The Role of Psychobiotics in Supporting the Treatment of Disturbances in the Functioning of the Nervous System—A Systematic Review
Abstract
1. Introduction
1.1. Psychobiotics
1.1.1. Microorganisms and Their Metabolites
1.1.2. The Intestinal Microbiome and Disorders of the Nervous System
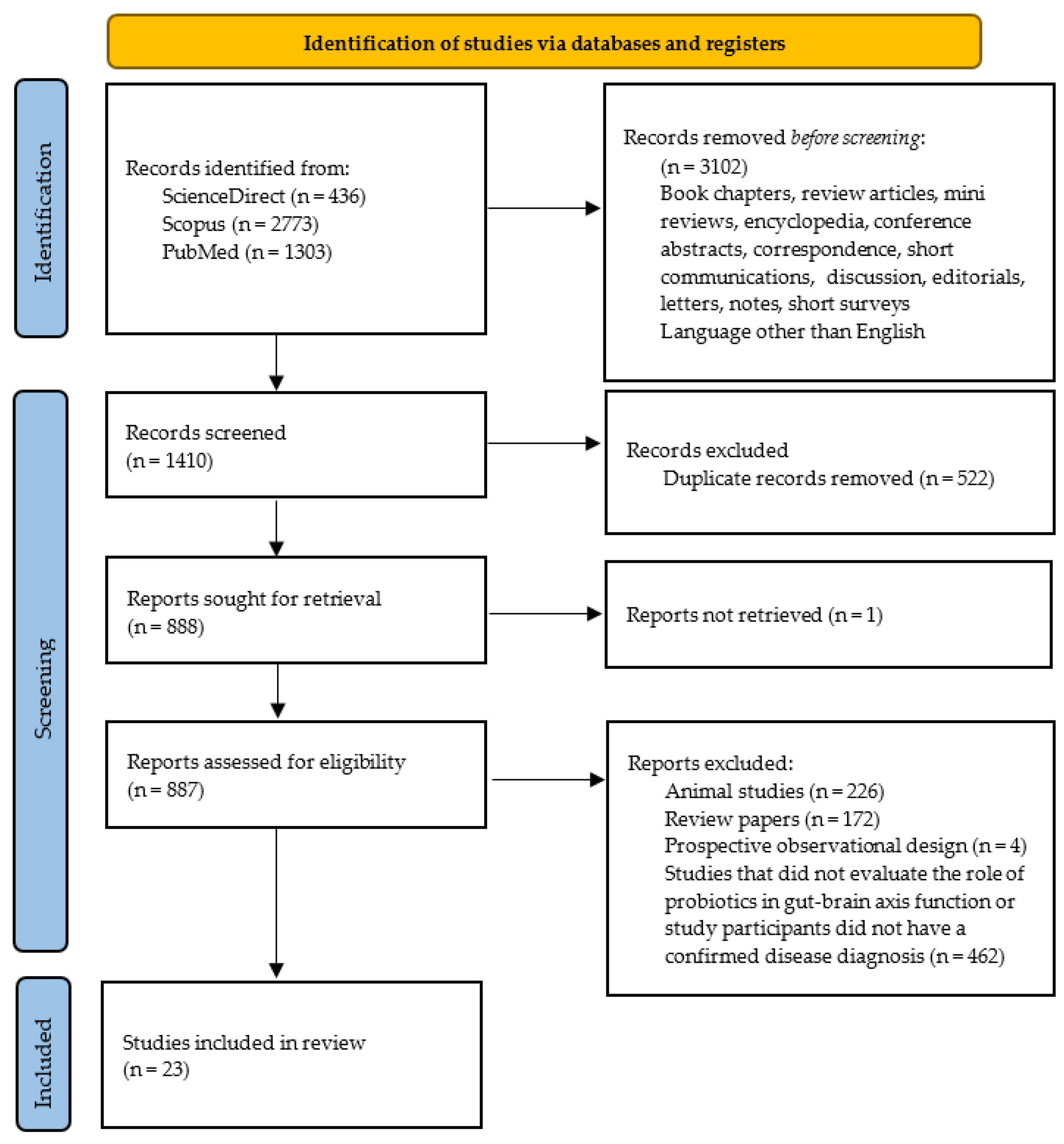
2. Materials and Methods
Search Strategy
3. Results and Discussion
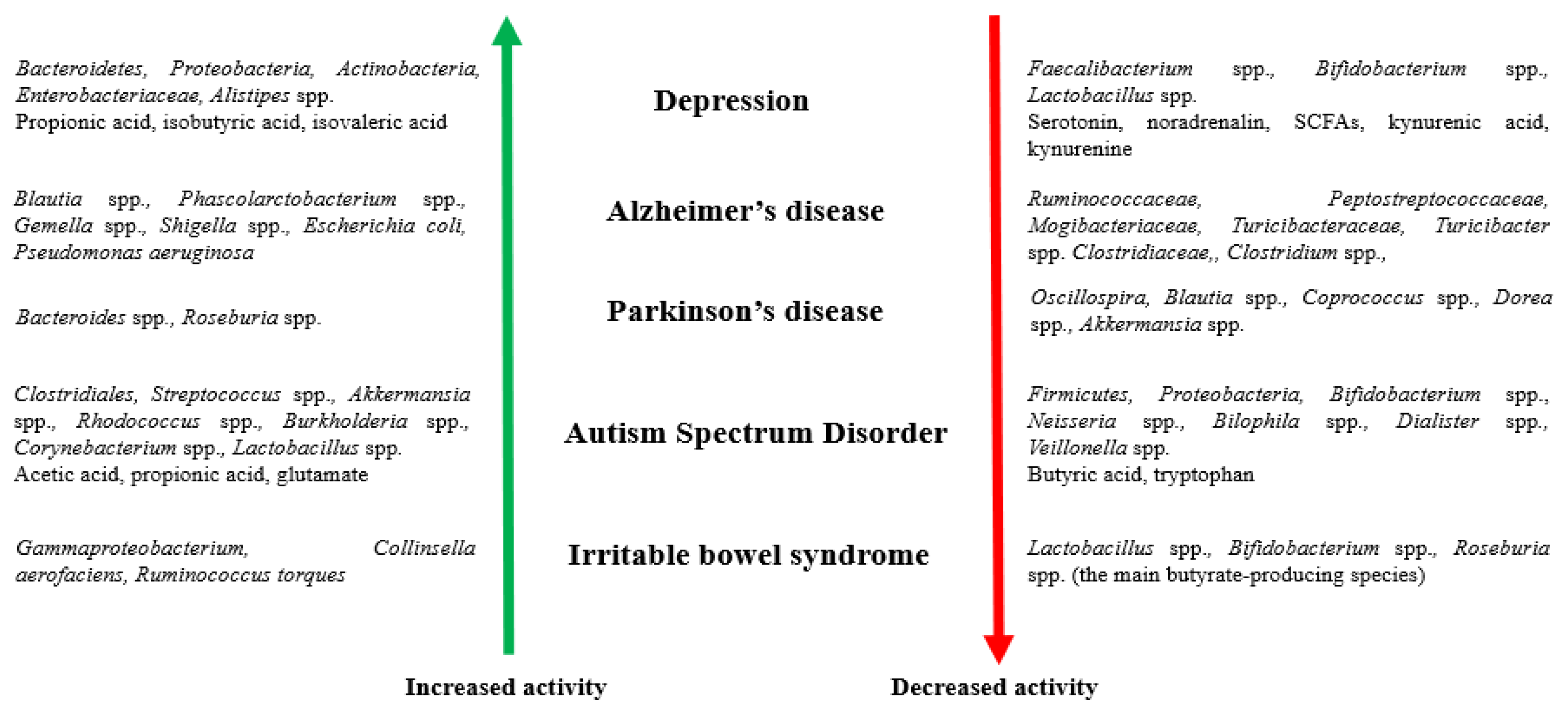
3.1. Depression
3.2. Alzheimer’s Disease
3.3. Parkinson’s Disease
3.4. Autism Spectrum Disorder
| Type of Examination | Study Population | Preparation/Probiotic Bacteria | Duration of the Intervention | Results | Statistical Significance | References | |
|---|---|---|---|---|---|---|---|
| DEPRESSION | Pilot study | 12 patients diagnosed with SSRI-treatment-resistant depression (mean age, 19.8 ± 5.7 years) | Magnesium orotate (1600 mg), and probiotics (Lactobacillus acidophilus, Bifidobacterium bifidum, amd Streptoccocus thermophiles) (total CFU 2 × 1010 divided between 2 daily doses) | 16 weeks (active intervention administered for 8 weeks) |
|
| [53] |
| Double-blind, placebo controlled, randomized, multi-centre, pilot clinical study | 40 patients with mild to moderate IBS and MDD (mean age, 40.36 ± 10.28 years) | Bacillus coagulans MTCC 5856 (2 × 109 CFU) (1 tablet per day) | 90 days |
|
| [55] | |
| Placebo-controlled, double-blind randomized controlled trial | 45 patients with mild to moderate IBS and MDD (mean age, 51.32 ± 16.11 years) | Bifidobacterium breve CCFM1025 (total CFU 1010) (1 sachet per day) | 4 weeks |
|
| [56] | |
| Randomized, double-blind, controlled placebo trial | 40 patients with a diagnosis of major depressive disorder (age range: 20–55) | Lactobacillus acidophilus (2 × 109 CFU/g), Lactobacillus casei (2 × 109 CFU/g), and Bifidobacterium bifidum (2 × 109 CFU/g) (1 capsule per day) | 8 weeks |
|
| [47] | |
| Prospective open-label trial | 40 patients with treatment-resistant major depressive disorder (mean age, 44.2 ± 15.6 years) | Clostridium butyricum MIYAIRI 588 (CBM588) (20 mg orally/twice a day for the first week; 20 mg orally/three times a day from weeks 2 to 8) | 8 weeks |
|
| [54] | |
| Open-label single-arm study | 29 outpatients with schizophrenia with anxiety and depressive symptoms (mean age, 45 (16) years) | Bifidobacterium breve A-1 (5.0 × 1010 CFU) (2 sachets per day) | 8 weeks (active intervention administered for 4 weeks) |
|
| [57] | |
| Three-arm parallel design, placebo-controlled, double-blind randomized controlled trial | 81 patients with mild to moderate major depression (mean age, 36.5 ± 8.03 years) | Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 (≥10 × 109 CFU) (1 sachet per day) | 8 weeks |
|
| [61] | |
| Double-blind, placebo-controlled, single-center, parallel design randomized controlled trial | 110 patients with a diagnosis of major depression (mean age, 36.15 ± 7.85 years) | Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 (≥10 × 109 CFU) (1 sachet per day) | 8 weeks |
|
| [60] | |
| Double-blind, randomized controlled trial | 78 patients with low to moderate depression (mean age, 36.0 ± 9.0 years) | Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 (≥10 × 109 CFU) (1 sachet per day) | 8 weeks |
|
| [62] | |
| Randomized, triple blind, controlled placebo trial | 71 participants with mild to severe depression (mean age, 36.65 ± 11.75 years in probiotic group) | Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivactocarius W56, Lactobacillus casei W56, Lactococcus lactis W19, and Lactococcus lactis W58 (total cell count 1 × 1010 CFU) (2 sachets per day) | 2 months |
|
| [43] | |
| Double-blind, randomized, placebo controlled trial | 79 participants with major depressive disorder (mean age, 39.13 ± 9.96 years in probiotic group) | Lactobacillus plantarum 299v (1 × 109 CFU) (2 capsules per day) | 8 weeks |
|
| [64] | |
| Single-center uncontrolled trial | 83 patients with symptoms suggesting anxiety/depression (mean age, 43.9 ± 12.3 years) | Bifidobacterium bifidum W23, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, Lactococcus lactis W19, and Lactococcus lactis W58 (over 2.5 × 109 CFU/g) (1 sachet per day) | 8 weeks |
|
| [58] | |
| Open-label exploratory study | 10 participants in a current episode of MDD (mean age, 25.2 ± 7.0 years) | Lactobacillus helveticus R0052 (90%) and Bifidobacterium longum R0175 (10%) (3 × 109 CFU) (1 sachet per day) | 8 weeks |
|
| [59] | |
