PLGA–TiO2 as a Carrier System for Drug Release
Abstract
:1. Introduction
2. Results and Discussion
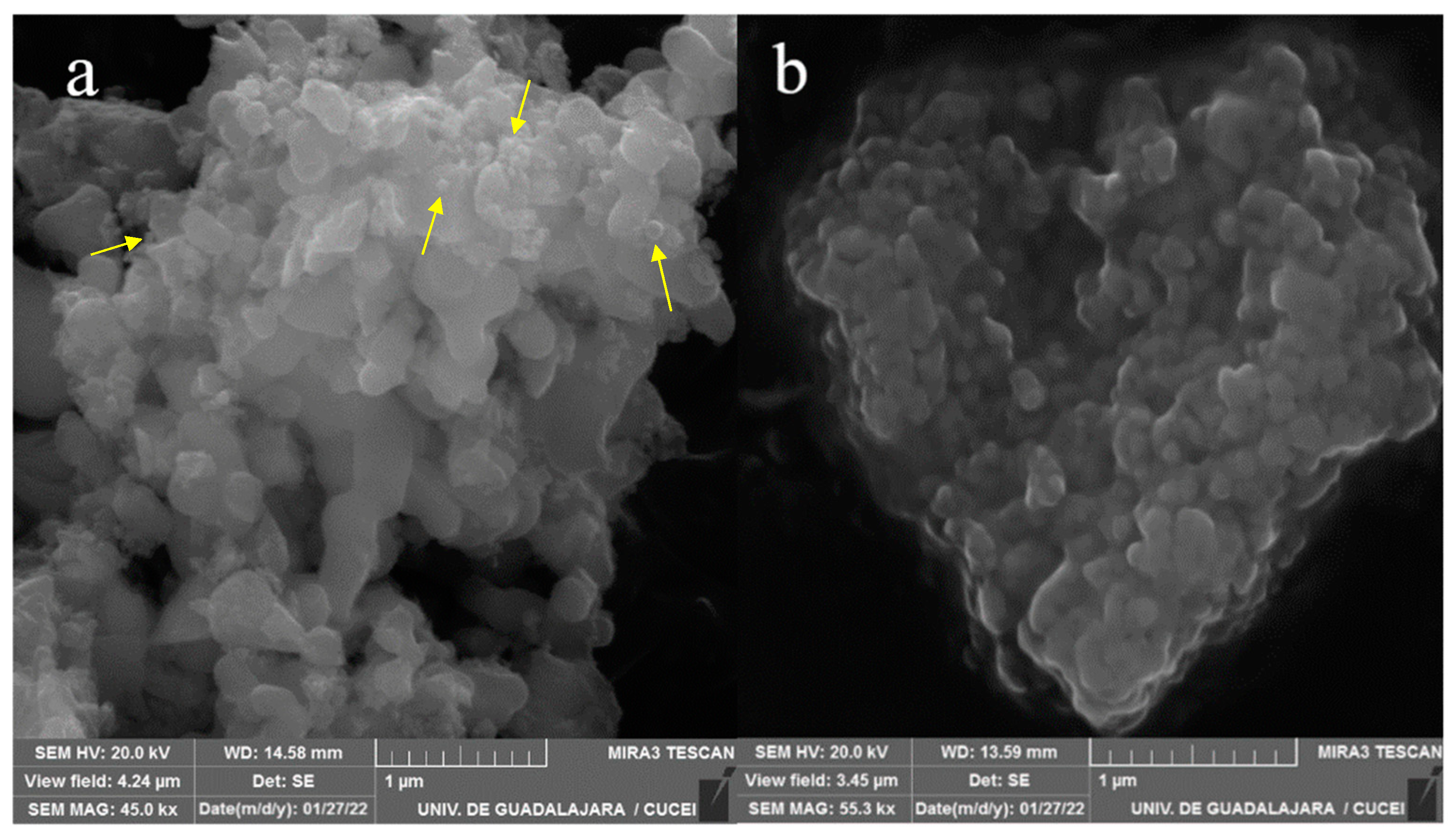
2.1. SEM Analysis
2.2. XRD Analysis
2.3. UV-Vis Analysis
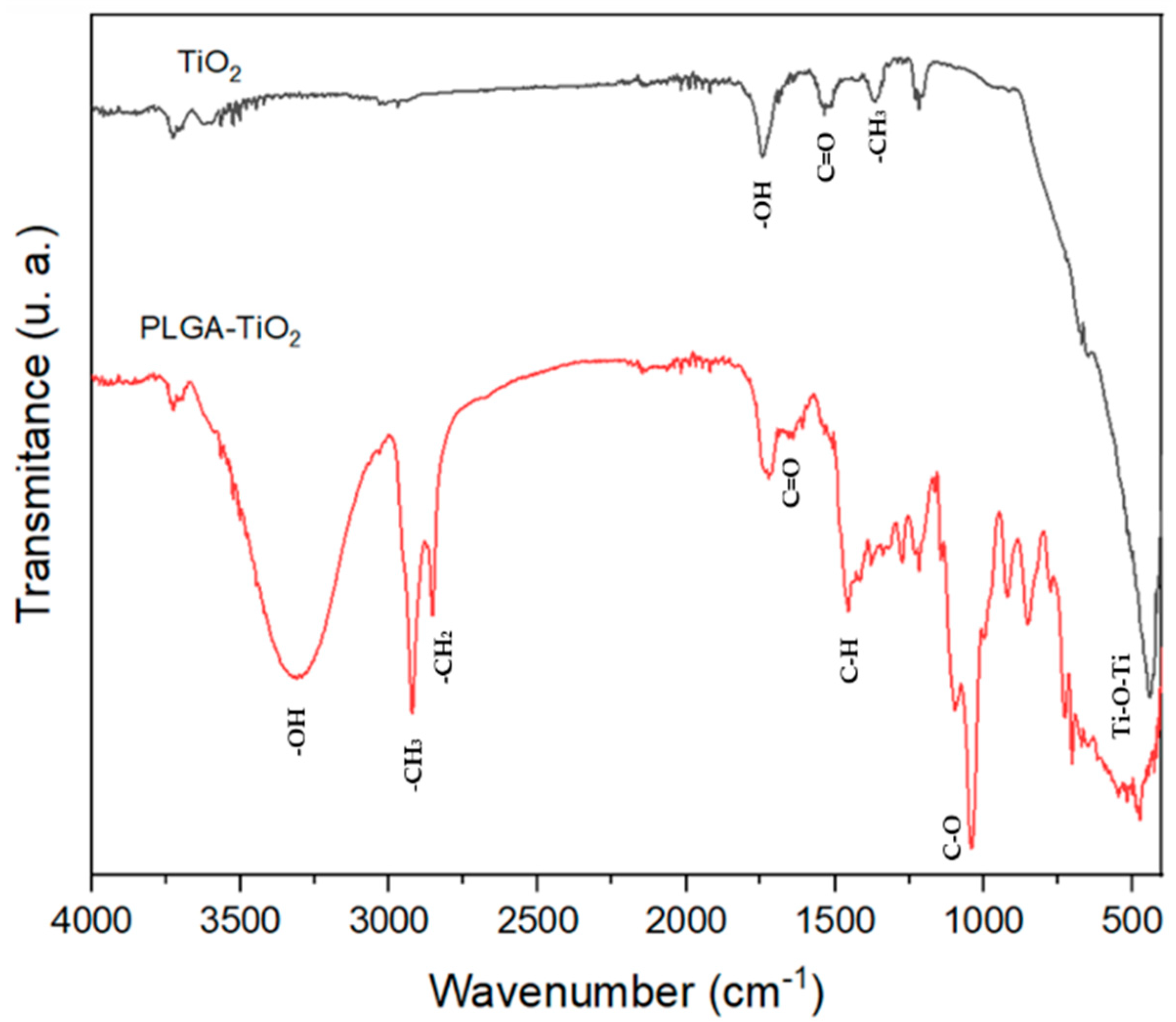
2.4. FT-IR
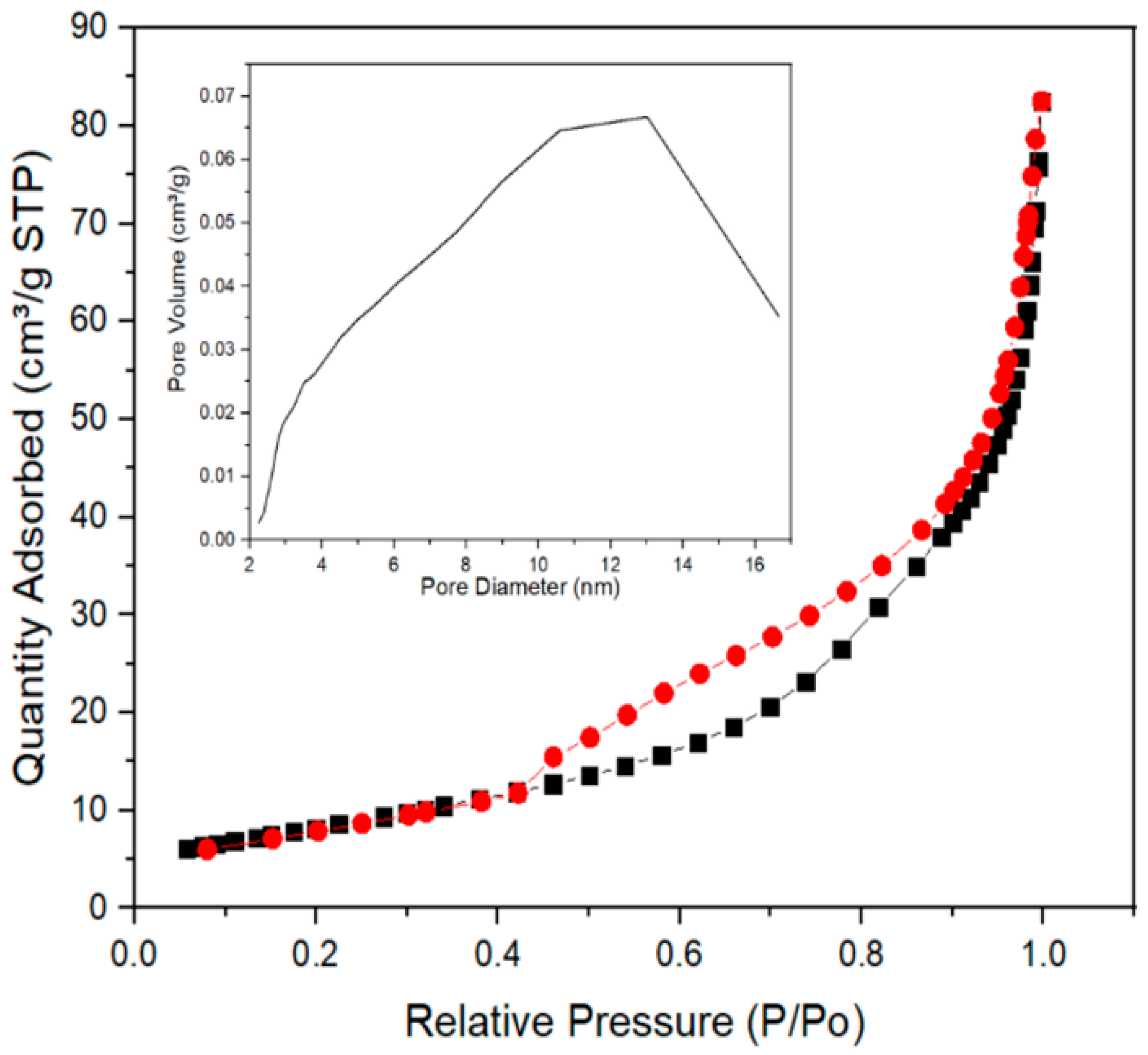
2.5. Physisorption Analysis
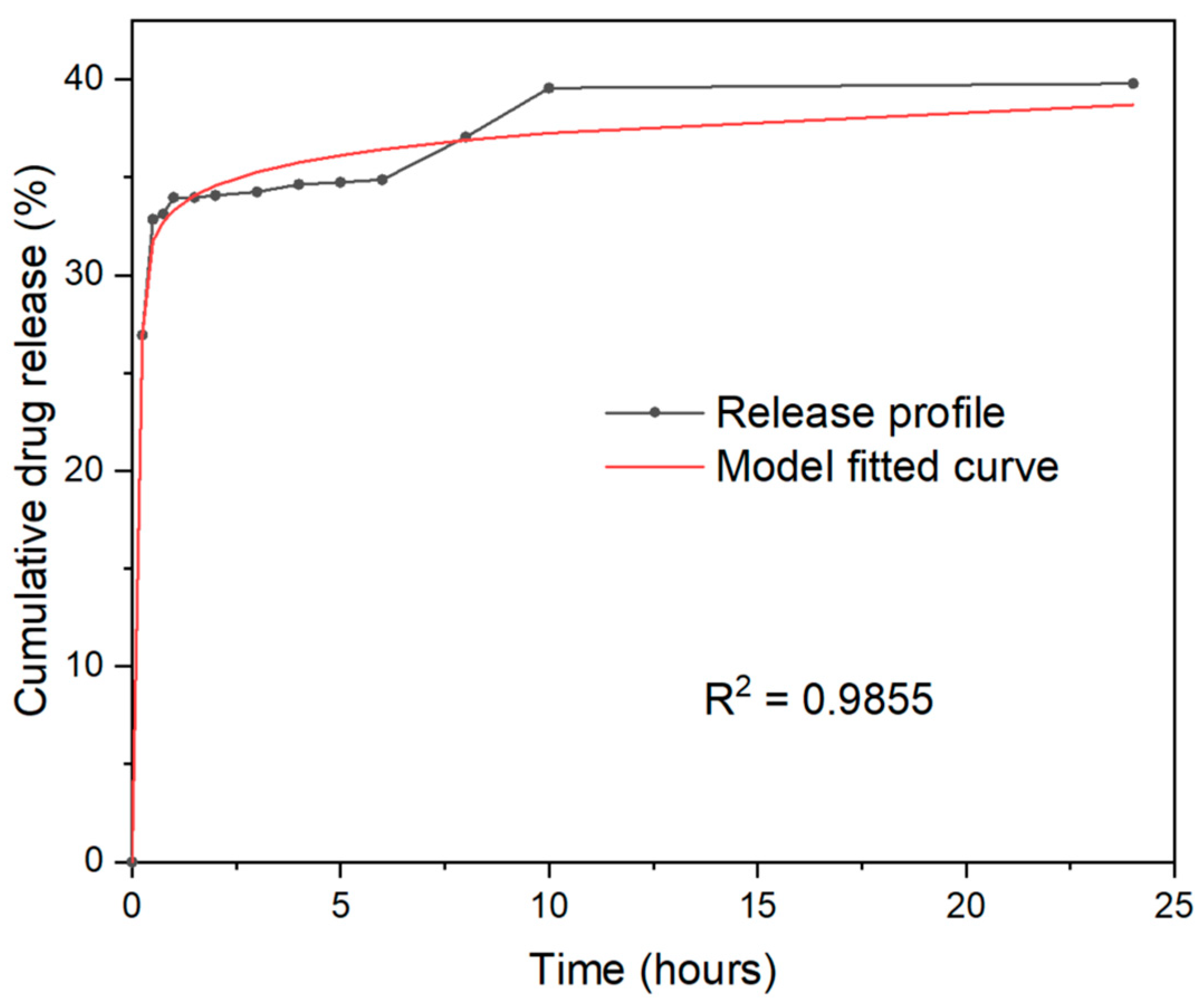
2.6. PLGA–TiO2 Release Profile
3. Materials and Methods
3.1. Chemical Reagents
3.2. Nanomaterial Synthesis
3.3. Nanoparticle Functionalization
3.4. Sample Characterization
3.5. Release Profile of the Extract in PLGA-TiO2
3.6. Evaluation of Extract Encapsulation Efficiency
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, S.; Chen, X.; Yu, M.; Li, J.; Liu, J.; Xie, Z.; Gao, F.; Liu, Y.; Liu, S.; Chen, X.; et al. Applications of Titanium Dioxide Nanostructure in Stomatology. Molecules 2022, 27, 3881. [Google Scholar] [CrossRef] [PubMed]
- Sandu, A.V.; Baltatu, S.; Doru, D.; Nergis, B.; Rodríguez-Barajas, N.; Becerra-Solano, L.; Karina Gutiérrez-Mercado, Y.; Macías-Carballo, M.; Gómez, C.M.; Pérez-Larios, A. Study of the Interaction of Ti-Zn as a Mixed Oxide at Different pH Values Synthesized by the Sol-Gel Method and Its Antibacterial Properties. Nanomaterials 2022, 12, 1948. [Google Scholar] [CrossRef]
