Procyanidin B2 Alleviates Heat-Induced Oxidative Stress through the Nrf2 Pathway in Bovine Mammary Epithelial Cells
Abstract
:1. Introduction
2. Results
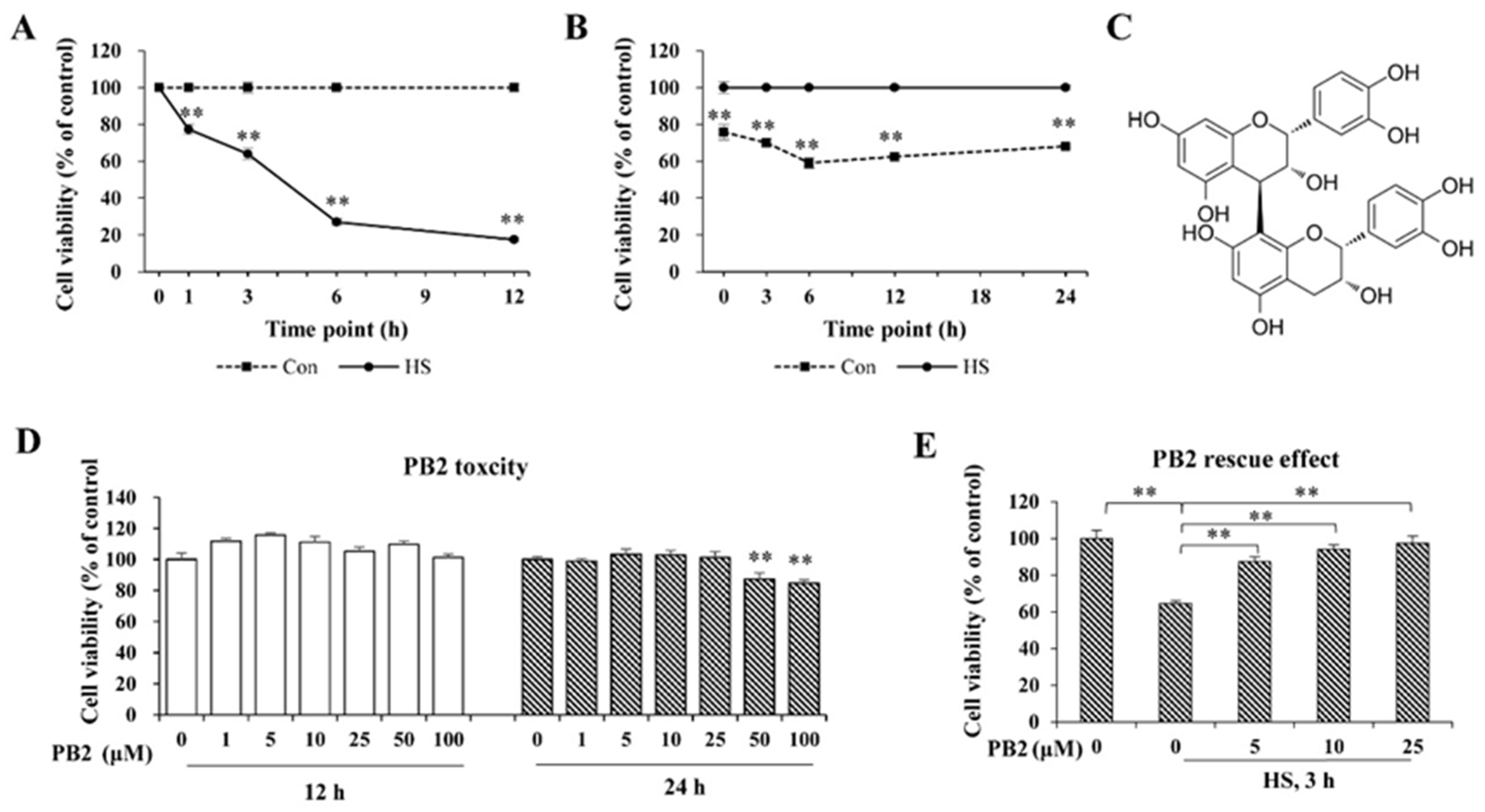
2.1. PB2 Ameliorates Acute HS-Induced Cell Viability Loss
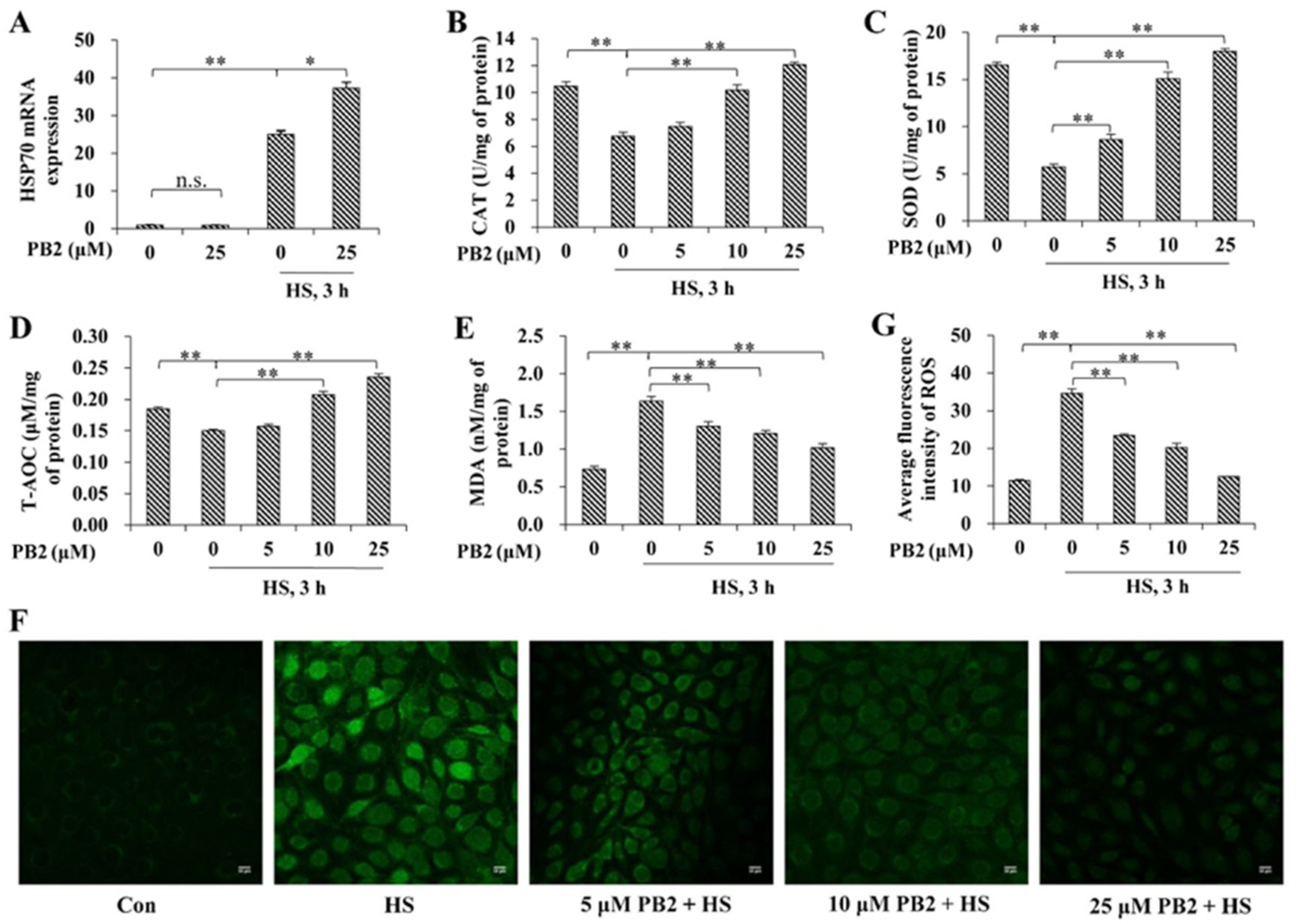
2.2. PB2 Alleviates Oxidative Stress Induced by HS
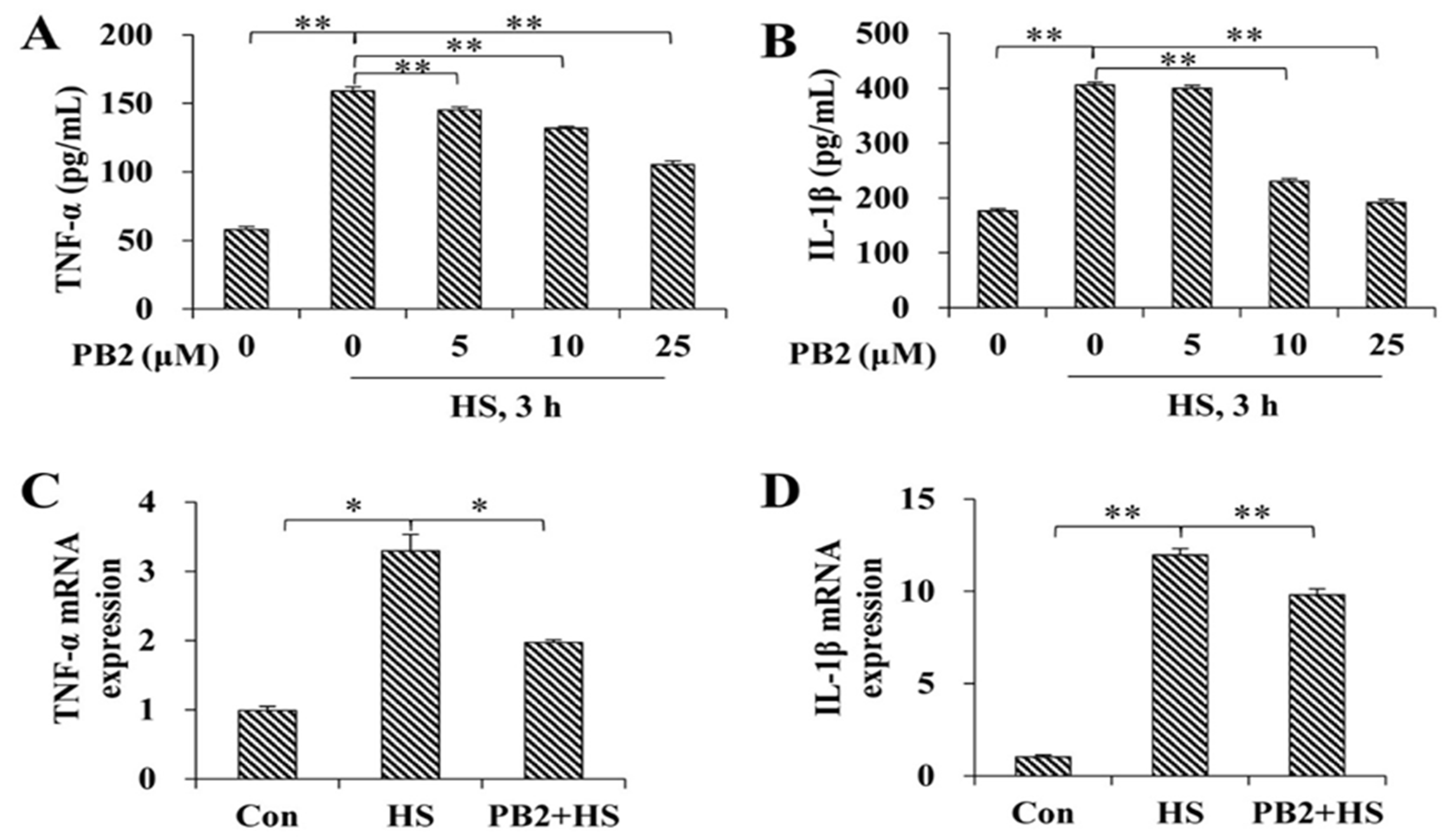
2.3. PB2 Protects against HS-Induced Inflammatory Response
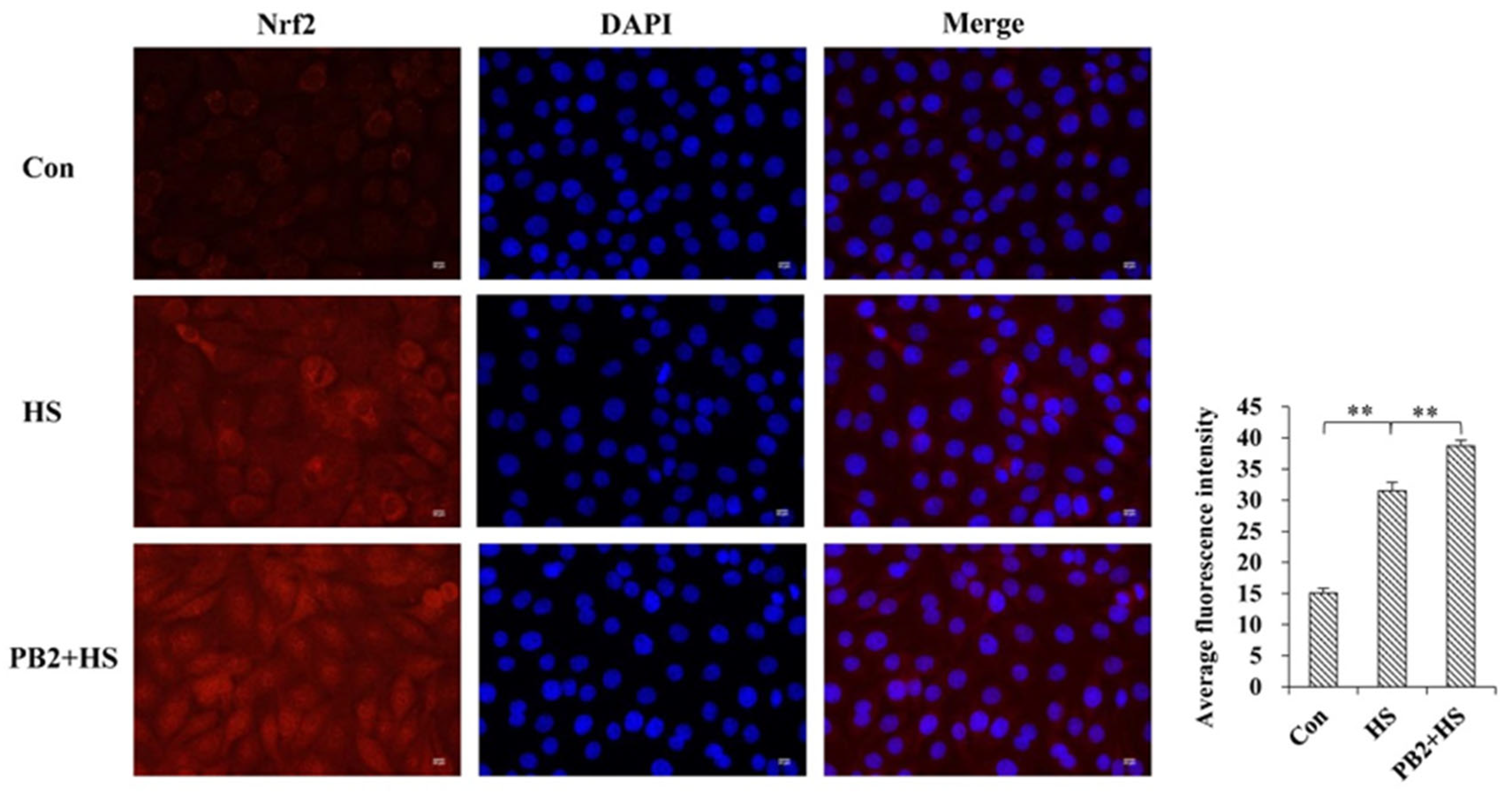
2.4. PB2 Further Promotes the Activation of Nrf2/HO-1 Signaling Pathway
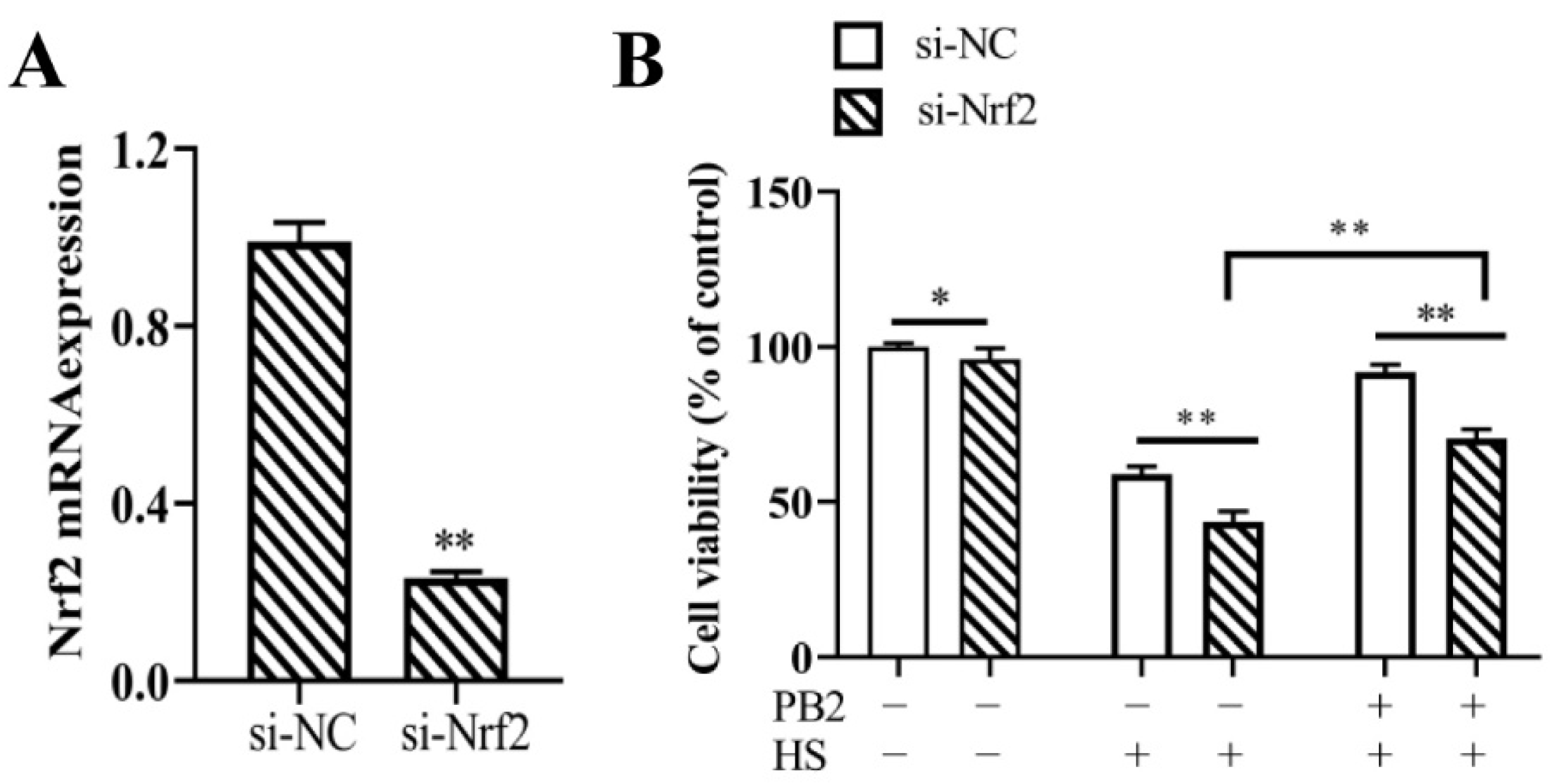
2.5. Nrf2 Knockdown and Its Effect on MAC-T Cell Viability
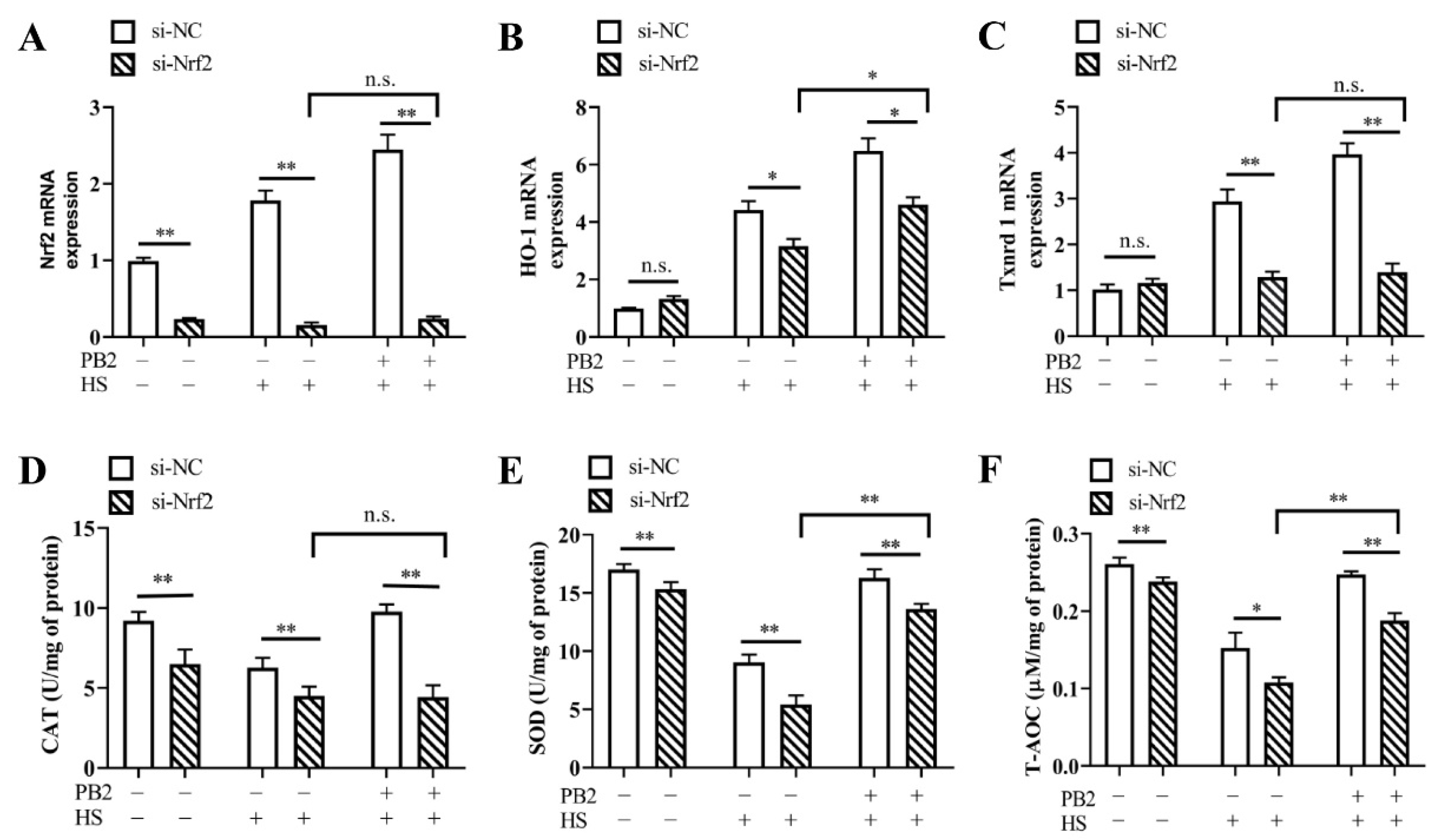
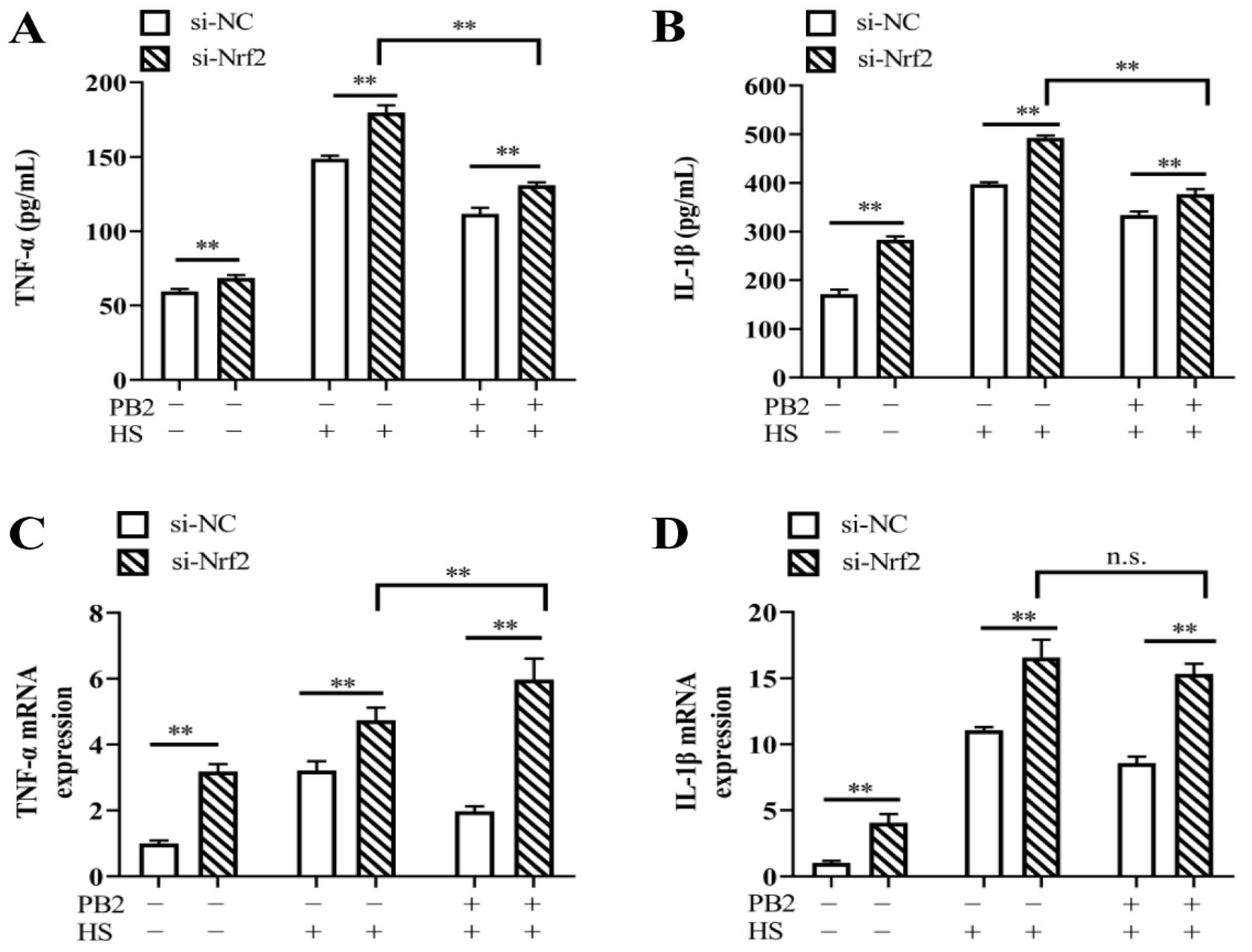
2.6. PB2 Alleviates HS-Induced Oxidative Stress and Inflammatory Response through the Nrf2 Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. Cell Viability Assay
4.3. Antioxidant Capacity Measurement
4.4. Detection of ROS
4.5. Determination of IL-1β and TNF-α
4.6. Transient Transfection of Nrf2
