Advances in the Therapeutic Effects of Apoptotic Bodies on Systemic Diseases
Abstract
1. Introduction
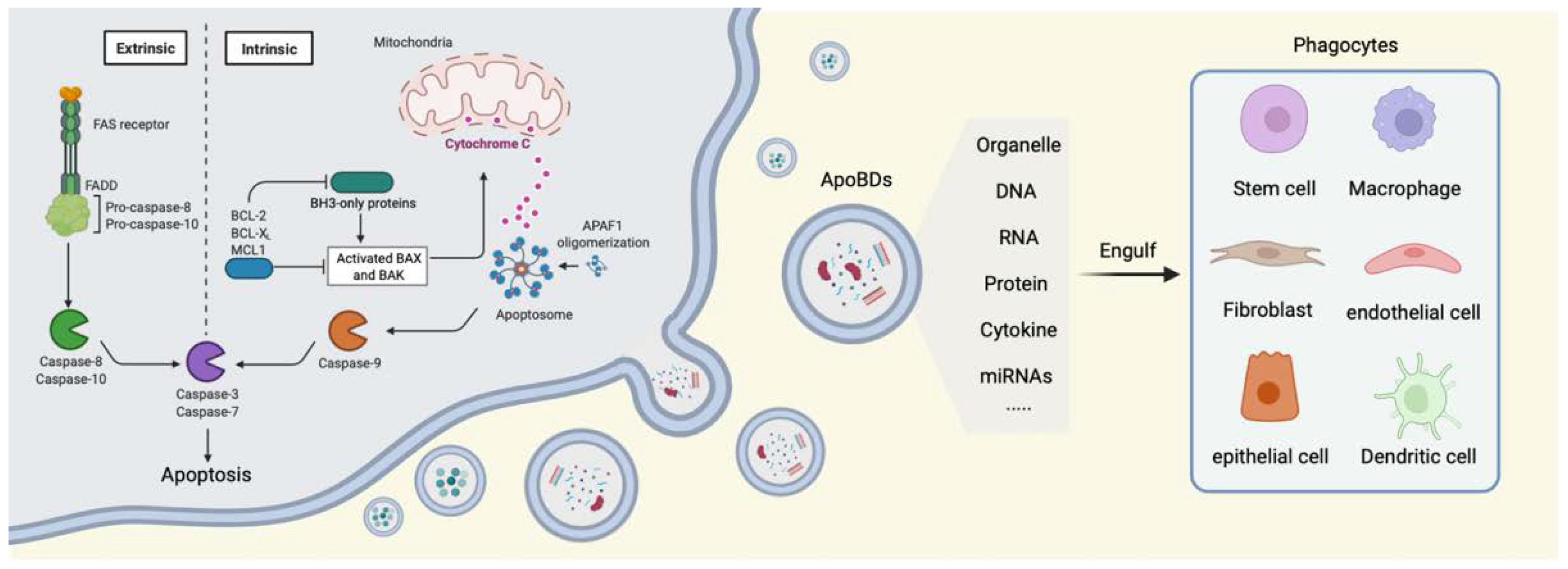
2. Cell Apoptosis
3. The Generation of ApoBDs
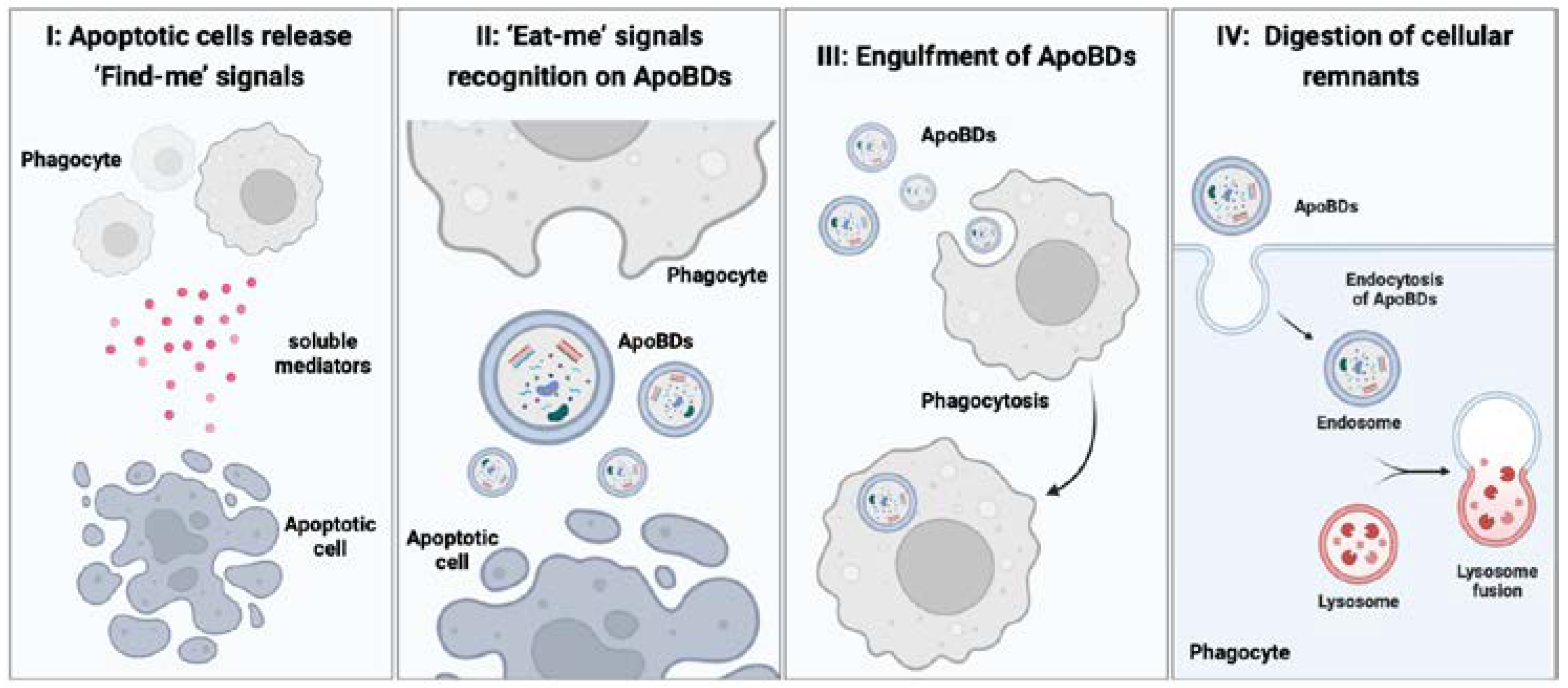
4. The Physiological Role of ApoBDs
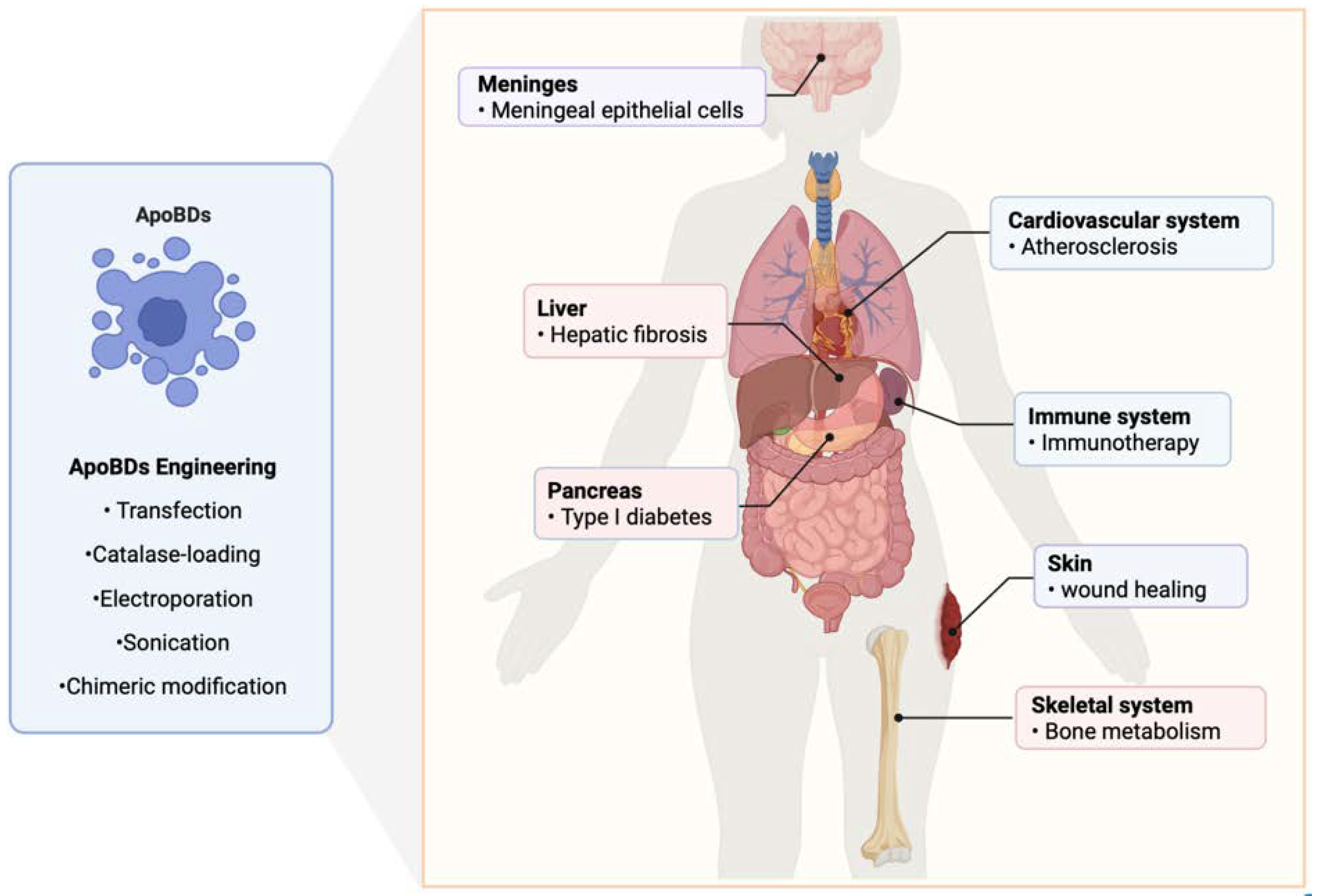
5. The Therapeutic Effect of ApoBDs on Systemic Diseases
5.1. Bacterial Infections in Cancer
5.2. Atherosclerosis
5.3. Bone Homeostasis
5.4. Hepatic Fibrosis
5.5. Enhancement of the Effect of Chemotherapy Drugs
5.6. Immunotherapy and Immune Defense
5.7. Diabetes
5.8. Wound Healing
6. Engineering and Recombination of ApoBDs
7. Concluding Remarks and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kerr, J.F.R. History of the events leading to the formulation of the apoptosis concept. Toxicology 2002, 181–182, 471–474. [Google Scholar] [CrossRef]
- Fuchs, Y.; Steller, H. Programmed cell death in animal development and disease. Cell 2011, 147, 742–758. [Google Scholar] [CrossRef] [PubMed]
- Davidson, F.; Steller, H. Blocking apoptosis prevents blindness in Drosophila retinal degeneration mutants. Nature 1998, 391, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Hochreiter-Hufford, A.; Lee, C.; Kinchen, J.; Sokolowski, J.; Arandjelovic, S.; Call, J.; Klibanov, A.; Yan, Z.; Mandell, J.; Ravichandran, K. Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion. Nature 2013, 497, 263–267. [Google Scholar] [CrossRef]
- Pellettieri, J.; Sánchez Alvarado, A. Cell turnover and adult tissue homeostasis: From humans to planarians. Annu. Rev. Genet. 2007, 41, 83–105. [Google Scholar] [CrossRef]
- Arandjelovic, S.; Ravichandran, K. Phagocytosis of apoptotic cells in homeostasis. Nat. Immunol. 2015, 16, 907–917. [Google Scholar] [CrossRef]
- Elliott, M.; Ravichandran, K. The Dynamics of Apoptotic Cell Clearance. Dev. Cell 2016, 38, 147–160. [Google Scholar] [CrossRef]
- Nagata, S. Apoptosis and Clearance of Apoptotic Cells. Annu. Rev. Immunol. 2018, 36, 489–517. [Google Scholar] [CrossRef]
- Zhan, S.; Jiang, J.; Wu, J.; Halsted, C.; Friedman, S.; Zern, M.; Torok, N. Phagocytosis of apoptotic bodies by hepatic stellate cells induces NADPH oxidase and is associated with liver fibrosis in vivo. Hepatology 2006, 43, 435–443. [Google Scholar] [CrossRef]
- Li, F.; Huang, Q.; Chen, J.; Peng, Y.; Roop, D.; Bedford, J.; Li, C. Apoptotic cells activate the "phoenix rising" pathway to promote wound healing and tissue regeneration. Sci. Signal. 2010, 3, ra13. [Google Scholar] [CrossRef]
- Buzas, E.; György, B.; Nagy, G.; Falus, A.; Gay, S. Emerging role of extracellular vesicles in inflammatory diseases. Nat. Rev. Rheumatol. 2014, 10, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Gray, E.; Heman-Ackah, S.; Mäger, I.; Talbot, K.; Andaloussi, S.; Wood, M.; Turner, M. Extracellular vesicles in neurodegenerative disease—Pathogenesis to biomarkers. Nat. Rev. Neurol. 2016, 12, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Karpman, D.; Ståhl, A.; Arvidsson, I. Extracellular vesicles in renal disease. Nat. Rev. Nephrol. 2017, 13, 545–562. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Thakur, B.; Weiss, J.; Kim, H.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef]
