Targeting VIP and PACAP Receptor Signaling: New Insights into Designing Drugs for the PACAP Subfamily of Receptors
Abstract
1. Introduction—Physiological Roles of the PACAP and VIP Receptors
2. Pharmacology of PACAP and VIP Receptors
2.1. Binding Characteristics of Natural Peptides Differentiating the PACAP Subfamily of Receptors
Selective Peptide Analogues of PAC1R, VPAC1R, and VPAC2R
2.2. Signaling Characteristics of PAC1R, VPAC1R, and VPAC2R
2.2.1. Alternative Splicing
2.2.2. Signaling via Gs Coupling
2.2.3. Signaling via Gq/11 and Gi/o Coupling
2.2.4. Non-G Protein Signaling
ADP-Ribosylation Factor
Endosomal Signaling
2.2.5. Additional Downstream Signal Transduction Pathways
Ion Channels
Transactivation of Epidermal Growth Factor Receptor (EGFR)
MAPK/ERK Signal Transduction
2.2.6. Receptor Activity-Modifying Proteins (RAMPs)
2.3. Receptor Desensitisation and Recycling
2.4. Understanding of PACAP and VIP Signaling and Regulation That Needs to Be Addressed for Disease-Focused Therapies
3. Molecular Activation of PACAP and VIP Receptors
3.1. Structural Characteristics of the PACAP Subfamily of Receptors

3.2. Characteristics of the PACAP-Bound VPAC1R, VPAC2R, and PAC1R Structures
3.3. Characteristics of the Maxadilan-Bound PAC1R Structure
3.4. Activation Characteristics of the PACAP Subfamily of Receptors
3.5. Allosteric Modulation
4. Concluding Remarks
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mutt, V.; Said, S.I. Structure of the porcine vasoactive intestinal octacosapeptide: The amino-acid sequence. Use of kallikrein in its determination. Eur. J. Biochem. 1974, 42, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Brenneman, D.E.; Eiden, L.E. Vasoactive intestinal peptide and electrical activity influence neuronal survival. Proc. Natl. Acad. Sci. USA 1986, 83, 1159–1162. [Google Scholar] [CrossRef] [PubMed]
- Sorg, O.; Magistretti, P.J. Vasoactive intestinal peptide and noradrenaline exert long-term control on glycogen levels in astrocytes: Blockade by protein synthesis inhibition. J. Neurosci. 1992, 12, 4923–4931. [Google Scholar] [CrossRef] [PubMed]
- Said, S.I.; Mutt, V. Isolation from Porcine-Intestinal Wall of a Vasoactive Octacosapeptide Related to Secretin and to Glucagon. Eur. J. Biochem. 1972, 28, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Fahrenkrug, J. Transmitter Role of Vasoactive Intestinal Peptide. Pharmacol. Toxicol. 1993, 72, 354–363. [Google Scholar] [CrossRef]
- Behar, J.; Guenard, V.; Walsh, J.H.; Biancani, P. VIP and acetylcholine: Neurotransmitters in esophageal circular smooth muscle. Am. J. Physiol. 1989, 257, G380–G385. [Google Scholar] [CrossRef]
- Lundberg, J.M.; Anggård, A.; Fahrenkrug, J.; Hökfelt, T.; Mutt, V. Vasoactive intestinal polypeptide in cholinergic neurons of exocrine glands: Functional significance of coexisting transmitters for vasodilation and secretion. Proc. Natl. Acad. Sci. USA 1980, 77, 1651–1655. [Google Scholar] [CrossRef]
- Matsushita, N.; Kato, Y.; Shimatsu, A.; Katakami, H.; Yanaihara, N.; Imura, H. Effects of VIP, TRH, GABA and dopamine on prolactin release from superfused rat anterior pituitary cells. Life Sci. 1983, 32, 1263–1269. [Google Scholar] [CrossRef]
- Malhotra, R.K.; Wakade, A.R. Vasoactive intestinal polypeptide stimulates the secretion of catecholamines from the rat adrenal gland. J. Physiol. 1987, 388, 285–294. [Google Scholar] [CrossRef]
- Ganea, D. Regulatory effects of vasoactive intestinal peptide on cytokine production in central and peripheral lymphoid organs. Adv. Neuroimmunol. 1996, 6, 61–74. [Google Scholar] [CrossRef]
- Coupar, I.M. Stimulation of sodium and water secretion without inhibition of glucose absorption in the rat jejunum by vasoactive intestinal peptide (VIP). Clin. Exp. Pharmacol. Physiol. 1976, 3, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Racusen, L.C.; Binder, H.J. Alteration of large intestinal electrolyte transport by vasoactive intestinal polypeptide in the rat. Gastroenterology 1977, 73, 790–796. [Google Scholar] [CrossRef]
- Steingart, R.A.; Solomon, B.; Brenneman, D.E.; Fridkin, M.; Gozes, I. VIP and peptides related to activity-dependent neurotrophic factor protect PC12 cells against oxidative stress. J. Mol. Neurosci. MN 2000, 15, 137–145. [Google Scholar] [CrossRef]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.C.; Hashimoto, H.; Galas, L.; et al. Pituitary Adenylate Cyclase-Activating Polypeptide and Its Receptors: 20 Years after the Discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef]
- Arimura, A.; Somogyvári-Vigh, A.; Miyata, A.; Mizuno, K.; Coy, D.H.; Kitada, C. Tissue Distribution of PACAP as Determined by RIA: Highly Abundant in the Rat Brain and Testes. Endocrinology 1991, 129, 2787–2789. [Google Scholar] [CrossRef]
- Shioda, S.; Shuto, Y.; Somogyvári-Vigh, A.; Legradi, G.; Onda, H.; Coy, D.H.; Nakajo, S.; Arimura, A. Localization and gene expression of the receptor for pituitary adenylate cyclase-activating polypeptide in the rat brain. Neurosci. Res. 1997, 28, 345–354. [Google Scholar] [CrossRef]
- Miyata, A.; Arimura, A.; Dahl, R.R.; Minamino, N.; Uehara, A.; Jiang, L.; Culler, M.D.; Coy, D.H. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem. Biophys. Res. Commun. 1989, 164, 567–574. [Google Scholar] [CrossRef]
- Miyata, A.; Jiang, L.; Dahl, R.D.; Kitada, C.; Kubo, K.; Fujino, M.; Minamino, N.; Arimura, A. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38). Biochem. Biophys. Res. Commun. 1990, 170, 643–648. [Google Scholar] [CrossRef]
- Telegdy, G.; Kokavszky, K. The action of pituitary adenylate cyclase activating polypeptide (PACAP) on passive avoidance learning. The role of transmitters. Brain Res. 2000, 874, 194–199. [Google Scholar] [CrossRef]
- Cagampang, F.R.; Piggins, H.D.; Sheward, W.J.; Harmar, A.J.; Coen, C.W. Circadian changes in PACAP type 1 (PAC1) receptor mRNA in the rat suprachiasmatic and supraoptic nuclei. Brain Res. 1998, 813, 218–222. [Google Scholar] [CrossRef]
- Ohtaki, H.; Satoh, A.; Nakamachi, T.; Yofu, S.; Dohi, K.; Mori, H.; Ohara, K.; Miyamoto, K.; Hashimoto, H.; Shintani, N.; et al. Regulation of oxidative stress by pituitary adenylate cyclase-activating polypeptide (PACAP) mediated by PACAP receptor. J. Mol. Neurosci. MN 2010, 42, 397–403. [Google Scholar] [CrossRef]
- Uchida, D.; Arimura, A.; Somogyvári-Vigh, A.; Shioda, S.; Banks, W.A. Prevention of ischemia-induced death of hippocampal neurons by pituitary adenylate cyclase activating polypeptide. Brain Res. 1996, 736, 280–286. [Google Scholar] [CrossRef]
- Miyamoto, K.; Tsumuraya, T.; Ohtaki, H.; Dohi, K.; Satoh, K.; Xu, Z.; Tanaka, S.; Murai, N.; Watanabe, J.; Sugiyama, K.; et al. PACAP38 Suppresses Cortical Damage in Mice with Traumatic Brain Injury by Enhancing Antioxidant Activity: MN. J. Mol. Neurosci. 2014, 54, 370–379. [Google Scholar] [CrossRef]
- Whalen, E.J.; Johnson, A.K.; Lewis, S.J. Hemodynamic actions of systemically injected pituitary adenylate cyclase activating polypeptide-27 in the rat. Eur. J. Pharmacol. 1999, 365, 205–215. [Google Scholar] [CrossRef]
- Bruch, L.; Bychkov, R.; Kästner, A.; Bülow, T.; Ried, C.; Gollasch, M.; Baumann, G.; Luft, F.C.; Haller, H. Pituitary adenylate-cyclase-activating peptides relax human coronary arteries by activating K(ATP) and K(Ca) channels in smooth muscle cells. J. Vasc. Res. 1997, 34, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Filipsson, K.; Pacini, G.; Scheurink, A.J.; Ahrén, B. PACAP stimulates insulin secretion but inhibits insulin sensitivity in mice. Am. J. Physiol. 1998, 274, E834–E842. [Google Scholar] [CrossRef]
- Arimura, A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn. J. Physiol. 1998, 48, 301–331. [Google Scholar] [CrossRef]
- Rawlings, S.R.; Hezareh, M. Pituitary adenylate cyclase-activating polypeptide (PACAP) and PACAP/vasoactive intestinal polypeptide receptors: Actions on the anterior pituitary gland. Endocr. Rev. 1996, 17, 4–29. [Google Scholar] [CrossRef][Green Version]
- Patton, A.P.; Edwards, M.D.; Smyllie, N.J.; Hamnett, R.; Chesham, J.E.; Brancaccio, M.; Maywood, E.S.; Hastings, M.H. The VIP-VPAC2 neuropeptidergic axis is a cellular pacemaking hub of the suprachiasmatic nucleus circadian circuit. Nat. Commun. 2020, 11, 3394. [Google Scholar] [CrossRef]
- Shen, S.; Spratt, C.; Sheward, W.J.; Kallo, I.; West, K.; Morrison, C.F.; Coen, C.W.; Marston, H.M.; Harmar, A.J. Overexpression of the human VPAC2 receptor in the suprachiasmatic nucleus alters the circadian phenotype of mice. Proc. Natl. Acad. Sci. USA 2000, 97, 11575–11580. [Google Scholar] [CrossRef]
- Harmar, A.J.; Marston, H.M.; Shen, S.; Spratt, C.; West, K.M.; Sheward, W.J.; Morrison, C.F.; Dorin, J.R.; Piggins, H.D.; Reubi, J.-C.; et al. The VPAC2 Receptor Is Essential for Circadian Function in the Mouse Suprachiasmatic Nuclei. Cell 2002, 109, 497–508. [Google Scholar] [CrossRef]
- Abad, C.; Jayaram, B.; Becquet, L.; Wang, Y.; O’Dorisio, M.S.; Waschek, J.A.; Tan, Y.V. VPAC1 receptor (Vipr1)-deficient mice exhibit ameliorated experimental autoimmune encephalomyelitis, with specific deficits in the effector stage. J. Neuroinflammation 2016, 13, 169. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.; Ganea, D. Vasoactive intestinal peptide: A neuropeptide with pleiotropic immune functions. Amino Acids 2013, 45, 25–39. [Google Scholar] [CrossRef]
- Tsutsumi, M.; Claus, T.H.; Liang, Y.; Li, Y.; Yang, L.; Zhu, J.; Dela Cruz, F.; Peng, X.; Chen, H.; Yung, S.L.; et al. A potent and highly selective VPAC2 agonist enhances glucose-induced insulin release and glucose disposal: A potential therapy for type 2 diabetes. Diabetes 2002, 51, 1453–1460. [Google Scholar] [CrossRef]
- Hirabayashi, T.; Nakamachi, T.; Shioda, S. Discovery of PACAP and its receptors in the brain. J. Headache Pain 2018, 19, 28. [Google Scholar] [CrossRef]
- Usdin, T.B.; Bonner, T.I.; Mezey, E. Two receptors for vasoactive intestinal polypeptide with similar specificity and complementary distributions. Endocrinology 1994, 135, 2662–2680. [Google Scholar] [CrossRef]
- Ishihara, T.; Shigemoto, R.; Mori, K.; Takahashi, K.; Nagata, S. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron 1992, 8, 811–819. [Google Scholar] [CrossRef]
- Stangerup, I.; Hannibal, J. Localization of Vasoactive Intestinal Polypeptide Receptor 1 (VPAC1) in Hypothalamic Neuroendocrine Oxytocin Neurons; A Potential Role in Circadian Prolactin Secretion. Front. Neuroanat. 2020, 14, 579466. [Google Scholar] [CrossRef]
- Nunan, R.; Sivasathiaseelan, H.; Khan, D.; Zaben, M.; Gray, W. Microglial VPAC1R mediates a novel mechanism of neuroimmune-modulation of hippocampal precursor cells via IL-4 release. Glia 2014, 62, 1313–1327. [Google Scholar] [CrossRef]
- Reubi, J.C. In vitro evaluation of VIP/PACAP receptors in healthy and diseased human tissues. Clinical implications. Ann. N. Y. Acad. Sci. 2000, 921, 1–25. [Google Scholar] [CrossRef]
- Yokota, C.; Kawai, K.; Ohashi, S.; Watanabe, Y.; Yamashita, K. PACAP stimulates glucose output from the perfused rat liver. Peptides 1995, 16, 55–60. [Google Scholar] [CrossRef]
- Sekiguchi, Y.; Kasai, K.; Hasegawa, K.; Suzuki, Y.; Shimoda, S.-I. Glycogenolytic activity of Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) in vivo and in vitro. Life Sci. 1994, 55, 1219–1228. [Google Scholar] [CrossRef]
- Ichikawa, S.; Sreedharan, S.P.; Owen, R.L.; Goetzl, E.J. Immunochemical localization of type I VIP receptor and NK-1-type substance P receptor in rat lung. Am. J. Physiol. 1995, 268, L584–L588. [Google Scholar] [CrossRef]
- Saga, T.; Said, S.I. Vasoactive intestinal peptide relaxes isolated strips of human bronchus, pulmonary artery, and lung parenchyma. Trans. Assoc. Am. Physicians 1984, 97, 304–310. [Google Scholar]
- Burian, B.; Storka, A.; Marzluf, B.A.; Yen, Y.-C.; Lambers, C.; Robibaro, B.; Vonbank, K.; Mosgoeller, W.; Petkov, V. Vasoactive intestinal peptide (VIP) receptor expression in monocyte-derived macrophages from COPD patients. Peptides 2010, 31, 603–608. [Google Scholar] [CrossRef]
- Beaubien, B.B.; Tippins, J.R.; Morris, H.R. Platelet-activating factor stimulation of peptidoleukotriene release: Inhibition by vasoactive polypeptide. Biochem. Biophys. Res. Commun. 1984, 125, 105–108. [Google Scholar] [CrossRef]
- Coles, S.J.; Said, S.I.; Reid, L.M. Inhibition by vasoactive intestinal peptide of glycoconjugate and lysozyme secretion by human airways in vitro. Am. Rev. Respir. Dis. 1981, 124, 531–536. [Google Scholar]
- Miotto, D.; Boschetto, P.; Bononi, I.; Zeni, E.; Cavallesco, G.; Fabbri, L.M.; Mapp, C.E. Vasoactive intestinal peptide receptors in the airways of smokers with chronic bronchitis. Eur. Respir. J. 2004, 24, 958–963. [Google Scholar] [CrossRef]
- Jayawardena, D.; Guzman, G.; Gill, R.K.; Alrefai, W.A.; Onyuksel, H.; Dudeja, P.K. Expression and localization of VPAC1, the major receptor of vasoactive intestinal peptide along the length of the intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G16–G25. [Google Scholar] [CrossRef]