4.7. RNA Isolation and Quantitative Real-Time PCR
4.8. Immunofluorescence Staining
4.9. Protein Extraction and Western Blotting
4.10. Statistical Analysis
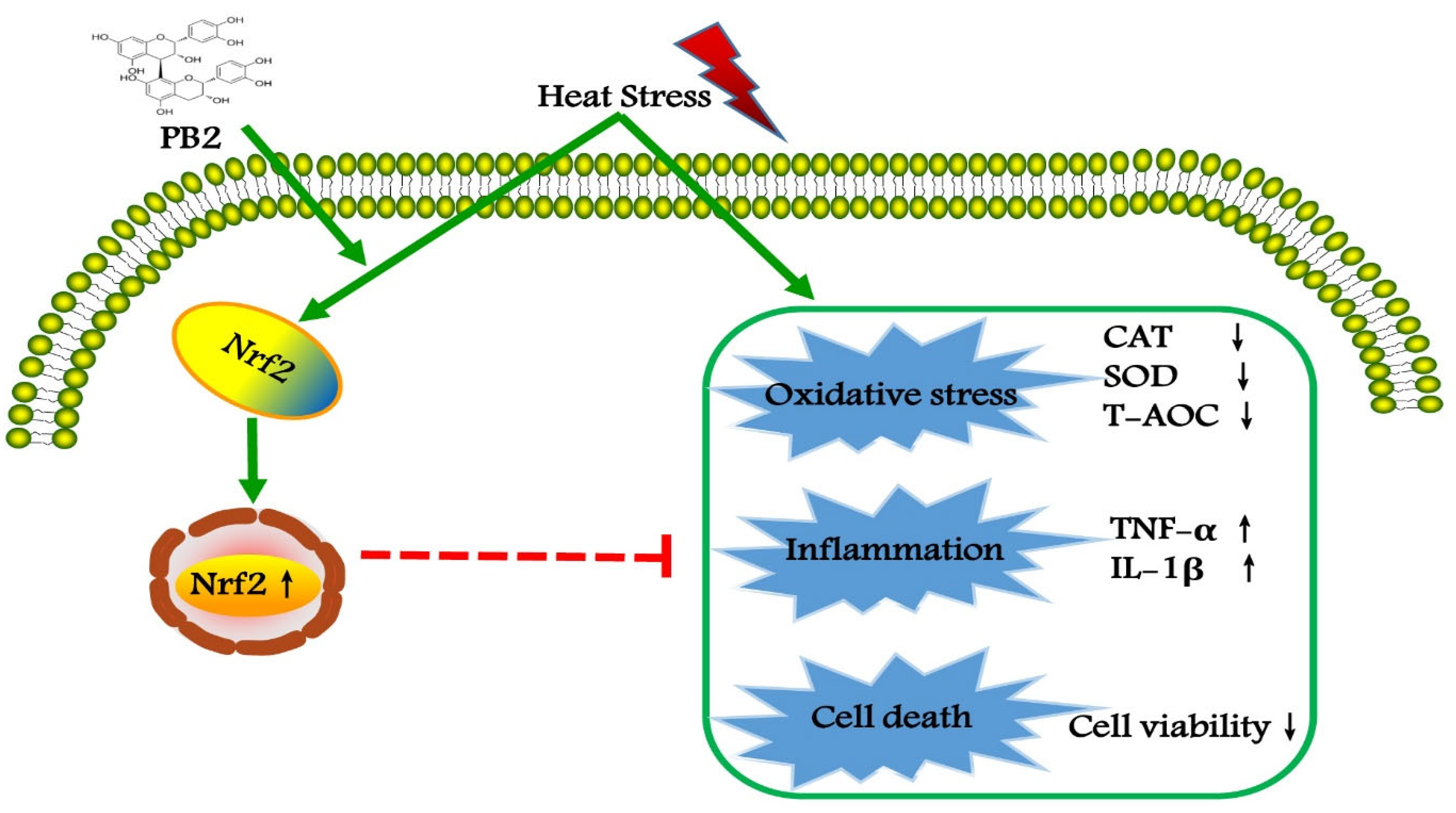
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Safa, S.; Kargar, S.; Moghaddam, G.A.; Ciliberti, M.G.; Caroprese, M. Heat stress abatement during the postpartum period: Effects on whole lactation milk yield, indicators of metabolic status, inflammatory cytokines, and biomarkers of the oxidative stress. J. Anim. Sci. 2019, 97, 122–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamel, J.; Zhang, Y.C.; Wente, N.; Kromker, V. Heat stress and cow factors affect bacteria shedding pattern from naturally infected mammary gland quarters in dairy cattle. J. Dairy Sci. 2021, 104, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Boustan, A.; Vahedi, V.; Abdi Farab, M.; Karami, H.; Seyedsharifi, R.; Hedayat, E.N.; Ghazaei, C.; Salem, A.Z.M. Effects of dry period length on milk yield and content and metabolic status of high-producing dairy cows under heat stress. Trop. Anim. Health Prod. 2021, 53, 205. