BcWRKY33A Enhances Resistance to Botrytis cinerea via Activating BcMYB51-3 in Non-Heading Chinese Cabbage
Abstract
:1. Introduction
2. Results
2.1. BcWRKY33A Expresses in Trichomes and Mediates Plant Resistance against B. cinerea Infection
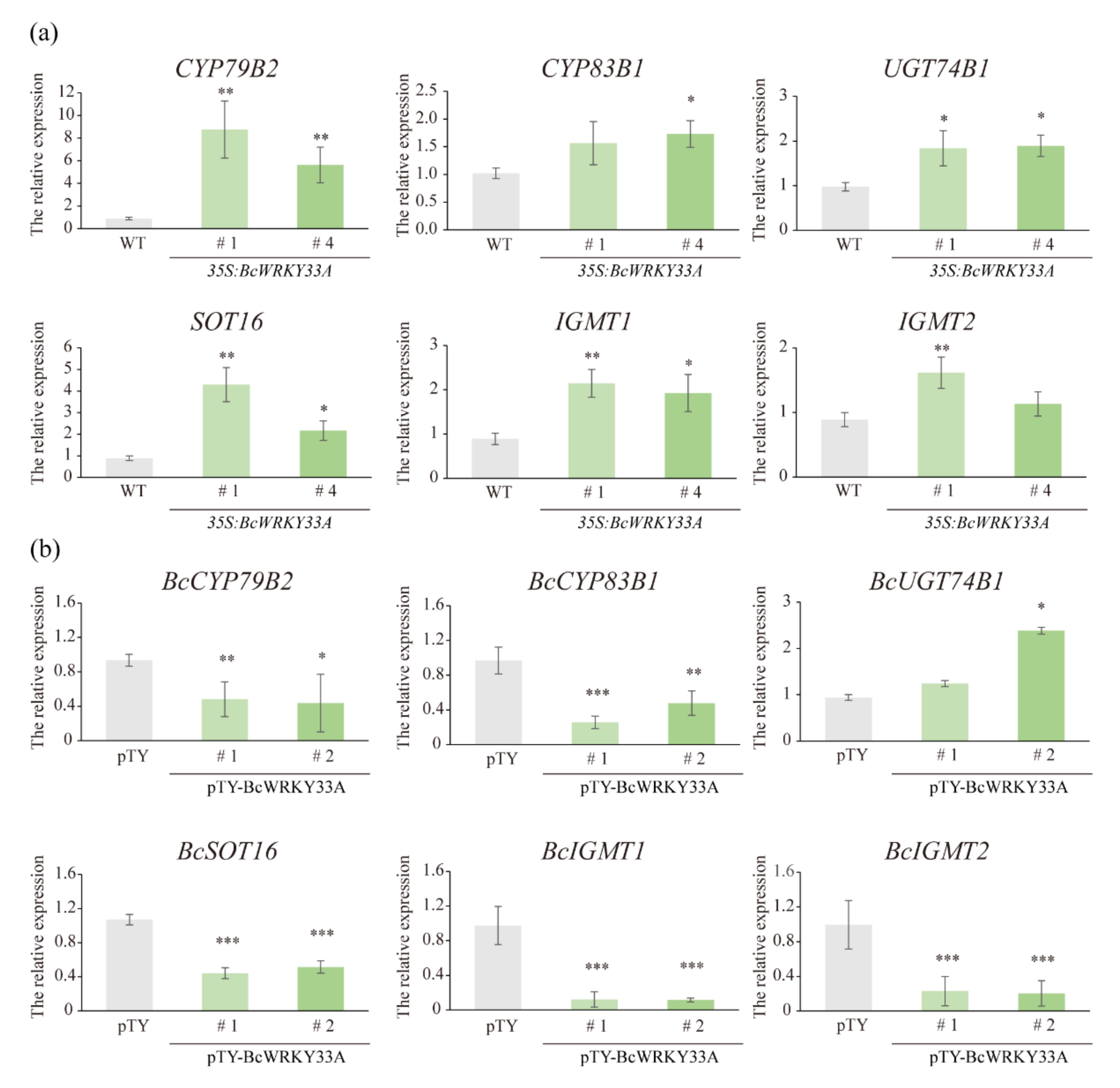
2.2. BcWRKY33A Modulates the Expression of IGSs’ Biosynthetic Genes
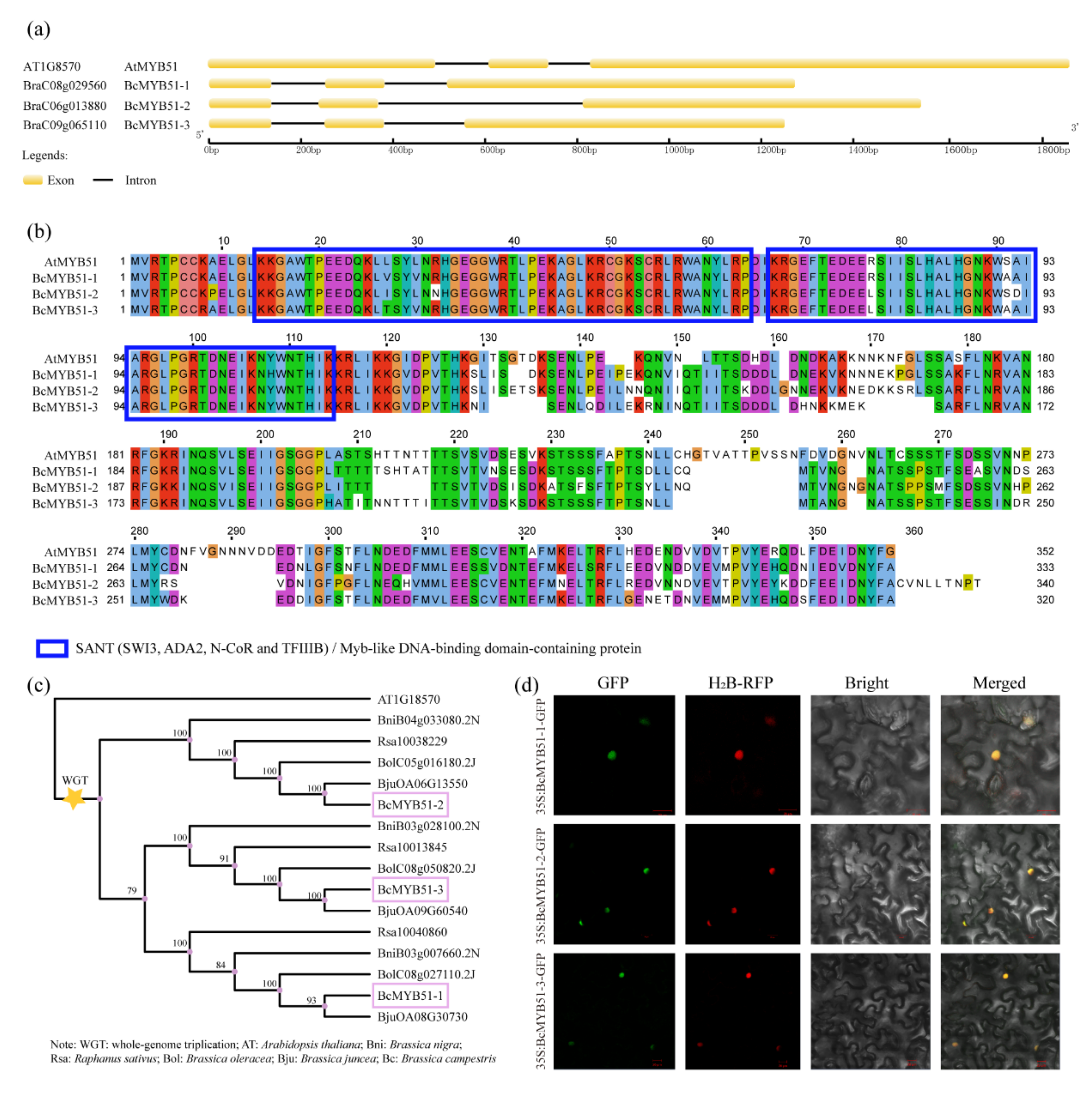
2.3. Identification of BcMYB51s Proteins in NHCC
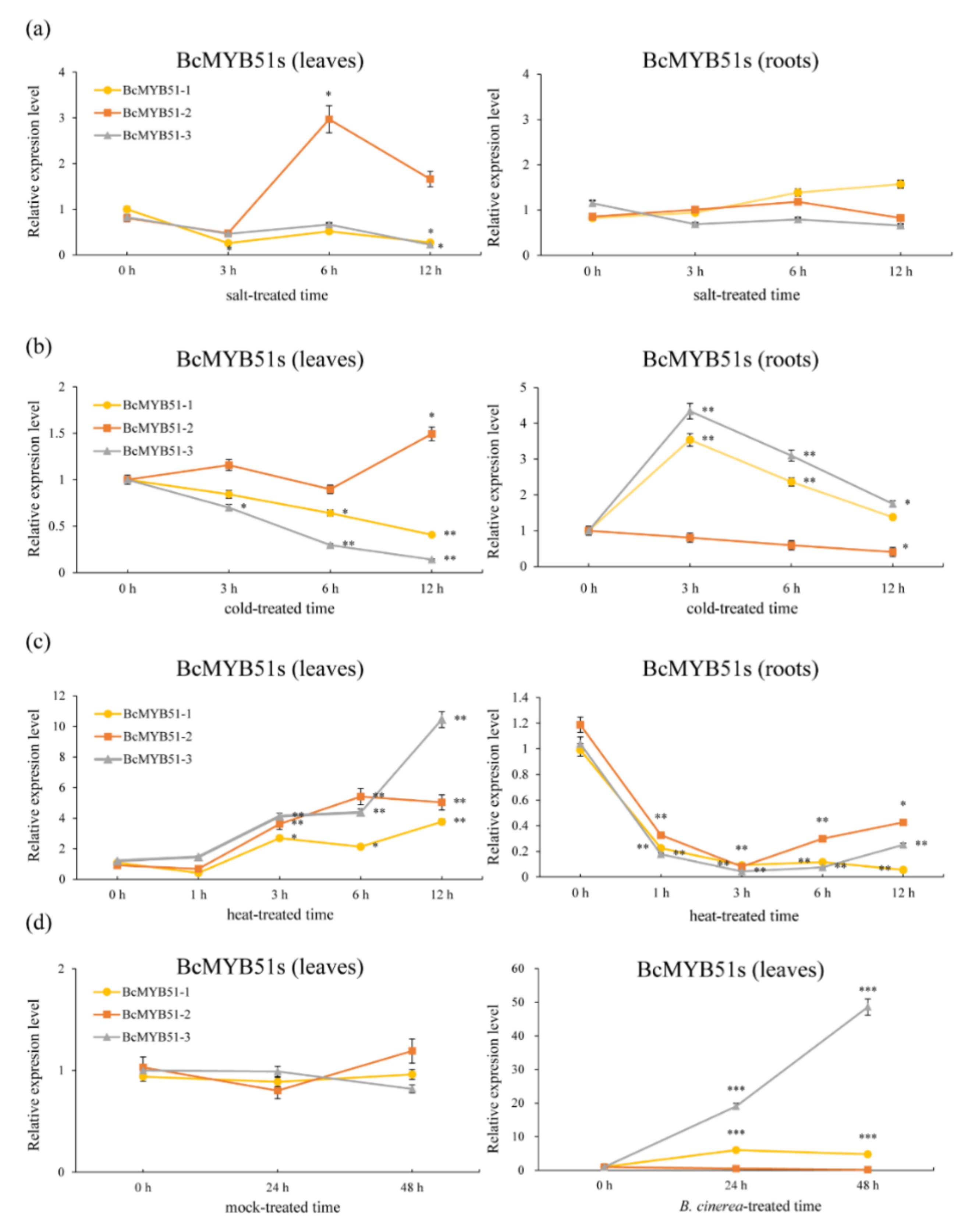
2.4. The Expression Patterns Analysis of BcMYB51s
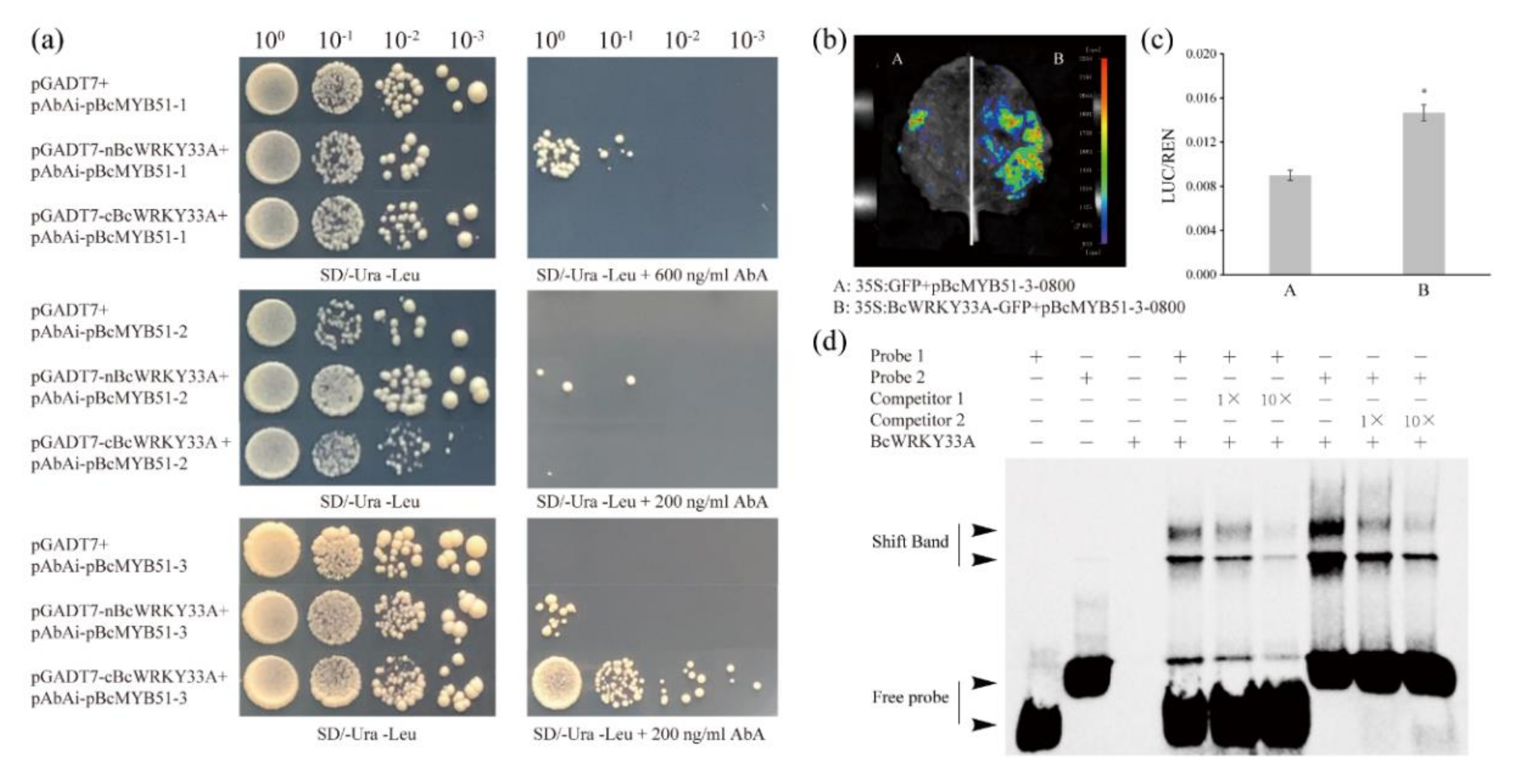
2.5. BcWRKY33A Binds to the W-Boxes in the BcMYB51-3 Promoter
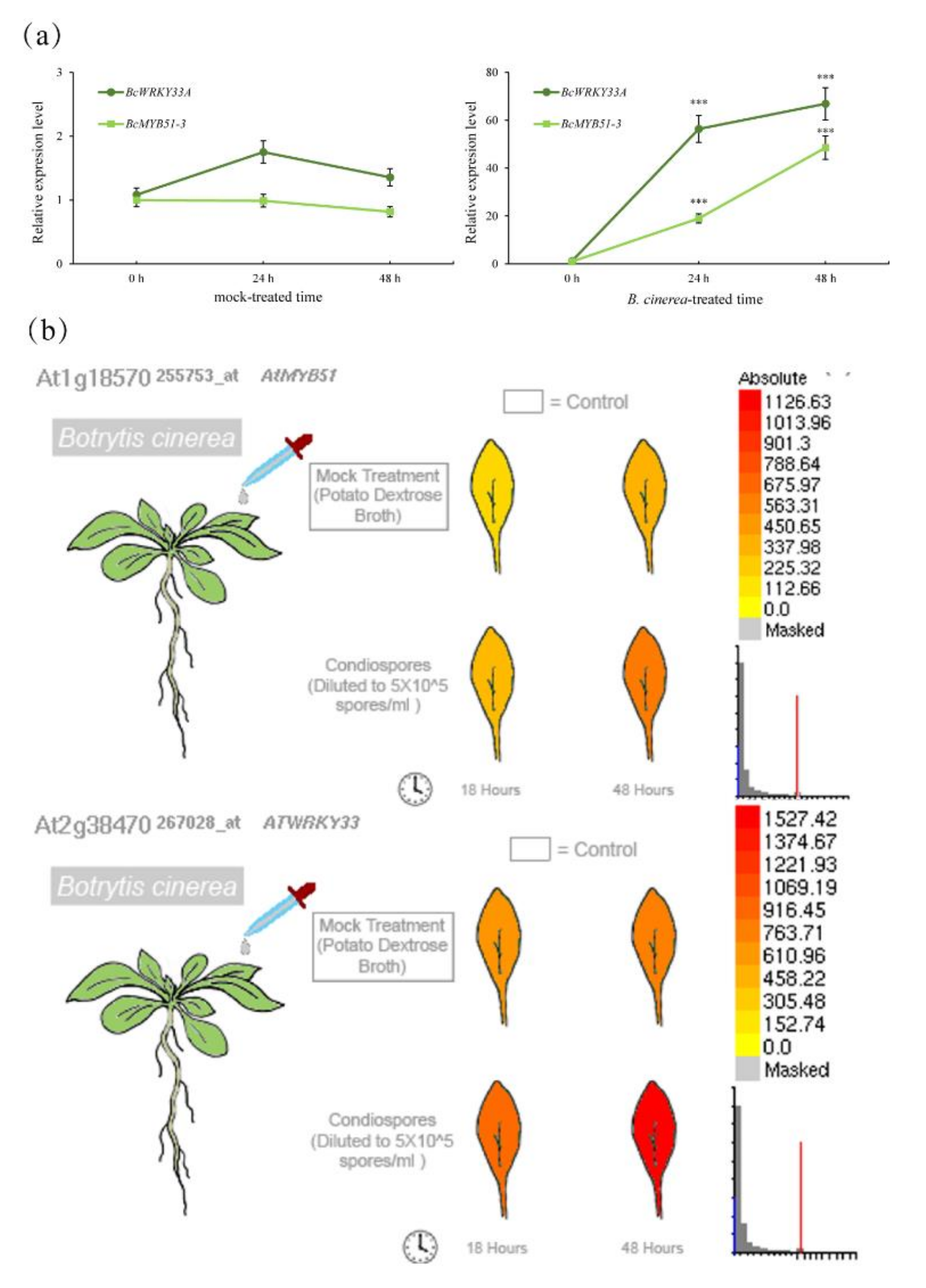
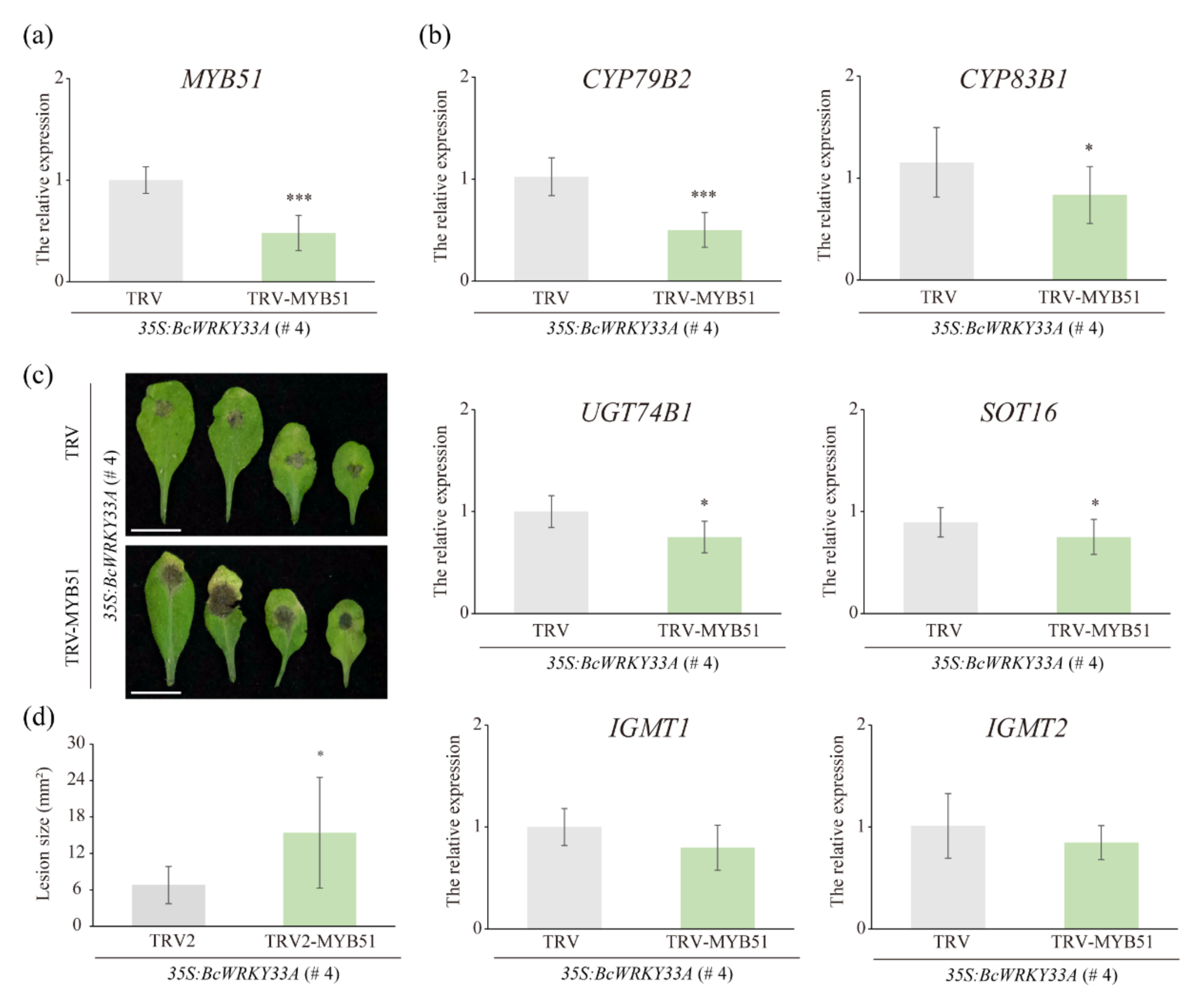
2.6. The Homolog of BcMYB51-3 Is Required for BcWRKY33A-Mediated Resistance to B. cinerea in Transgenic Arabidopsis
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Bioinformatics Analysis
4.3. Subcellular Localization
4.4. Infection Assay
4.5. Virus-Induced Gene Silencing (VIGS)
4.6. Yeast One-Hybrid Assay (Y1H)
4.7. Dual-Luciferase Reporter Assay (LUC)
4.8. Electrophoretic Mobility Shift Assay (EMSA)
4.9. Quantitative Real-Time PCR (qRT-PCR)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wani, S.H.; Anand, S.; Singh, B.; Bohra, A.; Joshi, R. WRKY transcription factors and plant defense responses: Latest discoveries and future prospects. Plant Cell Rep. 2021, 40, 1071–1085. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, J.; Guo, J.; Qiao, Q.; Guo, X.; Ma, Y. The WRKY transcription factor PlWRKY65 enhances the resistance of Paeonia lactiflora (herbaceous peony) to Alternaria tenuissima. Hortic. Res. 2020, 7, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, Q.; He, X.; Zhang, B.; Wang, Y.; Ma, Q. Identification and Characterization of WRKY41, a Gene Conferring Resistance to Powdery Mildew in Wild Tomato (Solanum habrochaites) LA1777. Int. J. Mol. Sci. 2022, 23, 1267. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, C.; Jiang, Y.; Luo, K.; Xu, C. Heterologous Expression of Poplar WRKY18/35 Paralogs in Arabidopsis Reveals Their Antagonistic Regulation on Pathogen Resistance and Abiotic Stress Tolerance via Variable Hormonal Pathways. Int. J. Mol. Sci. 2020, 21, 5440. [Google Scholar] [CrossRef]
