Facile Construction of Iron/Nickel Phosphide Nanocrystals Anchored on N-B-Doped Carbon-Based Composites with Advanced Catalytic Capacity for 4-Nitrophenol and Methylene Blue
Abstract
:1. Introduction
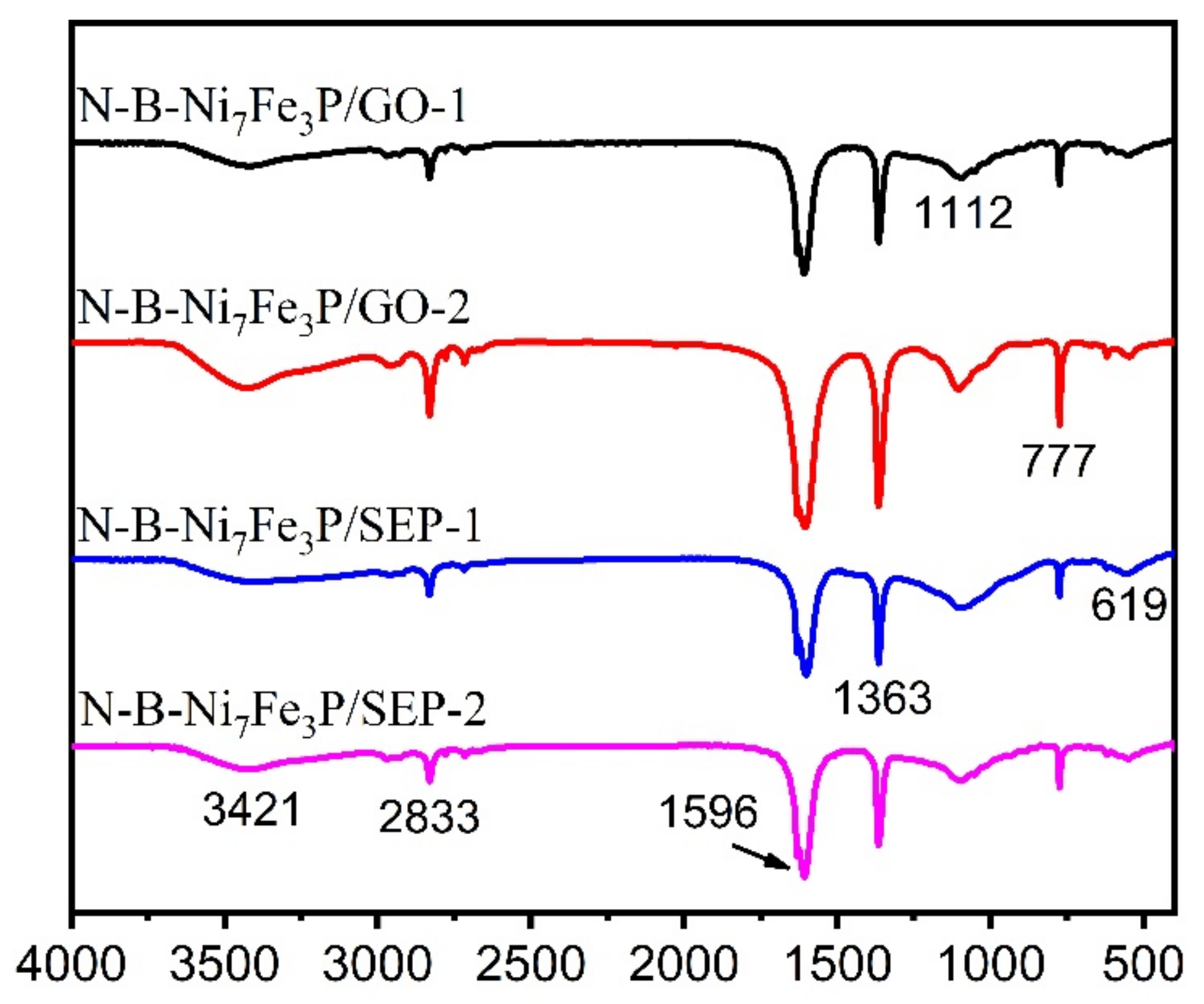
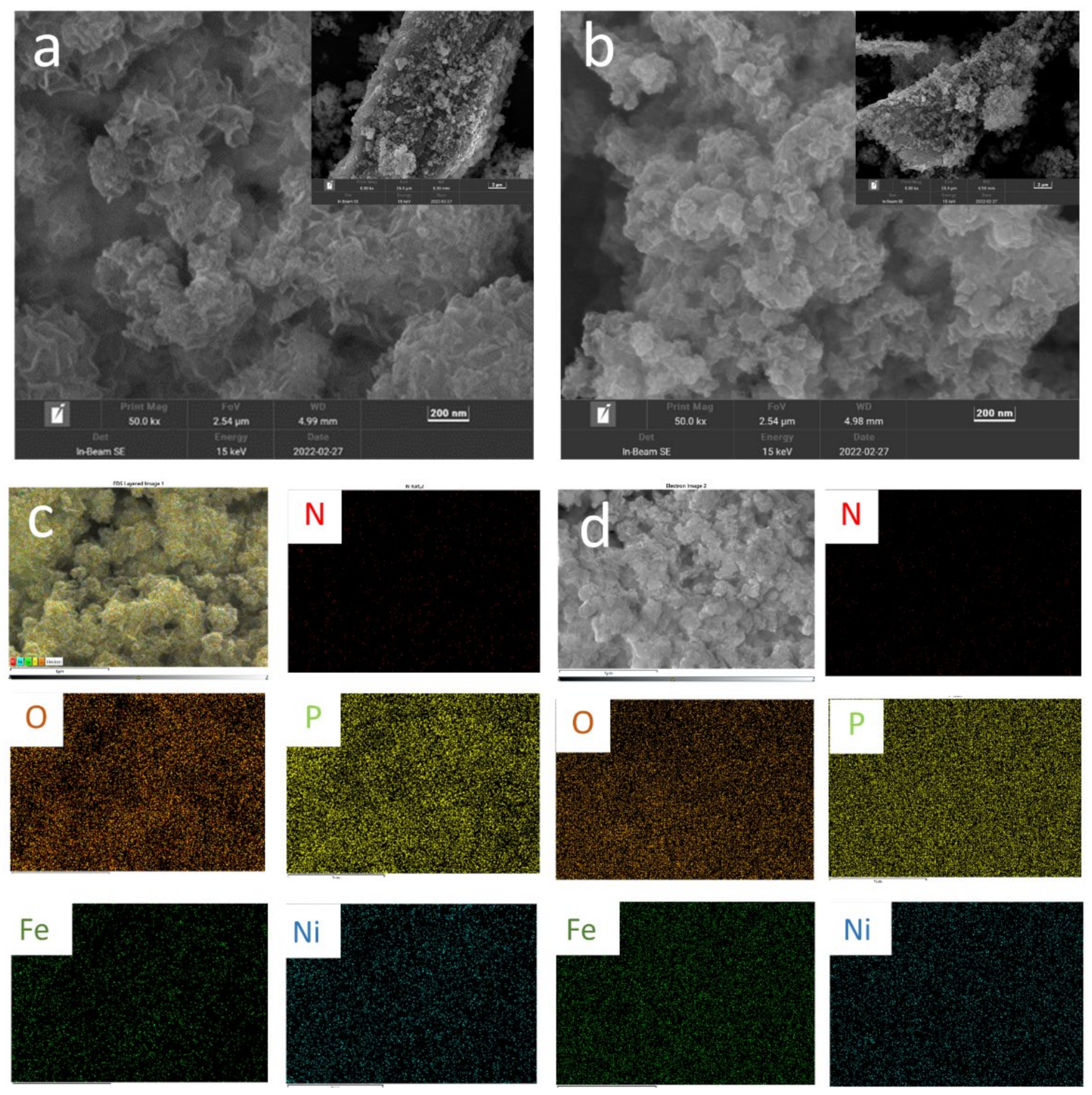
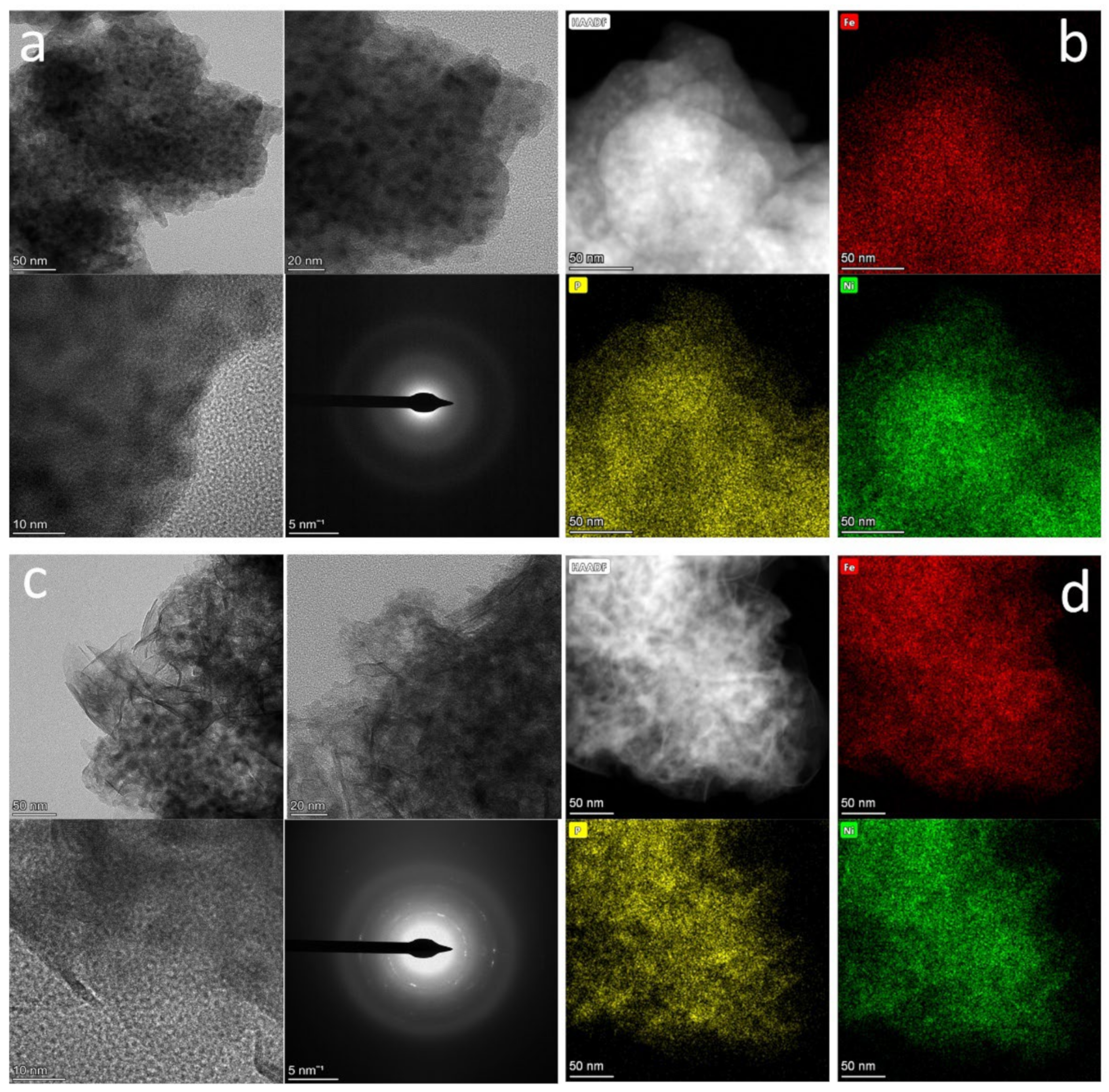
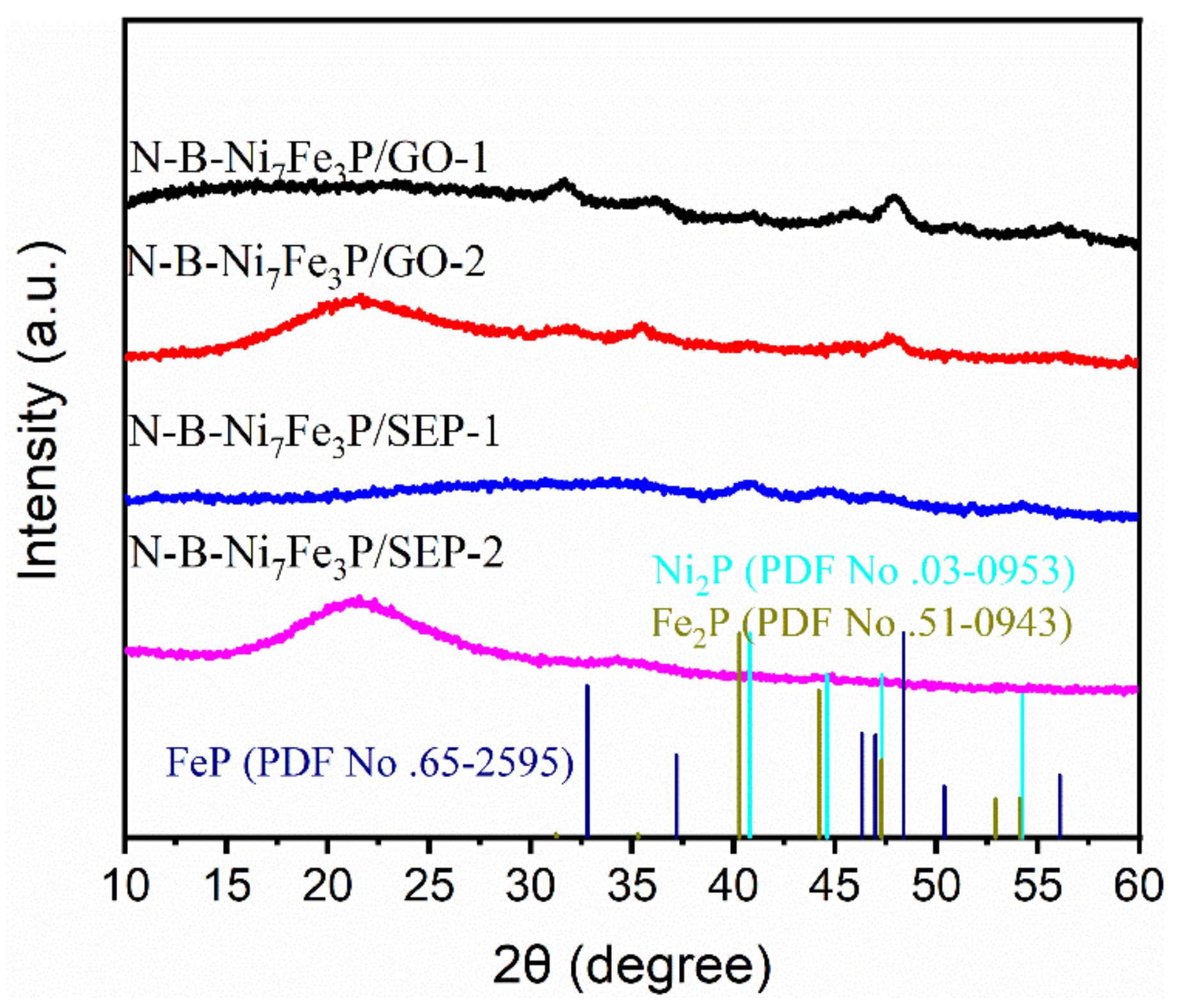
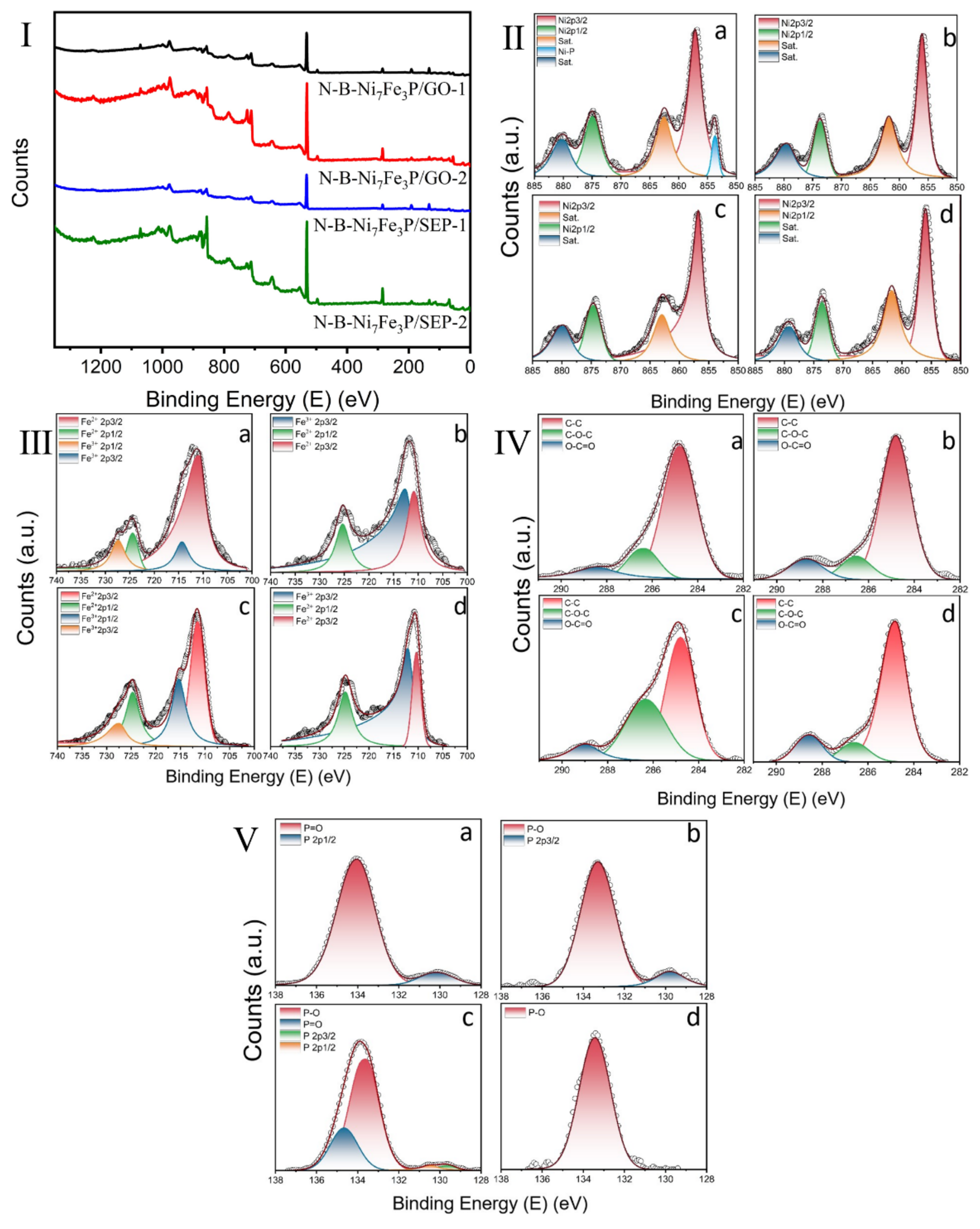
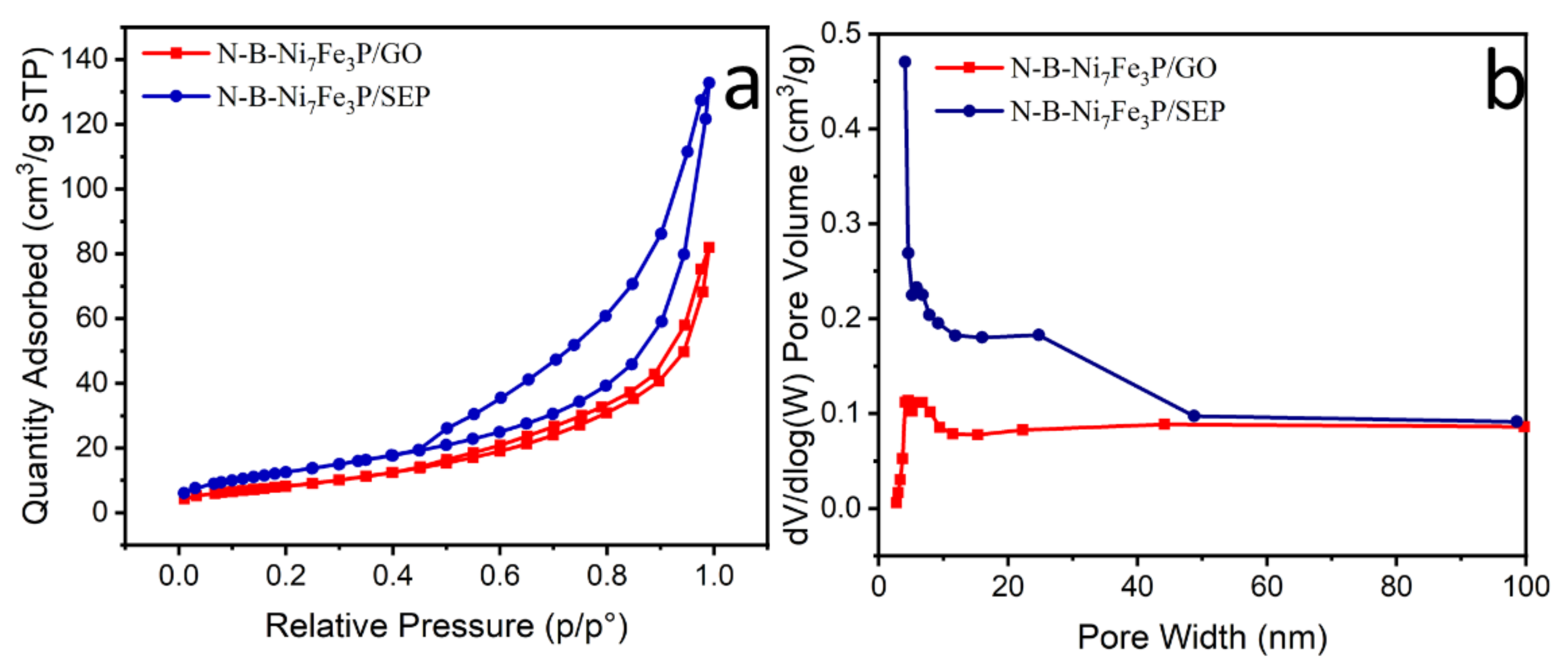
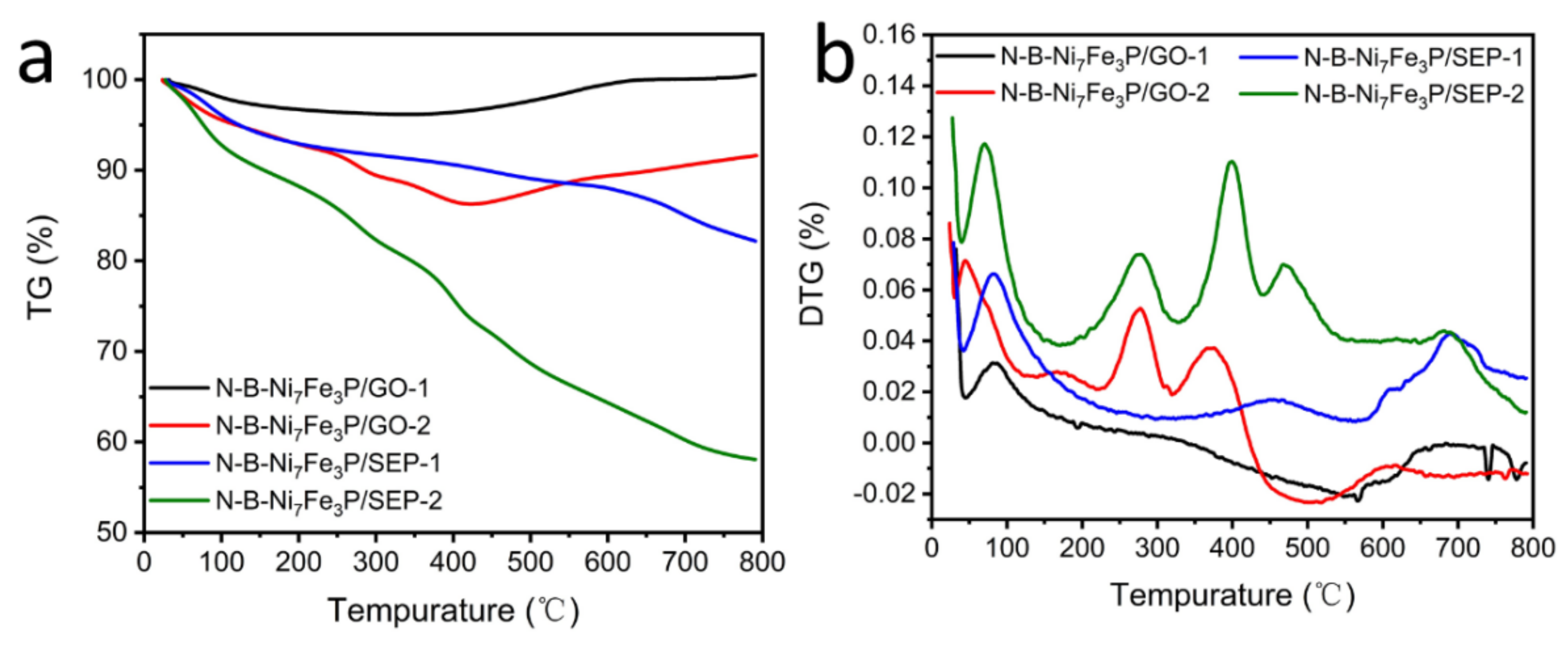
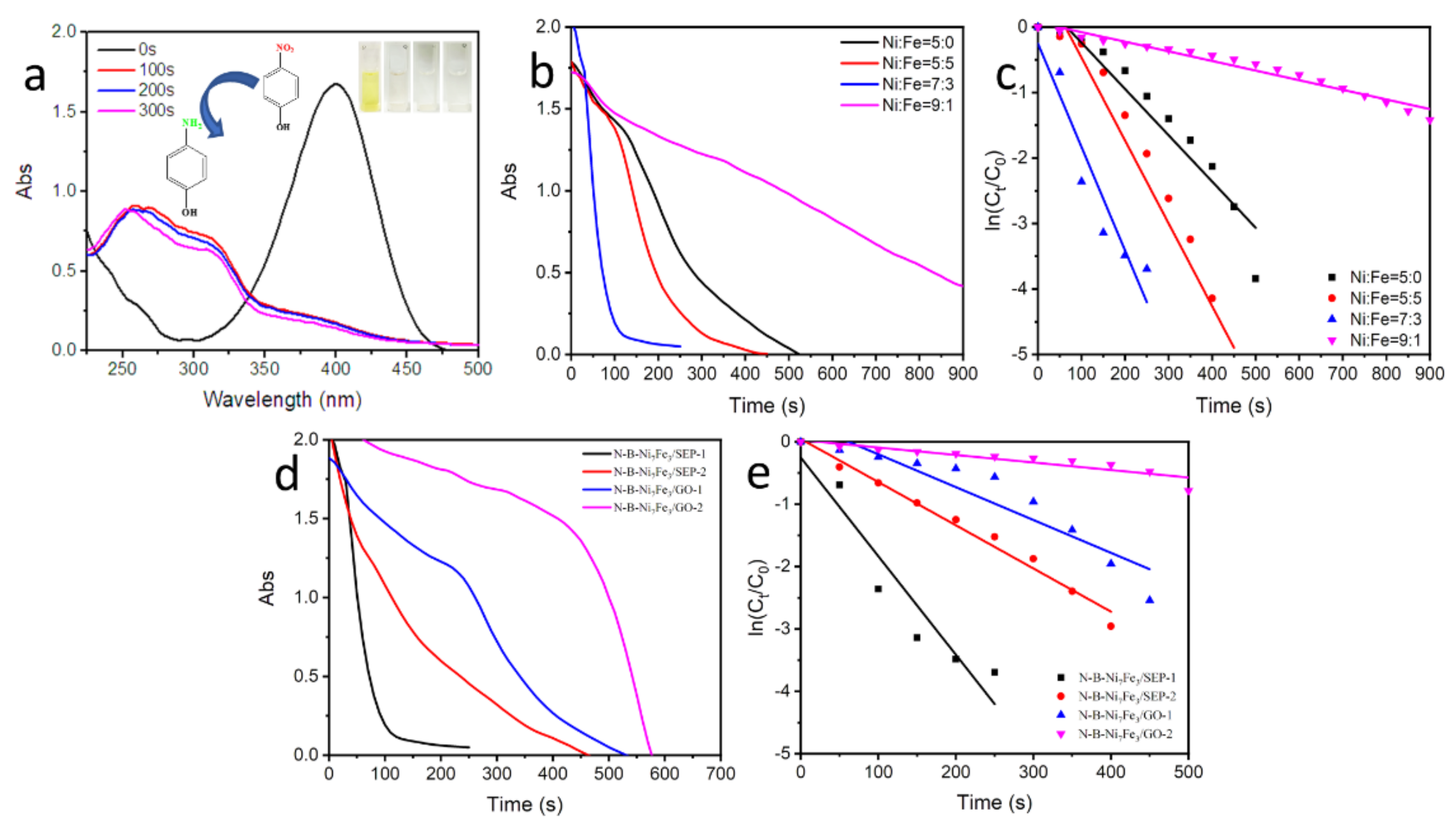
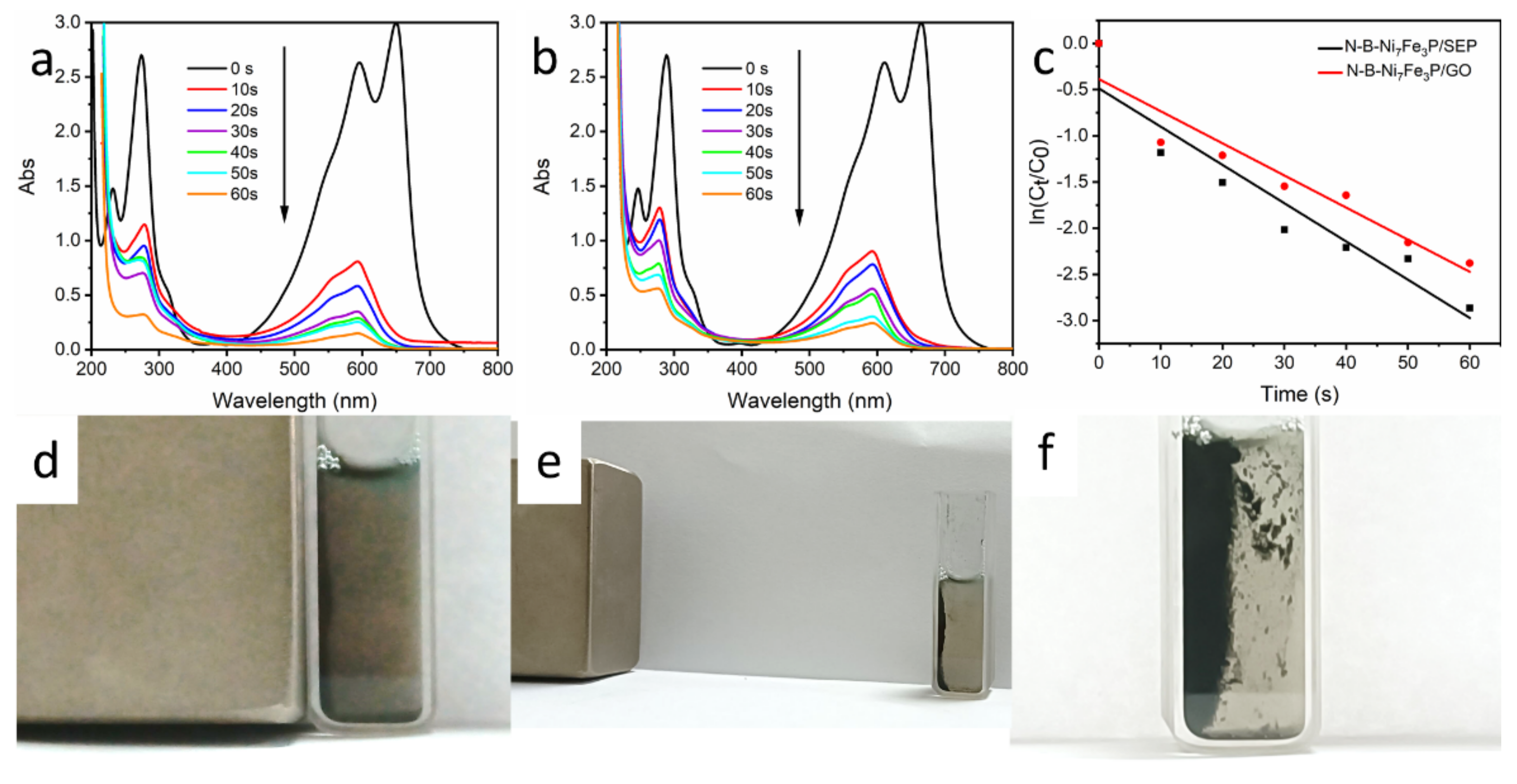
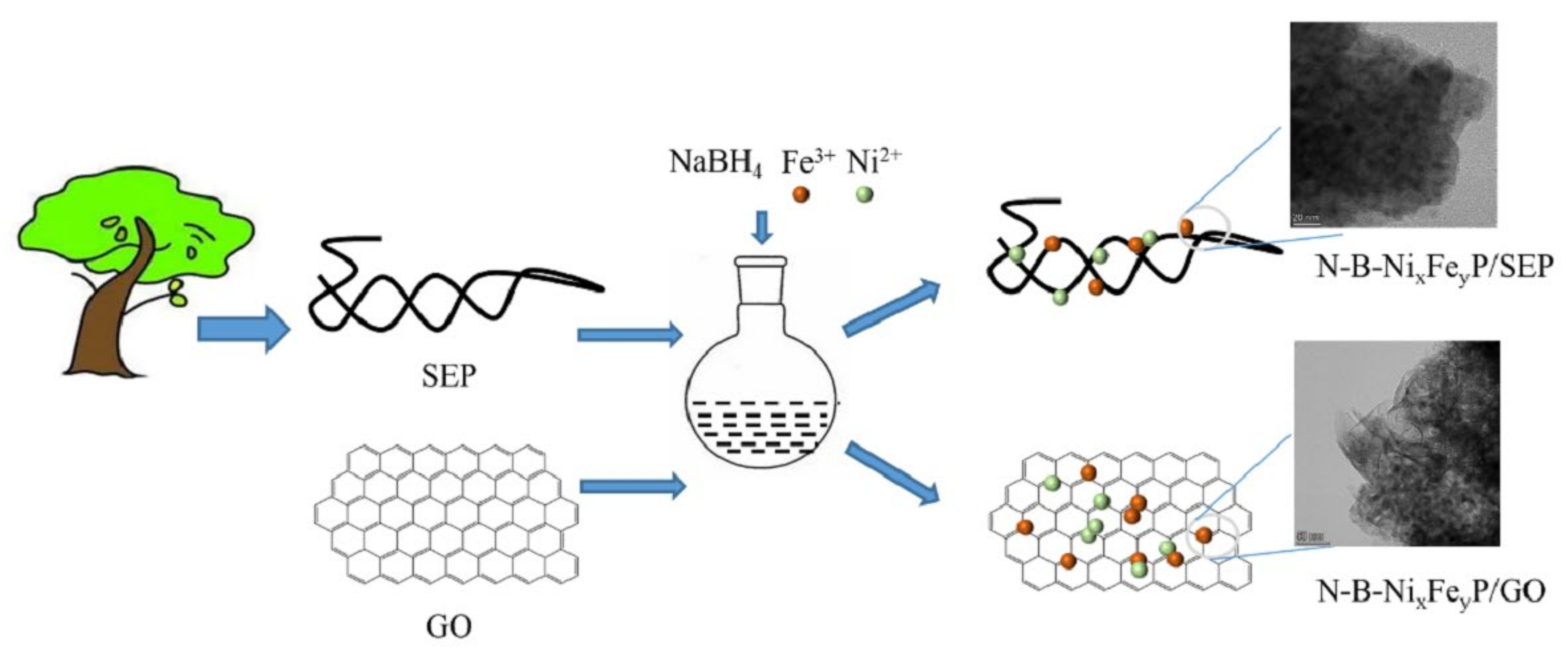
2. Results and Discussion
3. Methods and Materials
3.1. Materials
3.2. Preparation of Catalyst
3.3. Catalytic Reduction in Catalyst
3.4. Catalytic Ability Test of N-B-NixFeyP/SEP and N-B-NixFeyP/GO
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, E.B.; Vy, T.A.; Lee, S.W. Comparative release kinetics of small drugs (ibuprofen and acetaminophen) from multifunctional mesoporous silica nanoparticles. J. Mater. Chem. B 2020, 8, 2096–2106. [Google Scholar] [CrossRef] [PubMed]
- Doan, V.D.; Nguyen, N.V.; Nguyen, T.L.H.; Tran, V.A.; Le, V.T. High-efficient reduction of methylene blue and 4-nitrophenol by silver nanoparticles embedded in magnetic graphene oxide. Environ. Sci. Pollut. Res. 2021, 2021, 33741. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Rafiuddin; Khan, M.Z.; Umar, K.; Ahmed, A.S.; Shahadat, M. SnO2-SrO based nanocomposites and their photocatalytic activity for the treatment of organic pollutants. J. Mol. Struct. 2015, 1098, 393–399. [Google Scholar] [CrossRef]
- Malik, M.A.; Alshehri, A.A.; Patel, R. Facile one-pot green synthesis of Ag-Fe bimetallic nanoparticles and their catalytic capability for 4-nitrophenol reduction. J. Mater. Res. Technol. 2021, 12, 455–470. [Google Scholar] [CrossRef]
- Tran, V.A.; Phung, T.K.; Le, V.T.; Vo, T.K.; Nguyen, T.T.; Nguyen, T.A.N.; Viet, D.Q.; Hieu, V.Q.; Vo, T.T.T. Solar-light-driven photocatalytic degradation of methyl orange dye over Co3O4-ZnO nanoparticles. Mater. Lett. 2021, 284, 128902. [Google Scholar] [CrossRef]
- Wang, J.Y.; Niu, Y.L.; Teng, X.; Gong, S.Q.; Huang, J.H.; Xu, M.Z.; Chen, Z.F. Construction of three-dimensionally ordered macroporous bimetal phosphides as bifunctional electrocatalysts for highly efficient water splitting. J. Mater. Chem. A 2020, 8, 24572–24578. [Google Scholar] [CrossRef]
- Li, H.; Qiu, Y.F.; Wang, X.L.; Yang, J.; Yu, Y.J.; Chen, Y.Q.; Liu, Y.D. Biochar supported Ni/Fe bimetallic nanoparticles to remove 1,1,1-trichloroethane under various reaction conditions. Chemosphere 2017, 169, 534–541. [Google Scholar] [CrossRef]
