New Insights into TRP Ion Channels in Stem Cells
Abstract
:1. Introduction
2. TRPs in Stem Cells
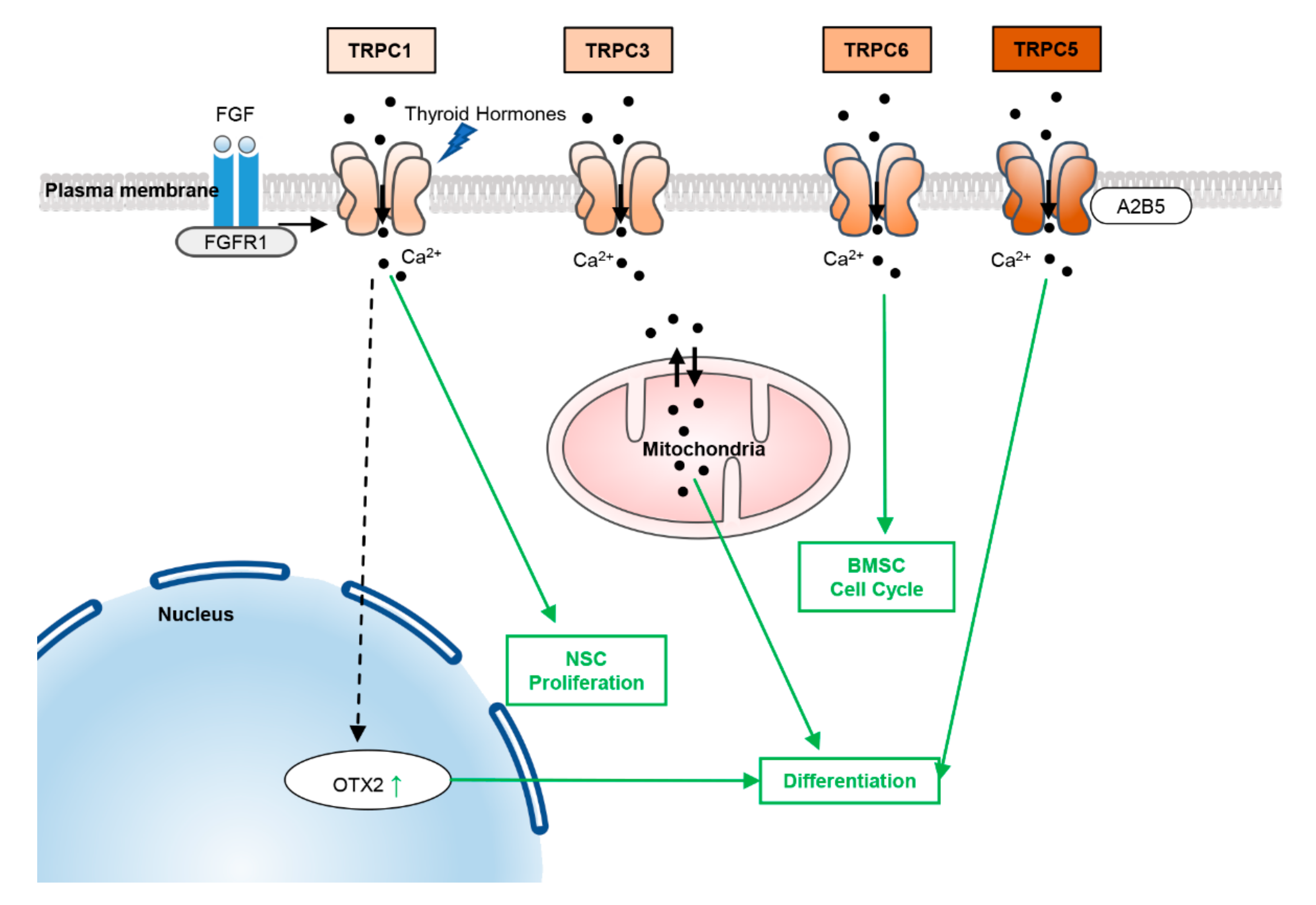
2.1. TRPCs
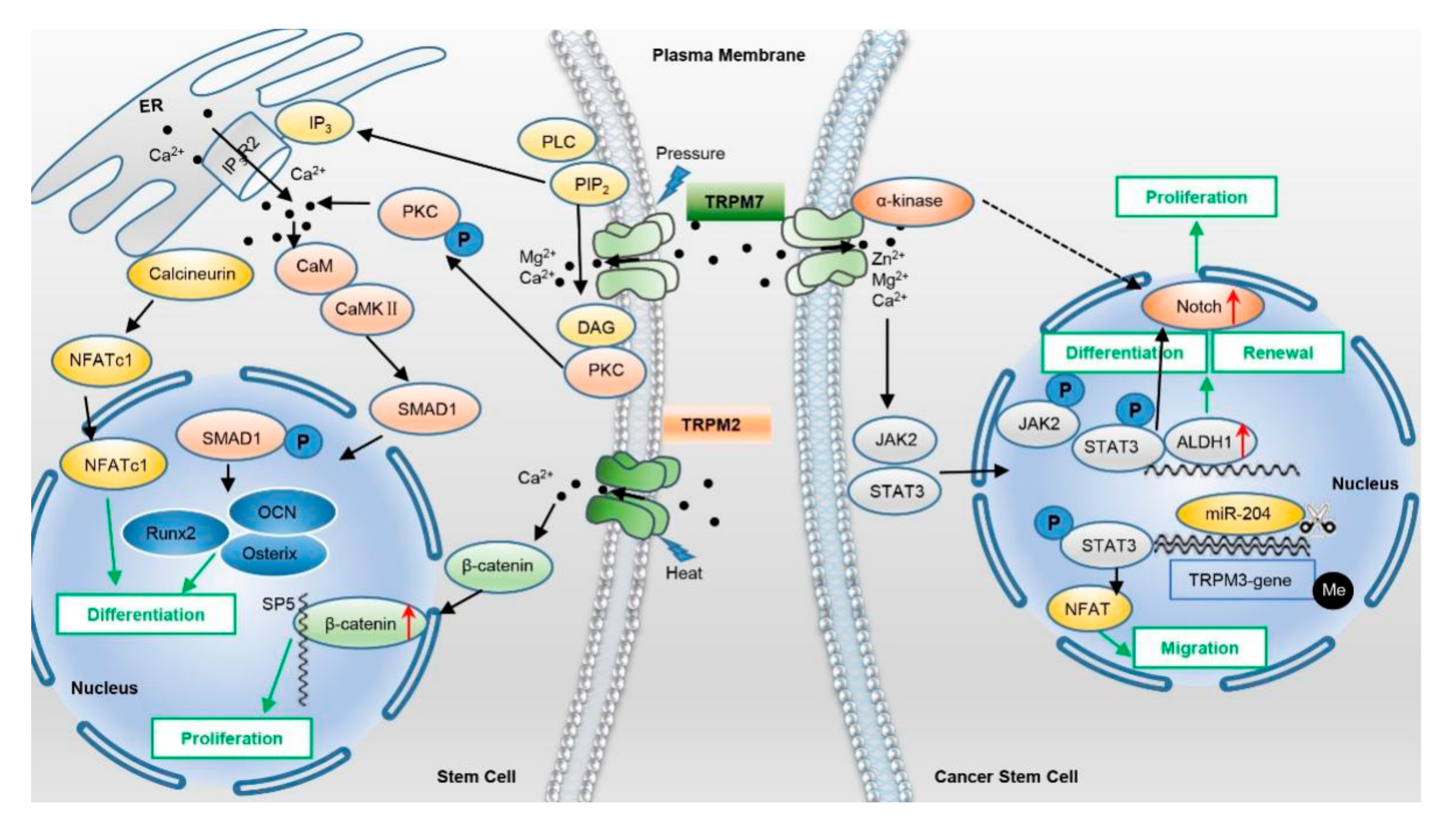
2.2. TRPMs
2.3. TRPVs
2.4. TRPA1
2.5. TRPMLs
| TRPs | Stem Cells | Origins | References |
|---|---|---|---|
| TRPC1 | MSCs | Rabbit bone marrow | [23] |
| MSCs | Human tissue and cells | [21,22] | |
| BMSCs | Rat bone marrow | [24] | |
| NSCs | Rat embryos | [16] | |
| NSCs | Mouse | [20] | |
| CD34+ stem cells | Human cord blood | [25] | |
| C2C12 myoblasts | Mouse skeleton | [18,19] | |
| TRPC2 | MSCs | Rabbit bone marrow | [23] |
| NSCs | Rat embryos | [16] | |
| TRPC3 | MSCs | Human tissues | [21] |
| NSCs | Rat embryos | [16] | |
| CD34+ stem cells | Human cord blood | [25] | |
| TRPC4 | MSCs | Human tissues | [21] |
| MSCs | Rabbit bone marrow | [23] | |
| NSCs | Rat embryos | [16] | |
| TRPC5 | MSCs | Human tissues | [21] |
| TRPC6 | MSCs | Human tissues | [21] |
| MSCs | Rabbit bone marrow | [24] | |
| NSCs | Rat embryos | [16] | |
| TRPC7 | CD34+ stem cells | Human cord blood | [25] |
| TRPM2 | NSCs | Mouse embryos | [27] |
| TRPM4 | DPSCs | Rat | [28] |
| TSCs | Mouse | [30] | |
| TRPM7 | DPSCs | Human | [29] |
| TSCs | Mouse | [30] | |
| ESCs | Mouse | [30] | |
| MSCs | Mouse bone marrow | [33] | |
| MSCs | Human bone marrow | [32,34,35,36] | |
| BMSCs | Mouse | [37] | |
| TRPV1 | NSCs | Rat | [40,44] |
| NSCs | Human | [41] | |
| Neural crest-like stem cells | Mouse | [42] | |
| DPSCs | Human | [45] | |
| Adipose-derived stem cells | Human | [47] | |
| Myogenic cells | Mouse | [46] | |
| TRPV2 | TSCs | Mouse | [30] |
| TRPV4 | TSCs | Mouse | [30] |
| MSCs | Mouse | [50,52] | |
| MSCs | Human | [51] | |
| Periodontal stem cells | Rat | [53] | |
| TRPA1 | ISCs | Drosophila | [54] |
| DPSCs | Human | [55] | |
| MSCs | Human | [21] | |
| NSCs | Human | [41] | |
| TRPML1 | MSCs | Human tissues | [21] |
| TRPML2 | NSCs | Human | [15] |
3. TRPs in Cancer Stem Cells (CSCs)
3.1. TRPCs
3.2. TRPMs
3.3. TRPVs
3.4. TRPA1
| TRPs | Cancer Stem Cells | Origins | References |
|---|---|---|---|
| TRPC1 | GSCs | Human tumor tissues | [56] |
| TRPM7 | GSCs | Human glioblastoma cells | [57] |
| Tumor spheres | Human lung cancer cells | [58] | |
| TRPV1 | GSCs | Human tumor tissues | [41] |
| TRPV2 | GSCs | Human Adult patients | [60,61] |
| Stem-like cells | Human liver cancer cells | [62] | |
| TRPA1 | GSCs | Human | [41] |
| MSCs | Human lung cancer tissues | [63] |
4. TRPs in Progenitor Cells
4.1. TRPCs
4.2. TRPVs
4.3. TRPMs and Other TRPs
| TRPs | Progenitor Cells | Origins | References |
|---|---|---|---|
| TRPC1 | Hematopoietic progenitors | Mouse bone marrow | [70] |
| Myeloid precursors | Mouse bone marrow | [70] | |
| Osteoclasts precursors | Mouse bone marrow | [70] | |
| NPCs | Rat | [64] | |
| NPCs | Mouse | [65] | |
| NPCs | Human | [67] | |
| EPCs | Rat | [68] | |
| EPCs | Mouse | [69] | |
| TRPC3 | NPCs | Rat | [64] |
| NPCs | Mouse | [65] | |
| NPCs | Human | [67] | |
| TRPC4 | NPCs | Rat | [64] |
| NPCs | Human | [67] | |
| TRPC5 | NPCs | Rat | [64] |
| NPCs | Human | [67] | |
| TRPC6 | NPCs | Rat | [64] |
| NPCs | Human | [67] | |
| TRPV1 | NPCs | Human | [71] |
| TRPV2 | NPCs | Human | [71] |
| TRPV3 | NPCs | Human | [71] |
| TRPV4 | NPCs | Human | [67] |
| EPCs | Human | [73] | |
| TRPV5 | NPCs | Human | [67] |
| TRPV6 | NPCs | Human | [67] |
| TRPM2 | NPCs | Human | [67] |
| NPCs | Mouse | [27] | |
| TRPM3 | NPCs | Human | [67] |
| TRPM4 | NPCs | Human | [67] |