- Schorey, J.; Cheng, Y.; Singh, P.; Smith, V. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 2015, 16, 24–43. [Google Scholar] [CrossRef]
- Yuana, Y.; Sturk, A.; Nieuwland, R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013, 27, 31–39. [Google Scholar] [CrossRef]
- Arienti, S.; Barth, N.; Dorward, D.; Rossi, A.; Dransfield, I. Regulation of Apoptotic Cell Clearance During Resolution of Inflammation. Front. Pharmacol. 2019, 10, 891. [Google Scholar] [CrossRef]
- Lynch, C.; Panagopoulou, M.; Gregory, C. Extracellular Vesicles Arising from Apoptotic Cells in Tumors: Roles in Cancer Pathogenesis and Potential Clinical Applications. Front. Immunol. 2017, 8, 1174. [Google Scholar] [CrossRef]
- Kooijmans, S.; Schiffelers, R.; Zarovni, N.; Vago, R. Modulation of tissue tropism and biological activity of exosomes and other extracellular vesicles: New nanotools for cancer treatment. Pharmacol. Res. 2016, 111, 487–500. [Google Scholar] [CrossRef]
- György, B.; Szabó, T.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, E.; Pap, E.; Kittel, A.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef]
- Morel, O.; Jesel, L.; Freyssinet, J.; Toti, F. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 15–26. [Google Scholar] [CrossRef]
- Gregory, C.; Dransfield, I. Apoptotic Tumor Cell-Derived Extracellular Vesicles as Important Regulators of the Onco-Regenerative Niche. Front. Immunol. 2018, 9, 1111. [Google Scholar] [CrossRef]
- Wickman, G.; Julian, L.; Olson, M. How apoptotic cells aid in the removal of their own cold dead bodies. Cell Death Differ. 2012, 19, 735–742. [Google Scholar] [CrossRef]
- Atkin-Smith, G.; Poon, I. Disassembly of the Dying: Mechanisms and Functions. Trends Cell Biol. 2017, 27, 151–162. [Google Scholar] [CrossRef]
- Muhsin-Sharafaldine, M.; McLellan, A. Tumor-Derived Apoptotic Vesicles: With Death They Do Part. Front. Immunol. 2018, 9, 957. [Google Scholar] [CrossRef]
- Hochreiter-Hufford, A.; Ravichandran, K. Clearing the dead: Apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb. Perspect. Biol. 2013, 5, a008748. [Google Scholar] [CrossRef]
- Elliott, M.; Zheng, S.; Park, D.; Woodson, R.; Reardon, M.; Juncadella, I.; Kinchen, J.; Zhang, J.; Lysiak, J.; Ravichandran, K. Unexpected requirement for ELMO1 in clearance of apoptotic germ cells in vivo. Nature 2010, 467, 333–337. [Google Scholar] [CrossRef]
- Pang, S.; D’Rozario, J.; Mendonca, S.; Bhuvan, T.; Payne, N.; Zheng, D.; Hisana, A.; Wallis, G.; Barugahare, A.; Powell, D.; et al. Mesenchymal stromal cell apoptosis is required for their therapeutic function. Nat. Commun. 2021, 12, 6495. [Google Scholar] [CrossRef]
- Weavers, H.; Evans, I.; Martin, P.; Wood, W. Corpse Engulfment Generates a Molecular Memory that Primes the Macrophage Inflammatory Response. Cell 2016, 165, 1658–1671. [Google Scholar] [CrossRef]
- Ullah, M.; Qiao, Y.; Concepcion, W.; Thakor, A. Stem cell-derived extracellular vesicles: Role in oncogenic processes, bioengineering potential, and technical challenges. Stem Cell Res. Ther. 2019, 10, 347. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef] [PubMed]
- King, K.; Cidlowski, J. Cell cycle regulation and apoptosis. Annu. Rev. Physiol. 1998, 60, 601–617. [Google Scholar] [CrossRef]
