Acclimation Strategy of Masson Pine (Pinus massoniana) by Limiting Flavonoid and Terpenoid Production under Low Light and Drought
Abstract
:1. Introduction
2. Results
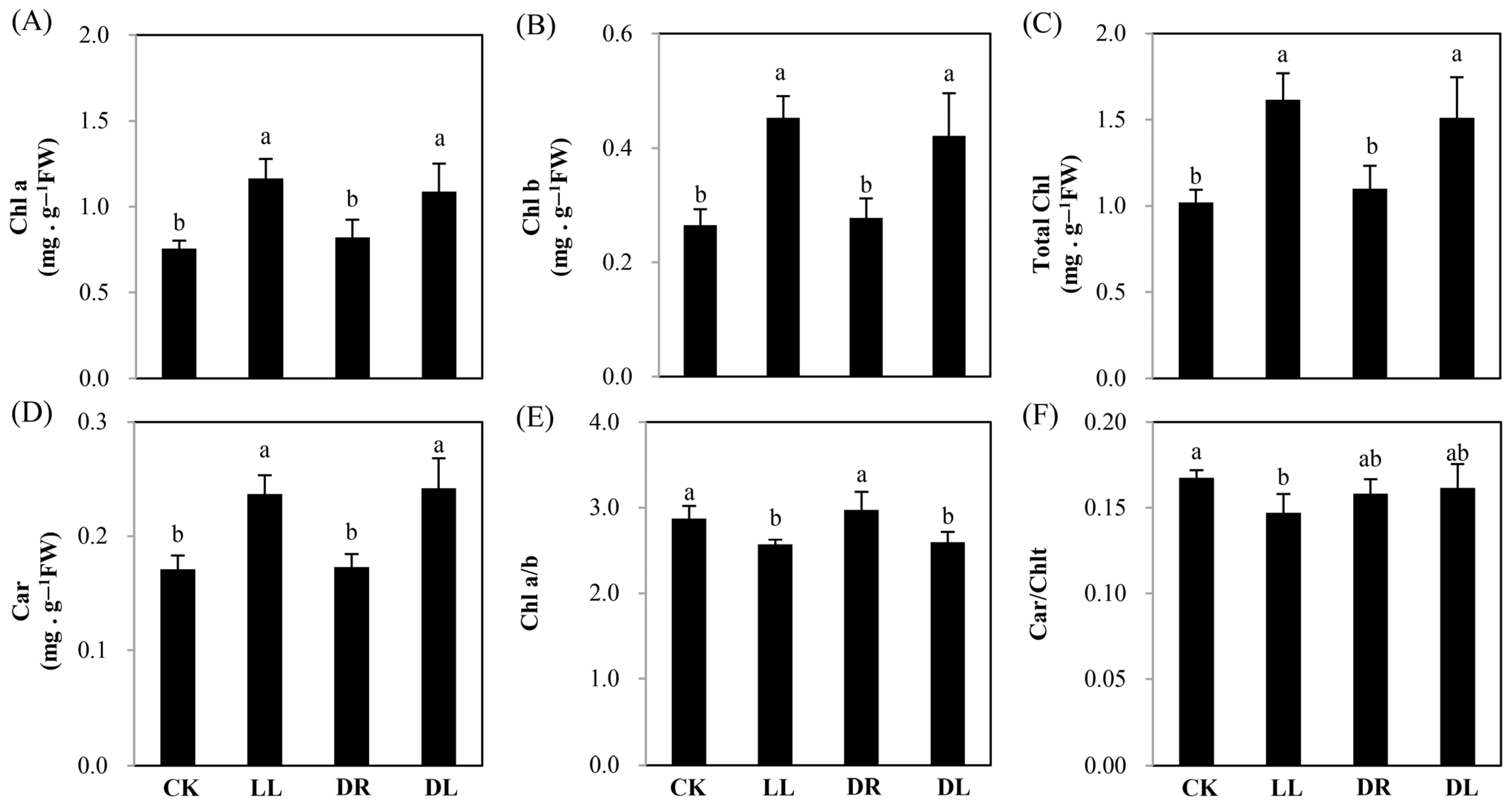
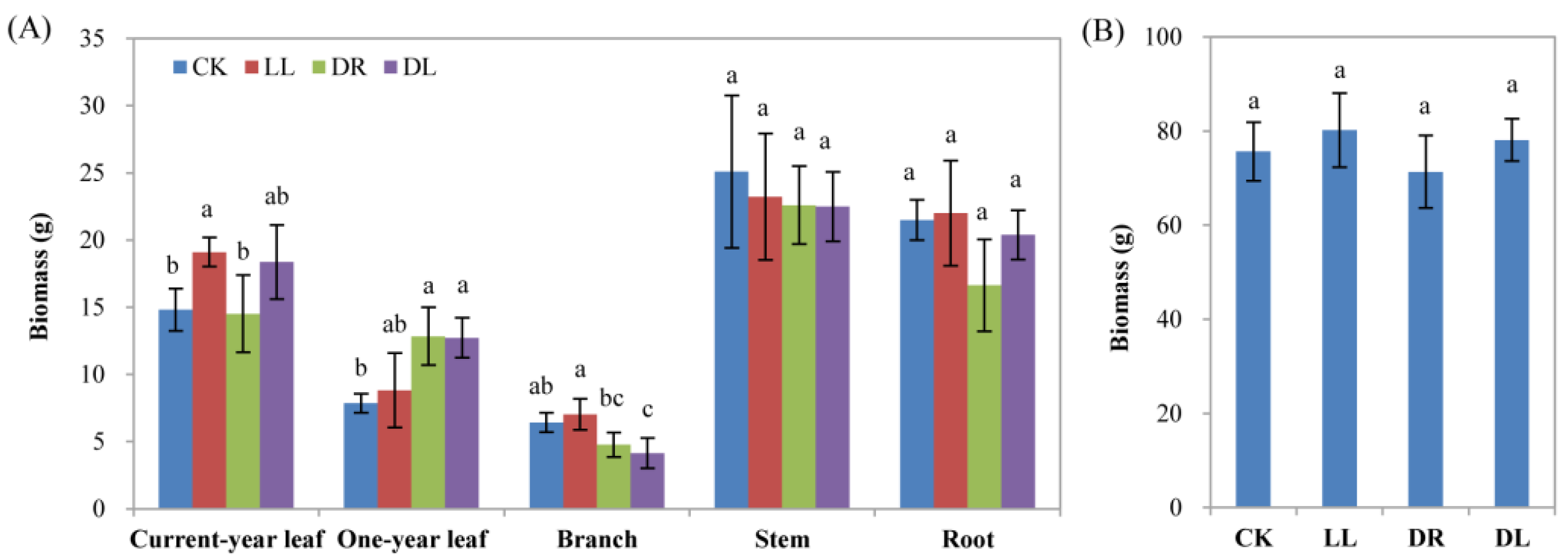
2.1. Effects of Low Light and Drought on the Growth and Pigment of Leaves
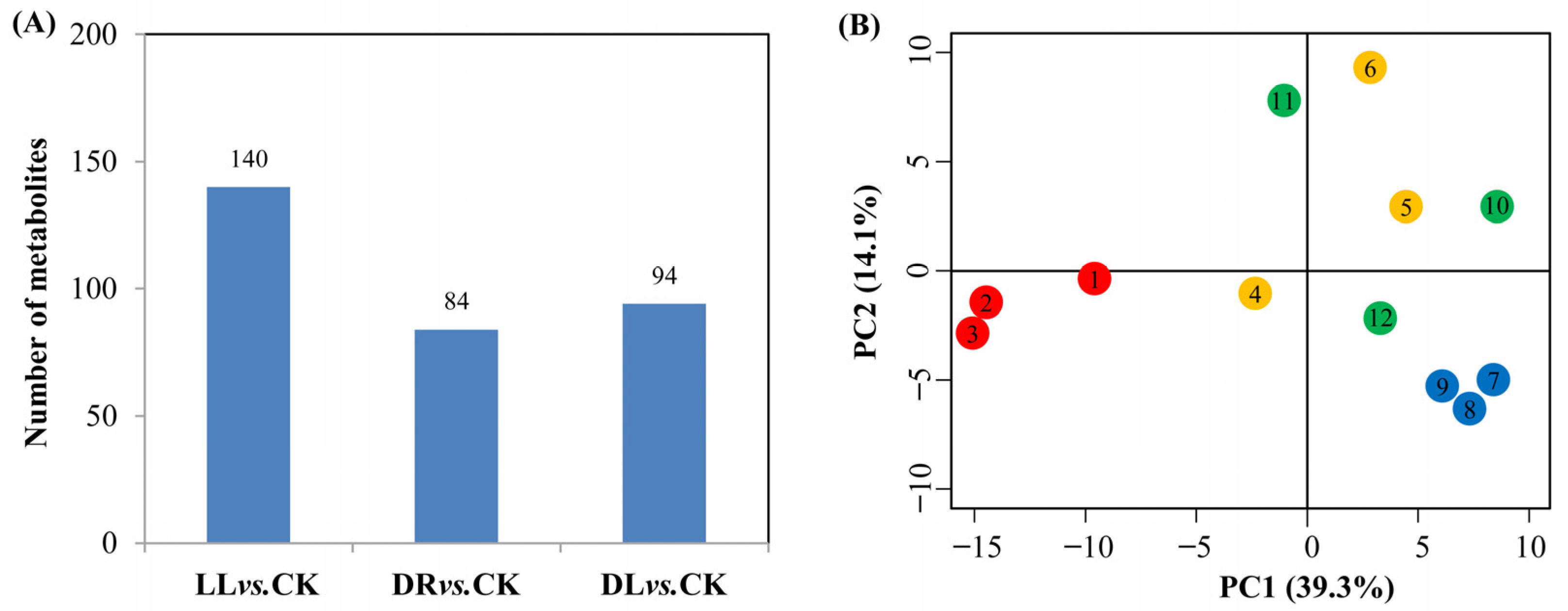
2.2. Effects of Low Light and Drought on Phenolics and Terpenoids in the Current-Year Leaves
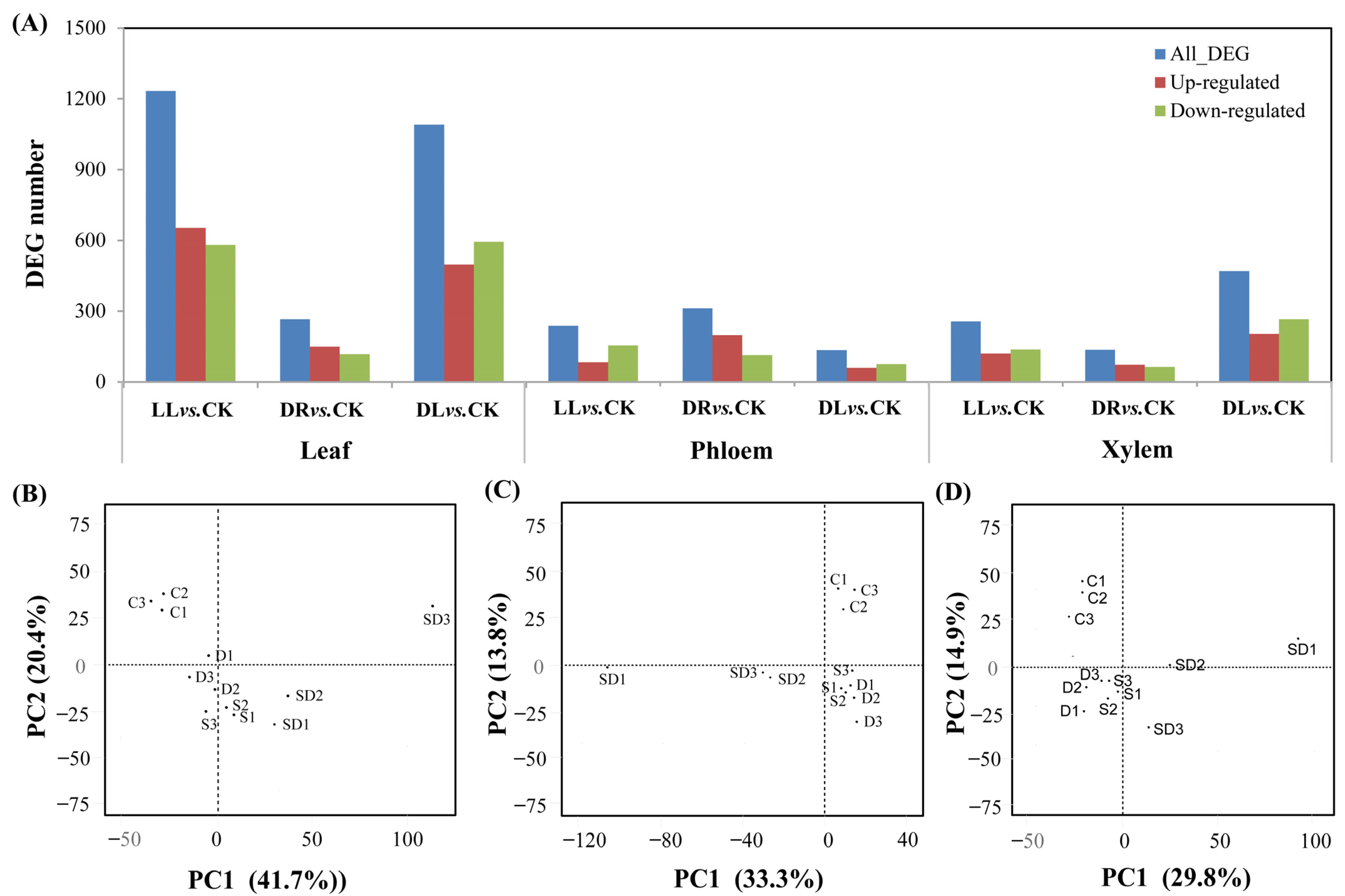
2.3. Transcriptomic Response under Low Light and Drought
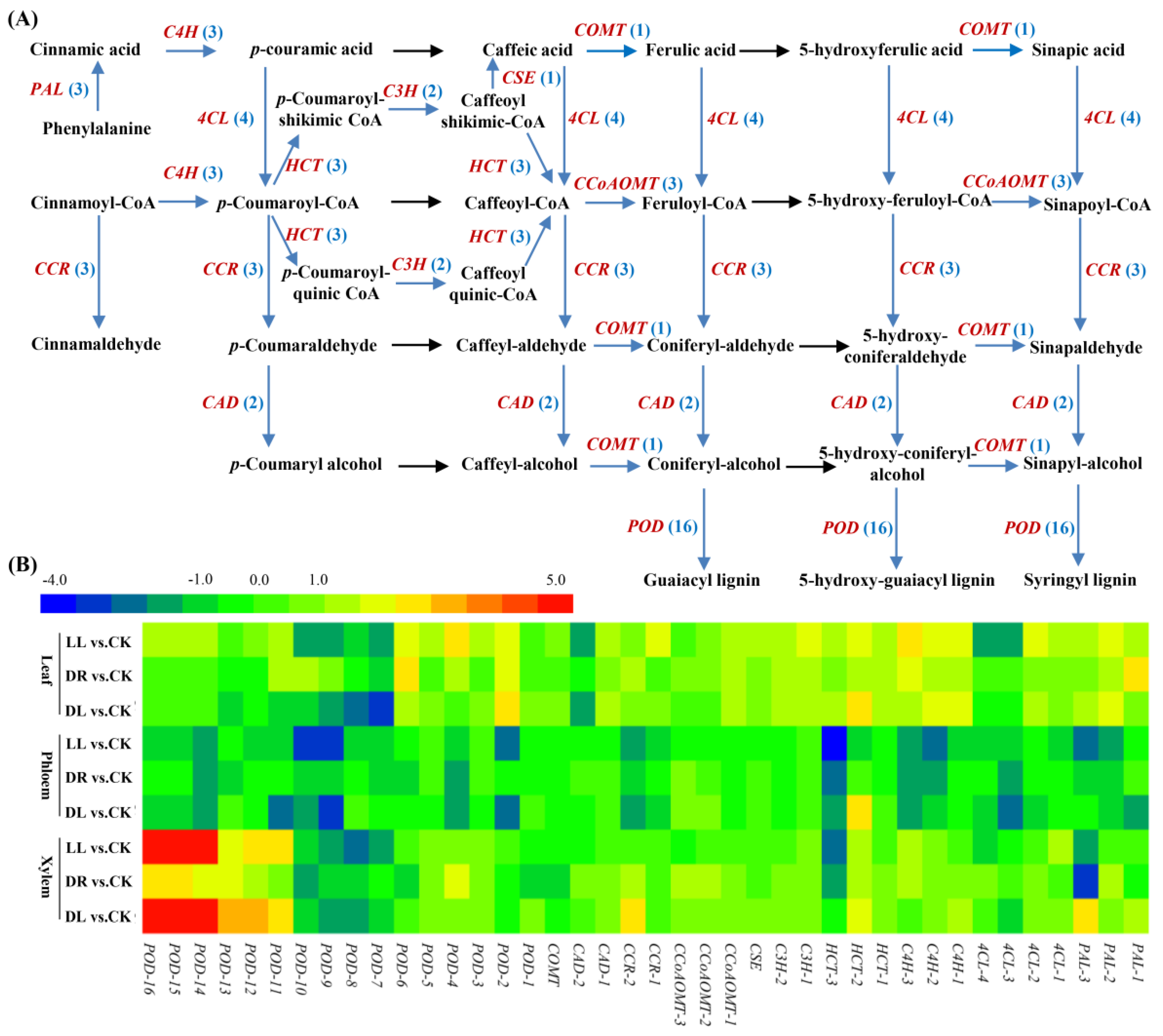
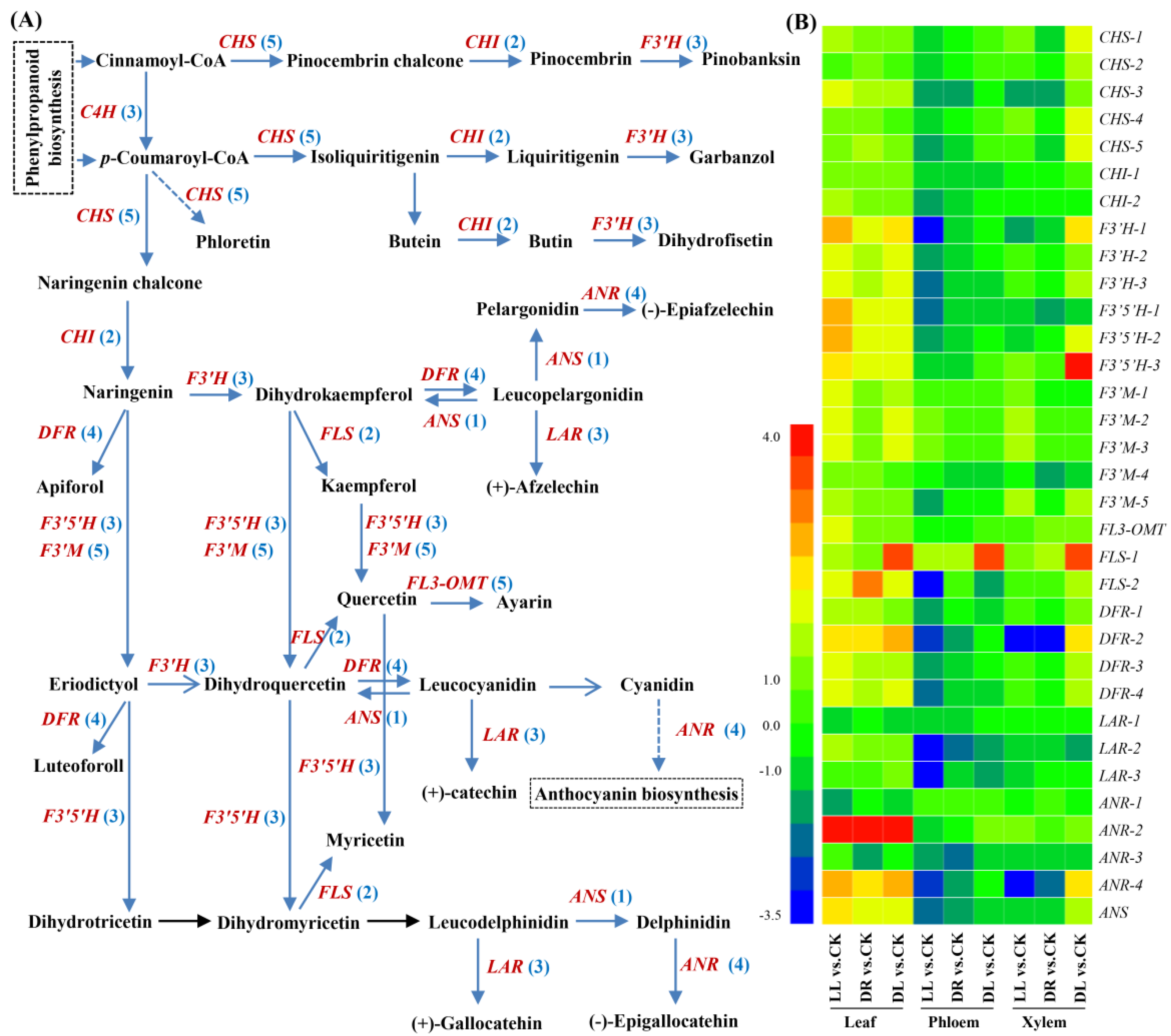
2.4. Expression Profiles of Phenolic-Related DEGs
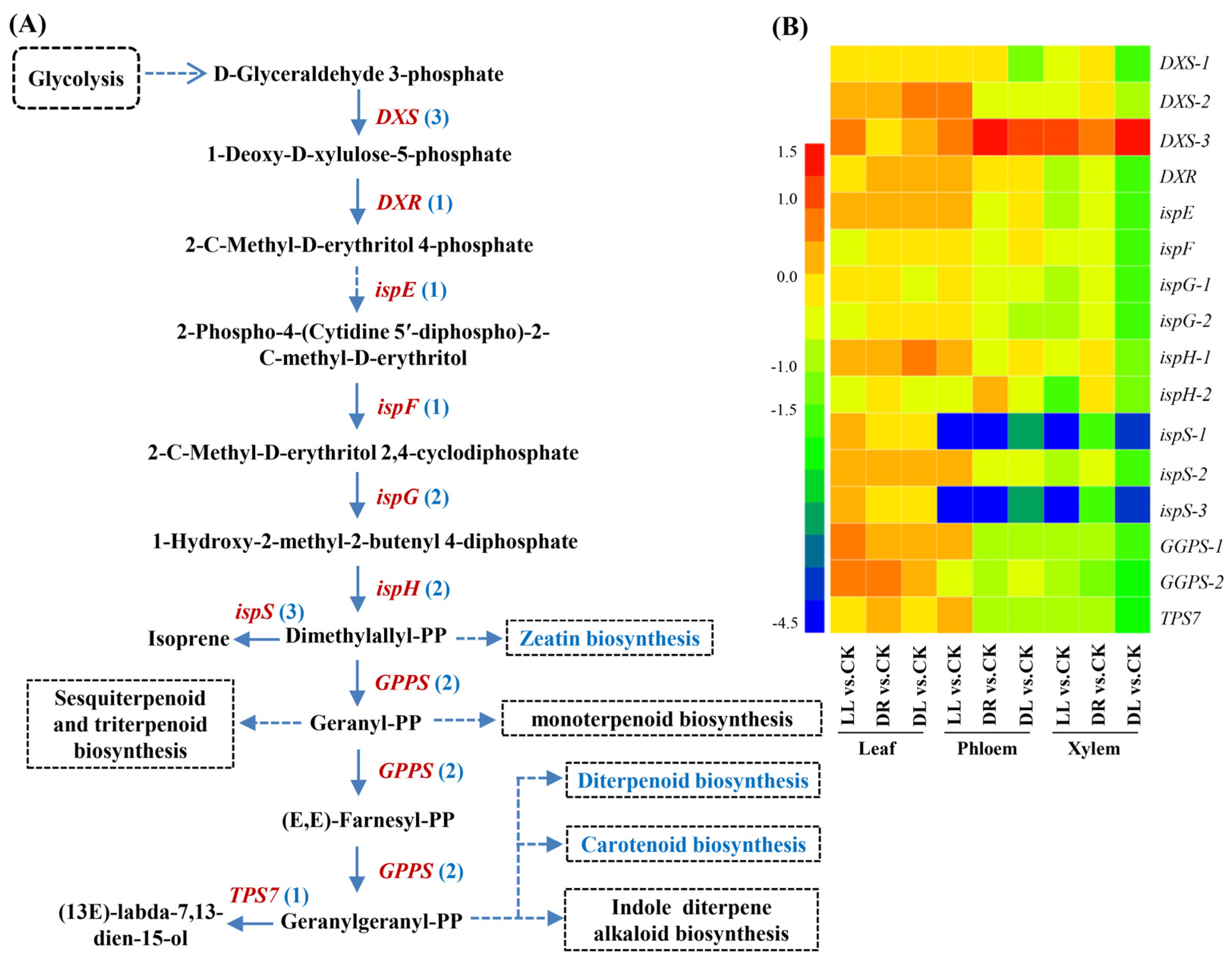
2.5. Expression Profiles of Terpenoid-Related DEGs
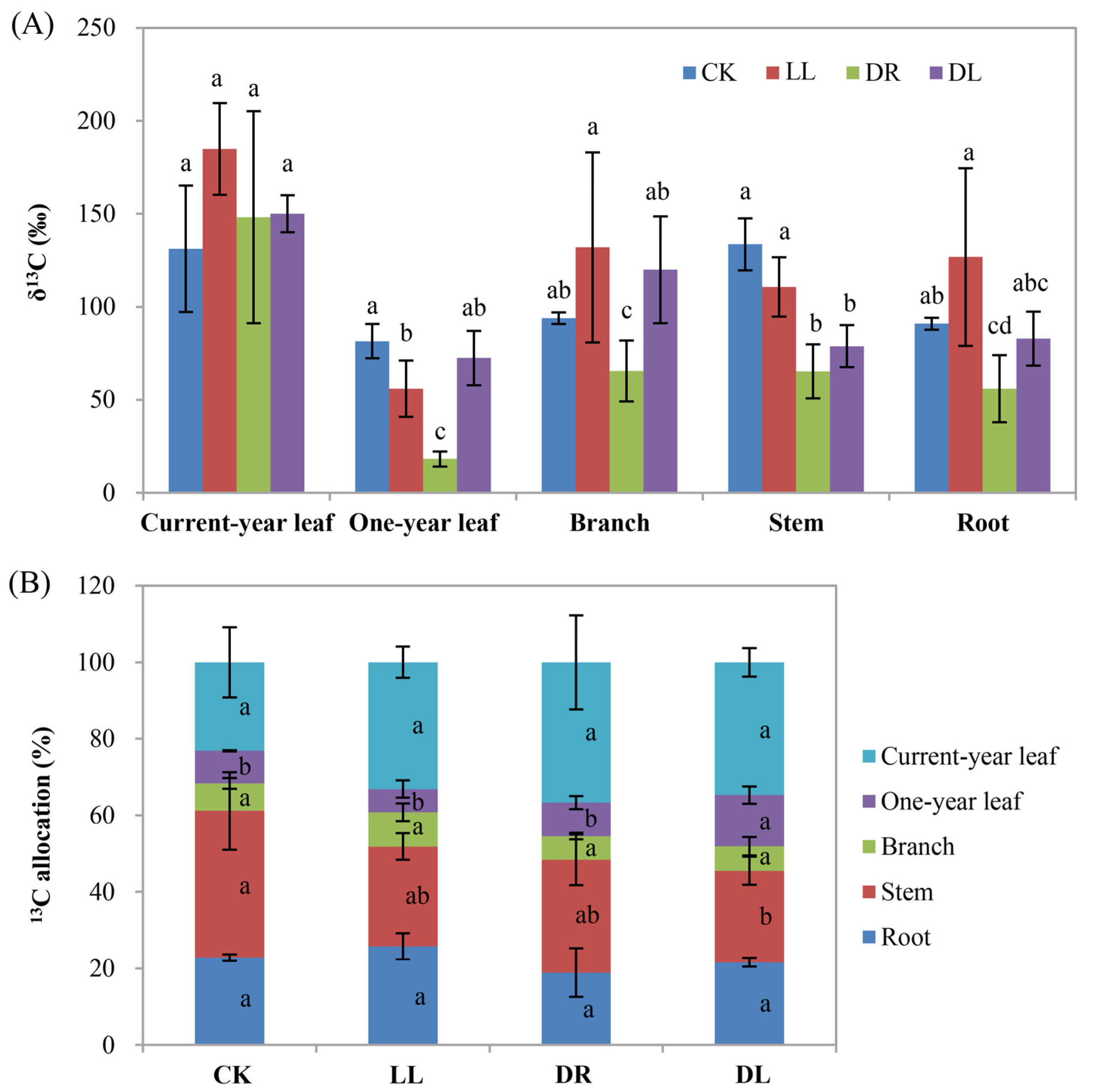
2.6. Effects of Low Light and Drought on Carbon Allocation
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Determination of Leaf Growth, Pigments, and Photosynthesis
4.3. Analysis of 13C-Labeling Experiment
4.4. Extraction, Identification, and Quantification of Metabolites
4.5. RNA-Seq and Gene Functional Annotation
4.6. Identifying Differentially Expressed Genes Involved in Pathways of Flavonoids and Terpenoids
4.7. Quantitative Real-Time PCR (qRT-PCR)
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pierik, R.; Testerink, C. The art of being flexible: How to escape from shade, salt, and drought. Plant Physiol. 2014, 166, 5–22. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Meng, C.; Ji, J.; Li, M.H.; Zhang, X.; Wu, Y.; Xie, T.; Du, C.; Sun, J.; Jiang, Z.; et al. Exogenous GABA promotes adaptation and growth by altering the carbon and nitrogen metabolic flux in poplar seedlings under low nitrogen conditions. Tree Physiol. 2020, 40, 1744–1761. [Google Scholar] [CrossRef] [PubMed]
- Puglielli, G.; Hutchings, M.J.; Laanisto, L. The triangular space of abiotic stress tolerance in woody species: A unified trade-off model. New Phytol. 2021, 229, 1354–1362. [Google Scholar] [CrossRef] [PubMed]
- Puglielli, G.; Laanisto, L.; Poorter, H.; Niinemets, Ü. Global patterns of biomass allocation in woody species with different tolerances of shade and drought: Evidence for multiple strategies. New Phytol. 2021, 229, 308–322. [Google Scholar] [CrossRef] [PubMed]
- Aranda, I.; Castro, L.; Pardos, M.; Gil, L.; Pardos, J.A. Effects of the interaction between drought and shade on water relations, gas exchange and morphological traits in cork oak Quercus suber L. seedlings. For. Ecol. Manag. 2005, 210, 117–129. [Google Scholar] [CrossRef]
- Yang, Y.; Han, C.; Liu, Q.; Lin, B.; Wang, J. Effect of drought and low light on growth and enzymatic antioxidant system of Picea asperata seedlings. Acta Physiol. Plant. 2008, 30, 433–440. [Google Scholar] [CrossRef]