| TRPM7 | NPCs | Human | [67] |
| TRPML1 | NPCs | Human | [67] |
| TRPML2 | NPCs | Human | [15] |
| TRPP2 | NPCs | Human | [67] |
5. TRP Roles in Stem Cells
5.1. TRPCs
5.2. TRPMs
5.3. TRPVs
5.4. TRPA1
6. Perspectives and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weissman, I.L. Stem cells: Units of development, units of regeneration, and units in evolution. Cell 2000, 100, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Chagastelles, P.C.; Nardi, N.B. Biology of stem cells: An overview. Kidney Int. Suppl. 2011, 1, 63–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alison, M.R.; Islam, S. Attributes of adult stem cells. J. Pathol. 2009, 217, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hu, K.; Smuga-Otto, K.; Tian, S.; Stewart, R.; Slukvin, I.I.; Thomson, J.A. Human induced pluripotent stem cells free of vector and transgene sequences. Science 2009, 324, 797–801. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.F.; Dirks, P.B. Cancer and stem cell biology: How tightly intertwined? Cell Stem Cell 2008, 3, 147–150. [Google Scholar] [CrossRef] [Green Version]
- Jordan, C.T. Cancer stem cell biology: From leukemia to solid tumors. Curr. Opin. Cell Biol. 2004, 16, 708–712. [Google Scholar] [CrossRef]
- Pardal, R.; Clarke, M.F.; Morrison, S.J. Applying the principles of stem-cell biology to cancer. Nat. Rev. Cancer 2003, 3, 895–902. [Google Scholar] [CrossRef]
- Dick, J.E. Stem cell concepts renew cancer research. Blood 2008, 112, 4793–4807. [Google Scholar] [CrossRef] [Green Version]
- Nilius, B.; Voets, T. TRP channels: A TR(I)P through a world of multifunctional cation channels. Pflug. Arch. 2005, 451, 1–10. [Google Scholar] [CrossRef]
- Clapham, D.E. SnapShot: Mammalian TRP channels. Cell 2007, 129, 220. [Google Scholar] [CrossRef] [Green Version]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef]
- Zheng, J. Molecular mechanism of TRP channels. Compr. Physiol. 2013, 3, 221–242. [Google Scholar] [CrossRef] [Green Version]
- Chinigo, G.; Castel, H.; Chever, O.; Gkika, D. TRP Channels in Brain Tumors. Front. Cell Dev. Biol. 2021, 9, 617801. [Google Scholar] [CrossRef]
- Henao, J.C.; Grismaldo, A.; Barreto, A.; Rodriguez-Pardo, V.M.; Mejia-Cruz, C.C.; Leal-Garcia, E.; Perez-Nunez, R.; Rojas, P.; Latorre, R.; Carvacho, I.; et al. TRPM8 Channel Promotes the Osteogenic Differentiation in Human Bone Marrow Mesenchymal Stem Cells. Front. Cell Dev. Biol. 2021, 9, 592946. [Google Scholar] [CrossRef]
- Morelli, M.B.; Nabissi, M.; Amantini, C.; Tomassoni, D.; Rossi, F.; Cardinali, C.; Santoni, M.; Arcella, A.; Oliva, M.A.; Santoni, A.; et al. Overexpression of transient receptor potential mucolipin-2 ion channels in gliomas: Role in tumor growth and progression. Oncotarget 2016, 7, 43654–43668. [Google Scholar] [CrossRef] [Green Version]
- Fiorio Pla, A.; Maric, D.; Brazer, S.C.; Giacobini, P.; Liu, X.; Chang, Y.H.; Ambudkar, I.S.; Barker, J.L. Canonical transient receptor potential 1 plays a role in basic fibroblast growth factor (bFGF)/FGF receptor-1-induced Ca2+ entry and embryonic rat neural stem cell proliferation. J. Neurosci. 2005, 25, 2687–2701. [Google Scholar] [CrossRef]
- Strubing, C.; Krapivinsky, G.; Krapivinsky, L.; Clapham, D.E. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J. Biol. Chem. 2003, 278, 39014–39019. [Google Scholar] [CrossRef] [Green Version]