- Fleisher, T. Apoptosis. Ann Allerg Astham Im 1997, 78, 245–249. [Google Scholar] [CrossRef]
- Humayun, A.; Fornace, A. GADD45 in Stress Signaling, Cell Cycle Control, and Apoptosis. Adv. Exp. Med. Biol. 2022, 1360, 1–22. [Google Scholar]
- Cory, S.; Adams, J. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef]
- Cheng, T.; Akey, I.; Yuan, S.; Yu, Z.; Ludtke, S.; Akey, C. A Near-Atomic Structure of the Dark Apoptosome Provides Insight into Assembly and Activation. Structure 2017, 25, 40–52. [Google Scholar] [CrossRef][Green Version]
- Czabotar, P.; Lessene, G.; Strasser, A.; Adams, J. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef]
- Yuan, S.; Akey, C. Apoptosome structure, assembly, and procaspase activation. Structure 2013, 21, 501–515. [Google Scholar] [CrossRef]
- Dorstyn, L.; Akey, C.; Kumar, S. New insights into apoptosome structure and function. Cell Death Differ. 2018, 25, 1194–1208. [Google Scholar] [CrossRef]
- Qi, H.; Jiang, Y.; Yin, Z.; Jiang, K.; Li, L.; Shuai, J. Optimal pathways for the assembly of the Apaf-1·cytochrome c complex into apoptosome. Phys. Chem. Chem. Phys. 2018, 20, 1964–1973. [Google Scholar] [CrossRef]
- Su, T.; Yang, C.; Kao, W.; Kuo, B.; Lin, S.; Lin, J.; Lo, Y.; Lin, S. Structural Insights into DD-Fold Assembly and Caspase-9 Activation by the Apaf-1 Apoptosome. Structure 2017, 25, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, M.; Hu, Q.; Bai, X.; Huang, W.; Scheres, S.; Shi, Y. Mechanistic insights into caspase-9 activation by the structure of the apoptosome holoenzyme. Proc. Natl. Acad. Sci. USA 2017, 114, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Harandi, H.; Falahati-Pour, S.; Mahmoodi, M.; Faramarz, S.; Maleki, H.; Nasab, F.; Shiri, H.; Fooladi, S.; Nematollahi, M. Nanoliposomal formulation of pistachio hull extract: Preparation, characterization and anti-cancer evaluation through Bax/Bcl2 modulation. Mol. Biol. Rep. 2022, 49, 2735–2743. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dorstyn, L.; Lim, Y. The role of caspases as executioners of apoptosis. Biochem. Soc. Trans. 2022, 50, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Prokhorova, E.; Kopeina, G.; Lavrik, I.; Zhivotovsky, B. Apoptosis regulation by subcellular relocation of caspases. Sci. Rep. 2018, 8, 12199. [Google Scholar] [CrossRef] [PubMed]
- Slee, E.; Adrain, C.; Martin, S. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J. Biol. Chem. 2001, 276, 7320–7326. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Kanneganti, T. Caspase-7: A protease involved in apoptosis and inflammation. Int. J. Biochem. Cell Biol. 2010, 42, 21–24. [Google Scholar] [CrossRef]
- Atkin-Smith, G.; Tixeira, R.; Paone, S.; Mathivanan, S.; Collins, C.; Liem, M.; Goodall, K.; Ravichandran, K.; Hulett, M.; Poon, I. A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure. Nat. Commun. 2015, 6, 7439. [Google Scholar] [CrossRef]
- Ravichandran, K. Beginnings of a good apoptotic meal: The find-me and eat-me signaling pathways. Immunity 2011, 35, 445–455. [Google Scholar] [CrossRef]
- Noori, A.; Tashakor, A.; Nikkhah, M.; Eriksson, L.; Hosseinkhani, S.; Fearnhead, H. Loss of WD2 subdomain of Apaf-1 forms an apoptosome structure which blocks activation of caspase-3 and caspase-9. Biochimie 2021, 180, 23–29. [Google Scholar] [CrossRef]
