Evaluation and Characterization of Post-Stroke Lung Damage in a Murine Model of Cerebral Ischemia
Abstract
:1. Introduction
2. Results
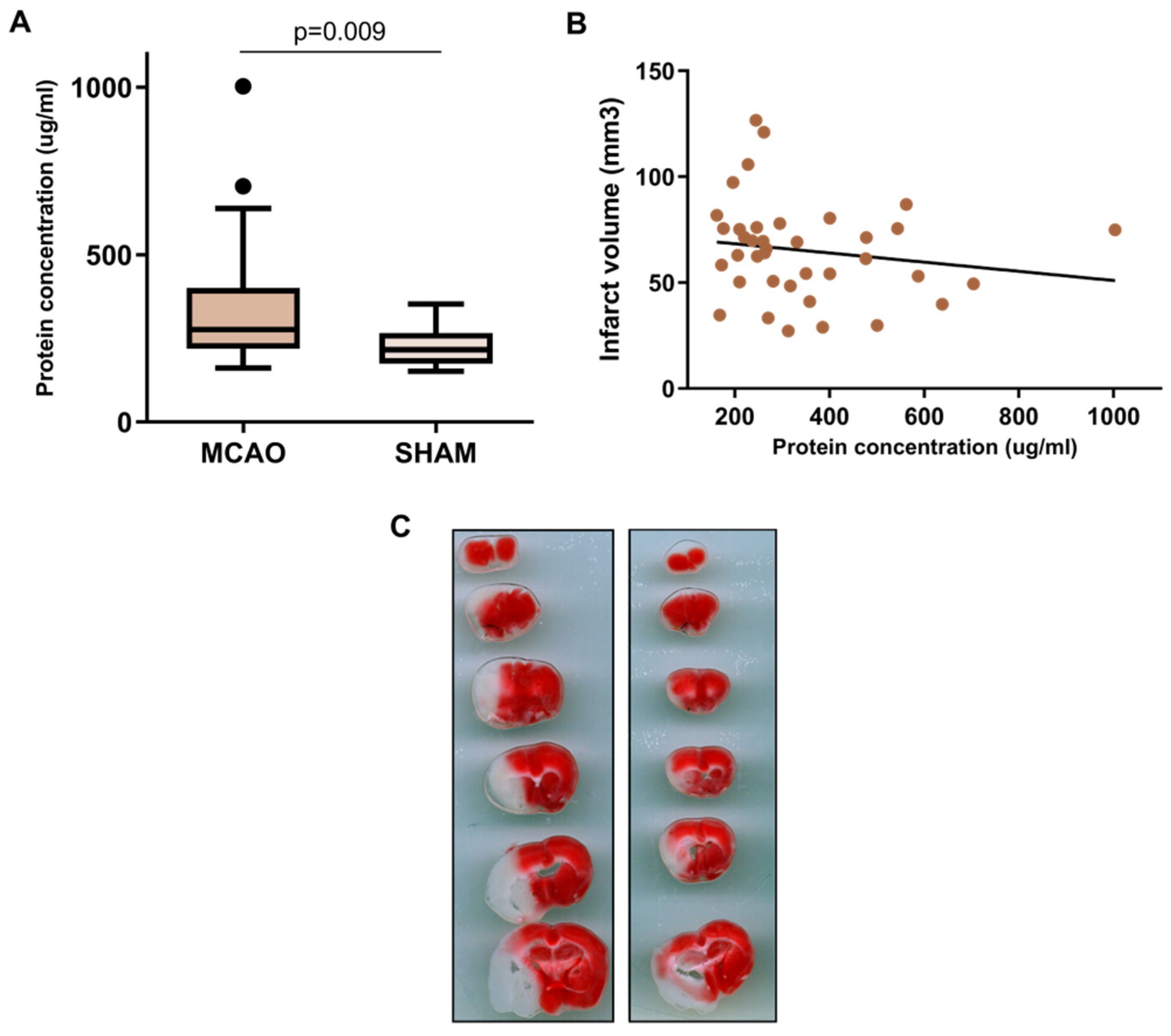
2.1. BALF Characterization after Cerebral Ischemia
2.2. Protein Expression Characterization in Lung
2.3. Alveolar-Capillary Barrier Permeability
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. MCAO Surgery
4.3. Sample Collection and Infarct Volume Quantification
4.4. BALF Protein Concentration Quantification
4.5. Lung Permeability Assay
4.6. Lung Homogenization
4.7. BALF and Lung Protein Characterization
4.8. ELISA Protein Measurement
4.9. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mrozek, S.; Constantin, J.-M.; Geeraerts, T. Brain-Lung Crosstalk: Implications for Neurocritical Care Patients. World J. Crit. Care Med. 2015, 4, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, W.; Li, W.; Ning, Y.L.; Li, P.; Zhao, Y.; Yang, N.; Jiang, Y.L.; Liang, Z.P.; Jiang, D.P.; Wang, Y.; et al. Blood Glutamate Levels Are Closely Related to Acute Lung Injury and Prognosis after Stroke. Front. Neurol. 2018, 8, 755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mascia, L. Acute Lung Injury in Patients with Severe Brain Injury: A Double Hit Model. Neurocrit. Care 2009, 11, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Samary, C.S.; Ramos, A.B.; Maia, L.A.; Rocha, N.N.; Santos, C.L.; Magalhães, R.F.; Clevelario, A.L.; Pimentel-Coelho, P.M.; Mendez-Otero, R.; Cruz, F.F.; et al. Focal Ischemic Stroke Leads to Lung Injury and Reduces Alveolar Macrophage Phagocytic Capability in Rats. Crit. Care 2018, 22, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, V.; Ku, J.M.; Miller, A.A.; Vlahos, R. Ischaemic Stroke in Mice Induces Lung Inflammation but Not Acute Lung Injury. Sci. Rep. 2019, 9, 3622. [Google Scholar] [CrossRef] [Green Version]
- Farris, B.Y.; Monaghan, K.L.; Zheng, W.; Amend, C.D.; Hu, H.; Ammer, A.G.; Coad, J.E.; Ren, X.; Wan, E.C.K. Ischemic Stroke Alters Immune Cell Niche and Chemokine Profile in Mice Independent of Spontaneous Bacterial Infection. Immunity Inflamm. Dis. 2019, 7, 326–341. [Google Scholar] [CrossRef] [Green Version]
- Badve, M.S.; Zhou, Z.; van de Beek, D.; Anderson, C.S.; Hackett, M.L. Frequency of Post-Stroke Pneumonia: Systematic Review and Meta-Analysis of Observational Studies. Int. J. Stroke 2019, 14, 125–136. [Google Scholar] [CrossRef]
- Teh, W.H.; Smith, C.J.; Barlas, R.S.; Wood, A.D.; Bettencourt-Silva, J.H.; Clark, A.B.; Metcalf, A.K.; Bowles, K.M.; Potter, J.F.; Myint, P.K. Impact of Stroke-Associated Pneumonia on Mortality, Length of Hospitalization, and Functional Outcome. Acta Neurol. Scand. 2018, 138, 293–300. [Google Scholar] [CrossRef]