- Fung, C.; Unterweger, P.; Parry, L.J.; Bornstein, J.C.; Foong, J.P. VPAC1 receptors regulate intestinal secretion and muscle contractility by activating cholinergic neurons in guinea pig jejunum. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G748–G758. [Google Scholar] [CrossRef]
- Moody, T.W.; Gozes, I. Vasoactive intestinal peptide receptors: A molecular target in breast and lung cancer. Curr. Pharm. Des. 2007, 13, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Zia, H.; Hida, T.; Jakowlew, S.; Birrer, M.; Gozes, Y.; Reubi, J.C.; Fridkin, M.; Gozes, I.; Moody, T.W. Breast cancer growth is inhibited by vasoactive intestinal peptide (VIP) hybrid, a synthetic VIP receptor antagonist. Cancer Res. 1996, 56, 3486–3489. [Google Scholar] [PubMed]
- Delgado, M.; Pozo, D.; Ganea, D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol. Rev. 2004, 56, 249–290. [Google Scholar] [CrossRef]
- Delgado, M.; Martinez, C.; Johnson, M.C.; Gomariz, R.P.; Ganea, D. Differential expression of vasoactive intestinal peptide receptors 1 and 2 (VIP-R1 and VIP-R2) mRNA in murine lymphocytes. J. Neuroimmunol. 1996, 68, 27–38. [Google Scholar] [CrossRef]
- Kaltreider, H.B.; Ichikawa, S.; Byrd, P.K.; Ingram, D.A.; Kishiyama, J.L.; Sreedharan, S.P.; Warnock, M.L.; Beck, J.M.; Goetzl, E.J. Upregulation of neuropeptides and neuropeptide receptors in a murine model of immune inflammation in lung parenchyma. Am. J. Respir. Cell Mol. Biol. 1997, 16, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Lara-Marquez, M.L.; O’Dorisio, M.S.; O’Dorisio, T.M.; Shah, M.H.; Karacay, B. Selective Gene Expression and Activation-Dependent Regulation of Vasoactive Intestinal Peptide Receptor Type 1 and Type 2 in Human T Cells. J. Immunol. 2001, 166, 2522–2530. [Google Scholar] [CrossRef]
- Sheward, W.J.; Lutz, E.M.; Harmar, A.J. The distribution of vasoactive intestinal peptide2 receptor messenger RNA in the rat brain and pituitary gland as assessed by in situ hybridization. Neuroscience 1995, 67, 409–418. [Google Scholar] [CrossRef]
- Lee, S.H.; Cox, C.L. Excitatory actions of vasoactive intestinal peptide on mouse thalamocortical neurons are mediated by VPAC2 receptors. J. Neurophysiol. 2006, 96, 858–871. [Google Scholar] [CrossRef]
- Lutz, E.M.; Sheward, W.J.; West, K.M.; Morrow, J.A.; Fink, G.; Harmar, A.J. The VIP2 receptor: Molecular characterisation of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett. 1993, 334, 3–8. [Google Scholar] [CrossRef]
- Ago, Y.; Hayata, A.; Hashimoto, H. Pathophysiological implication of the VPAC2 receptor in psychiatric disorders. Nihon Yakurigaku Zasshi. Folia Pharmacol. Jpn. 2018, 151, 249–253. [Google Scholar] [CrossRef][Green Version]
- Ago, Y.; Asano, S.; Hashimoto, H.; Waschek, J.A. Probing the VIPR2 Microduplication Linkage to Schizophrenia in Animal and Cellular Models. Front. Neurosci. 2021, 15, 717490. [Google Scholar] [CrossRef] [PubMed]
- Vacic, V.; McCarthy, S.; Malhotra, D.; Murray, F.; Chou, H.H.; Peoples, A.; Makarov, V.; Yoon, S.; Bhandari, A.; Corominas, R.; et al. Duplications of the neuropeptide receptor gene VIPR2 confer significant risk for schizophrenia. Nature 2011, 471, 499–503. [Google Scholar] [CrossRef]
- Rangon, C.M.; Goursaud, S.; Medja, F.; Lelièvre, V.; Mounien, L.; Husson, I.; Brabet, P.; Jégou, S.; Janet, T.; Gressens, P. VPAC2 receptors mediate vasoactive intestinal peptide-induced neuroprotection against neonatal excitotoxic brain lesions in mice. J. Pharmacol. Exp. Ther. 2005, 314, 745–752. [Google Scholar] [CrossRef]
- Ago, Y.; Hayata-Takano, A.; Kawanai, T.; Yamauchi, R.; Takeuchi, S.; Cushman, J.D.; Rajbhandari, A.K.; Fanselow, M.S.; Hashimoto, H.; Waschek, J.A. Impaired extinction of cued fear memory and abnormal dendritic morphology in the prelimbic and infralimbic cortices in VPAC2 receptor (VIPR2)-deficient mice. Neurobiol. Learn. Mem. 2017, 145, 222–231. [Google Scholar] [CrossRef]
- Harmar, A.J.; Sheward, W.J.; Morrison, C.F.; Waser, B.; Gugger, M.; Reubi, J.C. Distribution of the VPAC2 Receptor in Peripheral Tissues of the Mouse. Endocrinology 2004, 145, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Fizanne, L.; Sigaudo-Roussel, D.; Saumet, J.L.; Fromy, B. Evidence for the involvement of VPAC1 and VPAC2 receptors in pressure-induced vasodilatation in rodents. J. Physiol. 2004, 554, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Ottesen, B.; Wagner, G.; Virag, R.; Fahrenkrug, J. Penile erection: Possible role for vasoactive intestinal polypeptide as a neurotransmitter. Br. Med. J. (Clin. Res. Ed.) 1984, 288, 9–11. [Google Scholar] [CrossRef][Green Version]
- Inagaki, N.; Yoshida, H.; Mizuta, M.; Mizuno, N.; Fujii, Y.; Gonoi, T.; Miyazaki, J.; Seino, S. Cloning and functional characterization of a third pituitary adenylate cyclase-activating polypeptide receptor subtype expressed in insulin-secreting cells. Proc. Natl. Acad. Sci. USA 1994, 91, 2679–2683. [Google Scholar] [CrossRef]
- Groneberg, D.A.; Hartmann, P.; Dinh, Q.T.; Fischer, A. Expression and Distribution of Vasoactive Intestinal Polypeptide Receptor VPAC2 mRNA in Human Airways. Lab. Investig. 2001, 81, 749–755. [Google Scholar] [CrossRef]
- Yin, J.; Wang, L.; Yin, N.; Tabuchi, A.; Kuppe, H.; Wolff, G.; Kuebler, W.M. Vasodilatory Effect of the Stable Vasoactive Intestinal Peptide Analog RO 25-1553 in Murine and Rat Lungs. PLoS ONE 2013, 8, e75861. [Google Scholar] [CrossRef]
- St Hilaire, R.C.; Murthy, S.N.; Kadowitz, P.J.; Jeter, J.R., Jr. Role of VPAC1 and VPAC2 in VIP mediated inhibition of rat pulmonary artery and aortic smooth muscle cell proliferation. Peptides 2010, 31, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, E.J.; Voice, J.K.; Shen, S.; Dorsam, G.; Kong, Y.; West, K.M.; Morrison, C.F.; Harmar, A.J. Enhanced delayed-type hypersensitivity and diminished immediate-type hypersensitivity in mice lacking the inducible VPAC2 receptor for vasoactive intestinal peptide. Proc. Natl. Acad. Sci. USA 2001, 98, 13854–13859. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Nogi, H.; Mori, K.; Ohishi, H.; Shigemoto, R.; Yamamoto, K.; Matsuda, T.; Mizuno, N.; Nagata, S.; Baba, A. Distribution of the mRNA for a pituitary adenylate cyclase-activating polypeptide receptor in the rat brain: An in situ hybridization study. J. Comp. Neurol. 1996, 371, 567–577. [Google Scholar] [CrossRef]
- Shioda, S.; Nakai, Y.; Nakajo, S.; Nakaya, K.; Arimura, A. Pituitary Adenylate Cyclase-Activating Polypeptide and Its Type I Receptors in the Rat Hypothalamus: Neuroendocrine Interactionsa. Ann. N. Y. Acad. Sci. 1996, 805, 670–676. [Google Scholar] [CrossRef]
- Missig, G.; Mei, L.; Vizzard, M.A.; Braas, K.M.; Waschek, J.A.; Ressler, K.J.; Hammack, S.E.; May, V. Parabrachial Pituitary Adenylate Cyclase-Activating Polypeptide Activation of Amygdala Endosomal Extracellular Signal–Regulated Kinase Signaling Regulates the Emotional Component of Pain. Biol. Psychiatry 2017, 81, 671–682. [Google Scholar] [CrossRef]
- Hashimoto, H.; Kunugi, A.; Arakawa, N.; Shintani, N.; Fujita, T.; Kasai, A.; Kawaguchi, C.; Morita, Y.; Hirose, M.; Sakai, Y.; et al. Possible involvement of a cyclic AMP-dependent mechanism in PACAP-induced proliferation and ERK activation in astrocytes. Biochem. Biophys. Res. Commun. 2003, 311, 337–343. [Google Scholar] [CrossRef]
- Vu, J.P.; Goyal, D.; Luong, L.; Oh, S.; Sandhu, R.; Norris, J.; Parsons, W.; Pisegna, J.R.; Germano, P.M. PACAP intraperitoneal treatment suppresses appetite and food intake via PAC1 receptor in mice by inhibiting ghrelin and increasing GLP-1 and leptin. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G816–G825. [Google Scholar] [CrossRef]
- Morley, J.E.; Horowitz, M.; Morley, P.M.; Flood, J.F. Pituitary adenylate cyclase activating polypeptide (PACAP) reduces food intake in mice. Peptides 1992, 13, 1133–1135. [Google Scholar] [CrossRef]
- Otto, C.; Martin, M.; Wolfer, D.P.; Lipp, H.P.; Maldonado, R.; Schütz, G. Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Brain Res. Mol. Brain Res. 2001, 92, 78–84. [Google Scholar] [CrossRef]
- Roman, C.W.; Lezak, K.R.; Hartsock, M.J.; Falls, W.A.; Braas, K.M.; Howard, A.B.; Hammack, S.E.; May, V. PAC1 receptor antagonism in the bed nucleus of the stria terminalis (BNST) attenuates the endocrine and behavioral consequences of chronic stress. Psychoneuroendocrinology 2014, 47, 151–165. [Google Scholar] [CrossRef]
- Stroth, N.; Liu, Y.; Aguilera, G.; Eiden, L.E. Pituitary Adenylate Cyclase-Activating Polypeptide Controls Stimulus-Transcription Coupling in the Hypothalamic-Pituitary-Adrenal Axis to Mediate Sustained Hormone Secretion During Stress. J. Neuroendocrinol. 2011, 23, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Hammack, S.E.; Roman, C.W.; Lezak, K.R.; Kocho-Shellenberg, M.; Grimmig, B.; Falls, W.A.; Braas, K.; May, V. Roles for Pituitary Adenylate Cyclase-Activating Peptide (PACAP) Expression and Signaling in the Bed Nucleus of the Stria Terminalis (BNST) in Mediating the Behavioral Consequences of Chronic Stress. J. Mol. Neurosci. 2010, 42, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Ressler, K.J.; Mercer, K.B.; Bradley, B.; Jovanovic, T.; Mahan, A.; Kerley, K.; Norrholm, S.D.; Kilaru, V.; Smith, A.K.; Myers, A.J.; et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 2011, 470, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Toth, D.; Tamas, A.; Reglodi, D. The Neuroprotective and Biomarker Potential of PACAP in Human Traumatic Brain Injury. Int. J. Mol. Sci. 2020, 21, 827. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Beltrán, E.; Correnti, E.; Deen, M.; Kamm, K.; Kelderman, T.; Papetti, L.; Vigneri, S.; MaassenVanDenBrink, A.; Edvinsson, L.; On behalf of the European Headache Federation School of Advanced Studies (EHF-SAS). PACAP38 and PAC1 receptor blockade: A new target for headache? J. Headache Pain 2018, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Schaler, A.W.; Runyan, A.M.; Clelland, C.L.; Sydney, E.J.; Fowler, S.L.; Figueroa, H.Y.; Shioda, S.; Santa-Maria, I.; Duff, K.E.; Myeku, N. PAC1 receptor-mediated clearance of tau in postsynaptic compartments attenuates tau pathology in mouse brain. Sci. Transl. Med. 2021, 13, 595. [Google Scholar] [CrossRef]
- Han, P.; Caselli, R.J.; Baxter, L.; Serrano, G.; Yin, J.; Beach, T.G.; Reiman, E.M.; Shi, J. Association of pituitary adenylate cyclase-activating polypeptide with cognitive decline in mild cognitive impairment due to Alzheimer disease. JAMA Neurol. 2015, 72, 333–339. [Google Scholar] [CrossRef]
- Basille, M.; Cartier, D.; Vaudry, D.; Lihrmann, I.; Fournier, A.; Freger, P.; Gallo-Payet, N.; Vaudry, H.; Gonzalez, B. Localization and characterization of pituitary adenylate cyclase-activating polypeptide receptors in the human cerebellum during development. J. Comp. Neurol. 2006, 496, 468–478. [Google Scholar] [CrossRef]
- Basille, M.; Vaudry, D.; Coulouarn, Y.; Jegou, S.; Lihrmann, I.; Fournier, A.; Vaudry, H.; Gonzalez, B. Comparative distribution of pituitary adenylate cyclase-activating polypeptide (PACAP) binding sites and PACAP receptor mRNAs in the rat brain during development. J. Comp. Neurol. 2000, 425, 495–509. [Google Scholar] [CrossRef]
- Nicot, A.; DiCicco-Bloom, E. Regulation of Neuroblast Mitosis is Determined by PACAP Receptor Isoform Expression. Proc. Natl. Acad. Sci. USA 2001, 98, 4758–4763. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, X.; Pan, Z.; Ma, J.; Waschek, J.A.; DiCicco-Bloom, E. Pro- and anti-mitogenic actions of pituitary adenylate cyclase-activating polypeptide in developing cerebral cortex: Potential mediation by developmental switch of PAC1 receptor mRNA isoforms. J. Neurosci. 2013, 33, 3865–3878. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Hashimoto, H.; Iga, J.; Shintani, N.; Nakanishi, M.; Arakawa, N.; Shimada, T.; Baba, A. Inhibition of self-renewal and induction of neural differentiation by PACAP in neural progenitor cells. Ann. N. Y. Acad. Sci 2006, 1070, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D’Amico, A.G.; Castrogiovanni, P.; Saccone, S.; Federico, C.; Reibaldi, M.; Russo, A.; Bonfiglio, V.; Avitabile, T.; Longo, A.; et al. PACAP through EGFR transactivation preserves human corneal endothelial integrity. J. Cell. Biochem. 2019, 120, 10097–10105. [Google Scholar] [CrossRef] [PubMed]
- Nakamachi, T.; Matkovits, A.; Seki, T.; Shioda, S. Distribution and protective function of pituitary adenylate cyclase-activating polypeptide in the retina. Front. Endocrinol. 2012, 3, 145. [Google Scholar] [CrossRef] [PubMed]
- Nakamachi, T.; Ohtaki, H.; Seki, T.; Yofu, S.; Kagami, N.; Hashimoto, H.; Shintani, N.; Baba, A.; Mark, L.; Lanekoff, I.; et al. PACAP suppresses dry eye signs by stimulating tear secretion. Nat. Commun. 2016, 7, 12034. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Bucolo, C.; D’Agata, V. Protective effect of PACAP-38 on retinal pigmented epithelium in an in vitro and in vivo model of diabetic retinopathy through EGFR-dependent mechanism. Peptides 2019, 119, 170108. [Google Scholar] [CrossRef]