| Randomized placebo-controlled study | 119 participants with a mild or moderate depressive episode (mean age, 32.9 ± 6.1 years) | Lactobacillus casei PXN 37, Lactobacillus plantarum PXN 47, Lactobacillus rhamnosus PXN 54, Lactobacillus acidophilus PXN 35, Lactobacillus bulgaricus PXN 39, Lactobacillus helveticus PXN 45, Lactobacillus salivarius PXN 57, Lactobacillus fermentum PXN 44, Lactococcus lactis ssp. Lactis PXN 63, Streptococcus thermophilus PXN 66, Bifidobacterium bifidum PXN 23, Bifidobacterium breve PXN 25, Bifidobacterium longum PXN 30, and Bifidobacterium infantis PXN 27 (2 × 109 CFU) (3 capsules per day) | 6 weeks |
|
| [63] | |
| Open trial | 11 patients with major depressive disorder (mean age, 39.4 ± 12.0 years) | Lactobacillus plantarum PS128 (3 × 1010 CFU) (2 capsules per day) | 8 weeks |
|
| [65] | |
| Double-blind, randomized placebo-controlled trial | 61 depressed patients (mean age, 43 ± 14.31 years) | Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Bifidobacterium lactis W52, Lactobacillus acidophilus W22, Lactobacillus casei W56, Lactobacillus paracasei W20, Lactobacillus plantarum W62, Lactobacillus salivarius W24, and Lactobacillus lactis W19 (at least 7.5 × 1012 CFU), and 125 mg of D-Biotin (vitamin B7), 30 mg of common horsetail, 30 mg of fish collagen, and 30 mg of keratin (1 portion per day) | 28 days |
|
| [66] | |
| ALZHEIMER’S DISEASE | Randomized, double-blind, placebo-controlled trial | 60 patients (mean age, 77.67 ± 2.62 years in probiotic group) | Milk (200 mL per day) enriched with probiotic bacteria: Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus fermentum (2 × 109 CFU/g each) | 12 weeks |
|
| [88] |
| Randomized, double-blind, placebo-controlled | 79 patients (mean age, 76.2 ± 8.1 years in probiotic group) | Selenium (200 μg/day) and probiotic containing Lactobacillus acidophilus, Bifidobacterium bifidum, and Bifidobacterium longum (2 × 109 CFU/day each) | 12 weeks |
|
| [89] | |
| Randomized, double-blind, placebo-controlled trial | 48 patients (mean age, 79.70 ± 1.72 years in probiotic group) | Two variants of the preparation: Lactobacillus fermentum, Lactobacillus plantarum, and Bifidobacterium lactis, or Lactobacillus acidophilus, Bifidobacterium bifidum, and Bifidobacterium longum (each with a total dosage of 3 × 109 CFU) (1 of each capsule per day) | 12 weeks |
|
| [90] | |
| Uncontrolled clinical trial | 13 patients with AD exhibiting cognitive deficit (mean age of women, 78.7 ± 3 years; mean age of men, 78 ± 7 years) | Probiotic-fermented milk: pasteurized milk inoculated with 4% kefir grains containing the species Acetobacter aceti, Acetobacter sp., Lactobacillus delbrueckii delbrueckii, Lactobacillus fermentum, Lactobacillus fructivorans, Enterococcus faecium, Leuconostoc spp., Lactobacillus kefiranofaciens, Candida famata, and Candida krusei (2 mL/kg/daily) | 90 days |
|
| [91] | |
| PARKINSON’S DISEASE | Open-label, single-arm, baseline-controlled trial | 25 patients (mean age, 61.84 ± 5.74 years) | Lactobacillus plantarum PS128 (3 × 1013 CFU) (2 capsules per day) | 12 weeks |
|
| [108] |
| AUTISM SPECTRUM DISORDER | Real-world experience | 131 autistic children and adolescents (age: 86.1 ± 41.1 months) | Lactobacillus plantarum PS128 (105 patients) (6 × 1010 CFU or 3 × 1010 CFU if patient’s weight was <30 kg) or other probiotics (not listed in the publication) (dose in the recommended range according to age, weight, and specific product) | 6 months |
|
| [126] |
| Randomized, double-blind, controlled placebo pilot trial | 35 individuals with ASD (mean age, 9.85 ± 4.91 years in probiotic group) | Lactobacillus plantarum PS128 (6 × 1010 CFU) (2 capsules per day) and oxytocin from 16 weeks | 28 weeks |
|
| [75] | |
| Randomized, double-blind, controlled placebo trial | 63 preschoolers with ASD (mean age, 4.16 ± 1.17 years in probiotic group) | “Vivomixx” (Streptococcus thermophilus DSM 24731, Bifidobacterium breve DSM 24732, Bifidobacterium longum DSM 24736, Bifidobacterium infantis DSM 24737, Lactobacillus acidophilus DSM 24735, Lactobacillus plantarum DSM 24730, Lactobacillus paracasei DSM 24733, and Lactobacillus delbrueckii subsp. bulgaricus DSM 24734) (4.5 × 1011 CFU) (2 packets or 1 packet/day in the first and in the following 5 months, accordingly) | 6 months |
|
| [127] | |
| Randomized, double-blind, placebo-controlled study | 71 boys with ASD (mean age, 10.01 ± 2.32 years) | Lactobacillus plantarum PS128 (PS128) (3 × 1010 CFU/capsule) | 4 weeks |
|
| [76] | |
