Polyoxometalate Nanoparticles as a Potential Glioblastoma Therapeutic via Lipid-Mediated Cell Death
Abstract
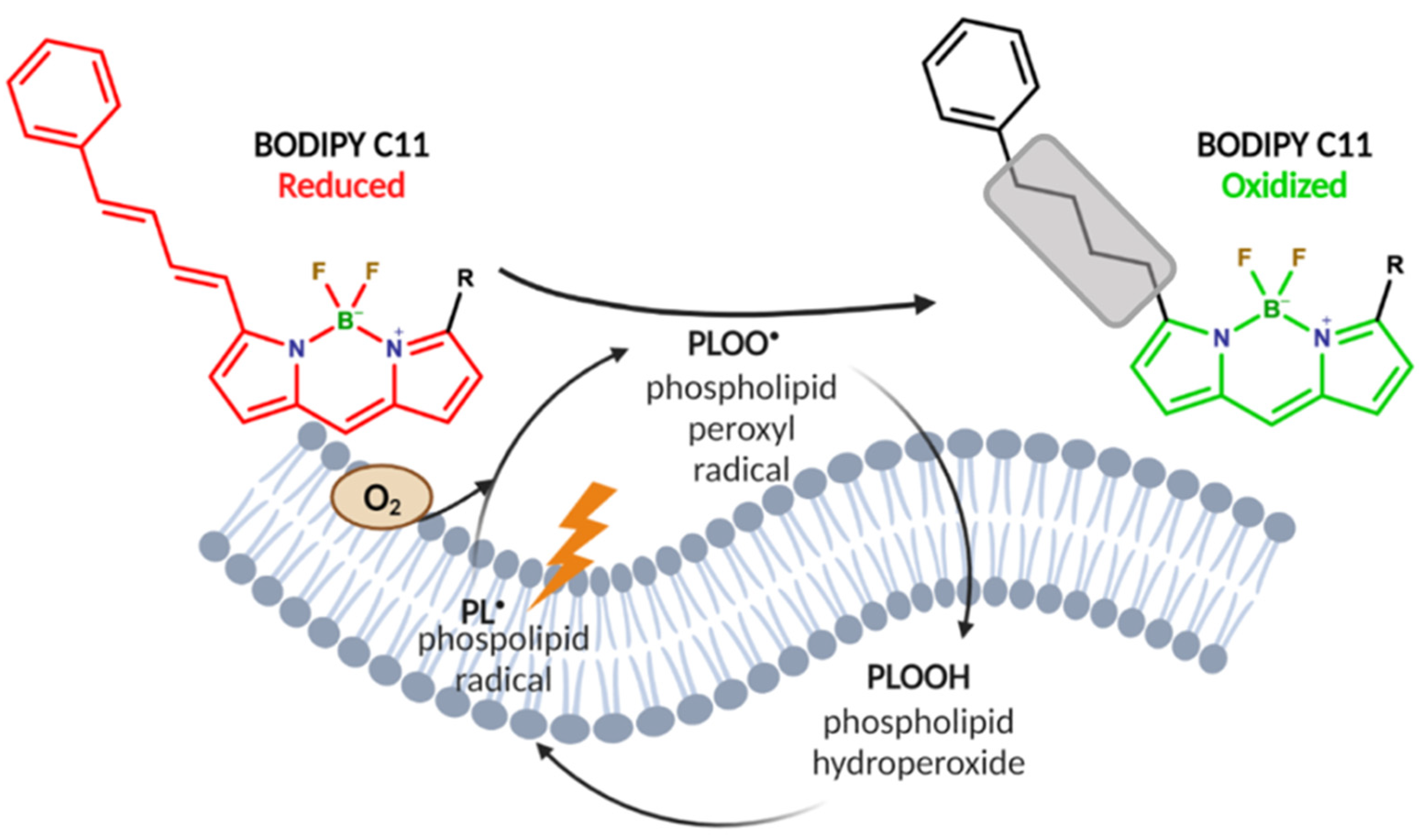
:1. Introduction
2. Results
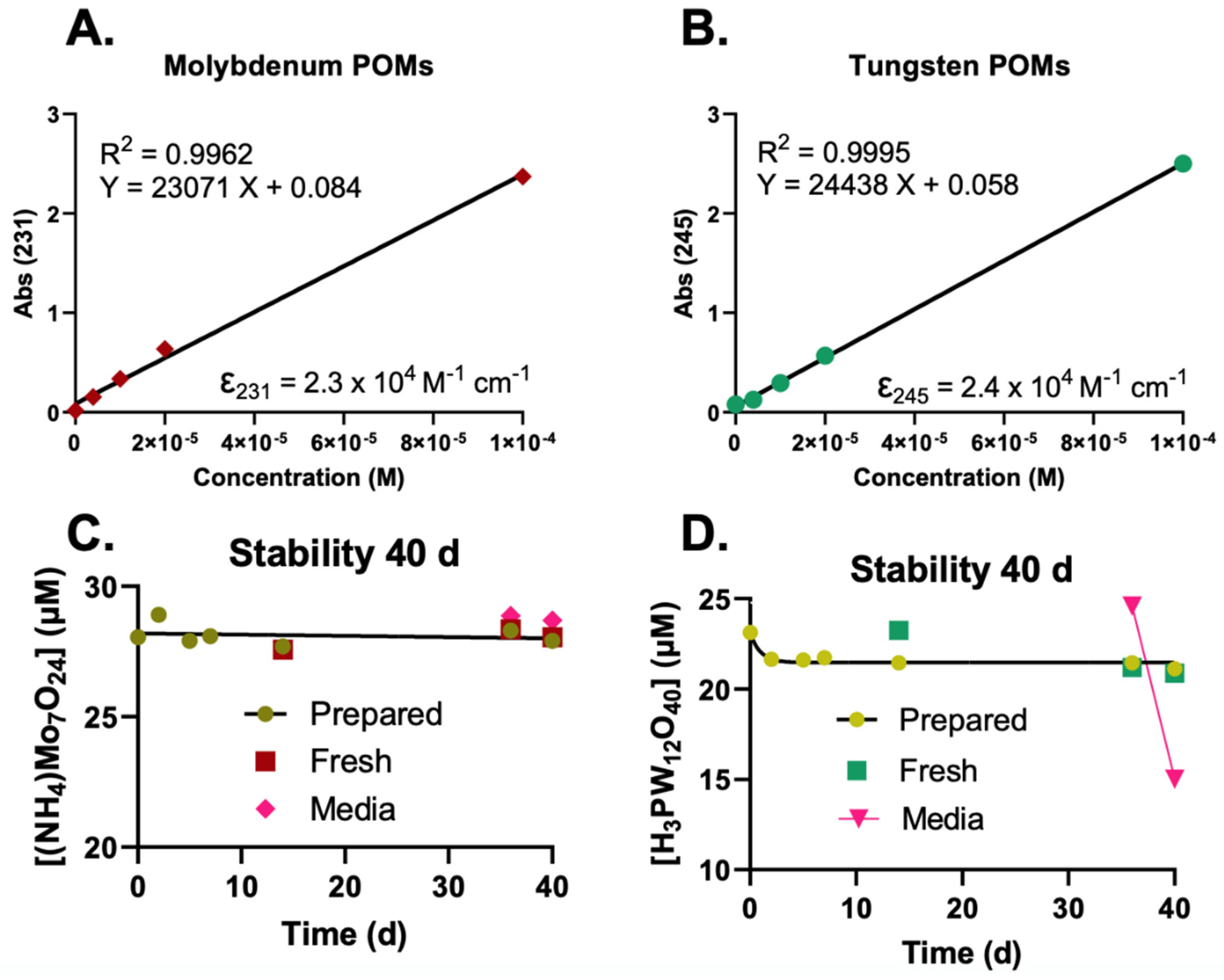
2.1. UV-Vis Determination and Stability of POMs
- 100 mM POMs in NanoPure water;
- 100 mM POMs in 50 mM KPO4 buffer, pH attempted to adjust;
- 100 mM POMs in 0.5 M HEPES buffer, pH adjusted to pH ≈ 6–7 (Table 1).