- Bortner, C.; Sifre, M.; Cidlowski, J. Cationic gradient reversal and cytoskeleton-independent volume regulatory pathways define an early stage of apoptosis. J. Biol. Chem. 2008, 283, 7219–7229. [Google Scholar] [CrossRef] [PubMed]
- Orlando, K.; Stone, N.; Pittman, R. Rho kinase regulates fragmentation and phagocytosis of apoptotic cells. Exp. Cell Res. 2006, 312, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Núñez, R.; Sancho-Martínez, S.; Novoa, J.; López-Hernández, F. Apoptotic volume decrease as a geometric determinant for cell dismantling into apoptotic bodies. Cell Death Differ. 2010, 17, 1665–1671. [Google Scholar] [CrossRef]
- López-Hernández, F. Cell Surface Area to Volume Relationship During Apoptosis and Apoptotic Body Formation. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2021, 55, 161–170. [Google Scholar] [CrossRef]
- Caruso, S.; Atkin-Smith, G.; Baxter, A.; Tixeira, R.; Jiang, L.; Ozkocak, D.; Santavanond, J.; Hulett, M.; Lock, P.; Phan, T.; et al. Defining the role of cytoskeletal components in the formation of apoptopodia and apoptotic bodies during apoptosis. Apoptosis Int. J. Program. Cell Death 2019, 24, 862–877. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lai, Y.; Hua, Z. Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci. Rep. 2019, 39, BSR20180992. [Google Scholar] [CrossRef]
- Santavanond, J.; Rutter, S.; Atkin-Smith, G.; Poon, I. Apoptotic Bodies: Mechanism of Formation, Isolation and Functional Relevance. Subcell. Biochem. 2021, 97, 61–88. [Google Scholar]
- Monier, B.; Suzanne, M. Orchestration of Force Generation and Nuclear Collapse in Apoptotic Cells. Int. J. Mol. Sci. 2021, 22, 10257. [Google Scholar] [CrossRef]
- Pontejo, S.; Murphy, P. Chemokines act as phosphatidylserine-bound "find-me" signals in apoptotic cell clearance. PLoS Biol. 2021, 19, e3001259. [Google Scholar] [CrossRef]
- Kinchen, J.; Doukoumetzidis, K.; Almendinger, J.; Stergiou, L.; Tosello-Trampont, A.; Sifri, C.; Hengartner, M.; Ravichandran, K. A pathway for phagosome maturation during engulfment of apoptotic cells. Nat. Cell Biol. 2008, 10, 556–566. [Google Scholar] [CrossRef]
- Gerke, V.; Moss, S. Annexins: From structure to function. Physiol. Rev. 2002, 82, 331–371. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, H.; Yang, D.; Cristofanilli, M.; Erwin, W.; Yu, D.; Kohanim, S.; Mendez, R.; Kim, E. Imaging and dosimetry of 99mTc EC annexin V: Preliminary clinical study targeting apoptosis in breast tumors. Appl. Radiat. Isot. Incl. Data Instrum. Methods Use Agric. Ind. Med. 2008, 66, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Rottey, S.; Loose, D.; Vakaet, L.; Lahorte, C.; Vermeersch, H.; Van Belle, S.; Van de Wiele, C. 99mTc-HYNIC Annexin-V imaging of tumors and its relationship to response to radiotherapy and/or chemotherapy. Q. J. Nucl. Med. Mol. Imaging 2007, 51, 182–188. [Google Scholar]
- Yang, D.; Azhdarinia, A.; Wu, P.; Yu, D.; Tansey, W.; Kalimi, S.; Kim, E.; Podoloff, D. In vivo and in vitro measurement of apoptosis in breast cancer cells using 99mTc-EC-annexin V. Cancer Biother. Radiopharm. 2001, 16, 73–83. [Google Scholar] [CrossRef]