- Xu, Z.; Ohtaki, H.; Watanabe, J.; Miyamoto, K.; Murai, N.; Sasaki, S.; Matsumoto, M.; Hashimoto, H.; Hiraizumi, Y.; Numazawa, S.; et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) contributes to the proliferation of hematopoietic progenitor cells in murine bone marrow via PACAP-specific receptor. Sci. Rep. 2016, 6, 22373. [Google Scholar] [CrossRef]
- Lamouche, S.; Yamaguchi, N. Role of PAC(1) receptor in adrenal catecholamine secretion induced by PACAP and VIP in vivo. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R510–R518. [Google Scholar] [CrossRef]
- Jamen, F.; Persson, K.; Bertrand, G.; Rodriguez-Henche, N.; Puech, R.; Bockaert, J.; Ahrén, B.; Brabet, P. PAC1 receptor–deficient mice display impaired insulinotropic response to glucose and reduced glucose tolerance. J. Clin. Investig. 2000, 105, 1307–1315. [Google Scholar] [CrossRef]
- Merriam, L.A.; Barstow, K.L.; Parsons, R.L. Pituitary adenylate cyclase-activating polypeptide enhances the hyperpolarization-activated nonselective cationic conductance, Ih, in dissociated guinea pig intracardiac neurons. Regul. Pept. 2004, 123, 123–133. [Google Scholar] [CrossRef]
- Ojala, J.; Tooke, K.; Hsiang, H.; Girard, B.M.; May, V.; Vizzard, M.A. PACAP/PAC1 Expression and Function in Micturition Pathways. J. Mol. Neurosci. 2019, 68, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Akerman, S.; Goadsby, P.J. Neuronal PAC1 receptors mediate delayed activation and sensitization of trigeminocervical neurons: Relevance to migraine. Sci. Transl. Med. 2015, 7, 308ra157. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L.; Tajti, J.; Szalárdy, L.; Vécsei, L. PACAP and its role in primary headaches. J. Headache Pain 2018, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Hensley, K.; Pretorius, J.; Chan, B.; Page, K.; Liu, H.; Choi, C.; Shi, D.; Xu, C.; Edvinsson, L.; Miller, S. PAC1 receptor mRNA and protein distribution in rat and human trigeminal and sphenopalatine ganglia, spinal trigeminal nucleus and in dura mater. Cephalalgia Int. J. Headache 2019, 39, 827–840. [Google Scholar] [CrossRef]
- Ross, R.A.; Hoeppner, S.S.; Hellberg, S.N.; O’Day, E.B.; Rosencrans, P.L.; Ressler, K.J.; May, V.; Simon, N.M. Circulating PACAP peptide and PAC1R genotype as possible transdiagnostic biomarkers for anxiety disorders in women: A preliminary study. Neuropsychopharmacology 2020, 45, 1125–1133. [Google Scholar] [CrossRef]
- Stroth, N.; Holighaus, Y.; Ait-Ali, D.; Eiden, L.E. PACAP: A master regulator of neuroendocrine stress circuits and the cellular stress response. Ann. N. Y. Acad. Sci. 2011, 1220, 49–59. [Google Scholar] [CrossRef]
- Agarwal, A.; Halvorson, L.M.; Legradi, G. Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: Evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene. Brain Res. Mol. Brain Res. 2005, 138, 45–57. [Google Scholar] [CrossRef]
- Norrholm, S.D.; Das, M.; Légrádi, G. Behavioral effects of local microinfusion of pituitary adenylate cyclase activating polypeptide (PACAP) into the paraventricular nucleus of the hypothalamus (PVN). Regul. Pept. 2005, 128, 33–41. [Google Scholar] [CrossRef]
- Hashimoto, H.; Shintani, N.; Tanaka, K.; Mori, W.; Hirose, M.; Matsuda, T.; Sakaue, M.; Miyazaki, J.; Niwa, H.; Tashiro, F.; et al. Altered psychomotor behaviors in mice lacking pituitary adenylate cyclase-activating polypeptide (PACAP). Proc. Natl. Acad. Sci. USA 2001, 98, 13355–13360. [Google Scholar] [CrossRef]
- Voice, J.K.; Dorsam, G.; Lee, H.; Kong, Y.; Goetzl, E.J. Allergic diathesis in transgenic mice with constitutive T cell expression of inducible vasoactive intestinal peptide receptor. FASEB J. 2001, 15, 2489–2496. [Google Scholar] [CrossRef]
- Villanueva-Romero, R.; Gutiérrez-Cañas, I.; Carrión, M.; Pérez-García, S.; Seoane, I.V.; Martínez, C.; Gomariz, R.P.; Juarranz, Y. The Anti-Inflammatory Mediator, Vasoactive Intestinal Peptide, Modulates the Differentiation and Function of Th Subsets in Rheumatoid Arthritis. J. Immunol. Res. 2018, 2018, 6043710. [Google Scholar] [CrossRef] [PubMed]
- Martínez, C.; Juarranz, Y.; Gutiérrez-Cañas, I.; Carrión, M.; Pérez-García, S.; Villanueva-Romero, R.; Castro, D.; Lamana, A.; Mellado, M.; González-Álvaro, I.; et al. A Clinical Approach for the Use of VIP Axis in Inflammatory and Autoimmune Diseases. Int. J. Mol. Sci. 2020, 21, 65. [Google Scholar] [CrossRef] [PubMed]
- Abad, C.; Martinez, C.; Juarranz, M.G.; Arranz, A.; Leceta, J.; Delgado, M.; Gomariz, R.P. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn’s disease. Gastroenterology 2003, 124, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Lodde, B.M.; Mineshiba, F.; Wang, J.; Cotrim, A.P.; Afione, S.; Tak, P.P.; Baum, B.J. Effect of human vasoactive intestinal peptide gene transfer in a murine model of Sjögren’s syndrome. Ann. Rheum. Dis. 2006, 65, 195–200. [Google Scholar] [CrossRef]
- Li, H.; Mei, Y.; Wang, Y.; Xu, L. Vasoactive intestinal polypeptide suppressed experimental autoimmune encephalomyelitis by inhibiting T helper 1 responses. J. Clin. Immunol. 2006, 26, 430–437. [Google Scholar] [CrossRef]
- Delgado, M.; Abad, C.; Martinez, C.; Leceta, J.; Gomariz, R.P. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat. Med. 2001, 7, 563–568. [Google Scholar] [CrossRef]
- Suda, K.; Smith, D.M.; Ghatei, M.A.; Bloom, S.R. Investigation of the interaction of VIP binding sites with VIP and PACAP in human brain. Neurosci. Lett. 1992, 137, 19–23. [Google Scholar] [CrossRef]
- Lam, H.-C.; Takahashi, K.; Ghatei, M.A.; Kanse, S.M.; Polak, J.M.; Bloom, S.R. Binding sites of a novel neuropeptide pituitary-adenylate-cyclase-activating polypeptide in the rat brain and lung. Eur. J. Biochem. 1990, 193, 725–729. [Google Scholar] [CrossRef]
- Gottschall, P.E.; Tatsuno, I.; Miyata, A.; Arimura, A. Characterization and Distribution of Binding Sites for the Hypothalamic Peptide, Pituitary Adenylate Cyclase-Activating Polypeptide. Endocrinology 1990, 127, 272–277. [Google Scholar] [CrossRef]
- Cauvin, A.; Buscail, L.; Gourlet, P.; De Neef, P.; Gossen, D.; Arimura, A.; Miyata, A.; Coy, D.H.; Robberecht, P.; Christophe, J. The novel VIP-like hypothalamic polypeptide PACAP interacts with high affinity receptors in the human neuroblastoma cell line NB-OK. Peptides 1990, 11, 773–777. [Google Scholar] [CrossRef]
- Robberecht, P.; Waelbroeck, M.; De Neef, P.; Tastenoy, M.; Gourlet, P.; Cogniaux, J.; Christophe, J. A new type of functional VIP receptor has an affinity for helodermin in human SUP-T1 lymphoblasts. FEBS Lett. 1988, 228, 351–355. [Google Scholar] [CrossRef]
- Robberecht, P.; De Neef, P.; Gourlet, P.; Cauvin, A.; Coy, D.H.; Christophe, J. Pharmacological characterization of the novel helodermin/VIP receptor present in human SUP-T1 lymphoma cell membranes. Regul. Pept. 1989, 26, 117–126. [Google Scholar] [CrossRef]
- Hoshino, M.; Yanaihara, C.; Hong, Y.M.; Kishida, S.; Katsumaru, Y.; Vandermeers, A.; Vandermeers-Piret, M.C.; Robberecht, P.; Christophe, J.; Yanaihara, N. Primary structure of helodermin, a VIP-secretin-like peptide isolated from Gila monster venom. FEBS Lett. 1984, 178, 233–239. [Google Scholar] [CrossRef]
- Raufman, J.P.; Jensen, R.T.; Sutliff, V.E.; Pisano, J.J.; Gardner, J.D. Actions of Gila monster venom on dispersed acini from guinea pig pancreas. Am. J. Physiol.-Gastrointest. Liver Physiol. 1982, 242, G470–G474. [Google Scholar] [CrossRef]
- Robberecht, P.; Waelbroeck, M.; Dehaye, J.-P.; Winand, J.; Vandermeers, A.; Vandermeers-Piret, M.-C.; Christophe, J. Evidence that helodermin, a newly extracted peptide from Gila monster venom, is a member of the secretin/VIP/PHI family of peptides with an original pattern of biological properties. FEBS Lett. 1984, 166, 277–282. [Google Scholar] [CrossRef]
- Amiranoff, B.; Vauclin-Jacques, N.; Boige, N.; Rouyer-Fessard, C.; Laburthe, M. Interaction of Gila monster venom with VIP receptors in intestinal epithelium of human: A comparison with rat. FEBS Lett. 1983, 164, 299–302. [Google Scholar] [CrossRef]
- Itoh, N.; Obata, K.-i.; Yanaihara, N.; Okamoto, H. Human preprovasoactive intestinal polypeptide contains a novel PHI-27-like peptide, PHM-27. Nature 1983, 304, 547–549. [Google Scholar] [CrossRef]
- Tatemoto, K.; Mutt, V. Isolation and characterization of the intestinal peptide porcine PHI (PHI-27), a new member of the glucagon--secretin family. Proc. Natl. Acad. Sci. USA 1981, 78, 6603–6607. [Google Scholar] [CrossRef]
- Bodner, M.; Fridkin, M.; Gozes, I. Coding sequences for vasoactive intestinal peptide and PHM-27 peptide are located on two adjacent exons in the human genome. Proc. Natl. Acad. Sci. USA 1985, 82, 3548–3551. [Google Scholar] [CrossRef]
- Laburthe, M.; Amiranoff, B.; Boige, N.; Rouyer-Fessard, C.; Tatemoto, K.; Moroder, L. Interaction of GRF with VIP receptors and stimulation of adenylate cyclase in rat and human intestinal epithelial membranes: Comparison with PHI and secretin. FEBS Lett. 1983, 159, 89–92. [Google Scholar] [CrossRef]
- Couvineau, A.; Rouyer-Fessard, C.; Maoret, J.J.; Gaudin, P.; Nicole, P.; Laburthe, M. Vasoactive intestinal peptide (VIP)1 receptor. Three nonadjacent amino acids are responsible for species selectivity with respect to recognition of peptide histidine isoleucineamide. J. Biol. Chem. 1996, 271, 12795–12800. [Google Scholar] [CrossRef]
- Moro, O.; Lerner, E.A. Maxadilan, the vasodilator from sand flies, is a specific pituitary adenylate cyclase activating peptide type I receptor agonist. J. Biol. Chem. 1997, 272, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Uchida, D.; Tatsuno, I.; Tanaka, T.; Hirai, A.; Saito, Y.; Moro, O.; Tajima, M. Maxadilan Is a Specific Agonist and Its Deleted Peptide (M65) Is a Specific Antagonist for PACAP Type 1 Receptor. Ann. N. Y. Acad. Sci. 1998, 865, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Watanabe, M.; Yada, T. Cytosolic Ca2+ responses to sub-picomolar and nanomolar PACAP in pancreatic beta-cells are mediated by VPAC2 and PAC1 receptors. Regul. Pept. 2004, 123, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-L.; Belousoff, M.J.; Zhao, P.; Koole, C.; Fletcher, M.M.; Truong, T.T.; Julita, V.; Christopoulos, G.; Xu, H.E.; Zhang, Y.; et al. Toward a Structural Understanding of Class B GPCR Peptide Binding and Activation. Mol. Cell 2020, 77, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Shen, D.-d.; Zhou, X.E.; Bi, P.; Liu, Q.-f.; Tan, Y.-x.; Zhuang, Y.-w.; Zhang, H.-b.; Xu, P.-y.; Huang, S.-J.; et al. Cryo-EM structure of an activated VIP1 receptor-G protein complex revealed by a NanoBiT tethering strategy. Nat. Commun. 2020, 11, 4121. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Feng, W.; Zhou, Q.; Liang, A.; Li, J.; Dai, A.; Zhao, F.; Yan, J.; Chen, C.-W.; Li, H.; et al. A distinctive ligand recognition mechanism by the human vasoactive intestinal polypeptide receptor 2. Nat. Commun. 2022, 13, 2272. [Google Scholar] [CrossRef]
- Wang, J.; Song, X.; Zhang, D.; Chen, X.; Li, X.; Sun, Y.; Li, C.; Song, Y.; Ding, Y.; Ren, R.; et al. Cryo-EM structures of PAC1 receptor reveal ligand binding mechanism. Cell Res. 2020, 30, 436–445. [Google Scholar] [CrossRef]
- Kobayashi, K.; Shihoya, W.; Nishizawa, T.; Kadji, F.M.N.; Aoki, J.; Inoue, A.; Nureki, O. Cryo-EM structure of the human PAC1 receptor coupled to an engineered heterotrimeric G protein. Nat. Struct. Mol. Biol. 2020, 27, 274–280. [Google Scholar] [CrossRef]
- Tams, J.W.; Jørgensen, R.M.; Holm, A.; Fahrenkrug, J. Creation of a Selective Antagonist and Agonist of the Rat VPAC1 Receptor Using a Combinatorial Approach with Vasoactive Intestinal Peptide 6–23 as Template. Mol. Pharmacol. 2000, 58, 1035–1041. [Google Scholar] [CrossRef]
- Gourlet, P.; Vandermeers-Piret, M.C.; Rathé, J.; De Neef, P.; Cnudde, J.; Robberecht, P.; Waelbroeck, M. Vasoactive intestinal peptide modification at position 22 allows discrimination between receptor subtypes. Eur. J. Pharmacol. 1998, 348, 95–99. [Google Scholar] [CrossRef]
- Nicole, P.; Lins, L.; Rouyer-Fessard, C.; Drouot, C.; Fulcrand, P.; Thomas, A.; Couvineau, A.; Martinez, J.; Brasseur, R.; Laburthe, M. Identification of key residues for interaction of vasoactive intestinal peptide with human VPAC1 and VPAC2 receptors and development of a highly selective VPAC1 receptor agonist. Alanine scanning and molecular modeling of the peptide. J. Biol. Chem. 2000, 275, 24003–24012. [Google Scholar] [CrossRef] [PubMed]
- Van Rampelbergh, J.; Juarranz, M.G.; Perret, J.; Bondue, A.; Solano, R.M.; Delporte, C.; De Neef, P.; Robberecht, P.; Waelbroeck, M. Characterization of a novel VPAC(1) selective agonist and identification of the receptor domains implicated in the carboxyl-terminal peptide recognition. Br. J. Pharmacol. 2000, 130, 819–826. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gourlet, P.; Vandermeers, A.; Vertongen, P.; Rathe, J.; De Neef, P.; Cnudde, J.; Waelbroeck, M.; Robberecht, P. Development of high affinity selective VIP1 receptor agonists. Peptides 1997, 18, 1539–1545. [Google Scholar] [CrossRef]
- Gourlet, P.; de Neef, P.; Cnudde, J.; Waelbroeck, M.; Robberecht, P. In Vitro Properties of a High Affinity Selective Antagonist of the VIP1 Receptor. Peptides 1997, 18, 1555–1560. [Google Scholar] [CrossRef]