- Ren, X.Y.; Tang, L.; Wang, J.J.; Almatrafi, E.; Feng, H.P.; Tang, X.; Yu, J.F.; Yang, Y.; Li, X.P.; Zhou, C.Y.; et al. Highly efficient catalytic hydrogenation of nitrophenols by sewage sludge derived biochar. Water Res. 2021, 201, 117360. [Google Scholar] [CrossRef]
- Liu, X.W.; Liu, Y.Y.; Zhang, X.; Miao, X.D. 3D N-doped graphene/bismuth composite as an efficient catalyst for the reduction of 4-nitrophenol efficient catalyst for reduction of 4-nitrophenol. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128098. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, K.; Hasija, V.; Sharma, V.; Sharma, S.; Raizada, P.; Singh, M.; Saini, A.K.; Hosseini-Bandegharaei, A. Systematic review on applicability of magnetic iron oxides–integrated photocatalysts for degradation of organic pollutants in water. Mater. Today Chem. 2019, 14, 100186. [Google Scholar] [CrossRef]
- Wang, L.; Fan, J.Y.; Liu, Y.; Chen, M.Y.; Lin, Y.; Bi, H.C.; Liu, B.X.; Shi, N.E.; Xu, D.D.; Bao, J.C.; et al. Phase-Modulation of Iron/Nickel Phosphides Nanocrystals “Armored” with Porous P-Doped Carbon and Anchored on P-Doped Graphene Nanohybrids for Enhanced Overall Water Splitting. Adv. Funct. Mater. 2021, 31, 2010912. [Google Scholar] [CrossRef]
- Niakan, M.; Asadi, Z.; Khosrozadeh, F. Palladium imine-pyridine-imine complex immobilized on graphene oxide as a recyclable catalyst for the carbonylative homocoupling of aryl halides. J. Coord. Chem. 2021, 74, 850–863. [Google Scholar] [CrossRef]