- Birkenbihl, R.P.; Diezel, C.; Somssich, I.E. Arabidopsis WRKY33 Is a Key Transcriptional Regulator of Hormonal and Metabolic Responses toward Botrytis cinerea Infection. Plant Physiol. 2012, 159, 266–285. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.A.; Kracher, B.; Ziegler, J.; Birkenbihl, R.P.; Somssich, I.E. Negative regulation of ABA signaling by WRKY33 is critical for Arabidopsis immunity towards Botrytis cinerea 2100. eLife 2015, 4, e07295. [Google Scholar] [CrossRef]
- Lai, Z.; Li, Y.; Wang, F.; Cheng, Y.; Fan, B.; Yu, J.Q.; Chen, Z. Arabidopsis sigma factor binding proteins are activators of the WRKY33 transcription factor in plant defense. Plant Cell 2011, 23, 3824–3841. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Xu, H.; Huang, J.Y.; Kong, Y.Z.; AbuQamar, S.; Yu, D.Q.; Liu, S.Y.; Zhou, G.K.; Chai, G.H. The Arabidopsis CCCH protein C3H14 contributes to basal defense against Botrytis cinerea mainly through the WRKY33-dependent pathway. Plant Cell Environ. 2020, 43, 1792–1806. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [Green Version]
- Plesken, C.; Pattar, P.; Reiss, B.; Noor, Z.N.; Zhang, L.; Klug, K.; Huettel, B.; Hahn, M. Genetic Diversity of Botrytis cinerea Revealed by Multilocus Sequencing, and Identification of B. cinerea Populations Showing Genetic Isolation and Distinct Host Adaptation. Front. Plant Sci. 2021, 12, 663027. [Google Scholar] [CrossRef]
- Xue, M.; Yi, H. Enhanced Arabidopsis disease resistance against Botrytis cinerea induced by sulfur dioxide. Ecotoxicol. Environ. Saf. 2018, 147, 523–529. [Google Scholar] [CrossRef]
- Ramirez, V.; Agorio, A.; Coego, A.; Garcia-Andrade, J.; Hernandez, M.J.; Balaguer, B.; Ouwerkerk, P.B.; Zarra, I.; Vera, P. MYB46 modulates disease susceptibility to Botrytis cinerea in Arabidopsis. Plant Physiol. 2011, 155, 1920–1935. [Google Scholar] [CrossRef] [Green Version]
- Vela-Corcia, D.; Srivastava, D.A.; Dafa-Berger, A.; Rotem, N.; Barda, O.; Levy, M. MFS transporter from Botrytis cinerea provides tolerance to glucosinolate-breakdown products and is required for pathogenicity. Nat. Commun. 2019, 10, 2886. [Google Scholar] [CrossRef] [Green Version]