- Xia, M.; Sreedharan, S.P.; Bolin, D.R.; Gaufo, G.O.; Goetzl, E.J. Novel cyclic peptide agonist of high potency and selectivity for the type II vasoactive intestinal peptide receptor. J. Pharmacol. Exp. Ther. 1997, 281, 629–633. [Google Scholar]
- Moreno, D.; Gourlet, P.; De Neef, P.; Cnudde, J.; Waelbroeck, M.; Robberecht, P. Development of selective agonists and antagonists for the human vasoactive intestinal polypeptide VPAC2 receptor. Peptides 2000, 21, 1543–1549. [Google Scholar] [CrossRef]
- Tatsuno, I.; Uchida, D.; Tanaka, T.; Saeki, N.; Hirai, A.; Saito, Y.; Moro, O.; Tajima, M. Maxadilan specifically interacts with PAC1 receptor, which is a dominant form of PACAP/VIP family receptors in cultured rat cortical neurons. Brain Res. 2001, 889, 138–148. [Google Scholar] [CrossRef]
- Gourlet, P.; Vandermeers, A.; Vandermeers-Piret, M.-C.; Rathé, J.; De Neef, P.; Robberecht, P. C-Terminally shortened pituitary adenylate cyclase-activating peptides (PACAP) discriminate PACAP I, PACAP II-VIP1 and PACAP II-VIP2 recombinant receptors. Regul. Pept. 1996, 62, 125–130. [Google Scholar] [CrossRef]
- Gourlet, P.; Vandermeers, A.; Vandermeers-Piret, M.-C.; Rathé, J.; De Neef, P.; Robberecht, P. Fragments of pituitary adenylate cyclase activating polypeptide discriminate between type I and II recombinant receptors. Eur. J. Pharmacol. 1995, 287, 7–11. [Google Scholar] [CrossRef]
- Robberecht, P.; Gourlet, P.; De Neef, P.; Woussen-Colle, M.-C.; Vandermeers-Piret, M.-C.; Vandermeers, A.; Christophe, J. Structural requirements for the occupancy of pituitary adenylate-cyclase-activating-peptide (PACAP) receptors and adenylate cyclase activation in human neuroblastoma NB-OK-1 cell membranes. Eur. J. Biochem. 1992, 207, 239–246. [Google Scholar] [CrossRef]
- Turner, J.T.; Jones, S.B.; Bylund, D.B. A fragment of vasoactive intestinal peptide, VIP(10-28), is an antagonist of VIP in the colon carcinoma cell line, HT29. Peptides 1986, 7, 849–854. [Google Scholar] [CrossRef]
- Göke, R.; Fehmann, H.C.; Linn, T.; Schmidt, H.; Krause, M.; Eng, J.; Göke, B. Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon-like peptide 1-(7-36)-amide receptor of insulin-secreting beta-cells. J. Biol. Chem. 1993, 268, 19650–19655. [Google Scholar] [CrossRef]
- Pozvek, G.; Hilton, J.M.; Quiza, M.; Houssami, S.; Sexton, P.M. Structure/function relationships of calcitonin analogues as agonists, antagonists, or inverse agonists in a constitutively activated receptor cell system. Mol. Pharmacol. 1997, 51, 658–665. [Google Scholar] [CrossRef]
- Carter, P.H.; Jüppner, H.; Gardella, T.J. Studies of the N-Terminal Region of a Parathyroid Hormone-Related Peptide(1–36) Analog: Receptor Subtype-Selective Agonists, Antagonists, and Photochemical Cross-Linking Agents1. Endocrinology 1999, 140, 4972–4981. [Google Scholar] [CrossRef] [PubMed]
- Ohta, N.; Mochizuki, T.; Hoshino, M.; Jun, L.; Kobayashi, H.; Yanaihara, N. Adrenocorticotropic hormone-releasing activity of urotensin I and its fragments in vitro. J. Pept. Res. Off. J. Am. Pept. Soc. 1997, 50, 178–183. [Google Scholar] [CrossRef]
- Rivier, J.; Rivier, C.; Vale, W. Synthetic competitive antagonists of corticotropin-releasing factor: Effect on ACTH secretion in the rat. Science 1984, 224, 889–891. [Google Scholar] [CrossRef]
- Yang, B.; Gelfanov, V.M.; Perez-Tilve, D.; DuBois, B.; Rohlfs, R.; Levy, J.; Douros, J.D.; Finan, B.; Mayer, J.P.; DiMarchi, R.D. Optimization of Truncated Glucagon Peptides to Achieve Selective, High Potency, Full Antagonists. J. Med. Chem. 2021, 64, 4697–4708. [Google Scholar] [CrossRef]
- Dong, M.; Harikumar, K.G.; Raval, S.R.; Milburn, J.E.; Clark, C.; Alcala-Torano, R.; Mobarec, J.C.; Reynolds, C.A.; Ghirlanda, G.; Christopoulos, A.; et al. Rational development of a high-affinity secretin receptor antagonist. Biochem. Pharm. 2020, 177, 113929. [Google Scholar] [CrossRef]
- O’Donnell, M.; Garippa, R.J.; Rinaldi, N.; Selig, W.M.; Simko, B.; Renzetti, L.; Tannu, S.A.; Wasserman, M.A.; Welton, A.; Bolin, D.R. Ro 25-1553: A novel, long-acting vasoactive intestinal peptide agonist. Part I: In vitro and in vivo bronchodilator studies. J. Pharmacol. Exp. Ther. 1994, 270, 1282–1288. [Google Scholar]
- Yung, S.L.; Dela Cruz, F.; Hamren, S.; Zhu, J.; Tsutsumi, M.; Bloom, J.W.; Caudle, M.; Roczniak, S.; Todd, T.; Lemoine, L.; et al. Generation of highly selective VPAC2 receptor agonists by high throughput mutagenesis of vasoactive intestinal peptide and pituitary adenylate cyclase-activating peptide. J. Biol. Chem. 2003, 278, 10273–10281. [Google Scholar] [CrossRef] [PubMed]
- Qiao, A.; Han, S.; Li, X.; Li, Z.; Zhao, P.; Dai, A.; Chang, R.; Tai, L.; Tan, Q.; Chu, X.; et al. Structural basis of Gs and Gi recognition by the human glucagon receptor. Science 2020, 367, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Mayo, K.E.; Miller, L.J.; Bataille, D.; Dalle, S.; Göke, B.; Thorens, B.; Drucker, D.J. International Union of Pharmacology. XXXV. The Glucagon Receptor Family. Pharmacol. Rev. 2003, 55, 167. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.H.; Gromada, J.; Bouchelouche, P.; Whitmore, T.; Jelinek, L.; Kindsvogel, W.; Nishimura, E. Glucagon-mediated Ca2+ signaling in BHK cells expressing cloned human glucagon receptors. Am. J. Physiol. 1998, 274, C1552–C1562. [Google Scholar] [CrossRef]
- Bringhurst, F.R.; Juppner, H.; Guo, J.; Urena, P.; Potts, J.T., Jr.; Kronenberg, H.M.; Abou-Samra, A.B.; Segre, G.V. Cloned, stably expressed parathyroid hormone (PTH)/PTH-related peptide receptors activate multiple messenger signals and biological responses in LLC-PK1 kidney cells. Endocrinology 1993, 132, 2090–2098. [Google Scholar] [CrossRef]
- Wootten, D.; Christopoulos, A.; Marti-Solano, M.; Babu, M.M.; Sexton, P.M. Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat. Reviews. Mol. Cell Biol. 2018, 19, 638–653. [Google Scholar] [CrossRef]
- Yuliantie, E.; van der Velden, W.J.C.; Labroska, V.; Dai, A.; Zhao, F.; Darbalaei, S.; Deganutti, G.; Xu, T.; Zhou, Q.; Yang, D.; et al. Insights into agonist-elicited activation of the human glucose-dependent insulinotropic polypeptide receptor. Biochem. Pharmacol. 2021, 192, 114715. [Google Scholar] [CrossRef]
- Christopoulos, A.; Christopoulos, G.; Morfis, M.; Udawela, M.; Laburthe, M.; Couvineau, A.; Kuwasako, K.; Tilakaratne, N.; Sexton, P.M. Novel Receptor Partners and Function of Receptor Activity-modifying Proteins. J. Biol. Chem. 2003, 278, 3293–3297. [Google Scholar] [CrossRef]
- Christopoulos, G.; Perry, K.J.; Morfis, M.; Tilakaratne, N.; Gao, Y.; Fraser, N.J.; Main, M.J.; Foord, S.M.; Sexton, P.M. Multiple Amylin Receptors Arise from Receptor Activity-Modifying Protein Interaction with the Calcitonin Receptor Gene Product. Mol. Pharmacol. 1999, 56, 235–242. [Google Scholar] [CrossRef]
- Hay, D.; Christopoulos, G.; Christopoulos, A.; Sexton, P. Amylin receptors: Molecular composition and pharmacology. Biochem. Soc. Trans. 2004, 32, 865–867. [Google Scholar] [CrossRef]
- McLatchie, L.M.; Fraser, N.J.; Main, M.J.; Wise, A.; Brown, J.; Thompson, N.; Solari, R.; Lee, M.G.; Foord, S.M. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 1998, 393, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Harikumar, K.G.; Simms, J.; Christopoulos, G.; Sexton, P.M.; Miller, L.J. Molecular Basis of Association of Receptor Activity-Modifying Protein 3 with the Family B G Protein-Coupled Secretin Receptor. Biochemistry 2009, 48, 11773–11785. [Google Scholar] [CrossRef] [PubMed]
- Furness, S.G.; Wootten, D.; Christopoulos, A.; Sexton, P.M. Consequences of splice variation on Secretin family G protein-coupled receptor function. Br. J. Pharmacol. 2012, 166, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Pan, Z.; Zhang, Z.; Lin, L.; Xing, Y. The Expanding Landscape of Alternative Splicing Variation in Human Populations. Am. J. Hum. Genet. 2018, 102, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Dautzenberg, F.M.; Mevenkamp, G.; Wille, S.; Hauger, R.L. N-terminal splice variants of the type I PACAP receptor: Isolation, characterization and ligand binding/selectivity determinants. J. Neuroendocrinol. 1999, 11, 941–949. [Google Scholar] [CrossRef]
- Lutz, E.M.; Ronaldson, E.; Shaw, P.; Johnson, M.S.; Holland, P.J.; Mitchell, R. Characterization of novel splice variants of the PAC1 receptor in human neuroblastoma cells: Consequences for signaling by VIP and PACAP. Mol. Cell. Neurosci. 2006, 31, 193–209. [Google Scholar] [CrossRef]
- Journot, L.; Spengler, D.; Pantaloni, C.; Dumuis, A.; Sebben, M.; Bockaert, J. The PACAP receptor: Generation by alternative splicing of functional diversity among G protein-coupled receptors in nerve cells. Semin. Cell Biol. 1994, 5, 263–272. [Google Scholar] [CrossRef]
- Spengler, D.; Waeber, C.; Pantaloni, C.; Holsboer, F.; Bockaert, J.; Seeburgt, P.H.; Journot, L. Differential signal transduction by five splice variants of the PACAP receptor. Nature 1993, 365, 170–175. [Google Scholar] [CrossRef]
- Pantaloni, C.; Brabet, P.; Bilanges, B.; Dumuis, A.; Houssami, S.; Spengler, D.; Bockaert, J.; Journot, L. Alternative Splicing in the N-terminal Extracellular Domain of the Pituitary Adenylate Cyclase-activating Polypeptide (PACAP) Receptor Modulates Receptor Selectivity and Relative Potencies of PACAP-27 and PACAP-38 in Phospholipase C Activation. J. Biol. Chem. 1996, 271, 22146–22151. [Google Scholar] [CrossRef]
- Blechman, J.; Levkowitz, G. Alternative Splicing of the Pituitary Adenylate Cyclase-Activating Polypeptide Receptor PAC1: Mechanisms of Fine Tuning of Brain Activity. Front. Endocrinol. 2013, 4, 55. [Google Scholar] [CrossRef]
- Pisegna, J.R.; Moody, T.W.; Wank, S.A. Differential signaling and immediate-early gene activation by four splice variants of the human pituitary adenylate cyclase-activating polypeptide receptor (hPACAP-R). Ann. N. Y. Acad. Sci. 1996, 805, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Tasma, Z.; Siow, A.; Harris, P.W.R.; Brimble, M.A.; Hay, D.L.; Walker, C.S. Characterisation of agonist signalling profiles and agonist-dependent antagonism at PACAP-responsive receptors: Implications for drug discovery. Br. J. Pharmacol. 2022, 179, 435–453. [Google Scholar] [CrossRef]
- Ushiyama, M.; Ikeda, R.; Yoshida, M.; Mori, K.; Kangawa, K.; Sugawara, H.; Inoue, K.; Yamada, K.; Miyata, A. Alternative Splicing of the Pituitary Adenylate Cyclase-activating Polypetide (PACAP) Receptor Contributes to Function of PACAP-27. J. Mol. Neurosci. 2010, 42, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, T.; Grimaldi, M.; Eiden, L.E. The hop cassette of the PAC1 receptor confers coupling to Ca2+ elevation required for pituitary adenylate cyclase-activating polypeptide-evoked neurosecretion. J. Biol. Chem. 2007, 282, 8079–8091. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Remington, J.M.; Liao, C.; Parsons, R.L.; Schneebeli, S.; Braas, K.M.; May, V.; Brewer, M. GPCR Intracellular Loop Regulation of Beta-Arrestin-Mediated Endosomal Signaling Dynamics. J. Mol. Neurosci. 2022, 72, 1358–1373. [Google Scholar] [CrossRef]
- Pisegna, J.R.; Wank, S.A. Cloning and characterization of the signal transduction of four splice variants of the human pituitary adenylate cyclase activating polypeptide receptor. Evidence for dual coupling to adenylate cyclase and phospholipase C. J. Biol. Chem. 1996, 271, 17267–17274. [Google Scholar] [CrossRef] [PubMed]
- Shneider, Y.; Shtrauss, Y.; Yadid, G.; Pinhasov, A. Differential expression of PACAP receptors in postnatal rat brain. Neuropeptides 2010, 44, 509–514. [Google Scholar] [CrossRef]
- Lakk, M.; Szabó, B.; Völgyi, B.; Gábriel, R.; Dénes, V. Development-Related Splicing Regulates Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Receptors in the Retina. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7825–7832. [Google Scholar] [CrossRef]
- Amir-Zilberstein, L.; Blechman, J.; Sztainberg, Y.; Norton, W.H.; Reuveny, A.; Borodovsky, N.; Tahor, M.; Bonkowsky, J.L.; Bally-Cuif, L.; Chen, A.; et al. Homeodomain protein otp and activity-dependent splicing modulate neuronal adaptation to stress. Neuron 2012, 73, 279–291. [Google Scholar] [CrossRef]
- Biran, J.; Gliksberg, M.; Shirat, I.; Swaminathan, A.; Levitas-Djerbi, T.; Appelbaum, L.; Levkowitz, G. Splice-specific deficiency of the PTSD-associated gene PAC1 leads to a paradoxical age-dependent stress behavior. Sci. Rep. 2020, 10, 9559. [Google Scholar] [CrossRef]