- Formigli, L.; Sassoli, C.; Squecco, R.; Bini, F.; Martinesi, M.; Chellini, F.; Luciani, G.; Sbrana, F.; Zecchi-Orlandini, S.; Francini, F.; et al. Regulation of transient receptor potential canonical channel 1 (TRPC1) by sphingosine 1-phosphate in C2C12 myoblasts and its relevance for a role of mechanotransduction in skeletal muscle differentiation. J. Cell Sci. 2009, 122, 1322–1333. [Google Scholar] [CrossRef] [Green Version]
- Louis, M.; Zanou, N.; Van Schoor, M.; Gailly, P. TRPC1 regulates skeletal myoblast migration and differentiation. J. Cell Sci. 2008, 121, 3951–3959. [Google Scholar] [CrossRef] [Green Version]
- Domenichini, F.; Terrie, E.; Arnault, P.; Harnois, T.; Magaud, C.; Bois, P.; Constantin, B.; Coronas, V. Store-Operated Calcium Entries Control Neural Stem Cell Self-Renewal in the Adult Brain Subventricular Zone. Stem Cells 2018, 36, 761–774. [Google Scholar] [CrossRef] [Green Version]
- Goralczyk, A.; van Vijven, M.; Koch, M.; Badowski, C.; Yassin, M.S.; Toh, S.A.; Shabbir, A.; Franco-Obregon, A.; Raghunath, M. TRP channels in brown and white adipogenesis from human progenitors: New therapeutic targets and the caveats associated with the common antibiotic, streptomycin. FASEB J. 2017, 31, 3251–3266. [Google Scholar] [CrossRef] [Green Version]
- Parate, D.; Franco-Obregon, A.; Frohlich, J.; Beyer, C.; Abbas, A.A.; Kamarul, T.; Hui, J.H.P.; Yang, Z. Enhancement of mesenchymal stem cell chondrogenesis with short-term low intensity pulsed electromagnetic fields. Sci. Rep. 2017, 7, 9421. [Google Scholar] [CrossRef]
- Torossian, F.; Bisson, A.; Vannier, J.P.; Boyer, O.; Lamacz, M. TRPC expression in mesenchymal stem cells. Cell. Mol. Biol. Lett. 2010, 15, 600–610. [Google Scholar] [CrossRef]
- Ichikawa, J.; Inoue, R. TRPC6 regulates cell cycle progression by modulating membrane potential in bone marrow stromal cells. Br. J. Pharm. 2014, 171, 5280–5294. [Google Scholar] [CrossRef] [Green Version]
- Ramanathan, G.; Mannhalter, C. Increased expression of transient receptor potential canonical 6 (TRPC6) in differentiating human megakaryocytes. Cell Biol. Int. 2016, 40, 223–231. [Google Scholar] [CrossRef]
- Raghuwanshi, S.; Dahariya, S.; Sharma, D.S.; Kovuru, N.; Sahu, I.; Gutti, R.K. RUNX1 and TGF-beta signaling cross talk regulates Ca(2+) ion channels expression and activity during megakaryocyte development. FEBS J. 2020, 287, 5411–5438. [Google Scholar] [CrossRef]
- Li, Y.; Jiao, J. Deficiency of TRPM2 leads to embryonic neurogenesis defects in hyperthermia. Sci. Adv. 2020, 6, eaay6350. [Google Scholar] [CrossRef] [Green Version]
- Ngoc Tran, T.D.; Stovall, K.E.; Suantawee, T.; Hu, Y.; Yao, S.; Yang, L.J.; Adisakwattana, S.; Cheng, H. Transient receptor potential melastatin 4 channel is required for rat dental pulp stem cell proliferation and survival. Cell Prolif. 2017, 50, e12360. [Google Scholar] [CrossRef]
- Cui, L.; Xu, S.M.; Ma, D.D.; Wu, B.L. The effect of TRPM7 suppression on the proliferation, migration and osteogenic differentiation of human dental pulp stem cells. Int. Endod. J. 2014, 47, 583–593. [Google Scholar] [CrossRef]
- De Clercq, K.; Perez-Garcia, V.; Van Bree, R.; Pollastro, F.; Peeraer, K.; Voets, T.; Vriens, J. Mapping the expression of transient receptor potential channels across murine placental development. Cell. Mol. Life Sci. 2021, 78, 4993–5014. [Google Scholar] [CrossRef]