- Xing, Y.Y.; Han, J.; Wang, L.; Li, C.M.; Wu, J.C.; Mao, Y.L.; Ni, L.; Wang, Y. Fabrication of dendrimeric phenylboronic acid-affinitive magnetic graphene oxide nanoparticles with excellent adsorption performance for separation and purification of Horseradish peroxidase. New J. Chem. 2020, 44, 5254–5264. [Google Scholar] [CrossRef]
- Brandi, F.; Baumel, M.; Molinari, V.; Shekova, I.; Lauermann, I.; Heil, T.; Antonietti, M.; Al-Naji, M. Nickel on nitrogen-doped carbon pellets for continuous flow catalytic hydrogenation of biomass-derived compounds in water. Green Chem. 2020, 22, 2755–2766. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.W.; Lui, Y.; Gaur, A.; Chen, B.L.; Tang, X.H.; Qi, Z.Y.; Hu, S. Hierarchical FeNiP@ultra-thin carbon nanoflakes as alkaline oxygen evolution and acidic hydrogen evolution catalyst for efficient water electrolysis and organic decomposition. ACS Appl. Mater. Interfaces 2018, 10, 8739–8748. [Google Scholar] [CrossRef]
- Li, L.Z.; Chen, M.X.; Huang, G.B.; Yang, N.; Zhang, L.; Wang, H.; Liu, Y.; Wang, W.; Gao, J.P. A green method to prepare Pd–Ag nanoparticles supported on reduced graphene oxide and their electrochemical catalysis of methanol and ethanol oxidation. J. Power Sources 2014, 263, 13–21. [Google Scholar] [CrossRef]