- Badve, M.S.; Zhou, Z.; Anderson, C.S.; Hackett, M.L. Effectiveness and Safety of Antibiotics for Preventing Pneumonia and Improving Outcome after Acute Stroke: Systematic Review and Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2018, 27, 3137–3147. [Google Scholar] [CrossRef]
- Faura, J.; Bustamante, A.; Miró-Mur, F.; Montaner, J. Stroke-Induced Immunosuppression: Implications for the Prevention and Prediction of Post-Stroke Infections. J. Neuroinflamm. 2021, 18, 127. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Xiao, W.; Song, T.; Wang, S. Incidence, Risk Factors, and Outcomes of Ventilator-Associated Pneumonia in Traumatic Brain Injury: A Meta-Analysis. Neurocrit. Care 2020, 32, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Crapser, J.; Ritzel, R.; Verma, R.; Venna, V.R.; Liu, F.; Chauhan, A.; Koellhoffer, E.; Patel, A.; Ricker, A.; Maas, K.; et al. Ischemic Stroke Induces Gut Permeability and Enhances Bacterial Translocation Leading to Sepsis in Aged Mice. Aging 2016, 8, 1049–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillot, L.; Nathan, N.; Tabary, O.; Thouvenin, G.; Le Rouzic, P.; Corvol, H.; Amselem, S.; Clement, A. Alveolar Epithelial Cells: Master Regulators of Lung Homeostasis. Int. J. Biochem. Cell Biol. 2013, 45, 2568–2573. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, E.Y.; Lauzon-Joset, J.F.; Debley, J.S.; Ziegler, S.F. Cross-Talk Between Alveolar Macrophages and Lung Epithelial Cells Is Essential to Maintain Lung Homeostasis. Front. Immunol. 2020, 11, 583042. [Google Scholar] [CrossRef]
- Panganiban, R.A.M.; Day, R.M. Hepatocyte Growth Factor in Lung Repair and Pulmonary Fibrosis. Acta Pharmacol. Sin. 2011, 32, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Stern, J.B.; Fierobe, L.; Paugam, C.; Rolland, C.; Dehoux, M.; Petiet, A.; Dombret, M.C.; Mantz, J.; Aubier, M.; Crestani, B. Keratinocyte Growth Factor and Hepatocyte Growth Factor in Bronchoalveolar Lavage Fluid in Acute Respiratory Distress Syndrome Patients. Crit. Care Med. 2000, 28, 2326–2333. [Google Scholar] [CrossRef]
- Van Zoelen, M.A.D.; Verstege, M.I.; Draing, C.; de Beer, R.; van’t Veer, C.; Florquin, S.; Bresser, P.; van der Zee, J.S.; te Velde, A.A.; von Aulock, S.; et al. Endogenous MCP-1 Promotes Lung Inflammation Induced by LPS and LTA. Mol. Immunol. 2011, 48, 1468–1476. [Google Scholar] [CrossRef]
- Hinkle, C.L.; Mohan, M.J.; Lin, P.; Yeung, N.; Rasmussen, F.; Milla, M.E.; Moss, M.L. Multiple Metalloproteinases Process Protransforming Growth Factor-Alpha (ProTGF-Alpha). Biochemistry 2003, 42, 2127–2136. [Google Scholar] [CrossRef]
- Madtes, D.K.; Busby, H.K.; Strandjord, T.P.; Clark, J.G. Expression of Transforming Growth Factor-Alpha and Epidermal Growth Factor Receptor Is Increased Following Bleomycin-Induced Lung Injury in Rats. Am. J. Respir. Cell Mol. Biol. 1994, 11, 540–551. [Google Scholar] [CrossRef]