| Prospective, open-label study | 30 autistic children (mean age, 84.77 ± 16.37 months) | Bifidobacterium longum, Lactobacillus rhamnosus, and Lactobacillus acidophilus (5 × 108 CFU) (1 sachet per day) | 3 months |
|
| [128] |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Alliance on Mental Illness (NAMI). Mental Health By the Numbers. 2021. Available online: https://www.nami.org/mhstats (accessed on 29 October 2021).
- Kong, X.-J.; Liu, J.; Li, J.; Kwong, K.; Koh, M.; Sukijthamapan, P.; Guo, J.J.; Sun, Z.J.; Song, Y. Probiotics and oxytocin nasal spray as neuro-social-behavioral interventions for patients with autism spectrum disorders: A pilot randomized controlled trial protocol. Pilot Feasibility Stud. 2020, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Gupta, D.; Mehrotra, R.; Mago, P. Psychobiotics: The Next-Generation Probiotics for the Brain. Curr. Microbiol. 2021, 78, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.-H.; Liu, Y.-W.; Wu, C.-C.; Wang, S.; Tsai, Y.-C. Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. J. Food Drug Anal. 2019, 27, 632–648. [Google Scholar] [CrossRef] [PubMed]
- Averina, O.V.; Zorkina, Y.A.; Yunes, R.A.; Kovtun, A.S.; Ushakova, V.M.; Morozova, A.Y.; Kostyuk, G.P.; Danilenko, V.N.; Chekhonin, V.P. Bacterial Metabolites of Human Gut Microbiota Correlating with Depression. Int. J. Mol. Sci. 2020, 21, 9234. [Google Scholar] [CrossRef]
- Fuller, R. Probiotics in man and animals. J. Appl. Bacteriol. 1989, 66, 365–378. [Google Scholar] [PubMed]
- Hutkins, R.W.; Krumbeck, J.A.; Bindels, L.B.; Cani, P.D.; Fahey, G., Jr.; Goh, Y.J.; Hamaker, B.; Martens, E.C.; Mills, D.A.; Rastal, R.A.; et al. Prebiotics: Why definitions matter. Curr. Opin. Biotechnol. 2016, 37, 1–7. [Google Scholar] [CrossRef]
- Gulas, E.; Wysiadecki, G.; Strzelecki, D.; Gawlik-Kotelnicka, O.; Polguj, M. Jak mikrobiologia może wpływać na psychiatrię? Powiązania między florą bakteryjną. Psychiatr. Pol. 2018, 52, 1023–1039. [Google Scholar]
- Żakowicz, J.; Bramorska, A.; Zarzycka, W.; Kovbasiuk, A.; Kuć, K.; Brzezicka, A. Wpływ mikrobioty jelitowej na mózg, funkcje poznawcze i emocje. KOSMOS Probl. Nauk. Biol. 2020, 1, 45–58. [Google Scholar] [CrossRef][Green Version]
- Staniak, N. Psychobiotyki w rekomendacji farmaceuty. Aptekarz Polski. 2018, 146, 124e. [Google Scholar]
- Wierzchanowska, W.M.; Iwanicki, T. Rola mikrobiomu jelitowego w funkcjonowaniu układu nerwowego. Kosmos. Probl. Nauk. Biol. 2020, 69, 301–311. [Google Scholar] [CrossRef]
- Cenit, M.C.; Sanz, Y.; Codoñer-Franch, P. Influence of gut microbiota on neuropsychiatric disorders. World J. Gastroenterol. 2017, 23, 5486–5498. [Google Scholar] [CrossRef]
- Zhu, X.; Han, Y.; Du, J.; Liu, R.; Jin, K.; Yi, W. Microbiota-gut-brain axis and the central nervous system. Oncotarget 2017, 8, 53829–53838. [Google Scholar] [CrossRef]
- Erny, D.; Hrabe de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, A.; Witecka, A.; Kiersztan, A. The role of gut microbiota in the pathogenesis of neuropsychiatric and neurodegenerative diseases. Postępy Hig. Med. Dośw. 2019, 73, 865–886. [Google Scholar] [CrossRef]
- Montiel-Castro, A.J.; González-Cervantes, R.M.; Ebravo-Ruiseco, G.; Epacheco-Lopez, G. The microbiota-gut-brain axis: Neurobehavioral correlates, health and sociality. Front. Integr. Neurosci. 2013, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria–Gut–Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef]
- Dobrowolska-Iwanek, J.; Zagrodzki, P.; Prochownik, E.; Jarkiewicz, A.; Paśko, P. Influence of brassica sprouts on short chain fatty acids concentration in stools of rats with thyroid dysfunction. Acta Pol. Pharm. Drug. Res. 2019, 76, 1005–1014. [Google Scholar] [CrossRef]
- Sandhu, K.V.; Sherwin, E.; Schellekens, H.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Feeding the microbiota-gut-brain axis: Diet, microbiome, and neuropsychiatry. Transl. Res. 2016, 179, 223–244. [Google Scholar] [CrossRef]
- Lyte, M. The microbial organ in the gut as a driver of homeostasis and disease. Med. Hypotheses 2010, 74, 634–638. [Google Scholar] [CrossRef]
- Glenny, E.M.; Bulik-Sullivan, E.C.; Tang, Q.; Bulik, C.; Carroll, I.M. Eating Disorders and the Intestinal Microbiota: Mechanisms of Energy Homeostasis and Behavioral Influence. Curr. Psychiatry Rep. 2017, 19, 51. [Google Scholar] [CrossRef] [PubMed]
- Eisenhofer, G.; Aneman, A.; Friberg, P.; Hooper, D.; Fandriks, L.; Lonroth, H.; Mezey, E. Substantial production of dopamine in the human gastrointestinal tract. J. Clin. Endocrinol. Metab. 1997, 82, 3864e71. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 2008, 43, 164–174. [Google Scholar] [CrossRef]