- Marizcal-Barba, A.; Sanchez-Burgos, J.A.; Zamora-Gasga, V.; Larios, A.P. Study of the Response Surface in the Photocatalytic Degradation of Acetaminophen Using TiO2. Photochem 2022, 2, 225–236. [Google Scholar] [CrossRef]
- Pérez-Larios, A.; Rico, J.L.; Anaya-Esparza, L.M.; Vargas, O.A.G.; González-Silva, N.; Gómez, R. Hydrogen Production from Aqueous Methanol Solutions Using Ti–Zr Mixed Oxides as Photocatalysts under UV Irradiation. Catalysts 2019, 9, 938. [Google Scholar] [CrossRef]
- Fonseca-Cervantes, O.R.; Pérez-Larios, A.; Romero Arellano, V.H.; Sulbaran-Rangel, B.; González, C.A.G. Effects in Band Gap for Photocatalysis in TiO2 Support by Adding Gold and Ruthenium. Processes 2020, 8, 1032. [Google Scholar] [CrossRef]
- Ruíz-Santoyo, V.; Marañon-Ruiz, V.F.; Romero-Toledo, R.; Arturo González Vargas, O.; Pérez-Larios, A. Photocatalytic Degradation of Rhodamine B and Methylene Orange Using TiO2-ZrO2 as Nanocomposite. Catalysts 2021, 11, 1035. [Google Scholar] [CrossRef]
- Pérez-Larios, A.; Torres-Ramos, I.; Zanella, R.; Rico, J.L. Ti-Co mixed oxide as photocatalysts in the generation of hydrogen from water. Int. J. Chem. React. Eng. 2022, 20, 129–140. [Google Scholar] [CrossRef]