- Qin, Q.; Jang, H.; Li, P.; Yuan, B.; Liu, X.; Cho, J. A Tannic Acid–Derived N-, P-Codoped Carbon-Supported Iron-Based Nanocomposite as an Advanced Trifunctional Electrocatalyst for the Overall Water Splitting Cells and Zinc–Air Batteries. Adv. Energy Mater. 2018, 9, 1803312. [Google Scholar] [CrossRef]
- Yan, H.J.; Xie, Y.; Wu, A.P.; Cai, Z.C.; Wang, L.; Tian, C.G.; Zhang, X.M.; Fu, H.G. Anion-Modulated HER and OER Activities of 3D Ni-V-Based Interstitial Compound Heterojunctions for High-Efficiency and Stable Overall Water Splitting. Adv. Mater. 2019, 31, 1901174. [Google Scholar] [CrossRef]
- Zhao, W.S.; Yang, S.; Guo, C.Z.; Yang, J.H.; Liu, Y. One-step fabrication of Fe3O4–Cu nanocomposites: High-efficiency and low-cost catalysts for reduction of 4-nitrophenol. Mater. Chem. Phys. 2021, 260, 124144. [Google Scholar] [CrossRef]
- Sawant, S.Y.; Kim, J.Y.; Hiep, H.T.; Ansari, S.A.; Cho, M.H. Electrochemically active biofilm-assisted biogenic synthesis of Ag-decorated ZnO@C coreshell ternary plasmonic photocatalyst with enhanced visible-photocatalytic activity. New J. Chem. 2017, 42, 1995–2005. [Google Scholar] [CrossRef]
- Ai, L.H.; Yue, H.T.; Jiang, J. Environmentally friendly light-driven synthesis of Ag nanoparticles in situ grown on magnetically separable biohydrogels as highly active and recyclable catalysts for 4-nitrophenol reduction. J. Mater. Chem. 2012, 22, 23447–23453. [Google Scholar] [CrossRef]