- Kobayashi, K. Role of Catecholamine Signaling in Brain and Nervous System Functions: New Insights from Mouse Molecular Genetic Study. J. Investig. Dermatol. Symp. Proc. 2001, 6, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-W.; Liu, W.-H.; Wu, C.-C.; Juan, Y.-C.; Wu, Y.-C.; Tsai, H.-P.; Wang, S.; Tsai, Y.-C. Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naïve adult mice. Brain Res. 2016, 1631, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Samardzic, J.; Jadzic, D.; Hencic, B.; Jancic, J.; Strac, D.S. Introductory Chapter: GABA/Glutamate Balance: A Key for Normal Brain Functioning. In GABA and Glutamate—New Developments in Neurotransmission Research; Samardzic, J., Ed.; InTech: London, UK, 2018; ISBN 978-953-51-3821-1. [Google Scholar]
- Girvin, G.T.; Stevenson, J.W. Cell free choline acetylase from Lactobacillus plantarum. Can. J. Biochem. Physiol. 1954, 32, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Nimgampalle, M. Anti-Alzheimer Properties of Probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer’s Disease induced Albino Rats. J. Clin. Diagn. Res. 2017, 11, KC01–KC05. [Google Scholar] [CrossRef]
- Laursen, M.F.; Sakanaka, M.; von Burg, N.; Mörbe, U.; Andersen, D.; Moll, J.M.; Pekmez, C.T.; Rivollier, A.; Michaelsen, K.F.; Mølgaard, C.; et al. Bifidobacterium species associated with breastfeeding produce aromatic lactic acids in the infant gut. Nat. Microbiol. 2021, 6, 1367–1382. [Google Scholar] [CrossRef]
- Sakurai, T.; Odamaki, T.; Xiao, J.-Z. Production of Indole-3-Lactic Acid by Bifidobacterium Strains Isolated from Human Infants. Microorganisms 2019, 7, 340. [Google Scholar] [CrossRef]
- Bjerre, K.; Cantor, M.D.; Nørgaard, J.V.; Poulsen, H.D.; Blaabjerg, K.; Canibe, N.; Jensen, B.B.; Stuer-Lauridsen, B.; Nielsen, B.; Derkx, P.M.F. Development of Bacillus subtilis mutants to produce tryptophan in pigs. Biotechnol. Lett. 2016, 39, 289–295. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna-Żydecka, K.; Marlicz, W.; Misera, A.; Koulaouzidis, A.; Łoniewski, I. Microbiome—The Missing Link in the Gut-Brain Axis: Focus on Its Role in Gastrointestinal and Mental Health. J. Clin. Med. 2018, 7, 521. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Ogyu, K.; Kubo, K.; Noda, Y.; Iwata, Y.; Tsugawa, S.; Omura, Y.; Wada, M.; Tarumi, R.; Plitman, E.; Moriguchi, S.; et al. Kynurenine pathway in depression: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2018, 90, 16–25. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Green, S.J.; Engen, P.A.; Voigt, R.M.; Naqib, A.; Forsyth, C.B.; Mutlu, E.; Shannon, K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015, 30, 1351–1360. [Google Scholar] [CrossRef]
- Kushak, R.I.; Winter, H.S.; Buie, T.M.; Cox, S.B.; Phillips, C.; Ward, N.L. Analysis of the Duodenal Microbiome in Autistic Individuals: Association With Carbohydrate Digestion. J. Pediatr. Gastroenterol. Nutr. 2017, 64, e110–e116. [Google Scholar] [CrossRef]
- Bryn, V.; Verkerk, R.; Skjeldal, O.H.; Saugstad, O.D.; Ormstad, H. Kynurenine Pathway in Autism Spectrum Disorders in Children. Neuropsychobiology 2017, 76, 82–88. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, M.; Li, M.; Bhaskar, Y.; Zhao, J.; Ji, Y.; Cui, H.; Zhang, H.; Sun, Z. Interactions between Human Gut Microbiome Dynamics and Sub-Optimal Health Symptoms during Seafaring Expeditions. Microbiol. Spectr. 2022, 10, e00925-21. [Google Scholar] [CrossRef]
- Chahwan, B.; Kwan, S.; Isik, A.; van Hemert, S.; Burke, C.; Roberts, L. Gut feelings: A randomised, triple-blind, placebo-controlled trial of probiotics for depressive symptoms. J. Affect. Disord. 2019, 253, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Andersson, H.; Tullberg, C.; Ahrné, S.; Hamberg, K.; Lazou Ahrén, I.; Molin, G.; Sonesson, M.; Håkansson, Å. Oral Administration of Lactobacillus plantarum 299v Reduces Cortisol Levels in Human Saliva during Examination Induced Stress: A Randomized, Double-Blind Controlled Trial. Int. J. Microbiol. 2016, 2016, 8469018. [Google Scholar] [CrossRef] [PubMed]
- Chassard, C.; Dapoigny, M.; Scott, K.P.; Crouzet, L.; Del’Homme, C.; Marquet, P.; Martin, J.C.; Pickering, G.; Ardid, D.; Eschalier, A.; et al. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment. Pharmacol. Ther. 2012, 35, 828–838. [Google Scholar] [CrossRef]