- Zou, Z.G.; Rios, F.J.; Montezano, A.C.; Touyz, R.M. TRPM7, Magnesium, and Signaling. Int. J. Mol. Sci. 2019, 20, 1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, F.; Wu, S.; Zhang, C.; Li, L.; Chen, J.; Fu, Y.; Wang, J. TRPM7 Upregulate the Activity of SMAD1 through PLC Signaling Way to Promote Osteogenesis of hBMSCs. BioMed Res. Int. 2020, 2020, 9458983. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Feng, J.M.; Figueiredo, M.L.; Zhang, H.; Nelson, P.L.; Marigo, V.; Beck, A. Transient receptor potential melastatin type 7 channel is critical for the survival of bone marrow derived mesenchymal stem cells. Stem Cells Dev. 2010, 19, 1393–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castiglioni, S.; Romeo, V.; Locatelli, L.; Zocchi, M.; Zecchini, S.; Maier, J.A.M. The simultaneous downregulation of TRPM7 and MagT1 in human mesenchymal stem cells in vitro: Effects on growth and osteogenic differentiation. Biochem. Biophys. Res. Commun. 2019, 513, 159–165. [Google Scholar] [CrossRef]
- Xiao, E.; Chen, C.; Zhang, Y. The mechanosensor of mesenchymal stem cells: Mechanosensitive channel or cytoskeleton? Stem Cell Res. Ther. 2016, 7, 140. [Google Scholar] [CrossRef] [Green Version]
- Xiao, E.; Yang, H.Q.; Gan, Y.H.; Duan, D.H.; He, L.H.; Guo, Y.; Wang, S.Q.; Zhang, Y. Brief reports: TRPM7 Senses mechanical stimulation inducing osteogenesis in human bone marrow mesenchymal stem cells. Stem Cells 2015, 33, 615–621. [Google Scholar] [CrossRef]
- Swain, S.M.; Parameswaran, S.; Sahu, G.; Verma, R.S.; Bera, A.K. Proton-gated ion channels in mouse bone marrow stromal cells. Stem Cell Res. 2012, 9, 59–68. [Google Scholar] [CrossRef]
- Bevan, S.; Quallo, T.; Andersson, D.A. Trpv1. In Mammalian Transient Receptor Potential (TRP) Cation Channels, Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 222, pp. 207–245. [Google Scholar] [CrossRef]
- Edwards, J.G. TRPV1 in the central nervous system: Synaptic plasticity, function, and pharmacological implications. Prog. Drug Res. 2014, 68, 77–104. [Google Scholar] [CrossRef]
- Ramirez-Barrantes, R.; Cordova, C.; Poblete, H.; Munoz, P.; Marchant, I.; Wianny, F.; Olivero, P. Perspectives of TRPV1 Function on the Neurogenesis and Neural Plasticity. Neural Plast. 2016, 2016, 1568145. [Google Scholar] [CrossRef] [Green Version]
- Santoni, G.; Nabissi, M.; Amantini, C.; Santoni, M.; Ricci-Vitiani, L.; Pallini, R.; Maggi, F.; Morelli, M.B. ERK Phosphorylation Regulates the Aml1/Runx1 Splice Variants and the TRP Channels Expression during the Differentiation of Glioma Stem Cell Lines. Cells 2021, 10, 2052. [Google Scholar] [CrossRef]
- Sviderskaya, E.V.; Easty, D.J.; Lawrence, M.A.; Sanchez, D.P.; Negulyaev, Y.A.; Patel, R.H.; Anand, P.; Korchev, Y.E.; Bennett, D.C. Functional neurons and melanocytes induced from immortal lines of postnatal neural crest-like stem cells. FASEB J. 2009, 23, 3179–3192. [Google Scholar] [CrossRef] [Green Version]
- Stein, A.T.; Ufret-Vincenty, C.A.; Hua, L.; Santana, L.F.; Gordon, S.E. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J. Gen. Physiol. 2006, 128, 509–522. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Han, H.; Tang, B.; Chen, J.; Mao, D.; Xiong, M. Transplantation of Recombinant Vascular Endothelial Growth Factor (VEGF)189-Neural Stem Cells Downregulates Transient Receptor Potential Vanilloid 1 (TRPV1) and Improves Motor Outcome in Spinal Cord Injury. Med. Sci. Monit. 2018, 24, 1089–1096. [Google Scholar] [CrossRef]
- Arimura, Y.; Shindo, Y.; Yamanaka, R.; Mochizuki, M.; Hotta, K.; Nakahara, T.; Ito, E.; Yoshioka, T.; Oka, K. Peripheral-neuron-like properties of differentiated human dental pulp stem cells (hDPSCs). PLoS ONE 2021, 16, e0251356. [Google Scholar] [CrossRef]
