: Cham, Switzerland, 2016; pp. 109–130. [Google Scholar]
- Thompson, R.E.; Pardieck, J.; Smith, L.; Kenny, P.; Crawford, L.; Shoichet, M.; Sakiyama-Elbert, S. Effect of hyaluronic acid hydrogels containing astrocyte-derived extracellular matrix and/or V2a interneurons on histologic outcomes following spinal cord injury. Biomaterials 2018, 162, 208–223. [Google Scholar] [CrossRef]
- Tam, R.Y.; Fisher, S.A.; Baker, A.E.G.; Shoichet, M.S. Transparent Porous Polysaccharide Cryogels Provide Biochemically Defined, Biomimetic Matrices for Tunable 3D Cell Culture. Chem. Mater. 2016, 28, 3762–3770. [Google Scholar] [CrossRef]
- Madl, C.M.; Heilshorn, S.C. Rapid Diels-Alder Cross-linking of Cell Encapsulating Hydrogels. Chem. Mater. 2019, 31, 8035–8043. [Google Scholar] [CrossRef]
- Smith, L.J.; Taimoory, S.M.; Tam, R.Y.; Baker, A.E.G.; Binth Mohammad, N.; Trant, J.F.; Shoichet, M.S. Diels-Alder Click-Cross-Linked Hydrogels with Increased Reactivity Enable 3D Cell Encapsulation. Biomacromolecules 2018, 19, 926–935. [Google Scholar] [CrossRef]
- Contessi Negrini, N.; Angelova Volponi, A.; Sharpe, P.T.; Celiz, A.D. Tunable Cross-Linking and Adhesion of Gelatin Hydrogels via Bioorthogonal Click Chemistry. ACS Biomater. Sci. Eng. 2021, 7, 4330–4346. [Google Scholar] [CrossRef] [PubMed]
- Nih, L.R.; Sideris, E.; Thomas Carmichael, S.; Segura, T.; Nih, L.R.; Sideris, E.; Segura, T.; Carmichael, S.T. Injection of Microporous Annealing Particle (MAP) Hydrogels in the Stroke Cavity Reduces Gliosis and Inflammation and Promotes NPC Migration to the Lesion. Adv. Mater. 2017, 29, 1606471. [Google Scholar] [CrossRef]
- Nih, L.R.; Sideris, E.; Carmichael, S.T.; Segura, T. Brain Transplantation of Microporous Particle Hydrogel After Stroke Promotes Tissue Repair and Endogenous NPC Recruitment to the Lesion. Adv. Mater. 2017, 29, 1606471. [Google Scholar] [CrossRef]
- Delplace, V.; Pickering, A.J.; Hettiaratchi, M.H.; Zhao, S.; Kivijärvi, T.; Shoichet, M.S. Inverse Electron-Demand Diels-Alder Methylcellulose Hydrogels Enable the Co-delivery of Chondroitinase ABC and Neural Progenitor Cells. Biomacromolecules 2020, 21, 2421–2431. [Google Scholar] [CrossRef]
- Ruskowitz, E.R.; Deforest, C.A. Photoresponsive biomaterials for targeted drug delivery and 4D cell culture. Nat. Rev. Mater. 2018, 3, 17087. [Google Scholar] [CrossRef]
- Fairbanks, B.D.; Macdougall, L.J.; Mavila, S.; Sinha, J.; Kirkpatrick, B.E.; Anseth, K.S.; Bowman, C.N. Photoclick Chemistry: A Bright Idea. Chem. Rev. 2021, 121, 6915–6990. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Lei, H.; Xie, X.; Zhou, L.; Zheng, P.; Cao, Y.; Zhang, Y. Self-sorting double network hydrogels with photo-definable biochemical cues as artificial synthetic extracellular matrix. Nano Res. 2022, 15, 4294–4301. [Google Scholar] [CrossRef]
- Grim, J.C.; Marozas, I.A.; Anseth, K.S. Thiol-ene and photo-cleavage chemistry for controlled presentation of biomolecules in hydrogels. J. Control. Release 2015, 219, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Kharkar, P.M.; Kiick, K.L.; Kloxin, A.M. Design of thiol- and light-sensitive degradable hydrogels using Michael-type addition reactions. Polym. Chem. 2015, 6, 5565–5574. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.D.; Liu, H.Y.; Hudson, B.N.; Lin, C.C. Enzymatic Cross-Linking of Dynamic Thiol-Norbornene Click Hydrogels. ACS Biomater. Sci. Eng. 2019, 5, 1247–1256. [Google Scholar] [CrossRef] [Green Version]