- The high concentration HEPES stock (100 mM POM in 0.5 M HEPES);
- 1 mM stock in water (100× dilution of the HEPES stock in water);
- 1 mM stock in medium (100× dilution of the HEPES stock in medium).
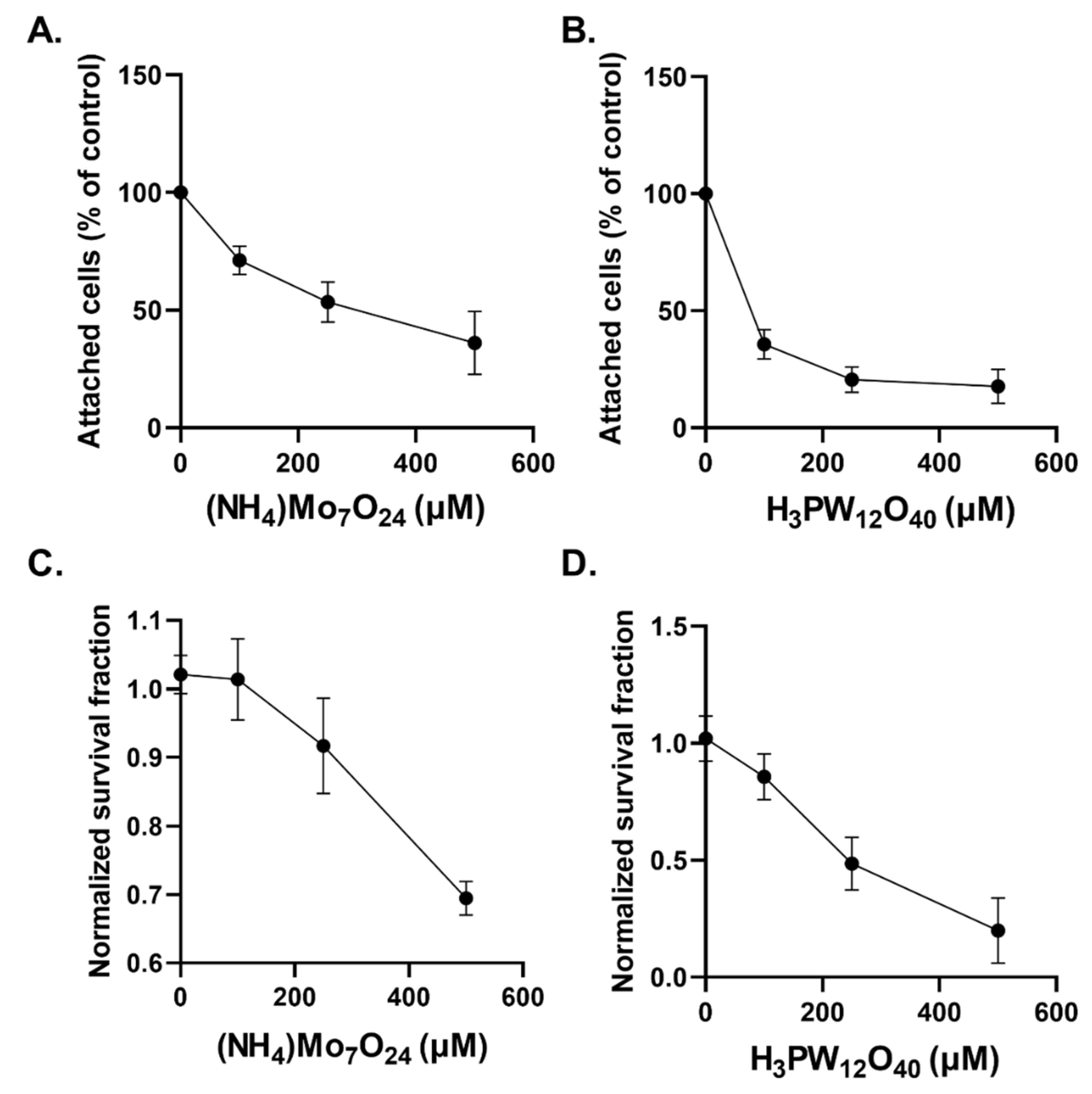
2.2. Cytostatic and Cytotoxic Effects of POMs in Glioblastoma Cells