- Cavatte, P.C.; Oliveira, A.A.; Morais, L.E.; Martins, S.C.; Sanglard, L.M.; DaMatta, F.M. Could shading reduce the negative impacts of drought on coffee? A morphophysiological analysis. Physiol. Plant. 2012, 144, 111–122. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, S.; Chi, Y.; Li, Q.; Huang, K.; Yu, Q. Responses of photosynthetic parameters to drought in subtropical forest ecosystem of China. Sci. Rep. 2015, 5, 18254. [Google Scholar] [CrossRef]
- Tomasella, M.; Casolo, V.; Natale, S.; Petruzzellis, F.; Kofler, W.; Beikircher, B.; Mayr, S.; Nardini, A. Shade-induced reduction of stem nonstructural carbohydrates increases xylem vulnerability to embolism and impedes hydraulic recovery in Populus nigra. New Phytol. 2021, 231, 108–121. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef] [Green Version]
- De Roo, L.; Salomón, R.L.; Oleksyn, J.; Steppe, K. Woody tissue photosynthesis delays drought stress in Populus tremula trees and maintains starch reserves in branch xylem tissues. New Phytol. 2020, 228, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Tamang, B.G.; Li, S.; Rajasundaram, D.; Lamichhane, S.; Fukao, T. Overlapping and stress-specific transcriptomic and hormonal responses to flooding and drought in soybean. Plant J. 2021, 107, 100–117. [Google Scholar] [CrossRef] [PubMed]
- Züst, T.; Agrawal, A.A. Trade-offs between plant growth and defense against insect herbivory: An emerging mechanistic synthesis. Annu. Rev. Plant Biol. 2017, 68, 513–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, C.M.; Rao, X.; Saxena, G.; Dixon, R.A. Growth-defense trade-offs and yield loss in plants with engineered cell walls. New Phytol. 2021, 231, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Kleiber, A.; Duan, Q.; Jansen, K.; Verena Junker, L.; Kammerer, B.; Rennenberg, H.; Ensminger, I.; Gessler, A.; Kreuzwieser, J. Drought effects on root and needle terpenoid content of a coastal and an interior Douglas fir provenance. Tree Physiol. 2017, 37, 1648–1658. [Google Scholar] [CrossRef]
- Furst-Jansen, J.M.R.; de Vries, S.; de Vries, J. Evo-physio: On stress responses and the earliest land plants. J. Exp. Bot. 2020, 71, 3254–3269. [Google Scholar] [CrossRef] [Green Version]
- Passon, M.; Weber, F.; Jung, N.U.; Bartels, D. Profiling of phenolic compounds in desiccation-tolerant and non-desiccation-tolerant Linderniaceae. Phytochem. Anal. 2021, 32, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Zoratti, L.; Karppinen, K.; Luengo Escobar, A.; Häggman, H.; Jaakola, L. Light-controlled flavonoid biosynthesis in fruits. Front. Plant Sci. 2014, 5, 534. [Google Scholar] [CrossRef]
- Smith, H. Phytochromes and light signal perception by plants—An emerging synthesis. Nature 2000, 407, 585–591. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Zou, H.; Qiu, L.; Zheng, Y.; Yang, D.; Wang, Y. Effects of light on secondary metabolite biosynthesis in medicinal plants. Front. Plant Sci. 2021, 12, 781236. [Google Scholar] [CrossRef]