- Fontana, A.; Falasconi, I.; Molinari, P.; Treu, L.; Basile, A.; Vezzi, A.; Campanaro, S.; Morelli, L. Genomic Comparison of Lactobacillus helveticus Strains Highlights Probiotic Potential. Front. Microbiol. 2019, 10, 1380. [Google Scholar] [CrossRef] [PubMed]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Stilling, R.M.; Dinan, T.G.; Cryan, J.F. Microbial genes, brain & behaviour—Epigenetic regulation of the gut-brain axis. Genes Brain. Behav. 2014, 13, 69–86. [Google Scholar] [PubMed]
- Schmidt, K.; Cowen, P.; Harmer, C.; Tzortzis, G.; Errington, S.; Burnet, P.W.J. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology 2014, 232, 1793–1801. [Google Scholar] [CrossRef]
- Huo, R.; Zeng, B.; Zeng, L.; Cheng, K.; Li, B.; Luo, Y.; Wang, H.; Zhou, C.; Fang, L.; Li, W.; et al. Microbiota Modulate Anxiety-Like Behavior and Endocrine Abnormalities in Hypothalamic-Pituitary-Adrenal Axis. Front. Cell. Infect. Microbiol. 2017, 7, 489. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Bambling, M.; Edwards, S.C.; Hall, S.; Vitetta, L. A combination of probiotics and magnesium orotate attenuate depression in a small SSRI resistant cohort: An intestinal anti-inflammatory response is suggested. Inflammopharmacology 2017, 25, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Miyaoka, T.; Kanayama, M.; Wake, R.; Hashioka, S.; Hayashida, M.; Nagahama, M.; Okazaki, S.; Yamashita, S.; Miura, S.; Miki, H.; et al. Clostridium butyricum MIYAIRI 588 as Adjunctive Therapy for Treatment-Resistant Major Depressive Disorder: A Prospective Open-Label Trial. Clin. Neuropharmacol. 2018, 41, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Nagabhushanam, K.; Arumugam, S.; Majeed, S.; Ali, F. Bacillus coagulans MTCC 5856 for the management of major depression with irritable bowel syndrome: A randomised, double-blind, placebo controlled, multi-centre, pilot clinical study. Food Nutr. Res. 2018, 62, 1218. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Chen, Y.; Zhu, H.; Wang, L.; Qian, X.; Zou, R.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav. Immun. 2021, 100, 233–241. [Google Scholar] [CrossRef]
- Okubo, R.; Koga, M.; Katsumata, N.; Odamaki, T.; Matsuyama, S.; Oka, M.; Narita, H.; Hashimoto, N.; Kusumi, I.; Xiao, J.; et al. Effect of Bifidobacterium breve A-1 on anxiety and depressive symptoms in schizophrenia: A proof-of-concept study. J. Affect. Disord. 2019, 245, 377–385. [Google Scholar] [CrossRef]
- Dao, V.H.; Hoang, L.B.; Trinh, T.O.; Tran, T.T.T.; Dao, V.L. Psychobiotics for Patients with Chronic Gastrointestinal Disorders Having Anxiety or Depression Symptoms. J. Multidiscip. Health 2021, 14, 1395–1402. [Google Scholar] [CrossRef]
- Wallace, C.J.K.; Milev, R.V. The Efficacy, Safety, and Tolerability of Probiotics on Depression: Clinical Results From an Open-Label Pilot Study. Front. Psychiatry 2021, 12, 618279. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Djafarian, K. Effect of prebiotic and probiotic supplementation on circulating pro-inflammatory cytokines and urinary cortisol levels in patients with major depressive disorder: A double-blind, placebo-controlled randomized clinical trial. J. Funct. Foods 2018, 52, 596–602. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr. 2019, 38, 522–528. [Google Scholar] [CrossRef]
- Heidarzadeh-Rad, N.; Gökmen-Özel, H.; Kazemi, A.; Almasi, N.; Djafarian, K. Effects of a Psychobiotic Supplement on Serum Brain-derived Neurotrophic Factor Levels in Depressive Patients: A Post Hoc Analysis of a Randomized Clinical Trial. J. Neurogastroenterol. Motil. 2020, 26, 486–495. [Google Scholar] [CrossRef]
- Alli, S.R.; Gorbovskaya, I.; Liu, J.C.W.; Kolla, N.J.; Brown, L.; Müller, D.J. The Gut Microbiome in Depression and Potential Benefit of Prebiotics, Probiotics and Synbiotics: A Systematic Review of Clinical Trials and Observational Studies. Int. J. Mol. Sci. 2022, 23, 4494. [Google Scholar] [CrossRef] [PubMed]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2018, 100, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-M.; Kuo, P.-H.; Hsu, C.-Y.; Chiu, Y.-H.; Liu, Y.-W.; Lu, M.-L.; Chen, C.-H. Psychophysiological Effects of Lactobacillus plantarum PS128 in Patients with Major Depressive Disorder: A Preliminary 8-Week Open Trial. Nutrients 2021, 13, 3731. [Google Scholar] [CrossRef] [PubMed]
- Reininghaus, E.Z.; Platzer, M.; Kohlhammer-Dohr, A.; Hamm, C.; Mörkl, S.; Bengesser, S.A.; Fellendorf, F.T.; Lahousen-Luxenberger, T.; Leitner-Afschar, B.; Schöggl, H.; et al. PROVIT: Supplementary Probiotic Treatment and Vitamin B7 in Depression—A Randomized Controlled Trial. Nutrients 2020, 12, 3422. [Google Scholar] [CrossRef] [PubMed]
- WHO. Depression. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 4 May 2022).