- Gao, P.; Zhang, H.; Yan, H.; Wang, Q.; Yan, B.; Jian, H.; Tang, K.; Qiu, X. RcTGA1 and glucosinolate biosynthesis pathway involvement in the defence of rose against the necrotrophic fungus Botrytis cinerea. BMC Plant Biol. 2021, 21, 223. [Google Scholar] [CrossRef]
- Bell, L.; Wagstaff, C. Glucosinolates, myrosinase hydrolysis products, and flavonols found in rocket (Eruca sativa and Diplotaxis tenuifolia). J. Agric. Food Chem. 2014, 62, 4481–4492. [Google Scholar] [CrossRef]
- Bell, L.; Wagstaff, C. Enhancement Of Glucosinolate and Isothiocyanate Profiles in Brassicaceae Crops: Addressing Challenges in Breeding for Cultivation, Storage, and Consumer-Related Traits. J. Agric. Food Chem. 2017, 65, 9379–9403. [Google Scholar] [CrossRef]
- Prieto, M.A.; Lopez, C.J.; Simal-Gandara, J. Glucosinolates: Molecular structure, breakdown, genetic, bioavailability, properties and healthy and adverse effects. Adv. Food Nutr. Res. 2019, 90, 305–350. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Xie, J.; Lv, J.; Zhang, G.; Hu, L.; Luo, S.; Li, L.; Yu, J. The Roles of Cruciferae Glucosinolates in Disease and Pest Resistance. Plants 2021, 10, 1097. [Google Scholar] [CrossRef]
- Sonderby, I.E.; Geu-Flores, F.; Halkier, B.A. Biosynthesis of glucosinolates—Gene discovery and beyond. Trends Plant Sci. 2010, 15, 283–290. [Google Scholar] [CrossRef]
- Essoh, A.P.; Monteiro, F.; Pena, A.R.; Pais, M.S.; Moura, M.; Romeiras, M.M. Exploring glucosinolates diversity in Brassicaceae: A genomic and chemical assessment for deciphering abiotic stress tolerance. Plant Physiol. Biochem. 2020, 150, 151–161. [Google Scholar] [CrossRef]
- Bekaert, M.; Edger, P.P.; Hudson, C.M.; Pires, J.C.; Conant, G.C. Metabolic and evolutionary costs of herbivory defense: Systems biology of glucosinolate synthesis. New Phytol. 2012, 196, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Tokuhisa, J.G.; Reichelt, M.; Gershenzon, J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 2003, 62, 471–4816. [Google Scholar] [CrossRef]