- Garrido, A.; Engel, J.; Mumm, R.; Conde, A.; Cunha, A.; De Vos, R.C.H. Metabolomics of photosynthetically active tissues in white grapes: Effects of light microclimate and stress mitigation strategies. Metabolites 2021, 11, 205. [Google Scholar] [CrossRef]
- Lorini, A.; Damin, F.M.; de Oliveira, D.N.; Crizel, R.L.; Godoy, H.T.; Galli, V.; Meinhart, A.D. Characterization and quantification of bioactive compounds from Ilex paraguariensis residue by HPLC-ESI-QTOF-MS from plants cultivated under different cultivation systems. J. Food Sci. 2021, 86, 1599–1619. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Lv, Z.; Zhang, G.; Li, J.; Zhang, J.; He, C. An ABA-flavonoid relationship contributes to the differences in drought resistance between different sea buckthorn subspecies. Tree Physiol. 2021, 41, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, N.; Lee, M.; Lee, H.; Adhikari, A.; Han, A.R.; Han, A.; Kim, H.S. Evaluation of morphological, physiological, and biochemical traits for assessing drought resistance in eleven tree species. Sci. Total Environ. 2021, 779, 146466. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Deng, X.; Bai, D.; Lei, J.; Li, M.; Zeng, L.; Xiao, W. Transcriptomic analysis reveals the mechanism of Picea crassifolia survival for alpine treeline condition. Forests 2020, 11, 156. [Google Scholar] [CrossRef] [Green Version]
- Blanch, J.-S.; Peñuelas, J.; Sardans, J.; Llusià, J. Drought, warming and soil fertilization effects on leaf volatile terpene concentrations in Pinus halepensis and Quercus ilex. Acta Physiol. Plant. 2009, 31, 207–218. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, H.; Tong, X.; Liu, Z.; Zhang, X.; Chen, Y.; Wu, F.; Jiang, X.; Yu, X. Effects of shading on triterpene saponin accumulation and related gene expression of Aralia elata (Miq.) Seem. Plant Physiol. Biochem. 2021, 160, 166–174. [Google Scholar] [CrossRef]
- Lv, Y.; Li, Y.; Liu, X.; Xu, K. Photochemistry and proteomics of ginger (Zingiber officinale Roscoe) under drought and shading. Plant Physiol. Biochem. 2020, 151, 188–196. [Google Scholar] [CrossRef]
- MacAllister, S.; Mencuccini, M.; Sommer, U.; Engel, J.; Hudson, A.; Salmon, Y.; Dexter, K.G. Drought-induced mortality in Scots pine: Opening the metabolic black box. Tree Physiol. 2019, 39, 1358–1370. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, Y.; Gong, J. Physiological mechanisms of the tolerance response to manganese stress exhibited by Pinus massoniana, a candidate plant for the phytoremediation of Mn-contaminated soil. Environ. Sci. Pollut. Res. 2021, 28, 45422–45433. [Google Scholar] [CrossRef]
- Xiao, F.; Zhao, Y.; Wang, X.R.; Liu, Q.; Ran, J. Transcriptome analysis of needle and root of Pinus Massoniana in response to continuous drought stress. Plants 2021, 10, 769. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.M.; Wen, X.F.; Yu, G.R.; Liu, Y.F.; Liu, Q.J. Seasonal drought effects on carbon sequestration of a mid-subtropical planted forest of southeastern China. Sci. China Ser. D Earth Sci. 2006, 49, 110–118. [Google Scholar] [CrossRef]