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Orellana, R.M.; Weng, X.; Marins, T.N.; Dahl, G.E.; Bernard, J.K. Symposium review: The influences of heat stress on bovine mammary gland function. J. Dairy Sci. 2018, 101, 5642–5654. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.C.; Lu, Q.Y.; Cai, J.Y.; Wang, Y.; Lai, X.F.; Qiu, Y.; Huang, Y.N.; Ke, Q.; Zhang, Y.N.; Guan, Y.J.; et al. Nestin regulates cellular redox homeostasis in lung cancer through the Keap1-Nrf2 feedback loop. Nat. Commun. 2019, 10, 5043. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Cao, K.; Liao, Y.; Zhang, W.; Zheng, J.; Li, X.; Huang, M.; Zhong, Y.; Hu, X.; Chen, D.; et al. CDCA2 protects against oxidative stress by promoting BRCA1-NRF2 signaling in hepatocellular carcinoma. Oncogene 2021, 40, 4368–4383. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, R.; Sepand, M.R.; Seyednejad, S.A.; Omidi, A.; Akbariani, M.; Gholami, M.; Sabzevari, O. Ellagic acid reduces methotrexate-induced apoptosis and mitochondrial dysfunction via up-regulating Nrf2 expression and inhibiting the IĸBα/NFĸB in rats. Daru 2019, 27, 721–733. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.L.; Xing, G.D.; Qian, Y.; Chen, K.L. S-allyl cysteine ameliorates heat stress-induced oxidative stress by activating Nrf2/HO-1 signaling pathway in BMECs. Toxicol. Appl. Pharmacol. 2021, 416, 115469. [Google Scholar] [CrossRef]
- Devi, P.; Singh, M.; Somagond, Y.M.; Aggarwal, A. Alleviation of heat stress by chlorophytum borivilianum: Impact on stress markers, antioxidant, and immune status in crossbred cows. Trop. Anim. Health Prod. 2021, 53, 351. [Google Scholar] [CrossRef]
- Li, C.M.; Wang, Y.R.; Li, L.; Han, Z.Y.; Mao, S.Y.; Wang, G.L. Betaine protects against heat exposure-induced oxidative stress and apoptosis in bovine mammary epithelial cells via regulation of ROS production. Cell Stress Chaperon. 2019, 24, 453–460. [Google Scholar] [CrossRef]