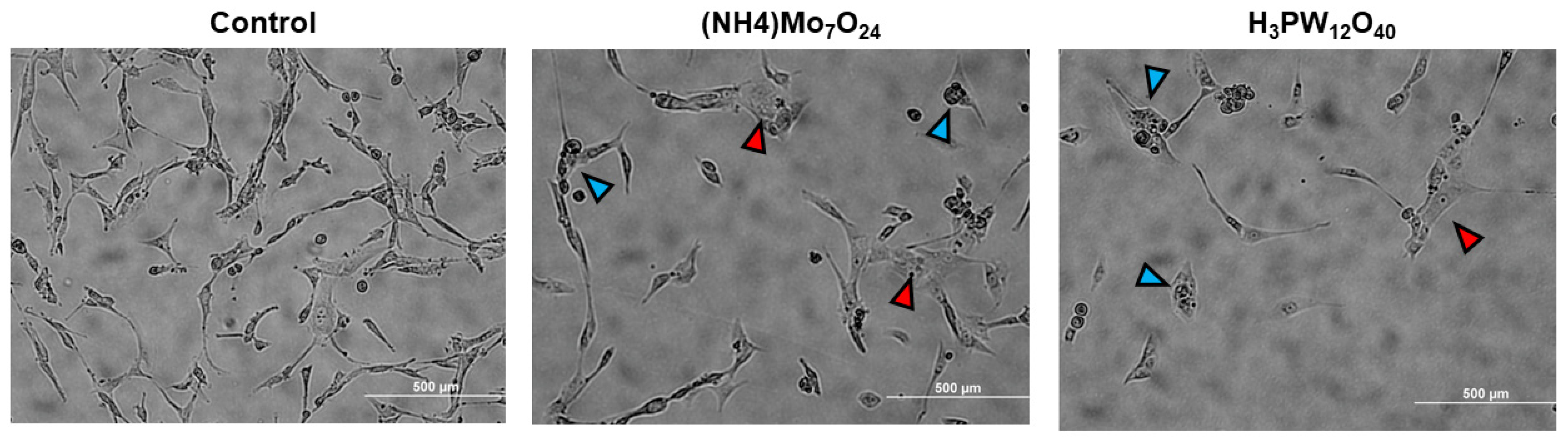
2.3. Morphological Changes Associated with POMs Treatment
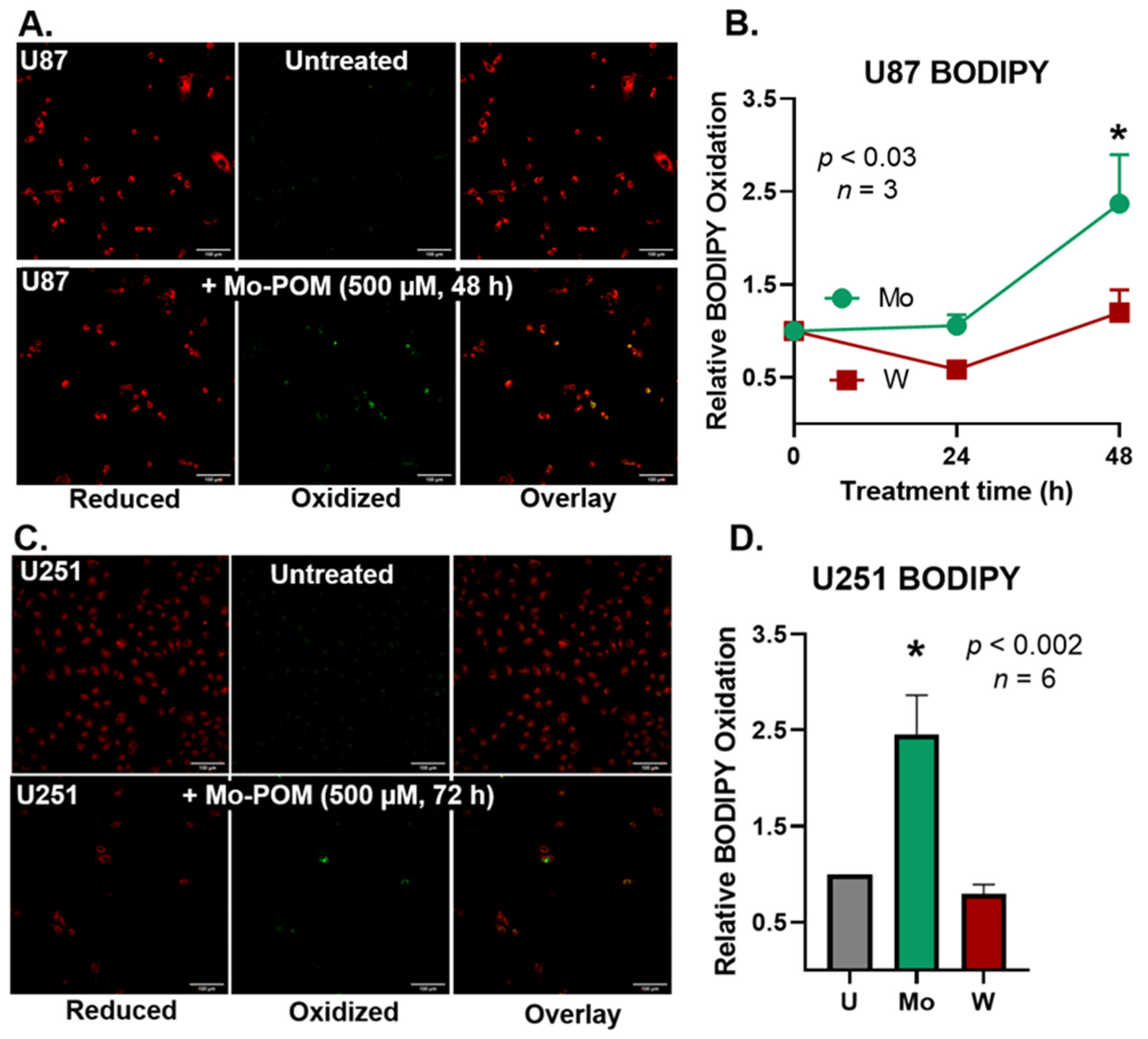
2.4. Mo-POMs Promote Lipid Peroxidation in GBM Cells
3. Discussion
4. Materials and Methods
4.1. POM Preparation
4.1.1. Reagents
- Gibco HEPES buffer 1 M (catalog#: 15630080).
- Tungsten POM (phosphotungstic acid hydrate, Sigma-Aldrich); molecular weight = 2880.05 g mol−1.
- Molybdenum POM (ammonium heptamolybdate tetrahydrate, Sigma-Aldrich) molecular weight = 1235.86 g mol−1.
- NaOH 5 N solution.
4.1.2. Materials
- Glass HPLC vials (1.5 mL).