- Orlando, K.; Pittman, R. Rho kinase regulates phagocytosis, surface expression of GlcNAc, and Golgi fragmentation of apoptotic PC12 cells. Exp. Cell Res. 2006, 312, 3298–3311. [Google Scholar] [CrossRef] [PubMed]
- Cockram, T.; Dundee, J.; Popescu, A.; Brown, G. The Phagocytic Code Regulating Phagocytosis of Mammalian Cells. Front. Immunol. 2021, 12, 629979. [Google Scholar] [CrossRef]
- Gozuacik, D.; Kimchi, A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene 2004, 23, 2891–2906. [Google Scholar] [CrossRef]
- Holmgren, L.; Bergsmedh, A.; Spetz, A. Horizontal transfer of DNA by the uptake of apoptotic bodies. Vox Sang. 2002, 83, 305–306. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Xu, A.; Zhou, D.; Zhang, B.; Qi, S.; Chen, Z.; Wang, X.; Ou, X.; Cao, B.; et al. Apoptosis-induced translocation of centromere protein F in its corresponding autoantibody production in hepatocellular carcinoma. Oncoimmunology 2021, 10, 1992104. [Google Scholar] [CrossRef]
- Moss, D.; Betin, V.; Malesinski, S.; Lane, J. A novel role for microtubules in apoptotic chromatin dynamics and cellular fragmentation. J. Cell Sci. 2006, 119, 2362–2374. [Google Scholar] [CrossRef]
- Atkin-Smith, G. Phagocytic clearance of apoptotic, necrotic, necroptotic and pyroptotic cells. Biochem. Soc. Trans. 2021, 49, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.; Tharmalingam, N.; Garcia Marques, F.; Sukumar, U.; Natarajan, A.; Zeng, Y.; Robinson, E.; Bermudez, A.; Chang, E.; Habte, F.; et al. Reconstructed Apoptotic Bodies as Targeted "Nano Decoys" to Treat Intracellular Bacterial Infections within Macrophages and Cancer Cells. ACS Nano 2020, 14, 5818–5835. [Google Scholar] [CrossRef] [PubMed]
- Zernecke, A.; Bidzhekov, K.; Noels, H.; Shagdarsuren, E.; Gan, L.; Denecke, B.; Hristov, M.; Köppel, T.; Jahantigh, M.; Lutgens, E.; et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci. Signal. 2009, 2, ra81. [Google Scholar] [CrossRef] [PubMed]
- Hristov, M.; Erl, W.; Linder, S.; Weber, P. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood 2004, 104, 2761–2766. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Liang, M.; Wu, Y.; Ding, N.; Duan, L.; Yu, T.; Bai, Y.; Kang, F.; Dong, S.; Xu, J.; et al. Mature osteoclast-derived apoptotic bodies promote osteogenic differentiation via RANKL-mediated reverse signaling. J. Biol. Chem. 2019, 294, 11240–11247. [Google Scholar] [CrossRef]
- Liu, D.; Kou, X.; Chen, C.; Liu, S.; Liu, Y.; Yu, W.; Yu, T.; Yang, R.; Wang, R.; Zhou, Y.; et al. Circulating apoptotic bodies maintain mesenchymal stem cell homeostasis and ameliorate osteopenia via transferring multiple cellular factors. Cell Res. 2018, 28, 918–933. [Google Scholar] [CrossRef]
- Jiang, J.; Mikami, K.; Venugopal, S.; Li, Y.; Török, N. Apoptotic body engulfment by hepatic stellate cells promotes their survival by the JAK/STAT and Akt/NF-kappaB-dependent pathways. J. Hepatol. 2009, 51, 139–148. [Google Scholar] [CrossRef]
- Zhao, D.; Tao, W.; Li, S.; Chen, Y.; Sun, Y.; He, Z.; Sun, B.; Sun, J. Apoptotic body-mediated intercellular delivery for enhanced drug penetration and whole tumor destruction. Sci. Adv. 2021, 7, eabg0880. [Google Scholar] [CrossRef]
- Schaible, U.; Winau, F.; Sieling, P.; Fischer, K.; Collins, H.; Hagens, K.; Modlin, R.; Brinkmann, V.; Kaufmann, S. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat. Med. 2003, 9, 1039–1046. [Google Scholar] [CrossRef]