- Wen, X.F.; Wang, H.M.; Wang, J.L.; Yu, G.R.; Sun, X.M. Ecosystem carbon exchanges of a subtropical evergreen coniferous plantation subjected to seasonal drought, 2003–2007. Biogeosciences 2010, 7, 357–369. [Google Scholar] [CrossRef] [Green Version]
- Du, M.; Ding, G.; Cai, Q. The transcriptomic responses of Pinus massoniana to drought stress. Forests 2018, 9, 326. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Xiao, W.; Shi, Z.; Zeng, L.; Lei, L. Combined effects of drought and shading on growth and non-structural carbohydrates in Pinus massoniana Lamb. seedlings. Forests 2020, 11, 18. [Google Scholar] [CrossRef] [Green Version]
- Harfouche, A.; Meilan, R.; Altman, A. Molecular and physiological responses to abiotic stress in forest trees and their relevance to tree improvement. Tree Physiol. 2014, 34, 1181–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palacio, S.; Hoch, G.; Sala, A.; Körner, C.; Millard, P. Does carbon storage limit tree growth? New Phytol. 2014, 201, 1096–1100. [Google Scholar] [CrossRef] [Green Version]
- Xie, T.; Ji, J.; Chen, W.; Yue, J.; Du, C.; Sun, J.; Chen, L.; Jiang, Z.; Shi, S. γ-Aminobutyric acid is closely associated with accumulation of flavonoids. Plant Signal. Behav. 2019, 14, 1604015. [Google Scholar] [CrossRef]
- Nogués, I.; Medori, M.; Calfapietra, C. Limitations of monoterpene emissions and their antioxidant role in Cistus sp. under mild and severe treatments of drought and warming. Environ. Exp. Bot. 2015, 119, 76–86. [Google Scholar] [CrossRef]
- Beggs, C.J.; Wellmann, E. Photocontrol of flavonoid biosynthesis. In Photomorphogenesis in Plants; Kendrick, R.E., Kronenberg, G.H., Eds.; Springer: Dordrecht, The Netherlands, 1994. [Google Scholar] [CrossRef]
- Xu, J.; Nie, S.; Xu, C.Q.; Liu, H.; Jia, K.H.; Zhou, S.S.; Zhao, W.; Zhou, X.Q.; El-Kassaby, Y.A.; Wang, X.R.; et al. UV-B-induced molecular mechanisms of stress physiology responses in the major northern Chinese conifer Pinus tabuliformis Carr. Tree Physiol. 2021, 41, 1247–1263. [Google Scholar] [CrossRef]
- Jun, J.H.; Lu, N.; Docampo-Palacios, M.; Wang, X.; Dixon, R.A. Dual activity of anthocyanidin reductase supports the dominant plant proanthocyanidin extension unit pathway. Sci. Adv. 2021, 7, eabg4682. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Bonn, B.; Kreuzwieser, J. Terpenoids are transported in the xylem sap of Norway spruce. Plant Cell Environ. 2020, 43, 1766–1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karunanithi, P.S.; Berrios, D.I.; Wang, S.; Davis, J.; Shen, T.; Fiehn, O.; Maloof, J.N.; Zerbe, P. The foxtail millet (Setaria italica) terpene synthase gene family. Plant J. 2020, 103, 781–800. [Google Scholar] [CrossRef]