- Scaldaferri, M.L.; Modesti, A.; Palumbo, C.; Ulisse, S.; Fabbri, A.; Piccione, E.; Frajese, G.; Moretti, C. Pituitary adenylate cyclase-activating polypeptide (PACAP) and PACAP-receptor type 1 expression in rat and human placenta. Endocrinol. 2000, 141, 1158–1167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sreedharan, S.P.; Patel, D.R.; Xia, M.H.; Ichikawa, S.; Goetzl, E.J. Human Vasoactive Intestinal Peptide1 Receptors Expressed by Stable Transfectants Couple to 2 Distinct Signaling Pathways. Biochem. Biophys. Res. Commun. 1994, 203, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Pugh, P.C.; Margiotta, J.F. PACAP support of neuronal survival requires MAPK- and activity-generated signals. Mol. Cell. Neurosci. 2006, 31, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.A.; Vaudry, D.; Basille, M.; Castel, H.; Fournier, A.; Vaudry, H.; Gonzalez, B.J. PACAP inhibits delayed rectifier potassium current via a cAMP/PKA transduction pathway: Evidence for the involvement of IK in the anti-apoptotic action of PACAP. Eur. J. Neurosci. 2004, 19, 1446–1458. [Google Scholar] [CrossRef]
- Falluel-Morel, A.; Vaudry, D.; Aubert, N.; Galas, L.; Benard, M.; Basille, M.; Fontaine, M.; Fournier, A.; Vaudry, H.; Gonzalez, B.J. PACAP and ceramides exert opposite effects on migration, neurite outgrowth, and cytoskeleton remodeling. Ann. N. Y. Acad. Sci. 2006, 1070, 265–270. [Google Scholar] [CrossRef]
- Yaka, R.; He, D.-Y.; Phamluong, K.; Ron, D. Pituitary Adenylate Cyclase-activating Polypeptide (PACAP(1–38)) Enhances N-Methyl-d-aspartate Receptor Function and Brain-derived Neurotrophic Factor Expression via RACK1. J. Biol. Chem. 2003, 278, 9630–9638. [Google Scholar] [CrossRef]
- Fila, T.; Trazzi, S.; Crochemore, C.; Bartesaghi, R.; Ciani, E. Lot1 is a key element of the pituitary adenylate cyclase-activating polypeptide (PACAP)/cyclic AMP pathway that negatively regulates neuronal precursor proliferation. J. Biol. Chem 2009, 284, 15325–15338. [Google Scholar] [CrossRef]
- Ster, J.; de Bock, F.; Guérineau, N.C.; Janossy, A.; Barrère-Lemaire, S.; Bos, J.L.; Bockaert, J.; Fagni, L. Exchange Protein Activated by cAMP (Epac) Mediates cAMP Activation of p38 MAPK and Modulation of Calcium-Dependent Potassium Channels in Cerebellar Neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 2519–2524. [Google Scholar] [CrossRef]
- Shi, G.-X.; Rehmann, H.; Andres, D.A. A Novel Cyclic AMP-Dependent Epac-Rit Signaling Pathway Contributes to PACAP38-Mediated Neuronal Differentiation. Mol. Cell. Biol. 2006, 26, 9136–9147. [Google Scholar] [CrossRef]
- Emery, A.C.; Eiden, L.E. Signaling through the neuropeptide GPCR PAC1 induces neuritogenesis via a single linear cAMP- and ERK-dependent pathway using a novel cAMP sensor. FASEB J. 2012, 26, 3199–3211. [Google Scholar] [CrossRef]
- Ravni, A.; Vaudry, D.; Gerdin, M.J.; Eiden, M.V.; Falluel-Morel, A.; Gonzalez, B.J.; Vaudry, H.; Eiden, L.E. A cAMP-dependent, protein kinase A-independent signaling pathway mediating neuritogenesis through Egr1 in PC12 cells. Mol. Pharmacol. 2008, 73, 1688–1708. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Zak, D.E.; Sauter, T.; Schwaber, J.; Ogunnaike, B.A. Modeling the VPAC2-Activated cAMP/PKA Signaling Pathway: From Receptor to Circadian Clock Gene Induction. Biophys. J. 2006, 90, 1560–1571. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Reis, D.; Ribeiro, J.A.; Sebastião, A.M. VIP enhances synaptic transmission to hippocampal CA1 pyramidal cells through activation of both VPAC1 and VPAC2 receptors. Brain Res. 2005, 1049, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Le Péchon-Vallée, C.; Magalon, K.; Rasolonjanahary, R.; Enjalbert, A.; Gérard, C. Vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptides stimulate mitogen-activated protein kinase in the pituitary cell line GH4C1 by a 3’,5’-cyclic adenosine monophosphate pathway. Neuroendocrinology 2000, 72, 46–56. [Google Scholar] [CrossRef]
- Delgado, M.; Munoz-Elias, E.J.; Kan, Y.; Gozes, I.; Fridkin, M.; Brenneman, D.E.; Gomariz, R.P.; Ganea, D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit tumor necrosis factor alpha transcriptional activation by regulating nuclear factor-kB and cAMP response element-binding protein/c-Jun. J. Biol. Chem. 1998, 273, 31427–31436. [Google Scholar] [CrossRef]
- El Zein, N.; Badran, B.; Sariban, E. VIP differentially activates β2 integrins, CR1, and matrix metalloproteinase-9 in human monocytes through cAMP/PKA, EPAC, and PI-3K signaling pathways via VIP receptor type 1 and FPRL1. J. Leukoc. Biol. 2008, 83, 972–981. [Google Scholar] [CrossRef]
- MacKenzie, C.J.; Lutz, E.M.; McCulloch, D.A.; Mitchell, R.; Harmar, A.J. Phospholipase C activation by VIP1 and VIP2 receptors expressed in COS 7 cells involves a pertussis toxin-sensitive mechanism. Ann. N. Y. Acad. Sci. 1996, 805, 579–584. [Google Scholar] [CrossRef]
- Holighaus, Y.; Mustafa, T.; Eiden, L.E. PAC1hop, null and hip receptors mediate differential signaling through cyclic AMP and calcium leading to splice variant-specific gene induction in neural cells. Peptides 2011, 32, 1647–1655. [Google Scholar] [CrossRef]
- Macdonald, D.S.; Weerapura, M.; Beazely, M.A.; Martin, L.; Czerwinski, W.; Roder, J.C.; Orser, B.A.; MacDonald, J.F. Modulation of NMDA receptors by pituitary adenylate cyclase activating peptide in CA1 neurons requires G alpha q, protein kinase C, and activation of Src. J. Neurosci. 2005, 25, 11374–11384. [Google Scholar] [CrossRef]
- May, V.; Buttolph, T.R.; Girard, B.M.; Clason, T.A.; Parsons, R.L. PACAP-induced ERK activation in HEK cells expressing PAC1 receptors involves both receptor internalization and PKC signaling. Am. J. Physiol. Cell Physiol. 2014, 306, C1068–C1079. [Google Scholar] [CrossRef]
- Bouschet, T.; Perez, V.; Fernandez, C.; Bockaert, J.; Eychene, A.; Journot, L. Stimulation of the ERK pathway by GTP-loaded Rap1 requires the concomitant activation of Ras, protein kinase C, and protein kinase A in neuronal cells. J. Biol. Chem. 2003, 278, 4778–4785. [Google Scholar] [CrossRef] [PubMed]
- Dejda, A.; Jozwiak-Bebenista, M.; Nowak, J.Z. PACAP, VIP, and PHI: Effects on AC-, PLC-, and PLD-driven signaling systems in the primary glial cell cultures. Ann. N. Y. Acad. Sci. 2006, 1070, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Van Rampelbergh, J.; Poloczek, P.; Françoys, I.; Christine, D.; Winand, J.; Robberecht, P.; Waelbroeck, M. The pituitary adenylate cyclase activating polypeptide (PACAP I) and VIP (PACAP II VIP1) receptors stimulate inositol phosphate synthesis in transfected CHO cells through interaction with different G proteins. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 1997, 1357, 249–255. [Google Scholar] [CrossRef][Green Version]
- Shreeve, S.M.; Sreedharan, S.P.; Hacker, M.P.; Gannon, D.E.; Morgan, M.J. VIP Activates Gs and Gi3 in Rat Alveolar Macrophages and Gs in HEK293 Cells Transfected with the Human VPAC1 Receptor. Biochem. Biophys. Res. Commun. 2000, 272, 922–928. [Google Scholar] [CrossRef]
- Murthy, K.S.; Makhlouf, G.M. Vasoactive intestinal peptide/pituitary adenylate cyclase-activating peptide-dependent activation of membrane-bound NO synthase in smooth muscle mediated by pertussis toxin-sensitive Gi1-2. J. Biol. Chem. 1994, 269, 15977–15980. [Google Scholar] [CrossRef]
- Luo, X.; Zeng, W.; Xu, X.; Popov, S.; Davignon, I.; Wilkie, T.M.; Mumby, S.M.; Muallem, S. Alternate Coupling of Receptors to Gs and Gi in Pancreatic and Submandibular Gland Cells*. J. Biol. Chem. 1999, 274, 17684–17690. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, D.A.; Lutz, E.M.; Johnson, M.S.; Robertson, D.N.; MacKenzie, C.J.; Holland, P.J.; Mitchell, R. ADP-Ribosylation Factor-Dependent Phospholipase D Activation by VPAC Receptors and a PAC1 Receptor Splice Variant. Mol. Pharmacol. 2001, 59, 1523. [Google Scholar] [CrossRef]
- McCulloch, D.A.; Lutz, E.M.; Johnson, M.S.; MacKenzie, C.J.; Mitchell, R. Differential Activation of Phospholipase D by VPAC and PAC1 Receptors. Ann. N. Y. Acad. Sci. 2000, 921, 175–185. [Google Scholar] [CrossRef]
- Broca, C.; Quoyer, J.; Costes, S.; Linck, N.; Varrault, A.; Deffayet, P.M.; Bockaert, J.; Dalle, S.; Bertrand, G. b-Arrestin 1 is required for PAC1 receptor-mediated potentiation of long-lasting ERK1/2 activation by glucose in pancreatic beta-cells. J. Biol. Chem. 2009, 284, 4332–4342. [Google Scholar] [CrossRef]
- Selvy, P.E.; Lavieri, R.R.; Lindsley, C.W.; Brown, H.A. Phospholipase D: Enzymology, Functionality, and Chemical Modulation. Chem. Rev. 2011, 111, 6064–6119. [Google Scholar] [CrossRef]
- Ronaldson, E.; Robertson, D.N.; Johnson, M.S.; Holland, P.J.; Mitchell, R.; Lutz, E.M. Specific interaction between the hop1 intracellular loop 3 domain of the human PAC1 receptor and ARF. Regul. Pept. 2002, 109, 193–198. [Google Scholar] [CrossRef]
- Khoury, E.; Nikolajev, L.; Simaan, M.; Namkung, Y.; Laporte, S.A. Differential regulation of endosomal GPCR/β-arrestin complexes and trafficking by MAPK. J. Biol. Chem. 2014, 289, 23302–23317. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, J.D.; Hardwick, J.C.; Locknar, S.A.; Merriam, L.A.; Parsons, R.L. Ca2+ Influx, But Not Ca2+ Release From Internal Stores, Is Required for the PACAP-Induced Increase in Excitability in Guinea Pig Intracardiac Neurons. J. Neurophysiol. 2006, 95, 2134–2142. [Google Scholar] [CrossRef] [PubMed]
- May, V.; Parsons, R.L. G Protein-Coupled Receptor Endosomal Signaling and Regulation of Neuronal Excitability and Stress Responses: Signaling Options and Lessons From the PAC1 Receptor. J. Cell. Physiol. 2017, 232, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Clason, T.A.; Girard, B.M.; May, V.; Parsons, R.L. Activation of MEK/ERK Signaling by PACAP in Guinea Pig Cardiac Neurons. J. Mol. Neurosci. MN 2016, 59, 309–316. [Google Scholar] [CrossRef]
- Maunze, B.; Bruckner, K.W.; Desai, N.N.; Chen, C.; Chen, F.; Baker, D.; Choi, S. Pituitary adenylate cyclase-activating polypeptide receptor activation in the hypothalamus recruits unique signaling pathways involved in energy homeostasis. Am. J. Physiol.-Endocrinol. Metab. 2022, 322, E199–E210. [Google Scholar] [CrossRef]
- May, V.; Lutz, E.; MacKenzie, C.; Schutz, K.C.; Dozark, K.; Braas, K.M. Pituitary adenylate cyclase-activating polypeptide (PACAP)/PAC1HOP1 receptor activation coordinates multiple neurotrophic signaling pathways: Akt activation through phosphatidylinositol 3-kinase gamma and vesicle endocytosis for neuronal survival. J. Biol. Chem. 2010, 285, 9749–9761. [Google Scholar] [CrossRef]
- Langer, I.; Jeandriens, J.; Couvineau, A.; Sanmukh, S.; Latek, D. Signal Transduction by VIP and PACAP Receptors. Biomedicines 2022, 10, 406. [Google Scholar] [CrossRef]
- Tanaka, K.; Shibuya, I.; Harayama, N.; Nomura, M.; Kabashima, N.; Ueta, Y.; Yamashita, H. Pituitary Adenylate Cyclase-Activating Polypeptide Potentiation of Ca2+ Entry via Protein Kinase C and A Pathways in Melanotrophs of the Pituitary Pars Intermedia of Rats*. Endocrinology 1997, 138, 4086–4095. [Google Scholar] [CrossRef][Green Version]
- Chatterjee, T.K.; Sharma, R.V.; Fisher, R.A. Molecular Cloning of a Novel Variant of the Pituitary Adenylate Cyclase-activating Polypeptide (PACAP) Receptor That Stimulates Calcium Influx by Activation of L-type Calcium Channels. J. Biol. Chem. 1996, 271, 32226–32232. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Lakhman, S.S.; Singh, S. Modulation of L-type calcium channels in Drosophila via a pituitary adenylyl cyclase-activating polypeptide (PACAP)-mediated pathway. J. Biol. Chem. 2004, 279, 37291–37297. [Google Scholar] [CrossRef] [PubMed]
- Dziema, H.; Obrietan, K. PACAP Potentiates L-Type Calcium Channel Conductance in Suprachiasmatic Nucleus Neurons by Activating the MAPK Pathway. J. Neurophysiol. 2002, 88, 1374–1386. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Reis, D.; Ribeiro, J.A.; de Almeida, R.F.M.; Sebastião, A.M. VPAC(1) and VPAC(2) receptor activation on GABA release from hippocampal nerve terminals involve several different signalling pathways. Br. J. Pharmacol. 2017, 174, 4725–4737. [Google Scholar] [CrossRef]
- Liu, M.; Yang, X.; Bai, T.; Liu, Z.; Liu, T.; Wang, Y.; Cui, L.; Liu, Y.; Zhang, Y. PACAP stimulates insulin secretion by PAC1 receptor and ion channels in β-cells. Cell. Signal. 2019, 61, 48–56. [Google Scholar] [CrossRef]
- Kurahashi, M.; Baker, S.A.; Kito, Y.; Bartlett, A.; Hara, M.; Takeyama, H.; Hashitani, H.; Sanders, K.M. PDGFRα+ Interstitial Cells are Effector Cells of PACAP Signaling in Mouse and Human Colon. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 357–373. [Google Scholar] [CrossRef] [PubMed]
- Delcourt, N.; Thouvenot, E.; Chanrion, B.; Galéotti, N.; Jouin, P.; Bockaert, J.; Marin, P. PACAP type I receptor transactivation is essential for IGF-1 receptor signalling and antiapoptotic activity in neurons. EMBO J. 2007, 26, 1542–1551. [Google Scholar] [CrossRef]
- Moody, T.W.; Osefo, N.; Nuche-Berenguer, B.; Ridnour, L.; Wink, D.; Jensen, R.T. Pituitary adenylate cyclase-activating polypeptide causes tyrosine phosphorylation of the epidermal growth factor receptor in lung cancer cells. J. Pharmacol. Exp. Ther. 2012, 341, 873–881. [Google Scholar] [CrossRef]
- Valdehita, A.; Bajo, A.M.; Schally, A.V.; Varga, J.L.; Carmena, M.J.; Prieto, J.C. Vasoactive intestinal peptide (VIP) induces transactivation of EGFR and HER2 in human breast cancer cells. Mol. Cell. Endocrinol. 2009, 302, 41–48. [Google Scholar] [CrossRef]