- Kurosaka, M.; Ogura, Y.; Funabashi, T.; Akema, T. Involvement of Transient Receptor Potential Cation Channel Vanilloid 1 (TRPV1) in Myoblast Fusion. J. Cell. Physiol. 2016, 231, 2275–2285. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Red (660 nm) or near-infrared (810 nm) photobiomodulation stimulates, while blue (415 nm), green (540 nm) light inhibits proliferation in human adipose-derived stem cells. Sci. Rep. 2017, 7, 7781. [Google Scholar] [CrossRef]
- Voets, T.; Prenen, J.; Vriens, J.; Watanabe, H.; Janssens, A.; Wissenbach, U.; Bodding, M.; Droogmans, G.; Nilius, B. Molecular determinants of permeation through the cation channel TRPV4. J. Biol. Chem. 2002, 277, 33704–33710. [Google Scholar] [CrossRef] [Green Version]
- Everaerts, W.; Nilius, B.; Owsianik, G. The vanilloid transient receptor potential channel TRPV4: From structure to disease. Prog. Biophys. Mol. Biol. 2010, 103, 2–17. [Google Scholar] [CrossRef]
- Corrigan, M.A.; Johnson, G.P.; Stavenschi, E.; Riffault, M.; Labour, M.N.; Hoey, D.A. TRPV4-mediates oscillatory fluid shear mechanotransduction in mesenchymal stem cells in part via the primary cilium. Sci. Rep. 2018, 8, 3824. [Google Scholar] [CrossRef]
- Gilchrist, C.L.; Leddy, H.A.; Kaye, L.; Case, N.D.; Rothenberg, K.E.; Little, D.; Liedtke, W.; Hoffman, B.D.; Guilak, F. TRPV4-mediated calcium signaling in mesenchymal stem cells regulates aligned collagen matrix formation and vinculin tension. Proc. Natl. Acad. Sci. USA 2019, 116, 1992–1997. [Google Scholar] [CrossRef] [Green Version]
- Das, R.; Goswami, C. TRPV4 expresses in bone cell lineages and TRPV4-R616Q mutant causing Brachyolmia in human reveals “loss-of-interaction” with cholesterol. Biochem. Biophys. Res. Commun. 2019, 517, 566–574. [Google Scholar] [CrossRef]
- Jin, S.S.; He, D.Q.; Wang, Y.; Zhang, T.; Yu, H.J.; Li, Z.X.; Zhu, L.S.; Zhou, Y.H.; Liu, Y. Mechanical force modulates periodontal ligament stem cell characteristics during bone remodelling via TRPV4. Cell Prolif. 2020, 53, e12912. [Google Scholar] [CrossRef]
- Xu, C.; Luo, J.; He, L.; Montell, C.; Perrimon, N. Oxidative stress induces stem cell proliferation via TRPA1/RyR-mediated Ca(2+) signaling in the Drosophila midgut. eLife 2017, 6, e22441. [Google Scholar] [CrossRef]
- Chen, C.; Huang, X.; Zhu, W.; Ding, C.; Huang, P.; Li, R. H2O2 gel bleaching induces cytotoxicity and pain conduction in dental pulp stem cells via intracellular reactive oxygen species on enamel/dentin disc. PLoS ONE 2021, 16, e0257221. [Google Scholar] [CrossRef]
- Terrie, E.; Deliot, N.; Benzidane, Y.; Harnois, T.; Cousin, L.; Bois, P.; Oliver, L.; Arnault, P.; Vallette, F.; Constantin, B.; et al. Store-Operated Calcium Channels Control Proliferation and Self-Renewal of Cancer Stem Cells from Glioblastoma. Cancers 2021, 13, 3428. [Google Scholar] [CrossRef]
- Liu, M.; Inoue, K.; Leng, T.; Guo, S.; Xiong, Z.G. TRPM7 channels regulate glioma stem cell through STAT3 and Notch signaling pathways. Cell. Signal. 2014, 26, 2773–2781. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Xu, S.H.; Chen, Z.; Zeng, Q.X.; Li, Z.J.; Chen, Z.M. TRPM7 overexpression enhances the cancer stem cell-like and metastatic phenotypes of lung cancer through modulation of the Hsp90alpha/uPA/MMP2 signaling pathway. BMC Cancer 2018, 18, 1167. [Google Scholar] [CrossRef]
- Nabissi, M.; Morelli, M.B.; Arcella, A.; Cardinali, C.; Santoni, M.; Bernardini, G.; Santoni, A.; Santoni, G.; Amantini, C. Post-transcriptional regulation of 5′-untranslated regions of human Transient Receptor Potential Vanilloid type-1 (TRPV-1) channels: Role in the survival of glioma patients. Oncotarget 2016, 7, 81541–81554. [Google Scholar] [CrossRef] [Green Version]