- Bednarek, P.; Pislewska-Bednarek, M.; Svatos, A.; Schneider, B.; Doubsky, J.; Mansurova, M.; Humphry, M.; Consonni, C.; Panstruga, R.; Sanchez-Vallet, A.; et al. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 2009, 323, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Madloo, P.; Lema, M.; Francisco, M.; Soengas, P. Changes in glucosinolates content in Brassica oleracea modulate disease severity caused by Xanthomonas campestris pv. campestris. Acta Hortic. 2018, 1202, 75–80. [Google Scholar] [CrossRef]
- Buxdorf, K.; Yaffe, H.; Barda, O.; Levy, M. The Effects of Glucosinolates and Their Breakdown Products on Necrotrophic Fungi. PLoS ONE 2013, 8, e70771. [Google Scholar] [CrossRef] [Green Version]
- Clay, N.K.; Adio, A.M.; Denoux, C.; Jander, G.; Ausubel, F.M. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 2009, 323, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Gigolashvili, T.; Berger, B.; Mock, H.P.; Muller, C.; Weisshaar, B.; Flugge, U.I. The transcription factor HIG1/MYB51 regulates indolic glucosinolate biosynthesis in Arabidopsis thaliana. Plant J. 2007, 50, 886–901. [Google Scholar] [CrossRef] [Green Version]
- Frerigmann, H.; Gigolashvili, T. MYB34, MYB51, and MYB122 distinctly regulate indolic glucosinolate biosynthesis in Arabidopsis thaliana. Mol. Plant 2014, 7, 814–828. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Kong, W.; Zhao, H.; Li, R.; Yang, Y.; Li, J. Transcriptome-wide identification of indole glucosinolate dependent flg22-response genes in Arabidopsis. Biochem. Biophys. Res. Commun. 2019, 520, 311–319. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Ren, H.; Zhang, C.; Xiao, D.; Li, Y.; Hou, X.; Liu, T. Regulatory interaction of BcWRKY33A and BcHSFA4A promotes salt tolerance in non-heading Chinese cabbage [Brassica campestris (syn. Brassica rapa) ssp. chinensis]. Hortic. Res. 2022, 9, uhac113. [Google Scholar] [CrossRef]
- Liu, S.; Ziegler, J.; Zeier, J.; Birkenbihl, R.P.; Somssich, I.E. Botrytis cinerea B05.10 promotes disease development in Arabidopsis by suppressing WRKY33-mediated host immunity. Plant Cell Environ. 2017, 40, 2189–2206. [Google Scholar] [CrossRef]
- Frerigmann, H.; Bottcher, C.; Baatout, D.; Gigolashvili, T. Glucosinolates are produced in trichomes of Arabidopsis thaliana. Front. Plant Sci. 2012, 3, 242. [Google Scholar] [CrossRef] [Green Version]