- Van Gastel, J.; Hendrickx, J.O.; Leysen, H.; Santos-Otte, P.; Luttrell, L.M.; Martin, B.; Maudsley, S. β-Arrestin Based Receptor Signaling Paradigms: Potential Therapeutic Targets for Complex Age-Related Disorders. Front. Pharmacol. 2018, 9, 1369. [Google Scholar] [CrossRef]
- Pelech, S.L.; Sanghera, J.S. MAP Kinases: Charting the Regulatory Pathways. Science 1992, 257, 1355–1356. [Google Scholar] [CrossRef]
- Gutiérrez-Cañas, I.; Juarranz, M.G.; Collado, B.; Rodríguez-Henche, N.; Chiloeches, A.; Prieto, J.C.; Carmena, M.J. Vasoactive intestinal peptide induces neuroendocrine differentiation in the LNCaP prostate cancer cell line through PKA, ERK, and PI3K. Prostate 2005, 63, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Moroo, I.; Tatsuno, I.; Uchida, D.; Tanaka, T.; Saito, J.; Saito, Y.; Hirai, A. Pituitary adenylate cyclase activating polypeptide (PACAP) stimulates mitogen-activated protein kinase (MAPK) in cultured rat astrocytes. Brain Res. 1998, 795, 191–196. [Google Scholar] [CrossRef]
- Lefkowitz Robert, J.; Shenoy Sudha, K. Transduction of Receptor Signals by ß-Arrestins. Science 2005, 308, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Frodin, M.; Sekine, N.; Roche, E.; Filloux, C.; Prentki, M.; Wollheim, C.B.; Van Obberghen, E. Glucose, other secretagogues, and nerve growth factor stimulate mitogen-activated protein kinase in the insulin-secreting beta-cell line, INS-1. J. Biol. Chem. 1995, 270, 7882–7889. [Google Scholar] [CrossRef]
- Sexton, P.M. Recent advances in our understanding of peptide hormone receptors and RAMPS. Curr. Opin. Drug Discov. Dev. 1999, 2, 440–448. [Google Scholar]
- Hay, D.L.; Pioszak, A.A. Receptor Activity-Modifying Proteins (RAMPs): New Insights and Roles. Annu. Rev Pharm. Toxicol 2016, 56, 469–487. [Google Scholar] [CrossRef]
- Poyner, D.R.; Sexton, P.M.; Marshall, I.; Smith, D.M.; Quirion, R.; Born, W.; Muff, R.; Fischer, J.A.; Foord, S.M. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol. Rev. 2002, 54, 233–246. [Google Scholar] [CrossRef]
- Wootten, D.L.; Simms, J.; Hay, D.L.; Christopoulos, A.; Sexton, P.M. Chapter 3—Receptor Activity Modifying Proteins and Their Potential as Drug Targets. In Progress in Molecular Biology and Translational Science; Lunn, C.A., Ed.; Academic Press: Cambridge, MA, USA, 2010; Volume 91, pp. 53–79. [Google Scholar]
- Lorenzen, E.; Dodig-Crnković, T.; Kotliar, I.B.; Pin, E.; Ceraudo, E.; Vaughan, R.D.; Uhlèn, M.; Huber, T.; Schwenk, J.M.; Sakmar, T.P. Multiplexed analysis of the secretin-like GPCR-RAMP interactome. Sci. Adv. 2019, 5, eaaw2778. [Google Scholar] [CrossRef]
- Wootten, D.; Lindmark, H.; Kadmiel, M.; Willcockson, H.; Caron, K.M.; Barwell, J.; Drmota, T.; Poyner, D.R. Receptor activity modifying proteins (RAMPs) interact with the VPAC2 receptor and CRF1 receptors and modulate their function. Br. J. Pharmacol. 2013, 168, 822–834. [Google Scholar] [CrossRef]
- Stachniak, T.; Krukoff, T.L. Receptor activity modifying protein 2 distribution in the rat central nervous system and regulation by changes in blood pressure. J. Neuroendocrinol. 2003, 15, 840–850. [Google Scholar] [CrossRef]
- Tilakaratne, N.; Christopoulos, G.; Zumpe, E.T.; Foord, S.M.; Sexton, P.M. Amylin receptor phenotypes derived from human calcitonin receptor/RAMP coexpression exhibit pharmacological differences dependent on receptor isoform and host cell environment. J. Pharmacol. Exp. Ther. 2000, 294, 61–72. [Google Scholar] [PubMed]
- Goodman, O.B.; Krupnick, J.G.; Santini, F.; Gurevich, V.V.; Penn, R.B.; Gagnon, A.W.; Keen, J.H.; Benovic, J.L. β-Arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature 1996, 383, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Laporte, S.A.; Oakley, R.H.; Zhang, J.; Holt, J.A.; Ferguson, S.S.; Caron, M.G.; Barak, L.S. The beta2-adrenergic receptor/betaarrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc. Natl. Acad. Sci. USA 1999, 96, 3712–3717. [Google Scholar] [CrossRef] [PubMed]
- Vilardaga, J.-P.; Jean-Alphonse, F.G.; Gardella, T.J. Endosomal generation of cAMP in GPCR signaling. Nat. Chem. Biol. 2014, 10, 700–706. [Google Scholar] [CrossRef]
- Lyu, R.-M.; Germano, P.M.; Choi, J.K.; Le, S.V.; Pisegna, J.R. Identification of an Essential Amino Acid Motif within the C Terminus of the Pituitary Adenylate Cyclase-activating Polypeptide Type I Receptor That Is Critical for Signal Transduction but Not for Receptor Internalization*. J. Biol. Chem. 2000, 275, 36134–36142. [Google Scholar] [CrossRef]
- Langlet, C.; Langer, I.; Vertongen, P.; Gaspard, N.; Vanderwinden, J.-M.; Robberecht, P. Contribution of the Carboxyl Terminus of the VPAC1 Receptor to Agonist-induced Receptor Phosphorylation, Internalization, and Recycling*. J. Biol. Chem. 2005, 280, 28034–28043. [Google Scholar] [CrossRef]
- Marie, J.C.; Rouyer-Fessard, C.; Couvineau, A.; Nicole, P.; Devaud, H.; El Benna, J.; Laburthe, M. Serine 447 in the carboxyl tail of human VPAC1 receptor is crucial for agonist-induced desensitization but not internalization of the receptor. Mol. Pharmacol. 2003, 64, 1565–1574. [Google Scholar] [CrossRef]
- Langer, I.; Langlet, C.; Robberecht, P. Effect of inactivating mutations on phosphorylation and internalization of the human VPAC2 receptor. J. Mol. Endocrinol. 2005, 34, 405–414. [Google Scholar] [CrossRef]
- Inglese, J.; Freedman, N.J.; Koch, W.J.; Lefkowitz, R.J. Structure and mechanism of the G protein-coupled receptor kinases. J. Biol. Chem. 1993, 268, 23735–23738. [Google Scholar] [CrossRef]
- Magalhaes, A.C.; Dunn, H.; Ferguson, S.S. Regulation of GPCR activity, trafficking and localization by GPCR-interacting proteins. Br. J. Pharmacol. 2012, 165, 1717–1736. [Google Scholar] [CrossRef]
- Dautzenberg, F.M.; Hauger, R.L. G-protein-coupled receptor kinase 3- and protein kinase C-mediated desensitization of the PACAP receptor type 1 in human Y-79 retinoblastoma cells. Neuropharmacology 2001, 40, 394–407. [Google Scholar] [CrossRef]
- Murthy, K.S.; Mahavadi, S.; Huang, J.; Zhou, H.; Sriwai, W. Phosphorylation of GRK2 by PKA augments GRK2-mediated phosphorylation, internalization, and desensitization of VPAC2 receptors in smooth muscle. Am. J. Physiology. Cell Physiol. 2008, 294, C477–C487. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Mahavadi, S.; Sriwai, W.; Grider, J.R.; Murthy, K.S. Cross-regulation of VPAC2 receptor desensitization by M3 receptors via PKC-mediated phosphorylation of RKIP and inhibition of GRK2. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 292, G867–G874. [Google Scholar] [CrossRef] [PubMed]
- Shetzline, M.A.; Walker, J.K.; Valenzano, K.J.; Premont, R.T. Vasoactive intestinal polypeptide type-1 receptor regulation. Desensitization, phosphorylation, and sequestration. J. Biol. Chem. 2002, 277, 25519–25526. [Google Scholar] [CrossRef]
- Langer, I. Mechanisms involved in VPAC receptors activation and regulation: Lessons from pharmacological and mutagenesis studies. Front. Endocrinol. 2012, 3, 129. [Google Scholar] [CrossRef]
- Langlet, C.; Gaspard, N.; Nachtergael, I.; Robberecht, P.; Langer, I. Comparative efficacy of VIP and analogs on activation and internalization of the recombinant VPAC2 receptor expressed in CHO cells. Peptides 2004, 25, 2079–2086. [Google Scholar] [CrossRef]
- Shintani, Y.; Hayata-Takano, A.; Moriguchi, K.; Nakazawa, T.; Ago, Y.; Kasai, A.; Seiriki, K.; Shintani, N.; Hashimoto, H. β-Arrestin1 and 2 differentially regulate PACAP-induced PAC1 receptor signaling and trafficking. PLoS ONE 2018, 13, e0196946. [Google Scholar] [CrossRef]
- Gupte, R.P.; Kadunganattil, S.; Shepherd, A.J.; Merrill, R.; Planer, W.; Bruchas, M.R.; Strack, S.; Mohapatra, D.P. Convergent phosphomodulation of the major neuronal dendritic potassium channel Kv4.2 by pituitary adenylate cyclase-activating polypeptide. Neuropharmacology 2016, 101, 291–308. [Google Scholar] [CrossRef]
- Moldovan Loomis, C.; Dutzar, B.; Ojala, E.W.; Hendrix, L.; Karasek, C.; Scalley-Kim, M.; Mulligan, J.; Fan, P.; Billgren, J.; Rubin, V.; et al. Pharmacologic Characterization of ALD1910, a Potent Humanized Monoclonal Antibody against the Pituitary Adenylate Cyclase-Activating Peptide. J. Pharmacol. Exp. Ther. 2019, 369, 26–36. [Google Scholar] [CrossRef]
- Hoffmann, J.; Miller, S.; Martins-Oliveira, M.; Akerman, S.; Supronsinchai, W.; Sun, H.; Shi, L.; Wang, J.; Zhu, D.; Lehto, S.; et al. PAC1 receptor blockade reduces central nociceptive activity: New approach for primary headache? Pain 2020, 161, 1670–1681. [Google Scholar] [CrossRef]
- Rustichelli, C.; Lo Castro, F.; Baraldi, C.; Ferrari, A. Targeting pituitary adenylate cyclase-activating polypeptide (PACAP) with monoclonal antibodies in migraine prevention: A brief review. Expert Opin. Investig. Drugs 2020, 29, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M.; Doležil, D.; Bonner, J.H.; Zhou, L.; Klatt, J.; Picard, H.; Mikol, D.D. A phase 2, randomized, double-blind, placebo-controlled trial of AMG 301, a pituitary adenylate cyclase-activating polypeptide PAC1 receptor monoclonal antibody for migraine prevention. Cephalalgia Int. J. Headache 2021, 41, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Cui, Z.; Li, M.; Yang, Y.; Zhong, J. Dimer-dependent intrinsic/basal activity of the class B G protein-coupled receptor PAC1 promotes cellular anti-apoptotic activity through Wnt/β-catenin pathways that are associated with dimer endocytosis. PLoS ONE 2014, 9, e113913. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Zhong, J.; Li, M.; Guo, X.; Zhang, H.; Chen, J. PACAP induces the dimerization of PAC1 on the nucleus associated with the cAMP increase in the nucleus. Neurosci. Lett. 2013, 549, 92–96. [Google Scholar] [CrossRef]
- Feher, M.; Gaszner, B.; Tamas, A.; Gil-Martinez, A.L.; Fernandez-Villalba, E.; Herrero, M.T.; Reglodi, D. Alteration of the PAC1 Receptor Expression in the Basal Ganglia of MPTP-Induced Parkinsonian Macaque Monkeys. Neurotox. Res. 2018, 33, 702–715. [Google Scholar] [CrossRef]
- Miura, A.; Kambe, Y.; Inoue, K.; Tatsukawa, H.; Kurihara, T.; Griffin, M.; Kojima, S.; Miyata, A. Pituitary adenylate cyclase-activating polypeptide type 1 receptor (PAC1) gene is suppressed by transglutaminase 2 activation. J. Biol. Chem. 2013, 288, 32720–32730. [Google Scholar] [CrossRef]
- Kenakin, T. Functional selectivity and biased receptor signaling. J. Pharmacol. Exp. Ther. 2011, 336, 296–302. [Google Scholar] [CrossRef]
- Kenakin, T.; Christopoulos, A. Signalling bias in new drug discovery: Detection, quantification and therapeutic impact. Nat. Rev. Drug Discov. 2013, 12, 205–216. [Google Scholar] [CrossRef]
- Wootten, D.; Miller, L.J.; Koole, C.; Christopoulos, A.; Sexton, P.M. Allostery and Biased Agonism at Class B G Protein-Coupled Receptors. Chem. Rev. 2017, 117, 111–138. [Google Scholar] [CrossRef]
- Koole, C.; Wootten, D.; Simms, J.; Valant, C.; Sridhar, R.; Woodman, O.L.; Miller, L.J.; Summers, R.J.; Christopoulos, A.; Sexton, P.M. Allosteric ligands of the glucagon-like peptide 1 receptor (GLP-1R) differentially modulate endogenous and exogenous peptide responses in a pathway-selective manner: Implications for drug screening. Mol. Pharmacol. 2010, 78, 456–465. [Google Scholar] [CrossRef]
- Liang, Y.-L.; Khoshouei, M.; Radjainia, M.; Zhang, Y.; Glukhova, A.; Tarrasch, J.; Thal, D.M.; Furness, S.G.B.; Christopoulos, G.; Coudrat, T.; et al. Phase-plate cryo-EM structure of a class B GPCR–G-protein complex. Nature 2017, 546, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.G.F.; DeVree, B.T.; Zou, Y.; Kruse, A.C.; Chung, K.Y.; Kobilka, T.S.; Thian, F.S.; Chae, P.S.; Pardon, E.; Calinski, D.; et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 2011, 477, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-L.; Zhao, P.; Draper-Joyce, C.; Baltos, J.-A.; Glukhova, A.; Truong, T.T.; May, L.T.; Christopoulos, A.; Wootten, D.; Sexton, P.M.; et al. Dominant Negative G Proteins Enhance Formation and Purification of Agonist-GPCR-G Protein Complexes for Structure Determination. ACS Pharmacol. Transl. Sci. 2018, 1, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Koehl, A.; Matile, H.; Hu, H.; Hilger, D.; Schertler, G.F.X.; Manglik, A.; Skiniotis, G.; Dawson, R.J.P.; Kobilka, B.K. Development of an antibody fragment that stabilizes GPCR/G-protein complexes. Nat. Commun. 2018, 9, 3712. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Okashah, N.; Inoue, A.; Nehmé, R.; Carpenter, B.; Tate, C.G.; Lambert, N.A. Mini G protein probes for active G protein-coupled receptors (GPCRs) in live cells. J. Biol. Chem. 2018, 293, 7466–7473. [Google Scholar] [CrossRef] [PubMed]
- Danev, R.; Baumeister, W. Cryo-EM single particle analysis with the Volta phase plate. eLife 2016, 5, e13046. [Google Scholar] [CrossRef]
- Danev, R.; Belousoff, M.; Liang, Y.L.; Zhang, X.; Eisenstein, F.; Wootten, D.; Sexton, P.M. Routine sub-2.5 Å cryo-EM structure determination of GPCRs. Nat. Commun. 2021, 12, 4333. [Google Scholar] [CrossRef]
- Zhang, X.; Johnson, R.M.; Drulyte, I.; Yu, L.; Kotecha, A.; Danev, R.; Wootten, D.; Sexton, P.M.; Belousoff, M.J. Evolving cryo-EM structural approaches for GPCR drug discovery. Structure 2021, 29, 963–974. [Google Scholar] [CrossRef]