- Small glass Erlenmeyer flask/beaker or volumetric flask (10–25 mL).
- pH paper or pH meter.
- Start by calculating the amount of POM powder needed. Use the basic molar calculation equations.
- Weigh out the amount calculated above.
- Dissolve the POMs in half the total volume of 1 M HEPES buffer, e.g., if you want to make 10 mL POM stock, dissolve the POMs in 5 mL of 1 M HEPES buffer.
- While stirring, gently add the 5 N NaOH. For 10 mL stock, 1 mL of NaOH is needed for Mo-POMs, and 1.2 mL for W-POMs.
- Add ddH2O totaling to your desired volume.
- Test the pH of the stock using pH paper or a pH meter. The pH should be around 6–7.
- Store aliquots and the main stock solution only in glass since the POMs may stick to plastic in high concentration solutions.
4.2. UV-Vis Spectroscopy
4.3. Cell Culture
4.4. BODIPY C11 Labeling of Cells
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gumerova, N.I.; Rompel, A. Synthesis, structures and applications of electron-rich polyoxometalates. Nat. Rev. Chem. 2018, 2, 0112. [Google Scholar] [CrossRef]
- Horn, M.R.; Singh, A.; Alomari, S.; Goberna-Ferrón, S.; Benages-Vilau, R.; Chodankar, N.; Motta, N.; Ostrikov, K.; MacLeod, J.; Sonar, P.; et al. Polyoxometalates (POMs): From electroactive clusters to energy materials. Energy Environ. Sci. 2021, 14, 1652–1700. [Google Scholar] [CrossRef]
- Hille, R. The Mononuclear Molybdenum Enzymes. Chem. Rev. 1996, 96, 2757–2816. [Google Scholar] [CrossRef]
- Ardan, T.; Kovaceva, J.; Cejková, J. Comparative histochemical and immunohistochemical study on xanthine oxidoreductase/xanthine oxidase in mammalian corneal epithelium. Acta Histochem. 2004, 106, 69–75. [Google Scholar] [CrossRef]
- White, H.; Strobl, G.; Feicht, R.; Simon, H. Carboxylic acid reductase: A new tungsten enzyme catalyses the reduction of non-activated carboxylic acids to aldehydes. Eur. J. Biochem. 1989, 184, 89–96. [Google Scholar] [CrossRef]
- Bijelic, A.; Aureliano, M.; Rompel, A. The antibacterial activity of polyoxometalates: Structures, antibiotic effects and future perspectives. Chem. Commun. 2018, 54, 1153–1169. [Google Scholar] [CrossRef] [Green Version]
- Čolović, B.M.; Lacković, M.; Lalatović, J.; Mougharbel, S.A.; Kortz, U.; Krstić, Z.D. Polyoxometalates in Biomedicine: Update and Overview. Curr. Med. Chem. 2020, 27, 362–379. [Google Scholar] [CrossRef] [PubMed]
- Rhule, J.T.; Hill, C.L.; Judd, D.A.; Schinazi, R.F. Polyoxometalates in Medicine. Chem. Rev. 1998, 98, 327–358. [Google Scholar] [CrossRef] [PubMed]
- She, S.; Bian, S.; Huo, R.; Chen, K.; Huang, Z.; Zhang, J.; Hao, J.; Wei, Y. Degradable Organically-Derivatized Polyoxometalate with Enhanced Activity against Glioblastoma Cell Line. Sci. Rep. 2016, 6, 33529. [Google Scholar] [CrossRef] [Green Version]
- Mao, G.; Xin, D.; Wang, Q.; Lai, D. Sodium molybdate inhibits the growth of ovarian cancer cells via inducing both ferroptosis and apoptosis. Free. Radic. Biol. Med. 2022, 182, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Bijelic, A.; Aureliano, M.; Rompel, A. Polyoxometalates as Potential Next-Generation Metallodrugs in the Combat against Cancer. Angew. Chem. Int. Ed. 2019, 58, 2980–2999. [Google Scholar] [CrossRef] [Green Version]