- Nair, D.P.; Podgórski, M.; Chatani, S.; Gong, T.; Xi, W.; Fenoli, C.R.; Bowman, C.N. The Thiol-Michael addition click reaction: A powerful and widely used tool in materials chemistry. Chem. Mater. 2014, 26, 724–744. [Google Scholar] [CrossRef]
- Zhao, T.; Sellers, D.L.; Cheng, Y.; Horner, P.J.; Pun, S.H. Tunable, Injectable Hydrogels Based on Peptide-Cross-Linked, Cyclized Polymer Nanoparticles for Neural Progenitor Cell Delivery. Biomacromolecules 2017, 18, 2723–2731. [Google Scholar] [CrossRef] [PubMed]
- Moshayedi, P.; Nih, L.R.; Llorente, I.L.; Berg, A.R.; Cinkornpumin, J.; Lowry, W.E.; Segura, T.; Carmichael, S.T. Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials 2016, 105, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Tam, R.Y.; Cooke, M.J.; Shoichet, M.S. A covalently modified hydrogel blend of hyaluronan-methyl cellulose with peptides and growth factors influences neural stem/progenitor cell fate. J. Mater. Chem. 2012, 22, 19402–19411. [Google Scholar] [CrossRef]
- Führmann, T.; Anandakumaran, P.N.; Payne, S.L.; Pakulska, M.M.; Varga, B.V.; Nagy, A.; Tator, C.; Shoichet, M.S. Combined delivery of chondroitinase ABC and human induced pluripotent stem cell-derived neuroepithelial cells promote tissue repair in an animal model of spinal cord injury. Biomed. Mater. 2018, 13, 024103. [Google Scholar] [CrossRef] [PubMed]
- Galarza, S.; Crosby, A.J.; Pak, C.H.; Peyton, S.R. Control of Astrocyte Quiescence and Activation in a Synthetic Brain Hydrogel. Adv. Healthc. Mater. 2020, 9, 1901419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glass, E.B.; Masjedi, S.; Dudzinski, S.O.; Wilson, A.J.; Duvall, C.L.; Yull, F.E.; Giorgio, T.D. Optimizing Mannose “click” Conjugation to Polymeric Nanoparticles for Targeted siRNA Delivery to Human and Murine Macrophages. ACS Omega 2019, 4, 16756–16767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, E.Y.; Kronenfeld, J.P.; Gattás-Asfura, K.M.; Bayer, A.L.; Stabler, C.L. Engineering an “infectious” Treg biomimetic through chemoselective tethering of TGF-β1 to PEG brush surfaces. Biomaterials 2015, 67, 20–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Sobral, M.C.; Zhang, D.K.Y.; Cartwright, A.N.; Li, A.W.; Dellacherie, M.O.; Tringides, C.M.; Koshy, S.T.; Wucherpfennig, K.W.; Mooney, D.J. Metabolic labeling and targeted modulation of dendritic cells. Nat. Mater. 2020, 19, 1244–1252. [Google Scholar] [CrossRef]
- Lim, H.J.; Perera, T.H.; Wilems, T.S.; Ghosh, S.; Zheng, Y.Y.; Azhdarinia, A.; Cao, Q.; Smith Callahan, L.A. Response to di-functionalized hyaluronic acid with orthogonal chemistry grafting at independent modification sites in rodent models of neural differentiation and spinal cord injury. J. Mater. Chem. B 2016, 4, 6865–6875. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.R.; Weaver, W.M.; Scumpia, P.O.; Di Carlo, D.; Segura, T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat. Mater. 2015, 14, 737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copeland, C.; Stabenfeldt, S.E. Leveraging the Dynamic Blood-Brain Barrier for Central Nervous System Nanoparticle-based Drug Delivery Applications. Curr. Opin. Biomed. Eng. 2020, 14, 1. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, K.; Wang, Y.; Zhou, Y.L.; Fu, Z.; Li, G.; Ling, J.; Yang, Y. Brain-Targeted Dual Site-Selective Functionalized Poly(β-Amino Esters) Delivery Platform for Nerve Regeneration. Nano Lett. 2021, 21, 3007–3015. [Google Scholar] [CrossRef]