- Rasmussen, S.E.; Frederiksen, H.; Krogholm, K.S.; Poulsen, L. Dietary proanthocyanidins: Occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. Mol. Nutr. Food Res. 2005, 49, 159–174. [Google Scholar] [CrossRef]
- Wn, J.; Huang, W.M.; Xiao, H.S.; Xie, Y.; Yuan, Z.H.; Yi, J.N.; Chen, J.S.; Tu, D.; Tian, Y.N. Procyanidins B2 reverses the T-2 toxin-induced mitochondrial apoptosis in TM3 Leydig cells. J. Funct. Foods 2018, 45, 118–128. [Google Scholar]
- Stoupi, S.; Williamson, G.; Viton, F.; Barron, D.; King, L.J.; Brown, J.E.; Clifford, M.N. In vivo bioavailability, absorption, excretion, and pharmacokinetics of [14C] procyanidin B2 in male rats. Drug Metab. Dispos. 2010, 38, 287–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottaviani, J.I.; Kwik-Uribe, C.; Keen, C.L.; Schroeter, H. Intake of dietary procyanidins does not contribute to the pool of circulating flavanols in humans. Am. J. Clin. Nutr. 2012, 95, 851–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Li, C.X.; Lian, S.; Xu, B.; Lv, H.M.; Liu, Y.Z.; Lu, J.J.; Ji, H.; Li, S.Z.; Guo, J.R.; et