- Li, R.; Chen, P.; Zhu, L.; Wu, F.; Chen, Y.; Zhu, P.; Ji, K. Characterization and function of the 1-deoxy-D-xylose-5-phosphate synthase (DXS) gene related to terpenoid synthesis in Pinus massoniana. Int. J. Mol. Sci. 2021, 22, 848. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Shi, Z.; Zeng, L.; Xin, X.; Lei, L.; Pei, S.; Xiao, W. Photosynthetic product allocations to the organs of Pinus massoniana are not affected by differences in synthesis or temporal variations in translocation rates. Forests 2021, 12, 471. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Jin, Y.; Tan, K.; Zheng, J.; Gao, T.; Zhang, Z.; Zhao, Y.; Ma, F.; Li, C. MdTyDc overexpression improves alkalinity tolerance in Malus domestica. Front. Plant Sci. 2021, 12, 625890. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [Green Version]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genom. Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Liu, Q.; Dong, H.; Zhou, Z.; Hao, Y.; Chen, X.; Xu, L. Selection of reference genes for real-time quantitative PCR in Pinus massoniana post nematode inoculation. PLoS ONE 2016, 11, e0147224. [Google Scholar] [CrossRef]

| #ID | Metabolites | LL vs.CK | p-Value | DR vs.CK | p-Value | DL vs.CK | p-Value |
|---|---|---|---|---|---|---|---|
| Phenylpropanoids | |||||||
| 281 | 4-Methylcoumarin | −1.8 | 0.08 | – | – | – | – |
| 858 | Chalepensin | −1.3 | 0.10 | – | – | – | – |
| 977 | Matairesinol | −1.4 | 0.07 | −2.1 | 0.04 | – | – |
| 893 | 4-Hydroxy-3-methoxycinnamaldehyde | −1.0 | 0.05 | −0.6 | 0.10 | −0.8 | 0.06 |
| 144 | Hydrocinnamic acid | −0.7 | 0.04 | −1.0 | 0.08 | −0.9 | 0.05 |
| 1404 | Dihydroconiferin | −0.5 | 0.07 | −0.7 | 0.08 | −0.7 | 0.04 |
| 791 | Propofol | −2.0 | 0.03 | – | – | −1.8 | 0.03 |
| 1447 | Europetin | −1.4 | 0.00 | – | – | −1.0 | 0.05 |
| 449 | Transferulic acid | – | – | – | – | 0.5 | 0.08 |
| Flavonoids and derivates | |||||||
| 150 | Petunidin 3-(6’’-acetylglucoside) | −2.4 | 0.06 | −3.1 | 0.05 | −1.3 | 0.08 |
| 1168 | Kaempferol 3-(2’’-rhamnosyl-6’’-acetylgalactoside) 7-rhamnoside | −1.7 | 0.04 | −1.1 | 0.07 | −1.4 | 0.06 |
| 299 | Quercetin-3-O-beta-glucopyranosyl-6’-acetate | −3.4 | 0.08 | −5.5 | 0.07 | −2.1 | 0.09 |
| 981 | Pinocembrin 5,7-dimethyl ether | −2.5 | 0.07 | −2.0 | 0.08 | −2.2 | 0.07 |
| 394 | Canthaxanthin | – | – | 1.7 | 0.01 | 2.4 | 0.03 |
| 1124 | Moracin I | – | – | −1.8 | 0.01 | −1.3 | 0.00 |
| 1521 | Alpinumisoflavone dimethyl ether | – | – | −1.6 | 0.02 | −1.2 | 0.00 |
| 1572 | Prodelphinidin A1 | – | – | 1.0 | 0.03 | 1.4 | 0.03 |
| 691 | Plantagoside | – | – | −1.0 | 0.04 | – | – |
| 852 | Quercetin-3-ramnoside | – | – | −1.0 | 0.02 | – | – |
| 701 | Taxifolin | – | – | −2.2 | 0.09 | – | – |
| 1012 | 4”-O-methyl-EGCG/(−)-epigallocatechin-3-O-gallate | – | – | 1.1 | 0.10 | – | – |
| 1563 | (−)-Epigallocatechin | – | – | 1.8 | 0.10 | – | – |
| 739 | 5,7,3’,4’,5’-Pentahydroxyflavanone | – | – | 0.9 | 0.07 | – | – |
| 1526 | 5-Carboxypyranopelargonidin 3-O-beta-glucopyranoside | – | – | −1.0 | 0.04 | – | – |
| 905 | 11b-Hydroxyaetiocholanolone | −1.4 | 0.08 | −1.0 | 0.09 | – | – |