- Belmaker, R.H.; Agam, G. Major depressive disorder. N. Engl. J. Med. 2008, 358, 55–68. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Tian, P.; Chen, Y.; Qian, X.; Zou, R.; Zhu, H.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Pediococcus acidilactici CCFM6432 mitigates chronic stress-induced anxiety and gut microbial abnormalities. Food Funct. 2021, 12, 11241–11249. [Google Scholar] [CrossRef]
- Bermúdez-Humarán, L.G.; Salinas, E.; Ortiz, G.G.; Ramírez-Jirano, L.J.; Morales, J.A.; Bitzer-Quintero, O.K. From Probiotics to Psychobiotics: Live Beneficial Bacteria Which Act on the Brain-Gut Axis. Nutrients 2019, 11, 890. [Google Scholar] [CrossRef]
- Dylus, E.; Buda, B.; Górska-Frączek, S.; Brzozowska, E.; Brzozowska, A. Białka powierzchniowe bakterii z rodzaju Bifidobacterium. Postępy Hig. Med. Dośw. 2013, 67, 402–412. [Google Scholar] [CrossRef]
- Zhu, H.; Tian, P.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. A psychobiotic approach to the treatment of depression: A systematic review and meta-analysis. J. Funct. Foods 2022, 91, 104999. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A Novel Class of Psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.-J.; Liu, J.; Liu, K.; Koh, M.; Sherman, H.; Liu, S.; Tian, R.; Sukijthamapan, P.; Wang, J.; Fong, M.; et al. Probiotic and Oxytocin Combination Therapy in Patients with Autism Spectrum Disorder: A Randomized, Double-Blinded, Placebo-Controlled Pilot Trial. Nutrients 2021, 13, 1552. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-W.; Liong, M.T.; Chung, Y.-C.E.; Huang, H.-Y.; Peng, W.-S.; Cheng, Y.-F.; Lin, Y.-S.; Wu, Y.-Y.; Tsai, Y.-C. Effects of Lactobacillus plantarum PS128 on Children with Autism Spectrum Disorder in Taiwan: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 820. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Y.; Wu, Y.-P.; Jia, X.-Z.; Lin, J.; Xiao, L.-F.; Liu, D.-M.; Liang, M.-H. Lactiplantibacillus plantarum DMDL 9010 alleviates dextran sodium sulfate (DSS)-induced colitis and behavioral disorders by facilitating microbiota-gut-brain axis balance. Food Funct. 2021, 13, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Bu, F.; Chen, T.; Shi, G.; Yuan, X.; Feng, Z.; Duan, Z.; Wang, R.; Zhang, S.; Wang, Q.; et al. A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress–induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl. Microbiol. Biotechnol. 2021, 105, 8411–8426. [Google Scholar] [CrossRef]
- Tian, P.; O’Riordan, K.J.; Lee, Y.-K.; Wang, G.; Zhao, J.; Zhang, H.; Cryan, J.F.; Chen, W. Towards a psychobiotic therapy for depression: Bifidobacterium breve CCFM1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol. Stress 2020, 12, 100216. [Google Scholar] [CrossRef]
- Birmann, P.T.; Casaril, A.M.; Pesarico, A.P.; Caballero, P.S.; Smaniotto, T.; Rodrigues, R.R.; Moreira, N.; Conceição, F.R.; Sousa, F.S.; Collares, T.; et al. Komagataella pastoris KM71H modulates neuroimmune and oxidative stress parameters in animal models of depression: A proposal for a new probiotic with antidepressant-like effect. Pharmacol. Res. 2021, 171, 105740. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, S.; Yang, X.; Li, W.; Si, J.; Yang, X. The antidepressant potential of lactobacillus casei in the postpartum depression rat model mediated by the microbiota-gut-brain axis. Neurosci. Lett. 2022, 774, 136474. [Google Scholar] [CrossRef]
- Abildgaard, A.; Kern, T.; Pedersen, O.; Hansen, T.; Lund, S.; Wegener, G. A diet-induced gut microbiota component and related plasma metabolites are associated with depressive-like behaviour in rats. Eur. Neuropsychopharmacol. 2020, 43, 10–21. [Google Scholar] [CrossRef]
- Daugé, V.; Philippe, C.; Mariadassou, M.; Rué, O.; Martin, J.-C.; Rossignol, M.-N.; Dourmap, N.; Svilar, L.; Tourniaire, F.; Monnoye, M.; et al. A Probiotic Mixture Induces Anxiolytic- and Antidepressive-Like Effects in Fischer and Maternally Deprived Long Evans Rats. Front. Behav. Neurosci. 2020, 14, 214. [Google Scholar] [CrossRef]
- Gu, F.; Wu, Y.; Liu, Y.; Dou, M.; Jiang, Y.; Liang, H. Lactobacillus casei improves depression-like behavior in chronic unpredictable mild stress-induced rats by the BDNF-TrkB signal pathway and the intestinal microbiota. Food Funct. 2020, 11, 6148–6157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, S.; Zhang, M.; Ren, F.; Ren, Y.; Li, Y.; Liu, N.; Zhang, Y.; Zhang, Q.; Wang, R. Effects of Fermented Milk Containing Lacticaseibacillus paracasei Strain Shirota on Constipation in Patients with Depression: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 2238. [Google Scholar] [CrossRef] [PubMed]
- Romijn, A.R.; Rucklidge, J.J.; Kuijer, R.G.; Frampton, C. A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust. N. Z. J. Psychiatry 2017, 51, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Otaka, M.; Kikuchi-Hayakawa, H.; Ogura, J.; Ishikawa, H.; Yomogida, Y.; Ota, M.; Hidese, S.; Ishida, I.; Aida, M.; Matsuda, K.; et al. Effect of Lacticaseibacillus paracasei Strain Shirota on Improvement in Depressive Symptoms, and Its Association with Abundance of Actinobacteria in Gut Microbiota. Microorganisms 2021, 9, 1026. [Google Scholar] [CrossRef]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Ali Hamidi, G.; Salami, M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef]
- Tamtaji, O.R.; Heidari-Soureshjani, R.; Mirhosseini, N.; Kouchaki, E.; Bahmani, F.; Aghadavod, E.; Tajabadi-Ebrahimi, M.; Asemi, Z. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: A randomized, double-blind, controlled trial. Clin. Nutr. 2018, 38, 2569–2575. [Google Scholar] [CrossRef]
- Agahi, A.; Hamidi, G.A.; Daneshvar, R.; Hamdieh, M.; Soheili, M.; Alinaghipour, A.; Esmaeili Taba, S.M.; Salami, M. Does Severity of Alzheimer’s Disease Contribute to Its Responsiveness to Modifying Gut Microbiota? A Double Blind Clinical Trial. Front. Neurol. 2018, 9, 662. [Google Scholar] [CrossRef]
- Ton, A.M.M.; Campagnaro, B.P.; Alves, G.A.; Aires, R.; Côco, L.Z.; Arpini, C.; E Oliveira, T.G.; Campos-Toimil, M.; Meyrelles, S.S.; Pereira, T.M.C.; et al. Oxidative Stress and Dementia in Alzheimer’s Patients: Effects of Synbiotic Supplementation. Oxid. Med. Cell. Longev. 2020, 2020, 2638703. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Albert, M.S.; Knopman, D.S.; McKhann, G.M.; Sperling, R.A.; Carrillo, M.C.; Thies, B.; Phelps, C.H. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 257–262. [Google Scholar] [CrossRef]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef]