- Nabissi, M.; Morelli, M.B.; Amantini, C.; Liberati, S.; Santoni, M.; Ricci-Vitiani, L.; Pallini, R.; Santoni, G. Cannabidiol stimulates Aml-1a-dependent glial differentiation and inhibits glioma stem-like cells proliferation by inducing autophagy in a TRPV2-dependent manner. Int. J. Cancer 2015, 137, 1855–1869. [Google Scholar] [CrossRef] [Green Version]
- Morelli, M.B.; Nabissi, M.; Amantini, C.; Farfariello, V.; Ricci-Vitiani, L.; di Martino, S.; Pallini, R.; Larocca, L.M.; Caprodossi, S.; Santoni, M.; et al. The transient receptor potential vanilloid-2 cation channel impairs glioblastoma stem-like cell proliferation and promotes differentiation. Int. J. Cancer 2012, 131, E1067–E1077. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Cao, X.; Fang, Y.; Liu, G.; Xie, C.; Qian, K.; Lei, X.; Cao, Z.; Du, H.; Cheng, X.; et al. Transient receptor potential vanilloid-type 2 targeting on stemness in liver cancer. Biomed. Pharmacother. 2018, 105, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Xiao, X.Y.; Wu, C.Y.; Li, D.; Chen, J.L.; Ding, X.C.; Cheng, C.; Chen, C.R.; Tong, S.; Wang, S.H. Clinical Roles of Risk Model Based on Differentially Expressed Genes in Mesenchymal Stem Cells in Prognosis and Immunity of Non-Small Cell Lung Cancer. Front. Genet. 2022, 13, 823075. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.Y.; Hong, Y.H.; Jang, S.S.; Chae, H.G.; Paek, S.L.; Moon, H.E.; Kim, D.G.; Kim, J.; Paek, S.H.; Kim, S.J. A role of canonical transient receptor potential 5 channel in neuronal differentiation from A2B5 neural progenitor cells. PLoS ONE 2010, 5, e10359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louhivuori, L.M.; Jansson, L.; Turunen, P.M.; Jantti, M.H.; Nordstrom, T.; Louhivuori, V.; Akerman, K.E. Transient receptor potential channels and their role in modulating radial glial-neuronal interaction: A signaling pathway involving mGluR5. Stem Cells Dev. 2015, 24, 701–713. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Chen, C.; Zhou, Z.; Xu, S.; Yu, Z. A TRPC1-mediated increase in store-operated Ca2+ entry is required for the proliferation of adult hippocampal neural progenitor cells. Cell Calcium 2012, 51, 486–496. [Google Scholar] [CrossRef]
- Stanslowsky, N.; Tharmarasa, S.; Staege, S.; Kalmbach, N.; Klietz, M.; Schwarz, S.C.; Leffler, A.; Wegner, F. Calcium, Sodium, and Transient Receptor Potential Channel Expression in Human Fetal Midbrain-Derived Neural Progenitor Cells. Stem Cells Dev. 2018, 27, 976–984. [Google Scholar] [CrossRef]
- Kuang, C.Y.; Yu, Y.; Wang, K.; Qian, D.H.; Den, M.Y.; Huang, L. Knockdown of transient receptor potential canonical-1 reduces the proliferation and migration of endothelial progenitor cells. Stem Cells Dev. 2012, 21, 487–496. [Google Scholar] [CrossRef]
- Du, L.L.; Shen, Z.; Li, Z.; Ye, X.; Wu, M.; Hong, L.; Zhao, Y. TRPC1 Deficiency Impairs the Endothelial Progenitor Cell Function via Inhibition of Calmodulin/eNOS Pathway. J. Cardiovasc. Transl. Res. 2018, 11, 339–345. [Google Scholar] [CrossRef]
- Ong, E.C.; Nesin, V.; Long, C.L.; Bai, C.X.; Guz, J.L.; Ivanov, I.P.; Abramowitz, J.; Birnbaumer, L.; Humphrey, M.B.; Tsiokas, L. A TRPC1 protein-dependent pathway regulates osteoclast formation and function. J. Biol. Chem. 2013, 288, 22219–22232. [Google Scholar] [CrossRef] [Green Version]