- Roeurn, S.; Hoshino, N.; Soejima, K.-t.; Yuuka, I.; Cushman, J.; Agarie, S. MYB and HD-ZIP IV homologs related to trichome formation are involved in epidermal bladder cell development in the halophyte: Mesembryanthemum crystallinum L. Plant Prod. Sci. 2017, 20, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Shafira, S.; Salamah, A. Analysis of leaves trichomes of Eclipta prostrata, Eleutheranthera ruderalis, Synedrella nodiflora, and Tridax procumbens (Asteraceae, Heliantheae). IOP Conf. Ser. Earth Environ. Sci. 2020, 524, 012001. [Google Scholar] [CrossRef]
- Li, Y.; Liu, G.F.; Ma, L.M.; Liu, T.K.; Zhang, C.W.; Xiao, D.; Zheng, H.K.; Chen, F.; Hou, X.L. A chromosome-level reference genome of non-heading Chinese cabbage [Brassica campestris (syn. Brassica rapa) ssp. chinensis]. Hortic. Res. 2020, 7, 212. [Google Scholar] [CrossRef]
- Goodin, M.M.; Dietzgen, R.G.; Schichnes, D.; Ruzin, S.; Jackson, A.O. pGD vectors: Versatile tools for the expression of green and red fluorescent protein fusions in agroinfiltrated plant leaves. Plant J. 2002, 31, 375–383. [Google Scholar] [CrossRef]
- Rombauts, S.; Dehais, P.; Van Montagu, M.; Rouze, P. PlantCARE, a plant cis-acting regulatory element database. Nucleic Acids Res. 1999, 27, 295–296. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Bi, H.; Liu, B.; Lou, S.; Song, Y.; Tong, S.; Chen, N.; Jiang, Y.; Liu, J.; Liu, H. WRKY33 interacts with WRKY12 protein to up-regulate RAP2.2 during submergence induced hypoxia response in Arabidopsis thaliana. New Phytol. 2021, 229, 106–125. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Vishal, B.; Ho, W.J.; Lok, F.C.J.; Lee, F.S.M.; Kumar, P.P. Regulation of a Cytochrome P450 gene CYP94B1 by WRKY33 transcription factor controls apoplastic barrier formation in roots to confer salt tolerance. Plant Physiol. 2020, 184, 2199–2215. [Google Scholar] [CrossRef]
- Li, P.; Song, A.; Gao, C.; Wang, L.; Wang, Y.; Sun, J.; Jiang, J.; Chen, F.; Chen, S. Chrysanthemum WRKY gene CmWRKY17 negatively regulates salt stress tolerance in transgenic chrysanthemum and Arabidopsis plants. Plant Cell Rep. 2015, 34, 1365–1378. [Google Scholar] [CrossRef]