- Li, J.; Loh, X.J. Cyclodextrin-based supramolecular architectures: Syntheses, structures, and applications for drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1000–1017. [Google Scholar] [CrossRef] [PubMed]
- Adepu, S.; Ramakrishna, S.; Costa-Pinto, R.; Oliveira, A.L. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Burmistrov, D.E.; Lednev, V.N.; Simakin, A.V.; Uvarov, O.V.; Kucherov, R.N.; Ivashkin, P.I.; Dorokhov, A.S.; Izmailov, A.Y. Biosafety Construction Composite Based on Iron Oxide Nanoparticles and PLGA. Inventions 2022, 7, 61. [Google Scholar] [CrossRef]
- Martín-Camacho, U.J.; Torres-Ramos, M.I.; Sánchez-Burgos, J.A.; Pérez-Larios, A. Drug delivery system of green synthesized Ti-Cu nanocomposite. Mater. Lett. 2022, 321, 132437. [Google Scholar] [CrossRef]
- Tsebriienko, T.; Popov, A.I. Effect of Poly(Titanium Oxide) on the Viscoelastic and Thermophysical Properties of Interpenetrating Polymer Networks. Crystals 2021, 11, 794. [Google Scholar] [CrossRef]
- Bonetta, S.; Bonetta, S.; Motta, F.; Strini, A.; Carraro, E. Photocatalytic bacterial inactivation by TiO2-coated surfaces. AMB Express 2013, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical Applications of TiO2 Nanostructures: Recent Advances. Int. J. Nanomed. 2020, 15, 3447–3470. [Google Scholar] [CrossRef]