- Morgan, P.J.; Hubner, R.; Rolfs, A.; Frech, M.J. Spontaneous calcium transients in human neural progenitor cells mediated by transient receptor potential channels. Stem Cells Dev. 2013, 22, 2477–2486. [Google Scholar] [CrossRef]
- Stock, K.; Kumar, J.; Synowitz, M.; Petrosino, S.; Imperatore, R.; Smith, E.S.; Wend, P.; Purfurst, B.; Nuber, U.A.; Gurok, U.; et al. Neural precursor cells induce cell death of high-grade astrocytomas through stimulation of TRPV1. Nat. Med. 2012, 18, 1232–1238. [Google Scholar] [CrossRef] [Green Version]
- Dragoni, S.; Guerra, G.; Fiorio Pla, A.; Bertoni, G.; Rappa, A.; Poletto, V.; Bottino, C.; Aronica, A.; Lodola, F.; Cinelli, M.P.; et al. A functional transient receptor potential vanilloid 4 (TRPV4) channel is expressed in human endothelial progenitor cells. J. Cell. Physiol. 2015, 230, 95–104. [Google Scholar] [CrossRef]
- Chen, C.; Ma, Q.; Deng, P.; Yang, J.; Yang, L.; Lin, M.; Yu, Z.; Zhou, Z. Critical role of TRPC1 in thyroid hormone-dependent dopaminergic neuron development. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1900–1912. [Google Scholar] [CrossRef]
- Hao, H.B.; Webb, S.E.; Yue, J.; Moreau, M.; Leclerc, C.; Miller, A.L. TRPC3 is required for the survival, pluripotency and neural differentiation of mouse embryonic stem cells (mESCs). Sci. China Life Sci. 2018, 61, 253–265. [Google Scholar] [CrossRef]
- Eapen, A.; Kulkarni, R.; Ravindran, S.; Ramachandran, A.; Sundivakkam, P.; Tiruppathi, C.; George, A. Dentin phosphophoryn activates Smad protein signaling through Ca2+-calmodulin-dependent protein kinase II in undifferentiated mesenchymal cells. J. Biol. Chem. 2013, 288, 8585–8595. [Google Scholar] [CrossRef] [Green Version]
- Nelson, P.; Ngoc Tran, T.D.; Zhang, H.; Zolochevska, O.; Figueiredo, M.; Feng, J.M.; Gutierrez, D.L.; Xiao, R.; Yao, S.; Penn, A.; et al. Transient receptor potential melastatin 4 channel controls calcium signals and dental follicle stem cell differentiation. Stem Cells 2013, 31, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.; Guo, A.A.; King, P.; Guo, S.; Saafir, T.; Jiang, Y.; Liu, M. TRPM7 Induces Tumorigenesis and Stemness through Notch Activation in Glioma. Front. Pharmacol. 2020, 11, 590723. [Google Scholar] [CrossRef]
- Middelbeek, J.; Visser, D.; Henneman, L.; Kamermans, A.; Kuipers, A.J.; Hoogerbrugge, P.M.; Jalink, K.; van Leeuwen, F.N. TRPM7 maintains progenitor-like features of neuroblastoma cells: Implications for metastasis formation. Oncotarget 2015, 6, 8760–8776. [Google Scholar] [CrossRef] [Green Version]
- Cobaleda, C.; Perez-Caro, M.; Vicente-Duenas, C.; Sanchez-Garcia, I. Function of the zinc-finger transcription factor SNAI2 in cancer and development. Annu. Rev. Genet. 2007, 41, 41–61. [Google Scholar] [CrossRef]
- Ying, Z.; Li, Y.; Wu, J.; Zhu, X.; Yang, Y.; Tian, H.; Li, W.; Hu, B.; Cheng, S.Y.; Li, M. Loss of miR-204 expression enhances glioma migration and stem cell-like phenotype. Cancer Res. 2013, 73, 990–999. [Google Scholar] [CrossRef] [Green Version]
- Courboulin, A.; Paulin, R.; Giguere, N.J.; Saksouk, N.; Perreault, T.; Meloche, J.; Paquet, E.R.; Biardel, S.; Provencher, S.; Cote, J.; et al. Role for miR-204 in human pulmonary arterial hypertension. J. Exp. Med. 2011, 208, 535–548. [Google Scholar] [CrossRef]
- Kim, H.Y.; Yu, Y.; Oh, S.Y.; Wang, K.K.; Kim, Y.R.; Jung, S.C.; Kim, H.S.; Jo, I. Far-Infrared Irradiation Inhibits Adipogenic Differentiation and Stimulates Osteogenic Differentiation of Human Tonsil-Derived Mesenchymal Stem Cells: Role of Protein Phosphatase 2B. Cell Physiol. Biochem. 2019, 52, 240–253. [Google Scholar] [CrossRef]