- Hardie, W.D.; Prows, D.R.; Piljan-Gentle, A.; Dunlavy, M.R.; Wesselkamper, S.C.; Leikauf, G.D.; Korfhagen, T.R. Dose-Related Protection from Nickel-Induced Lung Injury in Transgenic Mice Expressing Human Transforming Growth Factor-Alpha. Am. J. Respir. Cell Mol. Biol. 2002, 26, 430–437. [Google Scholar] [CrossRef]
- Hardie, W.D.; Prows, D.R.; Leikauf, G.D.; Korfhagen, T.R. Attenuation of Acute Lung Injury in Transgenic Mice Expressing Human Transforming Growth Factor-Alpha. Am. J. Physiol. 1999, 277, L1045–L1050. [Google Scholar] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The Arrive Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.M.; Lessov, N.S.; Dixon, M.P.; Eckenstein, F. Monofilament Intraluminal Middle Cerebral Artery Occlusion in the Mouse. Neurol. Res. 1997, 19, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Van Hoecke, L.; Job, E.R.; Saelens, X.; Roose, K. Bronchoalveolar Lavage of Murine Lungs to Analyze Inflammatory Cell Infiltration. J. Vis. Exp. 2017, 2017, e55398. [Google Scholar] [CrossRef]
- Bederson, J.B.; Pitts, L.H.; Germano, S.M.; Nishimura, M.C.; Davis, R.L.; Bartkowski, H.M. Evaluation of 2,3,5-Triphenyltetrazolium Chloride as a Stain for Detection and Quantification of Experimental Cerebral Infarction in Rats. Stroke 1986, 17, 1304–1308. [Google Scholar] [CrossRef] [Green Version]
- Morancho, A.; García-Bonilla, L.; Barceló, V.; Giralt, D.; Campos-Martorell, M.; Garcia, S.; Montaner, J.; Rosell, A. A New Method for Focal Transient Cerebral Ischaemia by Distal Compression of the Middle Cerebral Artery. Neuropathol. Appl. Neurobiol. 2012, 38, 617–627. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of Protein Using Bicinchoninic Acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Simats, A.; Ramiro, L.; Valls, R.; de Ramón, H.; García-Rodríguez, P.; Orset, C.; Artigas, L.; Sardon, T.; Rosell, A.; Montaner, J. Ceruletide and Alpha-1 Antitrypsin as a Novel Combination Therapy for Ischemic Stroke. Neurother. J. Am. Soc. Exp. Neurother. 2022, 19, 513–527. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]



| BALF | Lung Homogenate | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Protein | logFC | p-Value | FDR | Uniprot Code | Protein | logFC | p-Value | FDR | Uniprot Code |
| Hepatocyte growth factor (HGF) | 0.740 | >0.001 | 0.002 | Q08048 | Protransforming growth factor alpha (TGFA) | 1.296 | >0.001 | >0.001 | P48030 |
| NAD kinase (NADK) | 1.237 | >0.001 | 0.002 | P58058 | C-C motif chemokine 2 (CCL2) | 1.115 | >0.001 | >0.001 | P10148 |
| Protein phosphatase inhibitor 2 (PPP1R2) | 0.873 | >0.001 | 0.005 | Q9DCL8 | N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 (DDAH1) | 0.392 | 0.001 | 0.010 | Q9CWS0 |