- Li, J.; Fang, L.; Meyer, P.; Killer, H.; Flammer, J.; Neutzner, A. Anti-inflammatory response following uptake of apoptotic bodies by meningothelial cells. J. Neuroinflamm. 2014, 11, 35. [Google Scholar] [CrossRef]
- Marin-Gallen, S.; Clemente-Casares, X.; Planas, R.; Pujol-Autonell, I.; Carrascal, J.; Carrillo, J.; Ampudia, R.; Verdaguer, J.; Pujol-Borrell, R.; Borràs, F.; et al. Dendritic cells pulsed with antigen-specific apoptotic bodies prevent experimental type 1 diabetes. Clin. Exp. Immunol. 2010, 160, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiu, X.; Lv, Y.; Zheng, C.; Dong, Y.; Dou, G.; Zhu, B.; Liu, A.; Wang, W.; Zhou, J.; et al. Apoptotic bodies derived from mesenchymal stem cells promote cutaneous wound healing via regulating the functions of macrophages. Stem Cell Res. Ther. 2020, 11, 507. [Google Scholar] [CrossRef] [PubMed]
- Rolston, K. Infections in Cancer Patients with Solid Tumors: A Review. Infect. Dis. Ther. 2017, 6, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Ruczyński, J.; Rusiecka, I.; Turecka, K.; Kozłowska, A.; Alenowicz, M.; Gągało, I.; Kawiak, A.; Rekowski, P.; Waleron, K.; Kocić, I. Transportan 10 improves the pharmacokinetics and pharmacodynamics of vancomycin. Sci. Rep. 2019, 9, 3247. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.; Paulmurugan, R.; Moon, J.; Lee, S.; Park, H. Cell membrane-coated nanocarriers: The emerging targeted delivery system for cancer theranostics. Drug Discov. Today 2018, 23, 891–899. [Google Scholar] [CrossRef]
- Bose, R.; Uday Kumar, S.; Zeng, Y.; Afjei, R.; Robinson, E.; Lau, K.; Bermudez, A.; Habte, F.; Pitteri, S.; Sinclair, R.; et al. Tumor Cell-Derived Extracellular Vesicle-Coated Nanocarriers: An Efficient Theranostic Platform for the Cancer-Specific Delivery of Anti-miR-21 and Imaging Agents. ACS Nano 2018, 12, 10817–10832. [Google Scholar] [CrossRef]
- Berda-Haddad, Y.; Robert, S.; Salers, P.; Zekraoui, L.; Farnarier, C.; Dinarello, C.; Dignat-George, F.; Kaplanski, G. Sterile inflammation of endothelial cell-derived apoptotic bodies is mediated by interleukin-1α. Proc. Natl. Acad. Sci. USA 2011, 108, 20684–20689. [Google Scholar] [CrossRef]
- Soysa, N.; Alles, N. Positive and negative regulators of osteoclast apoptosis. Bone Rep. 2019, 11, 100225. [Google Scholar] [CrossRef]
- McDonald, M.; Kim, A.; Mulholland, B.; Rauner, M. New Insights Into Osteoclast Biology. JBMR Plus 2021, 5, e10539. [Google Scholar] [CrossRef]
- Tixeira, R.; Phan, T.; Caruso, S.; Shi, B.; Atkin-Smith, G.; Nedeva, C.; Chow, J.; Puthalakath, H.; Hulett, M.; Herold, M.; et al. ROCK1 but not LIMK1 or PAK2 is a key regulator of apoptotic membrane blebbing and cell disassembly. Cell Death Differ. 2020, 27, 102–116. [Google Scholar] [CrossRef]