- Buettner, G.R. The Pecking Order of Free Radicals and Antioxidants: Lipid Peroxidation, α-Tocopherol, and Ascorbate. Arch. Biochem. Biophys. 1993, 300, 535–543. [Google Scholar] [CrossRef]
- Wilcox, A.L.; Marnett, L.J. Polyunsaturated fatty acid alkoxyl radicals exist as carbon-centered epoxyallylic radicals: A key step in hydroperoxide-amplified lipid peroxidation. Chem. Res. Toxicol. 1993, 6, 413–416. [Google Scholar] [CrossRef]
- Marnett, L.J.; Wilcox, A.L. The chemistry of lipid alkoxyl radicals and their role in metal-amplified lipid peroxidation. Biochem. Soc. Symp. 1995, 61, 65–72. [Google Scholar] [CrossRef]
- Qian, S.Y.; Wang, H.P.; Schafer, F.Q.; Buettner, G.R. EPR detection of lipid-derived free radicals from PUFA, LDL, and cell oxidations. Free. Radic. Biol. Med. 2000, 29, 568–579. [Google Scholar] [CrossRef]
- Mc Gee, M.M. Targeting the Mitotic Catastrophe Signaling Pathway in Cancer. Mediat. Inflamm. 2015, 2015, 146282. [Google Scholar] [CrossRef] [Green Version]
- Saraste, A.; Pulkki, K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 2000, 45, 528–537. [Google Scholar] [CrossRef]
- Miller, M.; Zachary, J. Mechanisms and Morphology of Cellular Injury, Adaptation, and Death. Pathol. Basis Vet. Dis. 2017, 2–43.e19. [Google Scholar] [CrossRef]
- Wang, D. Redox chemistry of molybdenum in natural waters and its involvement in biological evolution. Front. Microbiol. 2012, 3, 427. [Google Scholar] [CrossRef] [Green Version]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [Green Version]
- Adamson, H.; Simonov, A.N.; Kierzek, M.; Rothery, R.A.; Weiner, J.H.; Bond, A.M.; Parkin, A. Electrochemical evidence that pyranopterin redox chemistry controls the catalysis of YedY, a mononuclear Mo enzyme. Proc. Natl. Acad. Sci. USA 2015, 112, 14506–14511. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.M.; Aust, S.D. Studies of ascorbate-dependent, iron-catalyzed lipid peroxidation. Arch. Biochem. Biophys. 1989, 271, 113–119. [Google Scholar] [CrossRef]
- Kostellow, A.B.; Morrill, G.A. Iron-catalyzed lipid peroxidation in aortic cells in vitro: Protective effect of extracellular magnesium. Atherosclerosis 2004, 175, 15–22. [Google Scholar] [CrossRef]
- Mendel, R.R. The Molybdenum Cofactor*. J. Biol. Chem. 2013, 288, 13165–13172. [Google Scholar] [CrossRef] [Green Version]
- Muravieva, V.K.; Loginov, I.P.; Sukhikh, T.S.; Ryzhikov, M.R.; Yanshole, V.V.; Nadolinny, V.A.; Dorcet, V.; Cordier, S.; Naumov, N.G. Synthesis, Structure, and Spectroscopic Study of Redox-Active Heterometallic Cluster-Based Complexes [Re5MoSe8(CN)6]n. Inorg. Chem. 2021, 60, 8838–8850. [Google Scholar] [CrossRef]
- Schnöller, L.E.; Albrecht, V.; Brix, N.; Nieto, A.E.; Fleischmann, D.F.; Niyazi, M.; Hess, J.; Belka, C.; Unger, K.; Lauber, K.; et al. Integrative analysis of therapy resistance and transcriptomic profiling data in glioblastoma cells identifies sensitization vulnerabilities for combined modality radiochemotherapy. Radiat. Oncol. 2022, 17, 79. [Google Scholar] [CrossRef] [PubMed]