| 416 | Apigenin 7-O-glucoside | −0.7 | 0.08 | −1.1 | 0.03 | – | – |
| 1362 | Herbacetin 7-methyl ether 3-(2’’-(E)-feruloylglucoside) | −2.4 | 0.02 | −2.2 | 0.02 | – | – |
| 81 | Oenin | −2.9 | 0.08 | −2.4 | 0.10 | – | – |
| 447 | Moracetin | −0.9 | 0.03 | – | – | – | – |
| 689 | Pinocembrin 7-O-benzoate | −1.0 | 0.08 | – | – | – | – |
| 1501 | 7-Hydroxy-2’,3’,4’-trimethoxyisoflavan | 1.3 | 0.10 | – | – | – | – |
| 500 | Praecanson A | −1.6 | 0.09 | – | – | −1.4 | 0.10 |
| 268 | Haginin B | −0.7 | 0.05 | – | – | −1.0 | 0.04 |
| 746 | 2-Ethoxycarbonyl-5,7-dihydroxy-8’,3’,4’,5’-tetramethoxyisoflavone | 0.5 | 0.01 | – | – | 0.3 | 0.09 |
| 528 | Laricitrin 3-galactoside | −1.3 | 0.00 | – | – | −0.9 | 0.08 |
| 646 | Piperaduncin A | −1.7 | 0.09 | – | – | −1.5 | 0.10 |
| 254 | 8-Prenylquercetin 4’-methyl ether 3-rhamnoside | – | – | – | – | 0.6 | 0.08 |
| 1658 | Kaempferol 3-(2”,6”-di-(E)-p-coumarylglucoside) | – | – | – | – | 0.9 | 0.09 |
| 1380 | Kaempferol 3-(3’’-p-coumaryl-6’’-ferulylglucoside) | – | – | – | – | 0.7 | 0.05 |
| 122 | Icariside II | – | – | – | – | 0.6 | 0.0 |
| 1204 | 4-Coumaroylshikimate | – | – | – | – | −2.0 | 0.10 |
| 718 | Kanzonol S | – | – | – | – | −1.0 | 0.04 |
| Lignas | |||||||
| 1585 | Silidianin | – | – | – | – | 0.9 | 0.04 |
| 1528 | Egonol | −1.9 | 0.07 | – | – | −1.6 | 0.08 |
| 644 | Dihydrocubebin | −0.5 | 0.02 | – | – | −0.6 | 0.02 |
| 98 | Justicidin A | −1.2 | 0.01 | – | – | ||
| 1238 | Divanillyltetrahydrofuran ferulate | −1.7 | 0.10 | – | – | – | – |
| #ID | Metabolites | LL vs.CK | p-Value | DR vs.CK | p-Value | DL vs.CK | p-Value |
|---|---|---|---|---|---|---|---|
| Monoterpenoids and derivatives | |||||||
| 656 | −1.0 | 0.09 | – | – | – | – | |
| 974 | loliolide | −1.5 | 0.10 | – | – | – | – |
| 665 | Safrole | −0.8 | 0.02 | – | – | – | – |
| Diterpenoids and derivatives | |||||||
| 1442 | 13-Acetyl-9-Dihydrobaccatin III | −1.4 | 0.04 | −0.7 | 0.10 | −1.2 | 0.04 |
| 631 | ent-15-Oxo-16-kauren-19-oic acid | −1.4 | 0.04 | −0.6 | 0.08 | – | – |
| Sesquiterpenoids and derivatives | |||||||
| 338 | 3,15-Epoxy-6-caryophyllene | −1.1 | 0.10 | – | – | – | – |
| 525 | gamma-Eudesmol rhamnoside | −2.7 | 0.01 | – | – | – | – |
| 640 | Dihydroisoalantolactone | −1.1 | 0.04 | −0.8 | 0.08 | −0.8 | 0.08 |
| 442 | Dihydronootkatone | −1.1 | 0.03 | −0.9 | 0.06 | −1.2 | 0.03 |
| 94 | Isocurcumenol | – | – | −1.5 | 0.01 | −0.8 | 0.07 |
| 1417 | (−)-Obtusadiene | – | – | −0.5 | 0.02 | – | – |
| 1650 | Taraxacolide 1-O-β-D-glucopyranoside | – | – | 0.6 | 0.05 | – | – |
| 91 | (+)-4,11-Eudesmadien-3-one | – | – | 0.7 | 0.03 | – | – |
| 86 | β-Atlantone | −0.6 | 0.06 | −0.9 | 0.03 | – | – |
| 219 | β-Sinensal | −0.6 | 0.05 | −0.8 | 0.03 | – | – |
| 919 | Citroside A | −1.5 | 0.05 | – | – | – | – |
| 612 | Cacalol | – | – | – | – | 1.1 | 0.07 |
| Triterpenoids and derivatives | |||||||
| 976 | Prosapogenin | – | – | – | – | −0.7 | 0.07 |
| 1205 | Ganoderic acid Mj | – | – | 0.5 | 0.03 | – | – |