- Walter, F.R.; Santa-Maria, A.R.; Mészáros, M.; Veszelka, S.; Dér, A.; Deli, M.A. Surface charge, glycocalyx, and blood-brain barrier function. Tissue Barriers 2021, 9, 1904773. [Google Scholar] [CrossRef] [PubMed]
- Critcher, M.; O’Leary, T.; Huang, M.L. Glycoengineering: Scratching the surface. Biochem. J. 2021, 478, 703–719. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Teng, X.; Yang, C.; Wang, Y.; Wang, L.; Dai, Y.; Sun, H.; Li, J. Ultrasound Controlled Anti-Inflammatory Polarization of Platelet Decorated Microglia for Targeted Ischemic Stroke Therapy. Angew. Chemie Int. Ed. 2021, 60, 5083–5090. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.C.; Jayasinghe, M.K.; Pham, T.T.; Yang, Y.; Wei, L.; Usman, W.M.; Chen, H.; Pirisinu, M.; Gong, J.; Kim, S.; et al. Covalent conjugation of extracellular vesicles with peptides and nanobodies for targeted therapeutic delivery. J. Extracell. Vesicles 2021, 10, e12057. [Google Scholar] [CrossRef] [PubMed]
- Gutowski, S.M.; Templeman, K.L.; South, A.B.; Gaulding, J.C.; Shoemaker, J.T.; LaPlaca, M.C.; Bellamkonda, R.V.; Lyon, L.A.; García, A.J. Host response to microgel coatings on neural electrodes implanted in the brain. J. Biomed. Mater. Res. Part A 2014, 102, 1486–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoffstall, A.J.; Ecker, M.; Danda, V.; Joshi-Imre, A.; Stiller, A.; Yu, M.; Paiz, J.E.; Mancuso, E.; Bedell, H.W.; Voit, W.E.; et al. Characterization of the Neuroinflammatory Response to Thiol-ene Shape Memory Polymer Coated Intracortical Microelectrodes. Micromachines 2018, 9, 486. [Google Scholar] [CrossRef] [Green Version]
- Oh, B.; Swaminathan, V.; Malkovskiy, A.; Santhanam, S.; McConnell, K.; George, P.M.; Oh, B.; Swaminathan, V.; Santhanam, S.; McConnell, K.; et al. Single-Cell Encapsulation via Click-Chemistry Alters Production of Paracrine Factors from Neural Progenitor Cells. Adv. Sci. 2020, 7, 1902573. [Google Scholar] [CrossRef]
- Kang, P.H.; Kumar, S.; Schaffer, D.V.; Schmidt, C.E. Novel biomaterials to study neural stem cell mechanobiology and improve cell-replacement therapies. Curr. Opin. Biomed. Eng. 2017, 4, 13–20. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornelison, C.; Fadel, S. Clickable Biomaterials for Modulating Neuroinflammation. Int. J. Mol. Sci. 2022, 23, 8496. https://doi.org/10.3390/ijms23158496
Cornelison C, Fadel S. Clickable Biomaterials for Modulating Neuroinflammation. International Journal of Molecular Sciences. 2022; 23(15):8496. https://doi.org/10.3390/ijms23158496
Chicago/Turabian StyleCornelison, Chase, and Sherly Fadel. 2022. "Clickable Biomaterials for Modulating Neuroinflammation" International Journal of Molecular Sciences 23, no. 15: 8496. https://doi.org/10.3390/ijms23158496
APA StyleCornelison, C., & Fadel, S. (2022). Clickable Biomaterials for Modulating Neuroinflammation. International Journal of Molecular Sciences, 23(15), 8496. https://doi.org/10.3390/ijms23158496