- Wu, J.Y.; Li, C.W.; Tsai, C.H.; Chou, C.W.; Chen, D.R.; Wang, G.J. Synthesis of antibacterial TiO2/PLGA composite biofilms. Nanomed. Nanotechnol. Biol. Med. 2014, 10, e1097–e1107. [Google Scholar] [CrossRef]
- Niu, B.; Wang, X.; Wu, K.; He, X.; Zhang, R. Mesoporous Titanium Dioxide: Synthesis and Applications in Photocatalysis, Energy and Biology. Materials 2018, 11, 1910. [Google Scholar] [CrossRef]
- Yi, W.; Yan, C.; Yan, P.; Li, F. A new perspective for effect of S and Cu on the photocatalytic activity of S, Cu-codoped nano-TiO2 under visible light irradiation. J. Sol. Gel. Sci. Technol. 2014, 69, 386–396. [Google Scholar] [CrossRef]
- Pelaseyed, S.S.; Hosseini, H.R.M.; Nokhbedehghan, Z.; Samadikuchaksaraei, A. PLGA/TiO2 nanocomposite scaffolds for biomedical applications: Fabrication, photocatalytic, and antibacterial properties. Bioimpacts 2021, 11, 45. [Google Scholar] [CrossRef]
- Basu, T.; Pal, B.; Singh, S. Fabrication of core–shell PLGA/PLA–pNIPAM nanocomposites for improved entrapment and release kinetics of antihypertensive drugs. Particuology 2018, 40, 169–176. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Krumina, A.; Romanova, M.; Kotomin, E.A.; Popov, A.I. Extraction–Pyrolytic Method for TiO2 Polymorphs Production. Crystals 2021, 11, 431. [Google Scholar] [CrossRef]
- Pelaseyed, S.S.; Madaah Hosseini, H.R.; Samadikuchaksaraei, A. A novel pathway to produce biodegradable and bioactive PLGA/TiO2 nanocomposite scaffolds for tissue engineering: Air–liquid foaming. J. Biomed. Mater. Res. Part A 2020, 108, 1390–1407. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, Y.; Hu, Y.; O’hayre, R.; Shao, Z. Facile Synthesis of Nanocrystalline TiO2 Mesoporous Microspheres for Lithium-Ion Batteries. J. Phys. Chem. C 2011, 115, 2529–2536. [Google Scholar] [CrossRef]
- Wilson, G.J.; Matijasevich, A.S.; Mitchell, D.R.G.; Schulz, J.C.; Will, G.D. Modification of TiO2 for Enhanced Surface Properties: Finite Ostwald Ripening by a Microwave Hydrothermal Process. Langmuir 2006, 22, 2016–2027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Dutta, A. Spectroscopic Methods for Nanomaterials Characterization. Spectrosc. Methods Nanomater. Charact. 2017, 2, 73–93. [Google Scholar] [CrossRef]


| Material | Lattice Parameters | Crystallite Size (nm) | |
|---|---|---|---|
| a = b (Å) | c (Å) | ||