| Caspase-3 (CASP3) | 1.209 | >0.001 | 0.005 | P70677 | Poly [ADP-ribose] polymerase 1 (PARP1) | 0.891 | 0.001 | 0.010 | P11103 |
| CNN family member 1 (CYR61) | 0.767 | 0.001 | 0.006 | P18406 | Calsyntenin-2 (CLSTN2) | 0.691 | 0.001 | 0.011 | Q9ER65 |
| Glial cell line-derived neurotrophic factor (GDNF) | 0.609 | 0.001 | 0.006 | P48540 | Integrin beta-1-binding protein 2 (ITGB1BP2) | −0.873 | 0.002 | 0.026 | Q9R000 |
| Tumor necrosis factor receptor superfamily member 6 (FAS) | 0.524 | 0.001 | 0.006 | P25446 | Interleukin-1 alpha (IL1A) | −0.928 | 0.003 | 0.029 | P01582 |
| Integrin beta-6 (ITGB6) | 0.573 | 0.002 | 0.015 | Q9Z0T9 | Peroxiredoxin-5 (PRDX5) | 0.510 | 0.004 | 0.033 | P99029 |
| Peroxiredoxin-5 (PRDX5) | 0.897 | 0.007 | 0.030 | P99029 | Disintegrin and metalloproteinase domain-containing protein 23 (ADAM23) | 0.182 | 0.008 | 0.063 | Q9R1V7 |
| Synaptosomal-associated protein 29 (SNAP29) | 1.007 | 0.007 | 0.030 | Q9ERB0 | Growth-regulated alpha protein (CXCL1) | 1.600 | 0.008 | 0.063 | P12850 |
| Dihydropteridine reductase (QDPR) | 0.634 | 0.007 | 0.030 | Q8BVI4 | Follistatin (FST) | 0.333 | 0.015 | 0.096 | P47931 |
| Legumain (LGMN) | 0.554 | 0.007 | 0.030 | O89017 | Matrilin-2 (MATN2) | −0.441 | 0.017 | 0.096 | O08746 |
| NAD kinase (NADK) | −0.256 | 0.017 | 0.096 | P58058 | |||||
| Transforming growth factor beta-1 proprotein (TGFB1) | 0.618 | 0.018 | 0.096 | P04202 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faura, J.; Ramiro, L.; Simats, A.; Ma, F.; Penalba, A.; Gasull, T.; Rosell, A.; Montaner, J.; Bustamante, A. Evaluation and Characterization of Post-Stroke Lung Damage in a Murine Model of Cerebral Ischemia. Int. J. Mol. Sci. 2022, 23, 8093. https://doi.org/10.3390/ijms23158093
Faura J, Ramiro L, Simats A, Ma F, Penalba A, Gasull T, Rosell A, Montaner J, Bustamante A. Evaluation and Characterization of Post-Stroke Lung Damage in a Murine Model of Cerebral Ischemia. International Journal of Molecular Sciences. 2022; 23(15):8093. https://doi.org/10.3390/ijms23158093
Chicago/Turabian StyleFaura, Júlia, Laura Ramiro, Alba Simats, Feifei Ma, Anna Penalba, Teresa Gasull, Anna Rosell, Joan Montaner, and Alejandro Bustamante. 2022. "Evaluation and Characterization of Post-Stroke Lung Damage in a Murine Model of Cerebral Ischemia" International Journal of Molecular Sciences 23, no. 15: 8093. https://doi.org/10.3390/ijms23158093
APA StyleFaura, J., Ramiro, L., Simats, A., Ma, F., Penalba, A., Gasull, T., Rosell, A., Montaner, J., & Bustamante, A. (2022). Evaluation and Characterization of Post-Stroke Lung Damage in a Murine Model of Cerebral Ischemia. International Journal of Molecular Sciences, 23(15), 8093. https://doi.org/10.3390/ijms23158093