- Brown, J.; Pengas, G.; Dawson, K.; A Brown, L.; Clatworthy, P. Self administered cognitive screening test (TYM) for detection of Alzheimer’s disease: Cross sectional study. BMJ 2009, 338, b2030. [Google Scholar] [CrossRef] [PubMed]
- Patterson, C. World Alzheimer Report 2018—The State of the Art of Dementia Research: New Frontiers; Alzheimer’s Disease International (ADI): London, UK, 2018. [Google Scholar]
- Newell, K.L.; Hyman, B.T.; Growdon, J.H.; Hedley-Whyte, E.T. Application of the National Institute on Aging (NIA)-Reagan Institute Criteria for the Neuropathological Diagnosis of Alzheimer Disease. J. Neuropathol. Exp. Neurol. 1999, 58, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.; Dunn, N.; Mullee, M.; Perry, H. P2-251 Association between dementia and systemic infectious disease: Evidence from a case-control study. Neurobiol. Aging 2004, 25, S303–S304. [Google Scholar] [CrossRef]
- Urosevic, N.; Martins, R.N. Infection and Alzheimer’s Disease: The APOE ε4 Connection and Lipid Metabolism. J. Alzheimers Dis. 2008, 13, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Repetto, M.G.; Reides, C.G.; Evelson, P.A.; Kohan, S.; De Lustig, E.S.; Llesuy, S.F. Peripheral markers of oxidative stress in probable Alzheimer patients. Eur. J. Clin. Investig. 1999, 29, 643–649. [Google Scholar] [CrossRef]
- Bonfili, L.; Cecarini, V.; Berardi, S.; Scarpona, S.; Suchodolski, J.S.; Nasuti, C.; Fiorini, D.; Boarelli, M.C.; Rossi, G.; Eleuteri, A.M. Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci. Rep. 2017, 7, 2426. [Google Scholar] [CrossRef]
- Haran, J.P.; Bhattarai, S.K.; Foley, S.E.; Dutta, P.; Ward, D.V.; Bucci, V.; McCormick, B.A. Alzheimer’s Disease Microbiome Is Associated with Dysregulation of the Anti-Inflammatory P-Glycoprotein Pathway. mBio 2019, 10, e00632-19. [Google Scholar] [CrossRef]
- Ho, L.; Ono, K.; Tsuji, M.; Mazzola, P.; Singh, R.; Pasinetti, G.M. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev. Neurother. 2017, 18, 83–90. [Google Scholar] [CrossRef]
- Song, X.; Zhao, Z.; Zhao, Y.; Jin, Q.; Li, Q.J.A.S. Protective Effects of Bacillus coagulans JA845 against D-Galactose/AlCl3-Induced Cognitive Decline, Oxidative Stress and Neuroinflammation. J. Microbiol. Biotechnol. 2022, 32, 212–219. [Google Scholar] [CrossRef]
- Abdelhamid, M.; Zhou, C.; Ohno, K.; Kuhara, T.; Taslima, F.; Abdullah, M.; Jung, C.-G.; Michikawa, M. Probiotic Bifidobacterium breve Prevents Memory Impairment Through the Reduction of Both Amyloid-β Production and Microglia Activation in APP Knock-In Mouse. J. Alzheimers Dis. Prepr. 2022, 85, 1555–1571. [Google Scholar] [CrossRef]
- Tan, C.; Liu, Y.; Zhang, H.; Di, C.; Xu, D.; Liang, C.; Zhang, N.; Han, B.; Lang, W. Neuroprotective Effects of Probiotic-Supplemented Diet on Cognitive Behavior of 3xTg-AD Mice. J. Healthc. Eng. 2022, 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Abdelhamid, M.; Zhou, C.; Taslima, F.; Abdullah, M.; Michikawa, M. Probiotic Bifidobacterium breve decreases Aβ production via the upregulation of ADAM10 level and attenuates microglia activation in an APP knock-in mouse model of Alzheimer’s disease. Alzheimers Dement. 2021, 17, e050965. [Google Scholar] [CrossRef]
- World Gastroenterology Organisation. WGO Practice Guideline. Probiotics and Prebiotics. Available online: https://www.worldgastroenterology.org/guidelines/probiotics-and-prebiotics (accessed on 13 June 2022).
- Lu, C.-S.; Chang, H.-C.; Weng, Y.-H.; Chen, C.-C.; Kuo, Y.-S.; Tsai, Y.-C. The Add-On Effect of Lactobacillus plantarum PS128 in Patients With Parkinson’s Disease: A Pilot Study. Front. Nutr. 2021, 8, 378. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2020, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Gathagan, R.J.; Covell, D.J.; Medellin, C.; Stieber, A.; Robinson, J.L.; Zhang, B.; Pitkin, R.M.; Olufemi, M.F.; Luk, K.C.; et al. Cellular milieu imparts distinct pathological α-synuclein strains in α-synucleinopathies. Nature 2018, 557, 558–563. [Google Scholar] [CrossRef]
- Hasegawa, S.; Goto, S.; Tsuji, H.; Okuno, T.; Asahara, T.; Nomoto, K.; Shibata, A.; Fujisawa, Y.; Minato, T.; Okamoto, A.; et al. Intestinal Dysbiosis and Lowered Serum Lipopolysaccharide-Binding Protein in Parkinson’s Disease. PLoS ONE 2015, 10, e0142164. [Google Scholar] [CrossRef]
- Cassani, E.; Privitera, G.; Pezzoli, G.; Pusani, C.; Madio, C.; Iorio, L.; Barichella, M. Use of probiotics for the treatment of constipation in Parkinson’s disease patients. Minerva Gastroenterol. Dietol. 2011, 57, 117–121. [Google Scholar]
- Georgescu, D.; Ancusa, O.E.; Georgescu, L.A.; Ionita, I.; Reisz, D. Nonmotor gastrointestinal disorders in older patients with Parkinson’s disease: Is there hope? Clin. Interv. Aging 2016, 11, 1601–1608. [Google Scholar] [CrossRef]
- Ghyselinck, J.; Verstrepen, L.; Moens, F.; Abbeele, P.V.D.; Bruggeman, A.; Said, J.; Smith, B.; Barker, L.A.; Jordan, C.; Leta, V.; et al. Influence of probiotic bacteria on gut microbiota composition and gut wall function in an in-vitro model in patients with Parkinson’s disease. Int. J. Pharm. X 2021, 3, 100087. [Google Scholar] [CrossRef]
- Tan, A.H.; Lim, S.-Y.; Chong, K.K.; A Manap, M.A.A.; Hor, J.W.; Lim, J.L.; Low, S.C.; Chong, C.W.; Mahadeva, S.; Lang, A.E. Probiotics for constipation in Parkinson’s disease: A randomized placebo-controlled study. Neurology 2020, 96, e772–e782. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Ali, R.A.R.; Manaf, M.R.A.; Ahmad, N.; Tajurruddin, F.W.; Qin, W.Z.; Desa, S.H.; Ibrahim, N.M. Multi-strain probiotics (Hexbio) containing MCP BCMC strains improved constipation and gut motility in Parkinson’s disease: A randomised controlled trial. PLoS ONE 2020, 15, e0244680. [Google Scholar] [CrossRef] [PubMed]
- Nurrahma, B.A.; Tsao, S.-P.; Wu, C.-H.; Yeh, T.-H.; Hsieh, P.-S.; Panunggal, B.; Huang, H.-Y. Probiotic Supplementation Facilitates Recovery of 6-OHDA-Induced Motor Deficit via Improving Mitochondrial Function and Energy Metabolism. Front. Aging Neurosci. 2021, 13, 668775. [Google Scholar] [CrossRef] [PubMed]