- Burch-Smith, T.M.; Schiff, M.; Liu, Y.; Dinesh-Kumar, S.P. Efficient virus-induced gene silencing in Arabidopsis. Plant Physiol. 2006, 142, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.J.; Liu, Z.X.; Guo, J.G.; Zhu, Z.N.; Zhou, Y.P.; Guo, C.X.; Hu, Y.H.; Li, J.A.; Yan, S.G.; Li, T.; et al. WRKY33-PIF4 loop is required for the regulation of H2O2 homeostasis. Biochem. Biophys. Res. Commun. 2020, 527, 922–928. [Google Scholar] [CrossRef]
- Li, C.N.; Ng, C.K.Y.; Fan, L.M. MYB transcription factors, active players in abiotic stress signaling. Environ. Exp. Bot. 2015, 114, 80–91. [Google Scholar] [CrossRef]
- Wilkins, O.; Nahal, H.; Foong, J.; Provart, N.J.; Campbell, M.M. Expansion and diversification of the Populus R2R3-MYB family of transcription factors. Plant Physiol. 2009, 149, 981–993. [Google Scholar] [CrossRef] [Green Version]
- Borg, M.; Brownfield, L.; Khatab, H.; Sidorova, A.; Lingaya, M.; Twell, D. The R2R3 MYB Transcription Factor DUO1 Activates a Male Germline-Specific Regulon Essential for Sperm Cell Differentiation in Arabidopsis. Plant Cell 2011, 23, 534–549. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.Y.; Wang, Y.Y.; Yang, A.; Zhang, W.H. OsMYB2P-1, an R2R3 MYB Transcription Factor, Is Involved in the Regulation of Phosphate-Starvation Responses and Root Architecture in Rice. Plant Physiol. 2012, 159, 169–183. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.C.; Barron, C.; Schiefelbein, J.; Chen, J.G. Distinct relationships between GLABRA2 and single-repeat R3 MYB transcription factors in the regulation of trichome and root hair patterning in Arabidopsis. New Phytol. 2010, 185, 387–400. [Google Scholar] [CrossRef] [Green Version]
- Song, S.S.; Qi, T.C.; Huang, H.; Ren, Q.C.; Wu, D.W.; Chang, C.Q.; Peng, W.; Liu, Y.L.; Peng, J.R.; Xie, D.X. The Jasmonate-ZIM Domain Proteins Interact with the R2R3-MYB Transcription Factors MYB21 and MYB24 to Affect Jasmonate-Regulated Stamen Development in Arabidopsis. Plant Cell 2011, 23, 1000–1013. [Google Scholar] [CrossRef] [Green Version]