- Kim, H.Y.; Oh, S.Y.; Choi, Y.M.; Park, J.H.; Kim, H.S.; Jo, I. Transient receptor potential vanilloid 2 mediates the inhibitory effect of far-infrared irradiation on adipogenic differentiation of tonsil-derived mesenchymal stem cells. Stem Cell Res. 2021, 53, 102291. [Google Scholar] [CrossRef]
- Hu, K.; Sun, H.; Gui, B.; Sui, C. TRPV4 functions in flow shear stress induced early osteogenic differentiation of human bone marrow mesenchymal stem cells. Biomed. Pharmacother. 2017, 91, 841–848. [Google Scholar] [CrossRef]
- Winslow, M.M.; Pan, M.; Starbuck, M.; Gallo, E.M.; Deng, L.; Karsenty, G.; Crabtree, G.R. Calcineurin/NFAT signaling in osteoblasts regulates bone mass. Dev. Cell 2006, 10, 771–782. [Google Scholar] [CrossRef] [Green Version]
- Masuyama, R.; Vriens, J.; Voets, T.; Karashima, Y.; Owsianik, G.; Vennekens, R.; Lieben, L.; Torrekens, S.; Moermans, K.; Vanden Bosch, A.; et al. TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab. 2008, 8, 257–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.H.; Liu, M.; He, Y.; Xiao, E.; Zhao, L.; Zhang, T.; Yang, H.Q.; Zhang, Y. TRPV1 deletion impaired fracture healing and inhibited osteoclast and osteoblast differentiation. Sci. Rep. 2017, 7, 42385. [Google Scholar] [CrossRef] [PubMed]
- Fiorio Pla, A.; Ong, H.L.; Cheng, K.T.; Brossa, A.; Bussolati, B.; Lockwich, T.; Paria, B.; Munaron, L.; Ambudkar, I.S. TRPV4 mediates tumor-derived endothelial cell migration via arachidonic acid-activated actin remodeling. Oncogene 2012, 31, 200–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thodeti, C.K.; Matthews, B.; Ravi, A.; Mammoto, A.; Ghosh, K.; Bracha, A.L.; Ingber, D.E. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ. Res. 2009, 104, 1123–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amcheslavsky, A.; Jiang, J.; Ip, Y.T. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell 2009, 4, 49–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohlstein, B.; Spradling, A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 2006, 439, 470–474. [Google Scholar] [CrossRef]
- Qian, F.; Germino, F.J.; Cai, Y.; Zhang, X.; Somlo, S.; Germino, G.G. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat. Genet. 1997, 16, 179–183. [Google Scholar] [CrossRef]
- Jung, S.H.; You, J.E.; Choi, S.W.; Kang, K.S.; Cho, J.Y.; Lyu, J.; Kim, P.H. Polycystin-1 Enhances Stemmness Potential of Umbilical Cord Blood-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 4868. [Google Scholar] [CrossRef]
- Cheng, W.; Zheng, J. Distribution and Assembly of TRP Ion Channels. Adv. Exp. Med. Biol. 2021, 1349, 111–138. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Shan, C.; Xu, J.; Li, M.; Zhao, J.; Cheng, W. New Insights into TRP Ion Channels in Stem Cells. Int. J. Mol. Sci. 2022, 23, 7766. https://doi.org/10.3390/ijms23147766
Guo J, Shan C, Xu J, Li M, Zhao J, Cheng W. New Insights into TRP Ion Channels in Stem Cells. International Journal of Molecular Sciences. 2022; 23(14):7766. https://doi.org/10.3390/ijms23147766
Chicago/Turabian StyleGuo, Jing, Chang Shan, Jiao Xu, Mei Li, Jiayu Zhao, and Wei Cheng. 2022. "New Insights into TRP Ion Channels in Stem Cells" International Journal of Molecular Sciences 23, no. 14: 7766. https://doi.org/10.3390/ijms23147766
APA StyleGuo, J., Shan, C., Xu, J., Li, M., Zhao, J., & Cheng, W. (2022). New Insights into TRP Ion Channels in Stem Cells. International Journal of Molecular Sciences, 23(14), 7766. https://doi.org/10.3390/ijms23147766