- Stavrovskaya, A.V.; Danilenko, V.N.; Voronkov, D.N.; Gushchina, A.S.; Marsova, M.V.; Olshansky, A.S.; Yamshikova, N.G.; Illarioshkin, S.N. Pharmabiotic Based on Lactobacillus fermentum Strain U-21 Modulates the Toxic Effect of 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine as Parkinsonism Inducer in Mice. Hum. Physiol. 2021, 47, 891–900. [Google Scholar] [CrossRef]
- Sun, J.; Li, H.; Jin, Y.; Yu, J.; Mao, S.; Su, K.-P.; Ling, Z.; Liu, J. Probiotic Clostridium butyricum ameliorated motor deficits in a mouse model of Parkinson’s disease via gut microbiota-GLP-1 pathway. Brain Behav. Immun. 2020, 91, 703–715. [Google Scholar] [CrossRef]
- Tsao, S.-P.; Nurrahma, B.A.; Kumar, R.; Wu, C.-H.; Yeh, T.-H.; Chiu, C.-C.; Lee, Y.-P.; Liao, Y.-C.; Huang, C.-H.; Yeh, Y.-T.; et al. Probiotic Enhancement of Antioxidant Capacity and Alterations of Gut Microbiota Composition in 6-Hydroxydopamin-Induced Parkinson’s Disease Rats. Antioxidants 2021, 10, 1823. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Z.; Zhao, L.; Zhao, Y.; Yang, G.; Wang, C.; Gao, L.; Niu, C.; Li, S. Lactobacillus plantarum DP189 Reduces α-SYN Aggravation in MPTP-Induced Parkinson’s Disease Mice via Regulating Oxidative Damage, Inflammation, and Gut Microbiota Disorder. J. Agric. Food Chem. 2022, 70, 1163–1173. [Google Scholar] [CrossRef]
- Liu, X.; Du, Z.R.; Wang, X.; Sun, X.R.; Zhao, Q.; Zhao, F.; Wong, W.T.; Wong, K.H.; Dong, X.-L. Polymannuronic acid prebiotic plus Lacticaseibacillus rhamnosus GG probiotic as a novel synbiotic promoted their separate neuroprotection against Parkinson’s disease. Food Res. Int. 2022, 155, 111067. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Jiang, Y.; Zhao, Z.; Shen, Y.; Zhang, J.; Zhao, L. Neuroprotective effect of Lactobacillus plantarum DP189 on MPTP-induced Parkinson’s disease model mice. J. Funct. Foods 2021, 85, 104635. [Google Scholar] [CrossRef]
- Ma, Y.-F.; Lin, Y.-A.; Huang, C.-L.; Hsu, C.-C.; Wang, S.; Yeh, S.-R.; Tsai, Y.-C. Lactiplantibacillus plantarum PS128 Alleviates Exaggerated Cortical Beta Oscillations and Motor Deficits in the 6-Hydroxydopamine Rat Model of Parkinson’s Disease. Probiotics Antimicrob. Proteins, 2021; 1–14, online ahead of print. [Google Scholar] [CrossRef]
- Mensi, M.; Rogantini, C.; Marchesi, M.; Borgatti, R.; Chiappedi, M. Lactobacillus plantarum PS128 and Other Probiotics in Children and Adolescents with Autism Spectrum Disorder: A Real-World Experience. Nutrients 2021, 13, 2036. [Google Scholar] [CrossRef]
- Santocchi, E.; Guiducci, L.; Prosperi, M.; Calderoni, S.; Gaggini, M.; Apicella, F.; Tancredi, R.; Billeci, L.; Mastromarino, P.; Grossi, E.; et al. Effects of Probiotic Supplementation on Gastrointestinal, Sensory and Core Symptoms in Autism Spectrum Disorders: A Randomized Controlled Trial. Front. Psychiatry 2020, 11, 550593. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, S.Y.; El Gendy, Y.G.; Mehanna, N.S.; El-Senousy, W.M.; El-Feki, H.S.A.; Saad, K.; El-Asheer, O.M. The role of probiotics in children with autism spectrum disorder: A prospective, open-label study. Nutr. Neurosci. 2018, 21, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Maenner, M.J.; Shaw, K.A.; Baio, J.; Washington, A.; Patrick, M.; DiRienzo, M.; Christensen, D.L.; Wiggins, L.D.; Pettygrove, S.; Andrews, J.G.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill. Summ. 2020, 69, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Herman, A. Zastosowanie suplementacji probiotykami w profilaktyce i leczeniu zaburzeń depresyjnych i lękowych—Przegląd dotychczasowych badań. Psychiatr. Pol. 2019, 53, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Van’t Hof, M.; Tisseur, C.; van Berckelear-Onnes, I.; van Nieuwenhuyzen, A.; Daniels, A.M.; Deen, M.; Hoek, H.W.; Ester, W.A. Age at autism spectrum disorder diagnosis: A systematic review and meta-analysis from 2012 to 2019. Autism 2021, 25, 862–873. [Google Scholar] [CrossRef]

| Gut Microbiota | Neurotransmitters Produced | Function | References |
|---|---|---|---|
| Bifidobacterium infantis, Candida spp., Streptococcus spp., Escherichia spp., Enterococcus spp. | Serotonin (5-HT; 5-hydroxytryptamine) |
| [24] |
| Bacillus spp., Lactobacillus spp. | Dopamine |
| [25,26] |
| Escherichia spp., Bacillus spp., Saccharomyces spp. | Norepinephrine | ||
| Noradrenaline | |||
| Lactobacillus spp., Bifidobacterium spp. | Gamma-aminobutyric acid (GABA) |
| [27] |
| Lactobacillus spp. | Acetylcholine |
| [28,29] |
| Bifidobacterium spp. | Gamma-aminobutyric acid (GABA) |
| [30,31,32] |
| Bifidobacterium spp., Bacillus spp. | Tryptophan |
|
| Randomized Studies | Non-Randomized Studies | |||
|---|---|---|---|---|
| Mean | Range | Mean | Range | |
| Reporting | 8.9 | 6–10 | 6.9 | 5–9 |
| External validity | 2.6 | 1–3 | 2.0 | 0–3 |
| Bias | 6.8 | 5–7 | 4.8 | 4–5 |
| Confounding | 4.5 | 1–6 | 0.9 | 0–2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skowron, K.; Budzyńska, A.; Wiktorczyk-Kapischke, N.; Chomacka, K.; Grudlewska-Buda, K.; Wilk, M.; Wałecka-Zacharska, E.; Andrzejewska, M.; Gospodarek-Komkowska, E. The Role of Psychobiotics in Supporting the Treatment of Disturbances in the Functioning of the Nervous System—A Systematic Review. Int. J. Mol. Sci. 2022, 23, 7820. https://doi.org/10.3390/ijms23147820
Skowron K, Budzyńska A, Wiktorczyk-Kapischke N, Chomacka K, Grudlewska-Buda K, Wilk M, Wałecka-Zacharska E, Andrzejewska M, Gospodarek-Komkowska E. The Role of Psychobiotics in Supporting the Treatment of Disturbances in the Functioning of the Nervous System—A Systematic Review. International Journal of Molecular Sciences. 2022; 23(14):7820. https://doi.org/10.3390/ijms23147820
Chicago/Turabian StyleSkowron, Krzysztof, Anna Budzyńska, Natalia Wiktorczyk-Kapischke, Karolina Chomacka, Katarzyna Grudlewska-Buda, Monika Wilk, Ewa Wałecka-Zacharska, Małgorzata Andrzejewska, and Eugenia Gospodarek-Komkowska. 2022. "The Role of Psychobiotics in Supporting the Treatment of Disturbances in the Functioning of the Nervous System—A Systematic Review" International Journal of Molecular Sciences 23, no. 14: 7820. https://doi.org/10.3390/ijms23147820
APA StyleSkowron, K., Budzyńska, A., Wiktorczyk-Kapischke, N., Chomacka, K., Grudlewska-Buda, K., Wilk, M., Wałecka-Zacharska, E., Andrzejewska, M., & Gospodarek-Komkowska, E. (2022). The Role of Psychobiotics in Supporting the Treatment of Disturbances in the Functioning of the Nervous System—A Systematic Review. International Journal of Molecular Sciences, 23(14), 7820. https://doi.org/10.3390/ijms23147820