| TiO2 | 3.774 a | 9.426 a | 11.97 b |
| TiO2 reported [19] | 3.784 | 9.478 | 21.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Ramos, M.I.; Martín-Marquez, M.F.; Leal-Moya, M.d.C.; Ghotekar, S.; Sánchez-Burgos, J.A.; Pérez-Larios, A. PLGA–TiO2 as a Carrier System for Drug Release. Int. J. Mol. Sci. 2022, 23, 10755. https://doi.org/10.3390/ijms231810755
Torres-Ramos MI, Martín-Marquez MF, Leal-Moya MdC, Ghotekar S, Sánchez-Burgos JA, Pérez-Larios A. PLGA–TiO2 as a Carrier System for Drug Release. International Journal of Molecular Sciences. 2022; 23(18):10755. https://doi.org/10.3390/ijms231810755
Chicago/Turabian StyleTorres-Ramos, M. I., M. F. Martín-Marquez, María del Carmen Leal-Moya, Suresh Ghotekar, Jorge Alberto Sánchez-Burgos, and Alejandro Pérez-Larios. 2022. "PLGA–TiO2 as a Carrier System for Drug Release" International Journal of Molecular Sciences 23, no. 18: 10755. https://doi.org/10.3390/ijms231810755
APA StyleTorres-Ramos, M. I., Martín-Marquez, M. F., Leal-Moya, M. d. C., Ghotekar, S., Sánchez-Burgos, J. A., & Pérez-Larios, A. (2022). PLGA–TiO2 as a Carrier System for Drug Release. International Journal of Molecular Sciences, 23(18), 10755. https://doi.org/10.3390/ijms231810755