- Wu, M.; Lander, G.C. Present and Emerging Methodologies in Cryo-EM Single-Particle Analysis. Biophys. J. 2020, 119, 1281–1289. [Google Scholar] [CrossRef]
- Nygaard, R.; Kim, J.; Mancia, F. Cryo-electron microscopy analysis of small membrane proteins. Curr. Opin. Struct. Biol. 2020, 64, 26–33. [Google Scholar] [CrossRef]
- Nakane, T.; Kotecha, A.; Sente, A.; McMullan, G.; Masiulis, S.; Brown, P.M.G.E.; Grigoras, I.T.; Malinauskaite, L.; Malinauskas, T.; Miehling, J.; et al. Single-particle cryo-EM at atomic resolution. Nature 2020, 587, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Yip, K.M.; Fischer, N.; Paknia, E.; Chari, A.; Stark, H. Atomic-resolution protein structure determination by cryo-EM. Nature 2020, 587, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Parthier, C.; Kleinschmidt, M.; Neumann, P.; Rudolph, R.; Manhart, S.; Schlenzig, D.; Fanghänel, J.; Rahfeld, J.U.; Demuth, H.U.; Stubbs, M.T. Crystal structure of the incretin-bound extracellular domain of a G protein-coupled receptor. Proc. Natl. Acad. Sci. USA 2007, 104, 13942–13947. [Google Scholar] [CrossRef] [PubMed]
- Runge, S.; Thøgersen, H.; Madsen, K.; Lau, J.; Rudolph, R. Crystal structure of the ligand-bound glucagon-like peptide-1 receptor extracellular domain. J. Biol. Chem. 2008, 283, 11340–11347. [Google Scholar] [CrossRef] [PubMed]
- Pike, A.C.W.; Barr, A.J.; Quigley, A.; Burgess Brown, N.; de Riso, A.; Bullock, A.; Berridge, G.; Muniz, J.R.C.; Chaikaud, A.; Vollmar, M.; et al. Crystal Structure of the Extracellular Domain of Human Vasoactive Intestinal Polypeptide Receptor 2. 2010. Available online: https://www.wwpdb.org/pdb?id=pdb_00002x57 (accessed on 22 June 2022).
- Kumar, S.; Pioszak, A.; Zhang, C.; Swaminathan, K.; Xu, H.E. Crystal structure of the PAC1R extracellular domain unifies a consensus fold for hormone recognition by class B G-protein coupled receptors. PLoS ONE 2011, 6, e19682. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Song, D.; Davis-Taber, R.A.; Barrett, L.W.; Scott, V.E.; Richardson, P.L.; Pereda-Lopez, A.; Uchic, M.E.; Solomon, L.R.; Lake, M.R.; et al. Solution Structure and Mutational Analysis of Pituitary Adenylate Cyclase-Activating Polypeptide Binding to the Extracellular Domain of PAC1-Rs. Proc. Natl. Acad. Sci. USA 2007, 104, 7875–7880. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Shen, Q.; Zhao, L.-H.; Mao, C.; Zhou, X.E.; Shen, D.-D.; de Waal, P.W.; Bi, P.; Li, C.; Jiang, Y.; et al. Molecular Basis for Hormone Recognition and Activation of Corticotropin-Releasing Factor Receptors. Mol. Cell 2020, 77, 669–680.e664. [Google Scholar] [CrossRef]
- Zhang, X.; Belousoff, M.J.; Zhao, P.; Kooistra, A.J.; Truong, T.T.; Ang, S.Y.; Underwood, C.R.; Egebjerg, T.; Šenel, P.; Stewart, G.D.; et al. Differential GLP-1R Binding and Activation by Peptide and Non-peptide Agonists. Mol. Cell 2020, 80, 485–500.e487. [Google Scholar] [CrossRef]
- Sun, W.; Chen, L.-N.; Zhou, Q.; Zhao, L.-H.; Yang, D.; Zhang, H.; Cong, Z.; Shen, D.-D.; Zhao, F.; Zhou, F.; et al. A unique hormonal recognition feature of the human glucagon-like peptide-2 receptor. Cell Res. 2020, 30, 1098–1108. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, C.; Zhou, Q.; Hang, K.; Zou, X.; Chen, Y.; Wu, F.; Rao, Q.; Dai, A.; Yin, W.; et al. Structural insights into hormone recognition by the human glucose-dependent insulinotropic polypeptide receptor. eLife 2021, 10, e68719. [Google Scholar] [CrossRef]
- Chang, R.; Zhang, X.; Qiao, A.; Dai, A.; Belousoff, M.J.; Tan, Q.; Shao, L.; Zhong, L.; Lin, G.; Liang, Y.L.; et al. Cryo-electron microscopy structure of the glucagon receptor with a dual-agonist peptide. J. Biol. Chem. 2020, 295, 9313–9325. [Google Scholar] [CrossRef]
- Dong, M.; Deganutti, G.; Piper, S.J.; Liang, Y.-L.; Khoshouei, M.; Belousoff, M.J.; Harikumar, K.G.; Reynolds, C.A.; Glukhova, A.; Furness, S.G.B.; et al. Structure and dynamics of the active Gs-coupled human secretin receptor. Nat. Commun. 2020, 11, 4137. [Google Scholar] [CrossRef]
- Liang, Y.-L.; Khoshouei, M.; Deganutti, G.; Glukhova, A.; Koole, C.; Peat, T.S.; Radjainia, M.; Plitzko, J.M.; Baumeister, W.; Miller, L.J.; et al. Cryo-EM structure of the active, Gs-protein complexed, human CGRP receptor. Nature 2018, 561, 492–497. [Google Scholar] [CrossRef]
- Cao, J.; Belousoff, M.J.; Liang, Y.-L.; Johnson, R.M.; Josephs, T.M.; Fletcher, M.M.; Christopoulos, A.; Hay, D.L.; Danev, R.; Wootten, D.; et al. A structural basis for amylin receptor phenotype. Science 2022, 375, eabm9609. [Google Scholar] [CrossRef]
- Liang, Y.-L.; Belousoff, M.J.; Fletcher, M.M.; Zhang, X.; Khoshouei, M.; Deganutti, G.; Koole, C.; Furness, S.G.B.; Miller, L.J.; Hay, D.L.; et al. Structure and Dynamics of Adrenomedullin Receptors AM1 and AM2 Reveal Key Mechanisms in the Control of Receptor Phenotype by Receptor Activity-Modifying Proteins. ACS Pharmacol. Transl. Sci. 2020, 3, 263–284. [Google Scholar] [CrossRef]
- Zhao, L.H.; Ma, S.; Sutkeviciute, I.; Shen, D.D.; Zhou, X.E.; de Waal, P.W.; Li, C.Y.; Kang, Y.; Clark, L.J.; Jean-Alphonse, F.G.; et al. Structure and dynamics of the active human parathyroid hormone receptor-1. Science 2019, 364, 148–153. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, X.; Zhao, L.; Wang, Y.; Ye, C.; Zou, X.; Dai, A.; Cong, Z.; Chen, J.; Zhou, Q.; et al. Molecular insights into differentiated ligand recognition of the human parathyroid hormone receptor 2. Proc. Natl. Acad. Sci. USA 2021, 118, e2101279118. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. A Publ. Protein Soc. 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Goddard, T.D.; Huang, C.C.; Meng, E.C.; Pettersen, E.F.; Couch, G.S.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci. A Publ. Protein Soc. 2018, 27, 14–25. [Google Scholar] [CrossRef]
- Doan, N.-D.; Bourgault, S.; Dejda, A.; Létourneau, M.; Detheux, M.; Vaudry, D.; Vaudry, H.; Chatenet, D.; Fournier, A. Design and in vitro characterization of PAC1/VPAC1-selective agonists with potent neuroprotective effects. Biochem. Pharmacol. 2011, 81, 552–561. [Google Scholar] [CrossRef]
- Bourgault, S.; Vaudry, D.; Ségalas-Milazzo, I.; Guilhaudis, L.; Couvineau, A.; Laburthe, M.; Vaudry, H.; Fournier, A. Molecular and Conformational Determinants of Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) for Activation of the PAC1 Receptor. J. Med. Chem. 2009, 52, 3308–3316. [Google Scholar] [CrossRef] [PubMed]
- Wootten, D.; Simms, J.; Miller, L.J.; Christopoulos, A.; Sexton, P.M. Polar transmembrane interactions drive formation of ligand-specific and signal pathway-biased family B G protein-coupled receptor conformations. Proc. Natl. Acad. Sci. USA 2013, 110, 5211–5216. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, H.; Ito, T.; Pradhan, T.K.; Mantey, S.A.; Hou, W.; Coy, D.H.; Jensen, R.T. Elucidation of the Vasoactive Intestinal Peptide Pharmacophore for VPAC2 Receptors in Human and Rat and Comparison to the Pharmacophore for VPAC1 Receptors. J. Pharmacol. Exp. Ther. 2002, 303, 445–460. [Google Scholar] [CrossRef]
- Liao, C.; de Molliens, M.P.; Schneebeli, S.T.; Brewer, M.; Song, G.; Chatenet, D.; Braas, K.M.; May, V.; Li, J. Targeting the PAC1 Receptor for Neurological and Metabolic Disorders. Curr. Top. Med. Chem. 2019, 19, 1399–1417. [Google Scholar] [CrossRef] [PubMed]
- Bourgault, S.; Vaudry, D.; Dejda, A.; Doan, D.N.; Vaudry, H.; Fournier, A. Pituitary Adenylate Cyclase-Activating Polypeptide: Focus on Structure- Activity Relationships of a Neuroprotective Peptide. Curr. Med. Chem. 2009, 16, 4462–4480. [Google Scholar] [CrossRef] [PubMed]
- Bourgault, S.; Chatenet, D.; Wurtz, O.; Doan, N.D.; Leprince, J.; Vaudry, H.; Fournier, A.; Vaudry, D. Strategies to convert PACAP from a hypophysiotropic neurohormone into a neuroprotective drug. Curr. Pharm. Des. 2011, 17, 1002–1024. [Google Scholar] [CrossRef]
- Schäfer, H.; Zheng, J.; Morys-Wortmann, C.; Fölsch, U.R.; Schmidt, W.E. Structural motifs of pituitary adenylate cyclase-activating polypeptide (PACAP) defining PAC1-receptor selectivity. Regul. Pept. 1999, 79, 83–92. [Google Scholar] [CrossRef]
- Wei, M.; Fujiki, K.; Ando, E.; Zhang, S.; Ozaki, T.; Ishiguro, H.; Kondo, T.; Nokihara, K.; Wray, V.; Naruse, S. Identification of key residues that cause differential gallbladder response to PACAP and VIP in the guinea pig. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 292, G76–G83. [Google Scholar] [CrossRef]
- Ando, E.; Nokihara, K.; Naruse, S.; Wray, V. Recognition of pituitary adenylate cyclase-activating polypeptide/vasoactive intestinal polypeptide (PACAP/VIP) hybrids and related peptides by rat brain membranes. Biomed. Pept. Proteins Nucleic Acids Struct. Synth. Biol. Act. 1996, 2, 41–46. [Google Scholar]
- Reddy, V.B.; Iuga, A.O.; Kounga, K.; Lerner, E.A. Functional analysis of recombinant mutants of maxadilan with a PAC1 receptor-expressing melanophore cell line. J. Biol. Chem. 2006, 281, 16197–16201. [Google Scholar] [CrossRef]
- Lerner, E.A.; Iuga, A.O.; Reddy, V.B. Maxadilan, a PAC1 receptor agonist from sand flies. Peptides 2007, 28, 1651–1654. [Google Scholar] [CrossRef]
- Moro, O.; Wakita, K.; Ohnuma, M.; Denda, S.; Lerner, E.A.; Tajima, M. Functional Characterization of Structural Alterations in the Sequence of the Vasodilatory Peptide Maxadilan Yields a Pituitary Adenylate Cyclase-activating Peptide Type 1 Receptor-specific Antagonist. J. Biol. Chem. 1999, 274, 23103–23110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, B.; Feng, D.; Hu, H.; Chu, M.; Qu, Q.; Tarrasch, J.T.; Li, S.; Sun Kobilka, T.; Kobilka, B.K.; et al. Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature 2017, 546, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Wootten, D.; Reynolds, C.A.; Koole, C.; Smith, K.J.; Mobarec, J.C.; Simms, J.; Quon, T.; Coudrat, T.; Furness, S.G.B.; Miller, L.J.; et al. A Hydrogen-Bonded Polar Network in the Core of the Glucagon-Like Peptide-1 Receptor Is a Fulcrum for Biased Agonism: Lessons from Class B Crystal Structures. Mol. Pharmacol. 2016, 89, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Wootten, D.; Savage, E.E.; Willard, F.S.; Bueno, A.B.; Sloop, K.W.; Christopoulos, A.; Sexton, P.M. Differential Activation and Modulation of the Glucagon-Like Peptide-1 Receptor by Small Molecule Ligands. Mol. Pharmacol. 2013, 83, 822–834. [Google Scholar] [CrossRef]
- Hollenstein, K.; Kean, J.; Bortolato, A.; Cheng, R.K.; Doré, A.S.; Jazayeri, A.; Cooke, R.M.; Weir, M.; Marshall, F.H. Structure of class B GPCR corticotropin-releasing factor receptor 1. Nature 2013, 499, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, A.; Doré, A.S.; Lamb, D.; Krishnamurthy, H.; Southall, S.M.; Baig, A.H.; Bortolato, A.; Koglin, M.; Robertson, N.J.; Errey, J.C.; et al. Extra-helical binding site of a glucagon receptor antagonist. Nature 2016, 533, 274–277. [Google Scholar] [CrossRef]
- Siu, F.Y.; He, M.; de Graaf, C.; Han, G.W.; Yang, D.; Zhang, Z.; Zhou, C.; Xu, Q.; Wacker, D.; Joseph, J.S.; et al. Structure of the human glucagon class B G-protein-coupled receptor. Nature 2013, 499, 444–449. [Google Scholar] [CrossRef]
- Wu, F.; Yang, L.; Hang, K.; Laursen, M.; Wu, L.; Han, G.W.; Ren, Q.; Roed, N.K.; Lin, G.; Hanson, M.A.; et al. Full-length human GLP-1 receptor structure without orthosteric ligands. Nat. Commun. 2020, 11, 1272. [Google Scholar] [CrossRef]
- Deganutti, G.; Liang, Y.-L.; Zhang, X.; Khoshouei, M.; Clydesdale, L.; Belousoff, M.J.; Venugopal, H.; Truong, T.T.; Glukhova, A.; Keller, A.N.; et al. Dynamics of GLP-1R peptide agonist engagement are correlated with kinetics of G protein activation. Nat. Commun. 2022, 13, 92. [Google Scholar] [CrossRef]
- Krumm, B.; Roth, B.L. A Structural Understanding of Class B GPCR Selectivity and Activation Revealed. Structure 2020, 28, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Do, H.N.; Haldane, A.; Levy, R.M.; Miao, Y. Unique features of different classes of G-protein-coupled receptors revealed from sequence coevolutionary and structural analysis. Proteins Struct. Funct. Bioinform. 2022, 90, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Liang, Y.-L.; Belousoff, M.J.; Deganutti, G.; Fletcher, M.M.; Willard, F.S.; Bell, M.G.; Christe, M.E.; Sloop, K.W.; Inoue, A.; et al. Activation of the GLP-1 receptor by a non-peptidic agonist. Nature 2020, 577, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, A.J.; Ma, A.K.; Fonseca, R.; Latorraca, N.R.; Kelly, B.; Betz, R.M.; Asawa, C.; Kobilka, B.K.; Dror, R.O. Diverse GPCRs exhibit conserved water networks for stabilization and activation. Proc. Natl. Acad. Sci. USA 2019, 116, 3288–3293. [Google Scholar] [CrossRef]
- Wootten, D.; Miller, L.J. Structural Basis for Allosteric Modulation of Class B G Protein-Coupled Receptors. Annu. Rev Pharm. Toxicol 2020, 60, 89–107. [Google Scholar] [CrossRef]
- Christopoulos, A.; Kenakin, T. G protein-coupled receptor allosterism and complexing. Pharmacol. Rev. 2002, 54, 323–374. [Google Scholar] [CrossRef]