- Leon, I.E.; Porro, V.; Astrada, S.; Egusquiza, M.G.; Cabello, C.I.; Bollati-Fogolin, M.; Etcheverry, S.B. Polyoxometalates as antitumor agents: Bioactivity of a new polyoxometalate with copper on a human osteosarcoma model. Chem.-Biol. Interact. 2014, 222, 87–96. [Google Scholar] [CrossRef]
- Moskovitz, B.L. Clinical trial of tolerance of HPA-23 in patients with acquired immune deficiency syndrome. Antimicrob. Agents Chemother. 1988, 32, 1300–1303. [Google Scholar] [CrossRef] [Green Version]
- Aymard, J.P.; Ferry, R.; Janot, C.; Schooneman, F.; Lfgras, B.; May, T.; Streiff, F. Toxicity of HPA-23 (am-monmm-21-tungsto-9-antimoniate) for normal human myeloid progenitor cells (GM-CFU) in vitro. Biomed. Pharmacother. 1989, 43, 451–454. [Google Scholar] [CrossRef]
- Borković-Mitić, S.; Stojsavljević, A.; Vujotić, L.; Matić, S.; Mitić, B.; Manojlović, D.; Pavlović, S. Differences between antioxidant defense parameters and specific trace element concentrations in healthy, benign, and malignant brain tissues. Sci. Rep. 2021, 11, 14766. [Google Scholar] [CrossRef]
- Drummen, G.P.C.; van Liebergen, L.C.M.; Op den Kamp, J.A.F.; Post, J.A. C11-BODIPY581/591, an oxidation-sensitive fluorescent lipid peroxidation probe: (micro) spectroscopic characterization and validation of methodology. Free. Radic. Biol. Med. 2002, 33, 473–490. [Google Scholar] [CrossRef]
- Wagner, B.A.; Buettner, G.R.; Burns, C.P. Free Radical-Mediated Lipid Peroxidation in Cells: Oxidizability Is a Function of Cell Lipid bis-Allylic Hydrogen Content. Biochemistry 1994, 33, 4449–4453. [Google Scholar] [CrossRef] [PubMed]
| Element | [HEPES] (mM) | [POM] (mM) | [NaOH] (mM) |
|---|---|---|---|
| Tungsten | 500 | 100 | 600 |
| Molybdenum | 500 | 100 | 500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petronek, M.S.; Allen, B.G.; Luthe, G.; Stolwijk, J.M. Polyoxometalate Nanoparticles as a Potential Glioblastoma Therapeutic via Lipid-Mediated Cell Death. Int. J. Mol. Sci. 2022, 23, 8263. https://doi.org/10.3390/ijms23158263
Petronek MS, Allen BG, Luthe G, Stolwijk JM. Polyoxometalate Nanoparticles as a Potential Glioblastoma Therapeutic via Lipid-Mediated Cell Death. International Journal of Molecular Sciences. 2022; 23(15):8263. https://doi.org/10.3390/ijms23158263
Chicago/Turabian StylePetronek, Michael S., Bryan G. Allen, Gregor Luthe, and Jeffrey M. Stolwijk. 2022. "Polyoxometalate Nanoparticles as a Potential Glioblastoma Therapeutic via Lipid-Mediated Cell Death" International Journal of Molecular Sciences 23, no. 15: 8263. https://doi.org/10.3390/ijms23158263
APA StylePetronek, M. S., Allen, B. G., Luthe, G., & Stolwijk, J. M. (2022). Polyoxometalate Nanoparticles as a Potential Glioblastoma Therapeutic via Lipid-Mediated Cell Death. International Journal of Molecular Sciences, 23(15), 8263. https://doi.org/10.3390/ijms23158263