- Yang, A.; Dai, X.Y.; Zhang, W.H. A R2R3-type MYB gene, OsMYB2, is involved in salt, cold, and dehydration tolerance in rice. J. Exp. Bot. 2012, 63, 2541–2556. [Google Scholar] [CrossRef]
- Tao, H.; Miao, H.; Chen, L.; Wang, M.; Xia, C.; Zeng, W.; Sun, B.; Zhang, F.; Zhang, S.; Li, C.; et al. WRKY33-mediated indolic glucosinolate metabolic pathway confers resistance against Alternaria brassicicola in Arabidopsis and Brassica crops. J. Integr. Plant Biol. 2022, 64, 1007–1019. [Google Scholar] [CrossRef]
- Ambawat, S.; Sharma, P.; Yadav, N.R.; Yadav, R.C. MYB transcription factor genes as regulators for plant responses: An overview. Physiol. Mol. Biol. Plants 2013, 19, 307–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheong, Y.H.; Chang, H.S.; Gupta, R.; Wang, X.; Zhu, T.; Luan, S. Transcriptional profiling reveals novel interactions between wounding, pathogen, abiotic stress, and hormonal responses in Arabidopsis. Plant Physiol. 2002, 129, 661–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, S.; Choudhury, S.R.; Singh, S.K.; Das, K.P. Functional analysis of light-regulated promoter region of AtPollambda gene. Planta 2012, 235, 411–432. [Google Scholar] [CrossRef]
- Hou, F.; Du, T.; Qin, Z.; Xu, T.; Li, A.; Dong, S.; Ma, D.; Li, Z.; Wang, Q.; Zhang, L. Genome-wide in silico identification and expression analysis of beta-galactosidase family members in sweetpotato [Ipomoea batatas (L.) Lam]. BMC Genom. 2021, 22, 140. [Google Scholar] [CrossRef] [PubMed]
- Gangappa, S.N.; Maurya, J.P.; Yadav, V.; Chattopadhyay, S. The Regulation of the Z- and G-Box Containing Promoters by Light Signaling Components, SPA1 and MYC2, in Arabidopsis. PLoS ONE 2013, 8, e62194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieberman-Lazarovich, M.; Yahav, C.; Israeli, A.; Efroni, I. Deep Conservation of cis-Element Variants Regulating Plant Hormonal Responses. Plant Cell 2019, 31, 2559–2572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priest, H.D.; Filichkin, S.A.; Mockler, T.C. Cis-regulatory elements in plant cell signaling. Curr. Opin. Plant Biol. 2009, 12, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, J.; Cai, X.; Chen, H.; Wu, J.; Lin, R.; Cheng, F.; Wang, X. Divergence of three BRX homoeologs in Brassica rapa and its effect on leaf morphology. Hortic. Res. 2021, 8, 68. [Google Scholar] [CrossRef]