- Atkin-Smith, G.; Miles, M.; Tixeira, R.; Lay, F.; Duan, M.; Hawkins, C.; Phan, T.; Paone, S.; Mathivanan, S.; Hulett, M.; et al. Plexin B2 is a Regulator of Monocyte Apoptotic Cell Disassembly. Cell Rep. 2019, 29, 1821–1831.e3. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Jiang, D.; Sindrilaru, A.; Stegemann, A.; Schatz, S.; Treiber, N.; Rojewski, M.; Schrezenmeier, H.; Vander Beken, S.; Wlaschek, M.; et al. TSG-6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine full-thickness skin wounds. J. Investig. Dermatol. 2014, 134, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Takanashi, M.; Sudo, K.; Ueda, S.; Ishikawa, A.; Matsuyama, N.; Fujita, K.; Mizutani, T.; Ohgi, T.; Ochiya, T.; et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. J. Am. Soc. Gene Ther. 2013, 21, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Wahlgren, J.; Karlson, T.D.L.; Brisslert, M.; Vaziri Sani, F.; Telemo, E.; Sunnerhagen, P.; Valadi, H. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012, 40, e130. [Google Scholar] [CrossRef]
- Liang, S.; Zuo, F.; Yin, B.; Ye, B. Delivery of siRNA based on engineered exosomes for glioblastoma therapy by targeting STAT3. Biomater. Sci. 2022, 10, 1582–1590. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, G.; Yu, T.; He, J.; Liu, J.; Chai, X.; Zhao, G.; Yin, D.; Zhang, C. Exosomes deliver lncRNA DARS-AS1 siRNA to inhibit chronic unpredictable mild stress-induced TNBC metastasis. Cancer Lett. 2022, 543, 215781. [Google Scholar] [CrossRef]
- Pofali, P.; Mondal, A.; Londhe, V. Exosome as a Natural Gene Delivery Vector for Cancer Treatment. Curr. Cancer Drug Targets 2020, 20, 821–830. [Google Scholar] [CrossRef]
- Didiot, M.; Hall, L.; Coles, A.; Haraszti, R.; Godinho, B.; Chase, K.; Sapp, E.; Ly, S.; Alterman, J.; Hassler, M.; et al. Exosome-mediated Delivery of Hydrophobically Modified siRNA for Huntingtin mRNA Silencing. Mol. Ther. J. Am. Soc. Gene Ther. 2016, 24, 1836–1847. [Google Scholar] [CrossRef]
- Whitley, J.; Kim, S.; Lou, L.; Ye, C.; Alsaidan, O.; Sulejmani, E.; Cai, J.; Desrochers, E.; Beharry, Z.; Rickman, C.; et al. Encapsulating Cas9 into extracellular vesicles by protein myristoylation. J. Extracell. Vesicles 2022, 11, e12196. [Google Scholar] [CrossRef]
- Izco, M.; Alvarez-Erviti, L. siRNA Loaded-Exosomes. Methods Mol. Biol. 2021, 2282, 395–401. [Google Scholar]
- Haney, M.; Klyachko, N.; Zhao, Y.; Gupta, R.; Plotnikova, E.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.; et al. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J. Control. Release Off. J. Control. Release Soc. 2015, 207, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, T.; Jeyaram, A.; Patel, D.; Parajuli, B.; Livingston, N.; Arumugasaamy, N.; Schardt, J.; Jay, S. Oncogene Knockdown via Active Loading of Small RNAs into Extracellular Vesicles by Sonication. Cell. Mol. Bioeng. 2016, 9, 315–324. [Google Scholar] [CrossRef]
- Dou, G.; Tian, R.; Liu, X.; Yuan, P.; Ye, Q.; Liu, J.; Liu, S.; Zhou, J.; Deng, Z.; Chen, X.; et al. Chimeric apoptotic bodies functionalized with natural membrane and modular delivery system for inflammation modulation. Sci. Adv. 2020, 6, eaba2987. [Google Scholar] [CrossRef]
- Bao, L.; Dou, G.; Tian, R.; Lv, Y.; Ding, F.; Liu, S.; Zhao, R.; Zhao, L.; Zhou, J.; Weng, L.; et al. Engineered neutrophil apoptotic bodies ameliorate myocardial infarction by promoting macrophage efferocytosis and inflammation resolution. Bioact. Mater. 2022, 9, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Capello, M.; Vykoukal, J.; Katayama, H.; Bantis, L.; Wang, H.; Kundnani, D.; Aguilar-Bonavides, C.; Aguilar, M.; Tripathi, S.; Dhillon, D.; et al. Exosomes harbor B cell targets in pancreatic adenocarcinoma and exert decoy function against complement-mediated cytotoxicity. Nat. Commun. 