- Strachan, J.; Barnett, C.; Anthony, F.M.; Thomas, M. 4-Nitrophenol reduction: Probing the putative mechanism of the model reaction. ACS Catal. 2020, 10, 5516–5521. [Google Scholar] [CrossRef]
- Ding, Y.; Miao, B.Q.; Li, S.N.; Jiang, Y.C.; Liu, Y.Y.; Yao, H.C.; Chen, Y. Benzylamine oxidation boosted electrochemical water-splitting: Hydrogen and benzonitrile co-production at ultra-thin NiP nanomeshes grown on nickel foam. Appl. Catal. B Environ. 2020, 268, 118393. [Google Scholar] [CrossRef]
- Huo, J.J.; Chen, Y.L.; Liu, Y.; Guo, J.J.; Lu, L.; Li, W.X.; Wang, Y.; Liu, H. Bifunctional iron nickel phosphide nanocatalysts supported on porous carbon for highly efficient overall water splitting. Sustainable Materials and Technologies. Sustain. Mater. Technol. SMT 2019, 22, e00117. [Google Scholar] [CrossRef]
- Fu, Y.; Qin, L.; Huang, D.; Zeng, G.; Lai, C.; Li, B.; He, J.; Yi, H.; Zhang, M.; Cheng, M. Chitosan functionalized activated coke for Au nanoparticles anchoring: Green synthesis and catalytic activities in hydrogenation of nitrophenols and azo dyes. Appl. Catal. B Environ. 2019, 255, 117740. [Google Scholar] [CrossRef]