- Zheng, Z.; Qamar, S.A.; Chen, Z.; Mengiste, T. Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J. 2006, 48, 592–605. [Google Scholar] [CrossRef]
- Chen, H.; Wang, T.; He, X.; Cai, X.; Lin, R.; Liang, J.; Wu, J.; King, G.; Wang, X. BRAD V3.0: An upgraded Brassicaceae database. Nucleic Acids Res. 2022, 50, D1432–D1441. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [Green Version]
- Clamp, M.; Cuff, J.; Searle, S.M.; Barton, G.J. The Jalview Java alignment editor. Bioinformatics 2004, 20, 426–427. [Google Scholar] [CrossRef] [Green Version]
- Lian, J.; Han, H.; Zhao, J.; Li, C. In-vitro and in-planta Botrytis cinerea Inoculation Assays for Tomato. Bio-Protocol 2018, 8, e2810. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Yang, X.D.; Wang, Q.; Gao, L.W.; Yang, Y.; Xiao, D.; Liu, T.K.; Li, Y.; Hou, X.L.; Zhang, C.W. Efficient virus-induced gene silencing in Brassica rapa using a turnip yellow mosaic virus vector. Biol. Plant. 2018, 62, 826–834. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zheng, Y.; Xiao, D.; Li, Y.; Liu, T.; Hou, X. BcWRKY33A Enhances Resistance to Botrytis cinerea via Activating BcMYB51-3 in Non-Heading Chinese Cabbage. Int. J. Mol. Sci. 2022, 23, 8222. https://doi.org/10.3390/ijms23158222
Wang H, Zheng Y, Xiao D, Li Y, Liu T, Hou X. BcWRKY33A Enhances Resistance to Botrytis cinerea via Activating BcMYB51-3 in Non-Heading Chinese Cabbage. International Journal of Molecular Sciences. 2022; 23(15):8222. https://doi.org/10.3390/ijms23158222
Chicago/Turabian StyleWang, Huiyu, Yushan Zheng, Dong Xiao, Ying Li, Tongkun Liu, and Xilin Hou. 2022. "BcWRKY33A Enhances Resistance to Botrytis cinerea via Activating BcMYB51-3 in Non-Heading Chinese Cabbage" International Journal of Molecular Sciences 23, no. 15: 8222. https://doi.org/10.3390/ijms23158222
APA StyleWang, H., Zheng, Y., Xiao, D., Li, Y., Liu, T., & Hou, X. (2022). BcWRKY33A Enhances Resistance to Botrytis cinerea via Activating BcMYB51-3 in Non-Heading Chinese Cabbage. International Journal of Molecular Sciences, 23(15), 8222. https://doi.org/10.3390/ijms23158222