2019, 10, 254. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Li, Z.; Xu, C.; Guo, B.; Guo, P. Folate-displaying exosome mediated cytosolic delivery of siRNA avoiding endosome trapping. J. Control. Release Off. J. Control. Release Soc. 2019, 311–312, 43–49. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, H.; He, C.; Fan, S.; Zhu, Y.; Qi, C.; Huang, N.; Xiao, Z.; Lu, Z.; Tannous, B.; et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef]
| Systematic Condition | ApoBDs | Regulatory Mechanism | Reference |
|---|---|---|---|
| Bacterial infection | Vancomycin loading cancer cell-derived ApoBDs | Targets the “eat me” signal on ApoBDs and vancomycin is delivered to kill Staphylococcus aureus | [72] |
| Atherosclerosis | Endothelial cell-derived ApoBDs | Promotes endothelial progenitor cell proliferation and differentiation | [73,74] |
| Bone homeostasis | 1. mOC-derived ApoBDs2. Circulating ApoBDs | 1. Induces osteoblast differentiation via the PI3K/Akt/mTOR/S6K signaling pathway2. Circulating ApoBDs maintain the self-renewal and osteogenic/adipogenic differentiation of BMMSCs via the Wnt/β-catenin pathway | [75,76] |
| Hepatic fibrosis | HepG2-derived ApoBDs | Promotes HSC survival via the JAK1/STAT3 and PI3K/Akt/NF-κB pathways | [77] |
| Chemotherapy | CPT+ PR104A loading cancer cell-derived ApoBDs | Enhances the ApoBD-based neighboring effect and facilitates the deep penetration of chemotherapeutic agents | [78] |
| Immunotherapy | 1. Macrophage-derived ApoBDs2. U-937/SH-SY5Y-derived ApoBDs | 1. Triggers dendritic cell-mediated cross presentation and CD8 + T cell activation through MHC-I and CD11b2. Inhibits the secretion of pro-inflammatory, chemoattractant cytokines and chemokines | [79,80] |
| Diabetes | NIT-1-derived ApoBDs | Reduces the expression of co-stimulatory molecules CD40, CD86, and proinflammatory cytokines of DCs, rebuilds peripheral immune tolerance | [81] |
| Wound healing | BMMSC-derived ApoBDs | Enhances the migration and proliferation of fibroblasts, inducing the polarization of macrophages to the M2 phenotype | [82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liu, Y.; Liu, X.; Du, J.; Bhawal, U.K.; Xu, J.; Guo, L.; Liu, Y. Advances in the Therapeutic Effects of Apoptotic Bodies on Systemic Diseases. Int. J. Mol. Sci. 2022, 23, 8202. https://doi.org/10.3390/ijms23158202
Li X, Liu Y, Liu X, Du J, Bhawal UK, Xu J, Guo L, Liu Y. Advances in the Therapeutic Effects of Apoptotic Bodies on Systemic Diseases. International Journal of Molecular Sciences. 2022; 23(15):8202. https://doi.org/10.3390/ijms23158202
Chicago/Turabian StyleLi, Xiaoyan, Yitong Liu, Xu Liu, Juan Du, Ujjal Kumar Bhawal, Junji Xu, Lijia Guo, and Yi Liu. 2022. "Advances in the Therapeutic Effects of Apoptotic Bodies on Systemic Diseases" International Journal of Molecular Sciences 23, no. 15: 8202. https://doi.org/10.3390/ijms23158202
APA StyleLi, X., Liu, Y., Liu, X., Du, J., Bhawal, U. K., Xu, J., Guo, L., & Liu, Y. (2022). Advances in the Therapeutic Effects of Apoptotic Bodies on Systemic Diseases. International Journal of Molecular Sciences, 23(15), 8202. https://doi.org/10.3390/ijms23158202