| 1445 | Pristimerol | 0.3 | 0.03 | 0.6 | 0.01 | – | – |
| 411 | Betavulgaroside II | −1.8 | 0.08 | – | – | – | – |
| 1372 | 25-Acetyl-6,7-didehydrofevicordin F 3-glucoside | −1.6 | 0.01 | – | – | – | – |
| 584 | Lucidenic acid M | −1.4 | 0.06 | – | – | – | – |
| 1667 | Notoginsenoside T2 | −0.8 | 0.03 | – | – | – | – |
| 1135 | Periandrin V | −2.6 | 0.00 | – | – | – | – |
| 821 | Dukunolide B | 0.6 | 0.04 | 0.8 | 0.02 | – | – |
| Tetraterpenoids and derivatives | |||||||
| 1194 | 3-Hydroxy-beta-zeacarotene | −2.4 | 0.08 | – | – | – | – |
| 1519 | Cucurbitaxanthin A | −0.9 | 0.10 | – | – | – | – |
| 1609 | 3’-Hydroxy-6,6-caroten-3-one | – | – | – | – | 0.4 | 0.02 |
| Pathway | Leaf | Phloem | Xylem | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LL vs. CK | DR vs. CK | DL vs. CK | LL vs. CK | DR vs. CK | DL vs. CK | LL vs. CK | DR vs. CK | DL vs. CK | ||||||||||
| DEG | p-Value | DEG | p-Value | DEG | p-Value | DEG | p-Value | DEG | p-Value | DEG | p-Value | DEG | p-Value | DEG | p-Value | DEG | p-Value | |
| Photosynthesis | 3 | 0.53 | 1 | 0.45 | 2 | 0.68 | 0 | – | 2 | 0.15 | 0 | – | 1 | 0.46 | 3 | 0.00 | 3 | 0.09 |
| Starch and sucrose metabolism | 13 | 0.52 | 1 | 0.94 | 12 | 0.38 | 3 | 0.69 | 6 | 0.10 | 1 | 0.72 | 4 | 0.32 | 2 | 0.42 | 7 | 0.21 |
| Porphyrin and chlorophyll metabolism | 5 | 0.08 | 1 | 0.39 | 9 | 0.00 | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – |
| Carbon fixation in photosynthetic organisms | 9 | 0.20 | 0 | – | 15 | 0.00 | 0 | – | 1 | 0.81 | 0 | – | 4 | 0.06 | 1 | 0.52 | 0 | – |
| Carbon metabolism | 24 | 0.40 | 2 | 0.96 | 34 | 0.00 | 0 | – | 3 | 0.93 | 0 | – | 6 | 0.39 | 2 | 0.73 | 7 | 0.75 |
| Pentose phosphate pathway | 3 | 0.84 | 0 | – | 7 | 0.09 | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 2 | 0.51 |
| Glycolysis and gluconeogenesis | 8 | 0.86 | 1 | 0.91 | 15 | 0.04 | 0 | – | 2 | 0.77 | 1 | 0.42 | 1 | 0.92 | 1 | 0.71 | 5 | 0.39 |
| Galactose metabolism | 7 | 0.12 | 2 | 0.22 | 11 | 0.00 | 2 | 0.31 | 6 | 0.00 | 0 | – | 0 | – | 0 | – | 2 | 0.46 |
| Glyoxylate and dicarboxylate metabolism | 14 | 0.02 | 2 | 0.47 | 13 | 0.01 | 0 | – | 3 | 0.29 | 0 | – | 3 | 0.23 | 2 | 0.20 | 1 | 0.94 |
| Pentose and glucuronate interconversions | 4 | 0.89 | 0 | – | 1 | 1.00 | 2 | 0.52 | 6 | 0.01 | 1 | 0.48 | 2 | 0.42 | 0 | – | 0 | – |
| Citrate cycle (TCA cycle) | 3 | 0.92 | 0 | – | 5 | 0.49 | 0 | – | 0 | – | 0 | – | 3 | 0.13 | 1 | 0.46 | 3 | 0.35 |
| Phenylalanine metabolism | 16 | 0.00 | 3 | 0.02 | 5 | 0.07 | 5 | 0.00 | 0 | – | 2 | 0.03 | 1 | 0.45 | 0 | – | 6 | 0.03 |
| Phenylalanine, tyrosine, and tryptophan biosynthesis | 14 | 0.00 | 4 | 0.00 | 6 | 0.03 | 4 | 0.01 | 0 | – | 0 | – | 0 | – | 1 | 0.27 | 1 | 0.64 |
| Phenylpropanoid biosynthesis | 26 | 0.00 | 4 | 0.15 | 16 | 0.01 | 23 | 0.00 | 2 | 0.71 | 5 | 0.00 | 4 | 0.17 | 2 | 0.30 | 1 | 0.65 |
| Flavonoid biosynthesis | 27 | 0.00 | 3 | 0.06 | 6 | 0.14 | 8 | 0.01 | 4 | 0.02 | 3 | 0.01 | 1 | 0.61 | 1 | 0.38 | 5 | 0.31 |