- Langer, I.; Latek, D. Drug Repositioning For Allosteric Modulation of VIP and PACAP Receptors. Front. Endocrinol. 2021, 12, 711906. [Google Scholar] [CrossRef]
- Latek, D.; Langer, I.; Krzysko, K.; Charzewski, L. A Molecular Dynamics Study of Vasoactive Intestinal Peptide Receptor 1 and the Basis of Its Therapeutic Antagonism. Int. J. Mol. Sci. 2019, 20, 4348. [Google Scholar] [CrossRef]
- Song, G.; Yang, D.; Wang, Y.; de Graaf, C.; Zhou, Q.; Jiang, S.; Liu, K.; Cai, X.; Dai, A.; Lin, G.; et al. Human GLP-1 receptor transmembrane domain structure in complex with allosteric modulators. Nature 2017, 546, 312–315. [Google Scholar] [CrossRef]
- Zhang, H.; Qiao, A.; Yang, D.; Yang, L.; Dai, A.; de Graaf, C.; Reedtz-Runge, S.; Dharmarajan, V.; Zhang, H.; Han, G.W.; et al. Structure of the full-length glucagon class B G-protein-coupled receptor. Nature 2017, 546, 259–264. [Google Scholar] [CrossRef]
- Yu, R.; Zheng, L.; Cui, Y.; Zhang, H.; Ye, H. Doxycycline exerted neuroprotective activity by enhancing the activation of neuropeptide GPCR PAC1. Neuropharmacology 2016, 103, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wang, L.; Li, J.; Huang, X.; Yu, R. The allosteric modulation effects of doxycycline, minocycline, and their derivatives on the neuropeptide receptor PAC1-R. Acta Biochim. Biophys. Sin. 2019, 51, 627. [Google Scholar] [CrossRef] [PubMed]
- Punjani, A.; Fleet, D.J. 3D variability analysis: Resolving continuous flexibility and discrete heterogeneity from single particle cryo-EM. J. Struct. Biol. 2021, 213, 107702. [Google Scholar] [CrossRef]
- Cary, B.P.; Deganutti, G.; Zhao, P.; Truong, T.T.; Piper, S.J.; Liu, X.; Belousoff, M.J.; Danev, R.; Sexton, P.M.; Wootten, D.; et al. Structural and functional diversity among agonist-bound states of the GLP-1 receptor. Nat. Chem. Biol. 2022, 18, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Deganutti, G.; Atanasio, S.; Rujan, R.-M.; Sexton, P.M.; Wootten, D.; Reynolds, C.A. Exploring Ligand Binding to Calcitonin Gene-Related Peptide Receptors. Front. Mol. Biosci. 2021, 8, 720561. [Google Scholar] [CrossRef]
- Josephs, T.M.; Belousoff, M.J.; Liang, Y.-L.; Piper, S.J.; Cao, J.; Garama, D.J.; Leach, K.; Gregory, K.J.; Christopoulos, A.; Hay, D.L.; et al. Structure and dynamics of the CGRP receptor in apo and peptide-bound forms. Science 2021, 372, eabf7258. [Google Scholar] [CrossRef]
- Asher, W.B.; Geggier, P.; Holsey, M.D.; Gilmore, G.T.; Pati, A.K.; Meszaros, J.; Terry, D.S.; Mathiasen, S.; Kaliszewski, M.J.; McCauley, M.D.; et al. Single-molecule FRET imaging of GPCR dimers in living cells. Nat. Methods 2021, 18, 397–405. [Google Scholar] [CrossRef]
- Dijkman, P.M.; Muñoz-García, J.C.; Lavington, S.R.; Kumagai, P.S.; Reis, R.I.d.; Yin, D.; Stansfeld, P.J.; Costa-Filho, A.J.; Watts, A. Conformational dynamics of a G protein–coupled receptor helix 8 in lipid membranes. Sci. Adv. 2020, 6, eaav8207. [Google Scholar] [CrossRef] [PubMed]
- Eps, N.V.; Altenbach, C.; Caro, L.N.; Latorraca, N.R.; Hollingsworth, S.A.; Dror, R.O.; Ernst, O.P.; Hubbell, W.L. Gi and Gs-coupled GPCRs show different modes of G-protein binding. Proc. Natl. Acad. Sci. USA 2018, 115, 2383–2388. [Google Scholar] [CrossRef]
- Yeliseev, A.A.; Zoretich, K.; Hooper, L.; Teague, W.; Zoubak, L.; Hines, K.G.; Gawrisch, K. Site-selective labeling and electron paramagnetic resonance studies of human cannabinoid receptor CB(2). Biochim. Biophys. Acta Biomembr. 2021, 1863, 183621. [Google Scholar] [CrossRef]
- Robertson, M.J.; Papasergi-Scott, M.; He, F.; Seven, A.B.; Meyerowitz, J.G.; Panova, O.; Peroto, M.C.; Che, T.; Skiniotis, G. Structure Determination of Inactive-State GPCRs with a Universal Nanobody. bioRxiv 2022, 2021, 466983. [Google Scholar] [CrossRef]
- Lei, S.; Clydesdale, L.; Dai, A.; Cai, X.; Feng, Y.; Yang, D.; Liang, Y.-L.; Koole, C.; Zhao, P.; Coudrat, T.; et al. Two distinct domains of the glucagon-like peptide-1 receptor control peptide-mediated biased agonism. J. Biol. Chem. 2018, 293, 9370–9387. [Google Scholar] [CrossRef] [PubMed]
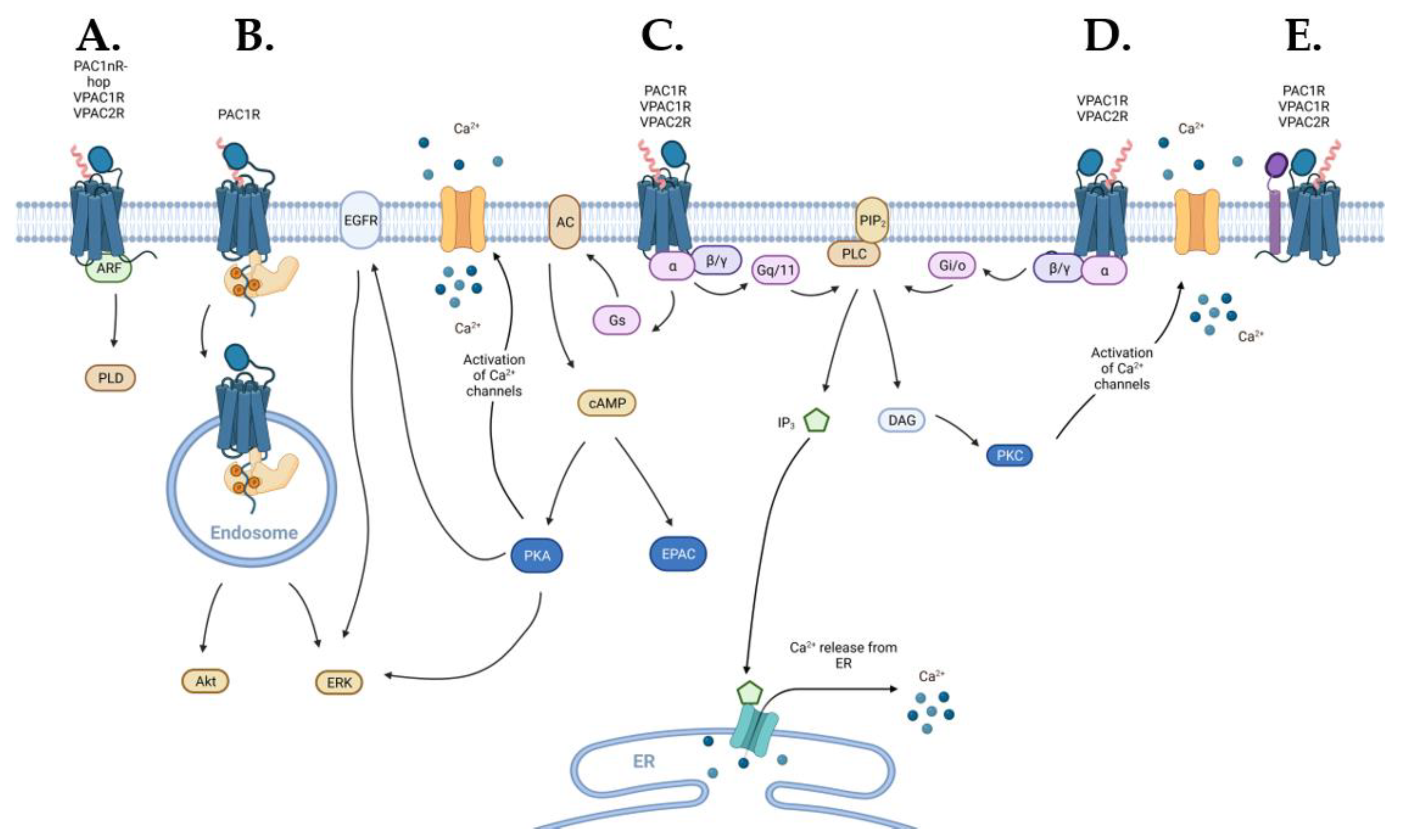
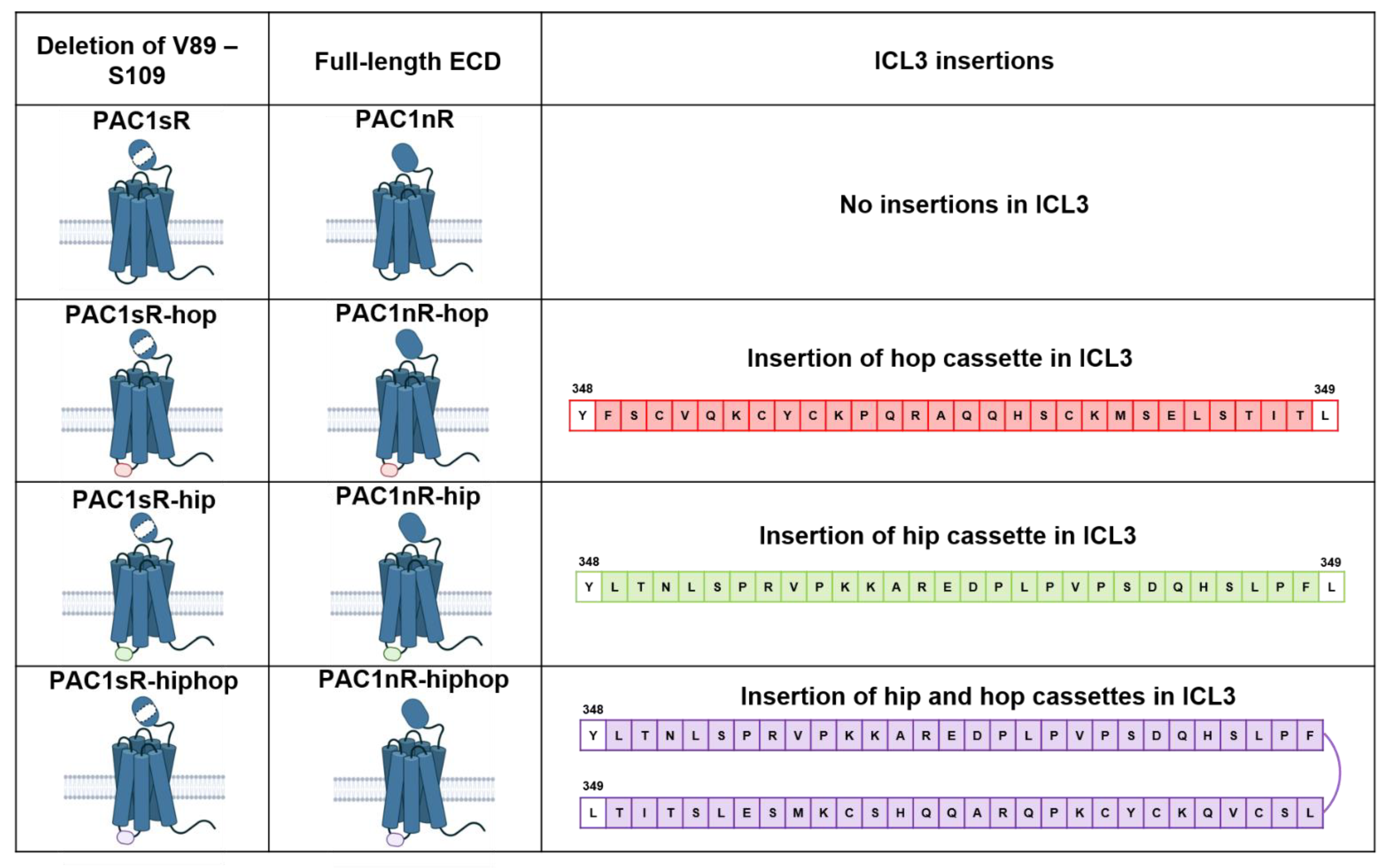
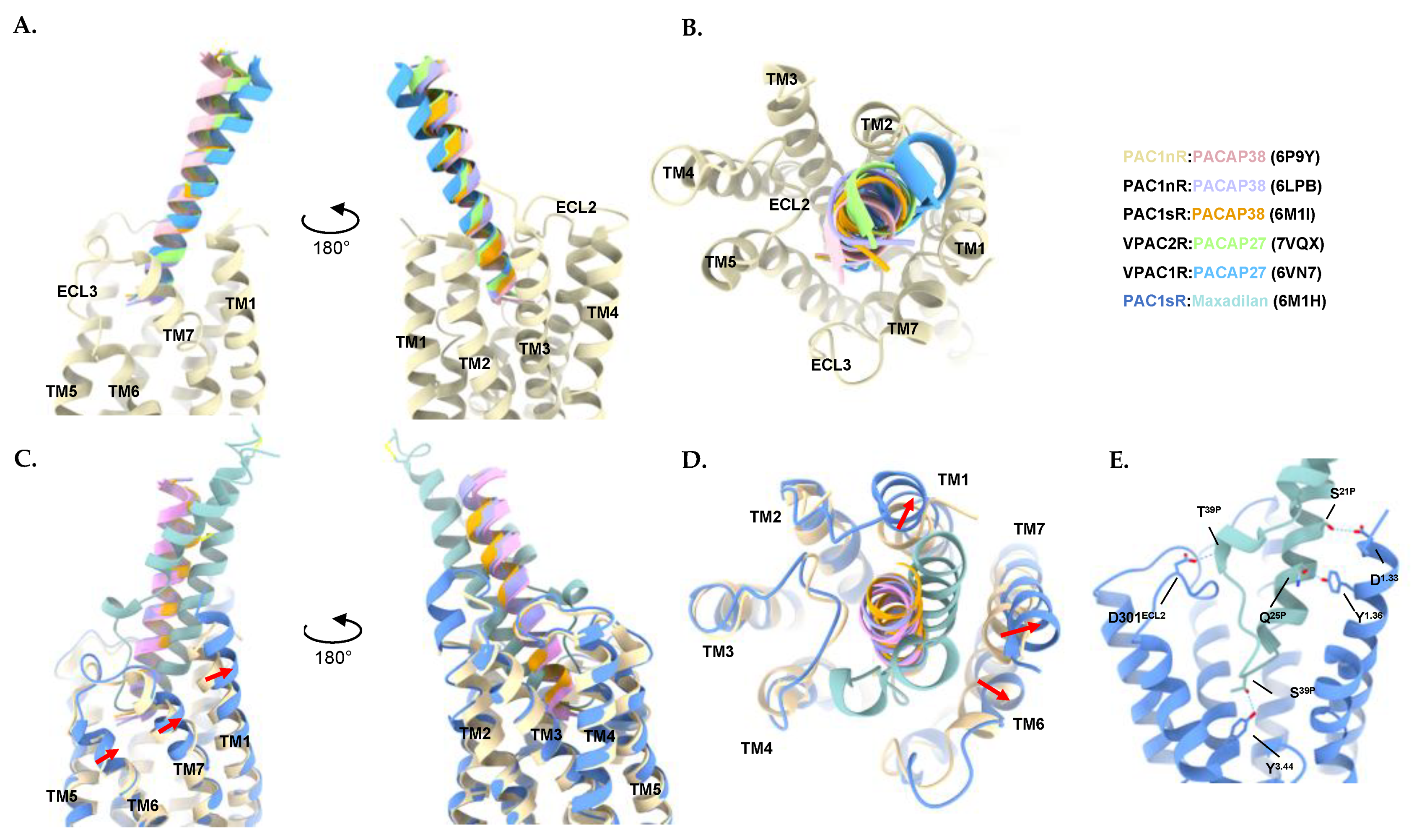

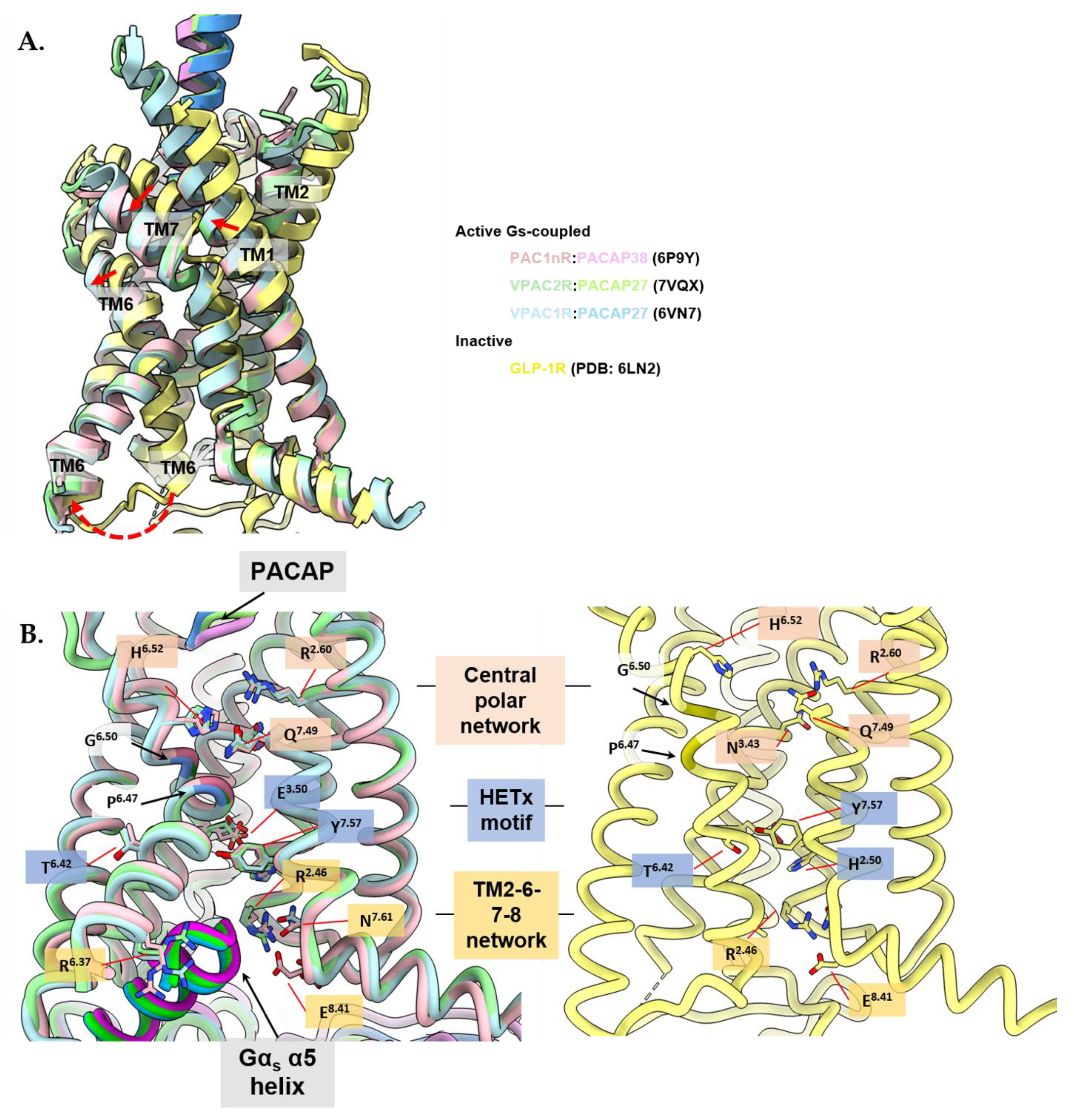
| Receptor | Distribution | Physiological/Therapeutic Role |
|---|---|---|
| VPAC1R | CNS [35] (cerebral cortex [36,37], hypothalamus [38], hippocampus [36,39]) | Control of circadian rhythm [38], learning, and memory [39] |
| Liver [40] | Glucose metabolism [41,42] | |
| Lung [40,43] | Asthma and COPD (relaxation of airway and vascular smooth muscles [44], anti-inflammatory effect [45,46], and regulation of mucus secretion [47]), chronic bronchitis [48] | |
| Intestine [40,49] | Peristalsis, ion transport and mucus secretion [11,12,50] | |
| Breast [40] | Cell proliferation in cancer [51,52] | |
| T-lymphocytes and macrophages (constitutively expressed) [53,54,55,56] | Immune regulation [32,33] | |
| VPAC2R | CNS [35] (thalamus [57,58,59], suprachiasmatic nucleus [29,31,57,59], dentate gyrus [59], amygdala [57]) | Schizophrenia [60,61,62], brain injury [63], control of circadian rhythm [29,30,31], processing of fear-related memory [64] |
| Smooth muscles [65] | Vasodilation (blood vessels) [66], erectile dysfunction (male reproductive system) [67] | |
| Pancreas [68] | Insulin secretion [34] | |
| Lungs [65,69] | Asthma and COPD (relaxation of airway and vascular smooth muscles [44,70], anti-inflammatory effect [45,46], and regulation of mucus secretion [47]), pulmonary arterial hypertension [71], chronic bronchitis [48] | |
| T-lymphocytes and macrophages (expressed upon cell activation) [53,54,56] | Immune regulation [72] | |
| PAC1R | CNS [35] (olfactory bulb [16,73], cerebral cortex, thalamus [73], hypothalamus [73,74], hippocampus, amygdala [73,75], substantia nigra [73], cerebellum [73] | Astrocyte proliferation [76], appetite and feeding behaviour [77,78], anxiety [79], stress response [80,81,82], control of circadian rhythm [20], post-traumatic stress disorder [83], traumatic brain injury [84], migraine [85], Alzheimer’s disease [86,87] |
| Embryonic nervous system [88,89] | Neuronal differentiation of neural progenitor and embryonic stem cells [90,91,92] | |
| Eyes (corneal endothelium [93], retina [94], lacrimal gland [95] | Maintenance of corneal endothelial barrier integrity [93] Protection against retinopathy [94,96] Stimulation of tear production [95] | |
| Bone marrow (haematopoietic progenitor cells) [97] | Haematopoiesis [97] | |
| Adrenal medulla | Adrenal catecholamine secretion [98] | |
| Pancreas | Insulin secretion [99] | |
| Cardiac neurons [100] | Modulates excitability—stimulatory effect on CV system [100] | |
| Bladder | Urinary bladder dysfunction [101] |
| Selective Receptor | Compound | Agonist/Antagonist | Peptide Modifications | Relative Selectivity * | Reference |
|---|---|---|---|---|---|
| VPAC1R | [Tyr9,Dip18]-VIP | Agonist | VIP analogue | VPAC1R ([125I]VIP Ki = 0.1 nM) VPAC2R ([125I]VIP Ki = 53 nM) PAC1R ([125I]PACAP27 Ki = 3 μM) | [140] |
| [Ala22]-VIP | Agonist | VIP analogue | VPAC1R ([125I]VIP IC50 = 10 nM), VPAC2R ([125I]RO 25-1553 IC50 = 1 μM) | [141,142] | |
| [Leu22]-VIP | Agonist | VIP analogue | VPAC1R ([125I]VIP IC50 = 11 nM), VPAC2R ([125I]RO 25-1553 IC50 = 700 nM) | [143] | |
| [Ala11,22,28]-VIP | Agonist | VIP analogue | VPAC1R (cAMP EC50 < 1 nM) VPAC2R (cAMP EC50 > 1 µM) | [142] | |
| [Arg16]-PACAP (1–23) | Agonist | C-terminal truncated PACAP analogue | VPAC1R ([125I]VIP IC50 = 2.5 nM), VPAC2R ([125I]RO 25-1553 IC50 = 1.2 µM) | [143] | |
| Chicken [Arg16]-secretin | Agonist | Secretin analogue | PAC1R ([125I]Ac-His1-PACAP27 IC50 = 30 µM) VPAC1R ([125I]VIP IC50 = 100 nM), VPAC2R ([125I]VIP IC50 = 10 µM) 1 | [144] | |
| [Lys15, Arg16, Leu27]-VIP(1-7)/GRF(8-27) | Agonist | Chimeric VIP/GRF analogue | VPAC1R ([125I]VIP IC50 = 1 nM), VPAC2R ([125I]VIP IC50 > 30 µM) 2 | [144] | |
| PG 97-269 | Antagonist | N-terminal modified VIP/GRF chimeric analogue | VPAC1R ([125I]VIP IC50 = 2 nM), VPAC2R ([125I]VIP IC50 = 3 μM) | [145] | |
| VPAC2R | RO 25-1392 | Agonist | Cyclic VIP analogue | VPAC1R ([125I]VIP Ki = 1 μM), VPAC2R ([125I]VIP Ki = 9.6 nM) | [146] |
| RO 25-1553 | Agonist | Cyclic VIP analogue | VPAC1R ([125I]VIP IC50 = 800 nM), VPAC2R ([125I]RO 25-1553 IC50 = 1 nM) | [147] | |
| PG 96-249 | Agonist | Linear RO 25-1553 analogue | VPAC1R ([125I]VIP IC50 = 3 μM), VPAC2R ([125I]RO 25-1553 IC50 = 10 nM) | [147] | |
| BAY 55-9837 | Agonist | PACAP/VIP analogue | PAC1R ([125I]PACAP27 IC50 = N/A) 3 VPAC1R ([125I]PACAP27 IC50 = 8.7 µM), VPAC2R ([125I]PACAP27 IC50 = 60 nM) | [34] | |
| PG 99–465 | Antagonist | N-terminal myristoylated, C-terminal elongated VIP analogue | VPAC1R ([125I]VIP IC50 = 200 nM), VPAC2R ([125I]RO 25-1553 IC50 = 1 nM) | [147] | |
| PAC1R | M65 | Antagonist | Maxadilan analogue | PAC1R ([125I]PACAP27 Kd = 0.6 nM), VPAC1R ([125I]VIP Kd = N/A), VPAC2R ([125I]VIP Kd = N/A) 4 | [133] |
| max.D.4 | Antagonist | Maxadilan analogue | PAC1R ([125I]PACAP27 Kd = 0.6 nM), VPAC1R ([125I]VIP Kd = N/A), VPAC2R ([125I]VIP Kd = N/A) 4 | [148] | |
| PACAP(6-38) | Antagonist | N-terminal truncated PACAP analogue | PAC1R ([125I]Ac-His1-PACAP27 Ki = 30 nM), VPAC1R ([125I]VIP Ki = 600 nM), VPAC2R ([125I]Ac-His1-PACAP27 Ki = 40 nM) 5 | [149,150] |
| N-Terminal Truncation/Modification | ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | |||||||||||||||||||||||||||||||
| PACAP38 | H | S | D | G | I | F | T | D | S | Y | S | R | Y | R | K | Q | M | A | V | K | K | Y | L | A | A | V | L | G | K | R | Y | K | Q | R | V | K | N | K |
| PACAP(6–38) | H | S | D | G | I | F | T | D | S | Y | S | R | Y | R | K | Q | M | A | V | K | K | Y | L | A | A | V | L | G | K | R | Y | K | Q | R | V | K | N | K |
| VIP | H | S | D | A | V | F | T | D | N | Y | T | R | L | R | K | Q | M | A | V | K | K | Y | L | N | S | I | L | N | ||||||||||
| PG 97-269 1 | H | F | D | A | V | F | T | N | S | Y | R | K | V | L | K | R | L | S | A | R | K | L | L | Q | D | I | L | |||||||||||
| PG 99-465 2 | H | S | D | A | V | F | T | D | N | Y | T | K | L | R | K | Q | M | A | V | K | K | Y | L | N | S | I | K | K | G | G | T | |||||||
| C-Terminal Truncation/Elongation | ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | |||||||||||||||||||||||||||||||
| PACAP38 | H | S | D | G | I | F | T | D | S | Y | S | R | Y | R | K | Q | M | A | V | K | K | Y | L | A | A | V | L | G | K | R | Y | K | Q | R | V | K | N | K |
| [R16]-PACAP(1–23) | H | S | D | G | I | F | T | D | S | Y | S | R | Y | R | R | Q | M | A | V | K | K | Y | L | A | A | V | L | G | K | R | Y | K | Q | R | V | K | N | K |
| VIP | H | S | D | A | V | F | T | D | N | Y | T | R | L | R | K | Q | M | A | V | K | K | Y | L | N | S | I | L | N | ||||||||||
| RO 25-1553 1 | H | S | D | A | V | F | T | E | N | Y | T | K | L | R | K | Q | L | A | A | K | K | Y | L | N | D | L | K | K | G | G | T | |||||||
| PG 96-249a 2 | H | S | D | A | V | F | T | E | N | Y | T | K | L | R | K | Q | L | A | A | K | K | Y | L | N | D | L | K | K | G | G | T | |||||||
| Receptor | Region | Agonist | PDB | Resolution | Method |
|---|---|---|---|---|---|
| VPAC2R [296] 1 | ECD | – | 2X57 | 2.1 Å | X-ray diffraction |
| PAC1sR [297] | ECD | – | 3N94 | 1.8 Å | X-ray diffraction |
| PAC1sR [298] | ECD | PACAP (6–38) | 2JOD | – | Solution NMR |
| Receptor | Agonist | G Protein 1 | PDB | Resolution |
|---|---|---|---|---|
| PAC1nR [135] | PACAP38 | DNGαs, Gβ1, Gγ2 | 6P9Y | 3.0 Å |
| PAC1nR [139] | PACAP38 | Mini-Gαs, Gβ1, Gγ2 | 6LPB | 3.9 Å |
| PAC1sR [138] | PACAP38 | DNGαs, Gβ1, Gγ2 | 6M1I | 3.5 Å |
| PAC1sR [138] | Maxadilan | DNGαs, Gβ1, Gγ2 | 6M1H | 3.6 Å |
| VPAC1R-LgBiT 2 [136] | PACAP27 | DNGαs, Gβ1-HiBiT, Gγ2 | 6VN7 | 3.2 Å |
| VPAC2R-LgBiT 2 [137] | PACAP27 | DNGαs, Gβ1-HiBiT, Gγ2 | 7VQX 3 | 2.7 Å |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Piper, S.J.; Zhao, P.; Miller, L.J.; Wootten, D.; Sexton, P.M. Targeting VIP and PACAP Receptor Signaling: New Insights into Designing Drugs for the PACAP Subfamily of Receptors. Int. J. Mol. Sci. 2022, 23, 8069. https://doi.org/10.3390/ijms23158069
Lu J, Piper SJ, Zhao P, Miller LJ, Wootten D, Sexton PM. Targeting VIP and PACAP Receptor Signaling: New Insights into Designing Drugs for the PACAP Subfamily of Receptors. International Journal of Molecular Sciences. 2022; 23(15):8069. https://doi.org/10.3390/ijms23158069
Chicago/Turabian StyleLu, Jessica, Sarah J. Piper, Peishen Zhao, Laurence J. Miller, Denise Wootten, and Patrick M. Sexton. 2022. "Targeting VIP and PACAP Receptor Signaling: New Insights into Designing Drugs for the PACAP Subfamily of Receptors" International Journal of Molecular Sciences 23, no. 15: 8069. https://doi.org/10.3390/ijms23158069
APA StyleLu, J., Piper, S. J., Zhao, P., Miller, L. J., Wootten, D., & Sexton, P. M. (2022). Targeting VIP and PACAP Receptor Signaling: New Insights into Designing Drugs for the PACAP Subfamily of Receptors. International Journal of Molecular Sciences, 23(15), 8069. https://doi.org/10.3390/ijms23158069