- Chettri, P.; Vendamani, V.S.; Tripathi, A.; Singh, M.K.; Pathak, A.P.; Tiwari, A. Green synthesis of silver nanoparticle-reduced graphene oxide using Psidium guajava and its application in SERS for the detection of methylene blue. Appl. Surf. Sci. 2017, 406, 312–318. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Panigrahy, B.; Thakur, D.; Bahadur, D. Ice-templating synthesis of macroporous noble metal/3D-graphene nanocomposites: Their fluorescence lifetimes and catalytic study. New J. Chem. 2017, 41, 7861–7869. [Google Scholar] [CrossRef]
- Yang, G.Y.; Pan, C.; Yang, H.T.; Feng, N.J. Carbon-supported Nickel Catalyst Prepared from Steam-exploded Poplar by Recovering Ni(II). Bioresources 2021, 16, 5481–5493. [Google Scholar] [CrossRef]
- Pan, C.; Yang, G.Y.; Yang, H.T.; Wang, L.; Jiang, J.G.; Zhang, Y.F.; Wu, F.F. Facile fabrication of steam-exploded poplar loaded with non-metal-doped Ni-Fe nanoparticles: Catalytic activities in 4-nitrophenol reduction and electrocatalytic reaction. Arab. J. Chem. 2022, 15, 103944. [Google Scholar] [CrossRef]
- Alam, M.S.; Gorman-Lewis, D.; Chen, N.; Flynn, S.L.; Ok, Y.S.; Konhauser, K.O.; Alessi, D.S. Thermodynamic Analysis of Nickel(II) and Zinc(II) Adsorption to Biochar. Environ. Sci. Technol. 2018, 52, 6246–6255. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, C.; Yang, G.; Yang, H.; Wu, F.; Wang, L.; Jiang, J.; Zhang, Y.; Yuan, J. Facile Construction of Iron/Nickel Phosphide Nanocrystals Anchored on N-B-Doped Carbon-Based Composites with Advanced Catalytic Capacity for 4-Nitrophenol and Methylene Blue. Int. J. Mol. Sci. 2022, 23, 8408. https://doi.org/10.3390/ijms23158408
Pan C, Yang G, Yang H, Wu F, Wang L, Jiang J, Zhang Y, Yuan J. Facile Construction of Iron/Nickel Phosphide Nanocrystals Anchored on N-B-Doped Carbon-Based Composites with Advanced Catalytic Capacity for 4-Nitrophenol and Methylene Blue. International Journal of Molecular Sciences. 2022; 23(15):8408. https://doi.org/10.3390/ijms23158408
Chicago/Turabian StylePan, Cheng, Guangying Yang, Haitao Yang, Feifan Wu, Lei Wang, Jungang Jiang, Yifan Zhang, and Junxia Yuan. 2022. "Facile Construction of Iron/Nickel Phosphide Nanocrystals Anchored on N-B-Doped Carbon-Based Composites with Advanced Catalytic Capacity for 4-Nitrophenol and Methylene Blue" International Journal of Molecular Sciences 23, no. 15: 8408. https://doi.org/10.3390/ijms23158408
APA StylePan, C., Yang, G., Yang, H., Wu, F., Wang, L., Jiang, J., Zhang, Y., & Yuan, J. (2022). Facile Construction of Iron/Nickel Phosphide Nanocrystals Anchored on N-B-Doped Carbon-Based Composites with Advanced Catalytic Capacity for 4-Nitrophenol and Methylene Blue. International Journal of Molecular Sciences, 23(15), 8408. https://doi.org/10.3390/ijms23158408