| Flavone and flavonol biosynthesis | 7 | 0.00 | 1 | 0.19 | 2 | 0.20 | 3 | 0.00 | 2 | 0.03 | 0 | – | 1 | 0.20 | 1 | 0.11 | 1 | 0.80 |
| Terpenoid backbone biosynthesis | 2 | 0.72 | 0 | – | 1 | 0.88 | 1 | 0.50 | 1 | 0.47 | 0 | – | 9 | 0.00 | 1 | 0.24 | 0 | – |
| Diterpenoid biosynthesis | 2 | 0.30 | 1 | 0.21 | 1 | 0.60 | 0 | – | 0 | – | 0 | – | 3 | 0.00 | 2 | 0.01 | 15 | 0.00 |
| Monoterpenoid biosynthesis | 0 | – | 0 | – | 1 | 0.11 | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 5 | 0.00 |
| Sesquiterpenoid and triterpenoid biosynthesis | 1 | 0.37 | 0 | – | 0 | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | |
| Ubiquinone and other terpenoid-quinone biosynthesis | 12 | 0.00 | 1 | 0.40 | 4 | 0.14 | 2 | 0.14 | 1 | 0.46 | 0 | – | 0 | – | 0 | – | 0 | – |
| Plant hormone signal transduction | 18 | 0.00 | 8 | 0.00 | 14 | 0.02 | 5 | 0.09 | 7 | 0.01 | 4 | 0.01 | 3 | 0.32 | 3 | 0.07 | 1 | 0.60 |
| Carotenoid biosynthesis | 6 | 0.00 | 1 | 0.24 | 6 | 0.00 | 0 | – | 0 | – | 1 | 0.12 | 0 | – | 0 | – | 4 | 0.43 |
| Zeatin biosynthesis | 4 | 0.02 | 0 | – | 5 | 0.00 | 1 | 0.26 | 1 | 0.25 | 0 | – | 0 | – | 0 | – | 3 | 0.01 |
| Brassinosteroid biosynthesis | 1 | 0.50 | 0 | – | 1 | 0.44 | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 1 | 0.35 |
| Cysteine and methionine metabolism | 11 | 0.07 | 1 | 0.76 | 11 | 0.02 | 3 | 0.27 | 0 | – | 0 | – | 5 | 0.02 | 1 | 0.52 | 0 | – |
| Tryptophan metabolism | 4 | 0.43 | 0 | – | 4 | 0.31 | 0 | – | 0 | – | 0 | – | 1 | 0.53 | 1 | 0.53 | 3 | 0.45 |
| Alpha-linolenic acid metabolism | 4 | 0.54 | 4 | 0.01 | 4 | 0.40 | 3 | 0.09 | 2 | 0.25 | 0 | – | 0 | – | 1 | 0.35 | 2 | 0.36 |
| Total number | 248 | 41 | 211 | 65 | 49 | 18 | 52 | 26 | 78 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Z.; Deng, X.; Zeng, L.; Shi, S.; Lei, L.; Xiao, W. Acclimation Strategy of Masson Pine (Pinus massoniana) by Limiting Flavonoid and Terpenoid Production under Low Light and Drought. Int. J. Mol. Sci. 2022, 23, 8441. https://doi.org/10.3390/ijms23158441
Shi Z, Deng X, Zeng L, Shi S, Lei L, Xiao W. Acclimation Strategy of Masson Pine (Pinus massoniana) by Limiting Flavonoid and Terpenoid Production under Low Light and Drought. International Journal of Molecular Sciences. 2022; 23(15):8441. https://doi.org/10.3390/ijms23158441
Chicago/Turabian StyleShi, Zheng, Xiuxiu Deng, Lixiong Zeng, Shengqing Shi, Lei Lei, and Wenfa Xiao. 2022. "Acclimation Strategy of Masson Pine (Pinus massoniana) by Limiting Flavonoid and Terpenoid Production under Low Light and Drought" International Journal of Molecular Sciences 23, no. 15: 8441. https://doi.org/10.3390/ijms23158441
APA StyleShi, Z., Deng, X., Zeng, L., Shi, S., Lei, L., & Xiao, W. (2022). Acclimation Strategy of Masson Pine (Pinus massoniana) by Limiting Flavonoid and Terpenoid Production under Low Light and Drought. International Journal of Molecular Sciences, 23(15), 8441. https://doi.org/10.3390/ijms23158441