al. Procyanidin B2 alleviates liver injury caused by cold stimulation through sonic hedgehog signalling and autophagy. J. Cell. Mol. Med. 2021, 25, 8015–8027. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Lu, X.L.; Tian, P.Y.; Wang, K.; Shi, J.P. Procyanidin B2 induces apoptosis and autophagy in gastric cancer cells by inhibiting Akt/mTOR signaling pathway. BMC Complement. Med. Ther. 2021, 21, 76. [Google Scholar] [CrossRef] [PubMed]
- Sakano, K.; Mizutani, M.; Murata, M.; Oikawa, S.; Hiraku, Y.; Kawanishi, S. Procyanidin B2 has anti- and pro-oxidant effects on metal-mediated DNA damage. Free Radic. Biol. Med. 2005, 39, 1041–1049. [Google Scholar] [CrossRef]
- Wang, Y.R.; Yang, C.X.; Elsheikh, N.A.H.; Li, C.M.; Yang, F.X.; Wang, G.L.; Li, L. HO-1 reduces heat stress-induced apoptosis in bovine granulosa cells by suppressing oxidative stress. Aging 2019, 11, 5535–5547. [Google Scholar] [CrossRef]
- Yue, L.A.; Cheng, Z.Q.; Wang, K.; Zhu, X.; Ali, Y.M.; Shu, W.Y.; Bao, X.F.; Zhu, L.; Fan, X.H.; Murray, M.; et al. Procyanidin B2 and rutin in Ginkgo biloba extracts protect human retinal pigment epithelial (RPE) cells from oxidative stress by modulating Nrf2 and Erk1/2 signalling. Exp. Eye Res. 2021, 207, 108586. [Google Scholar]
- Qiao, C.Y.; Li, Y.; Shang, Y.; Jiang, M.; Lian, L.H. Management of Gout-associated MSU crystals-induced NLRP3 inflammasome activation by procyanidin B2: Targeting IL-1β and Cathepsin B in macrophages. Inflammopharmacology 2020, 28, 1481–1493. [Google Scholar] [CrossRef]
- Xiao, Y.; Rungruang, S.; Hall, L.W.; Collier, J.L.; Dunshea, F.R.; Collier, R.J. Effects of niacin and betaine on bovine mammary and uterine cells exposed to thermal shock in vitro. J. Dairy Sci. 2017, 100, 4025–4037. [Google Scholar] [CrossRef]
- Zou, Y.; Shao, J.; Li, Y.; Zhao, F.Q.; Liu, J.X.; Liu, H. Protective effects of inorganic and organic selenium on heat stress in bovine mammary epithelial cells. Oxid. Med. Cell Longev. 2019, 26, 1503478. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chang, J.; Gao, Y.; Wang, C. Procyanidin B2 protects neurons from cypermethrin-induced oxidative stress through the P13K/Akt/Nrf2 signaling pathway. Nan Fang Yi Ke Da Xue Xue Bao 2021, 41, 1158–1164. [Google Scholar] [PubMed]
- Song, D.Q.; Liu, J.; Wang, F.; Li, X.F.; Liu, M.H.; Zhang, Z.; Cao, S.S.; Jiang, X. ProcyanidinB2 inhibits lipopolysaccharideinduced apoptosis by suppressing the Bcl2/Bax and NFκB signalling pathways in human umbilical vein endothelial cells. Mol. Med. Rep. 2021, 23, 267. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Ma, F.T.; Wei, J.Y.; Li, H.Y.; Ma, H.; Sun, P. Physiological functions of heat shock proteins. Curr. Protein Pept. Sci. 2019, 21, 751–760. [Google Scholar] [CrossRef]
- Mosser, D.D.; Caron, A.W.; Bourget, L.; Meriin, A.B.; Sherman, M.Y.; Morimoto, R.I.; Massie, B. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol. Cell. Biol. 2000, 20, 7146–7159. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Zhang, W.; Dang, W.; Mou, Y.; Gao, Y.; Sun, B.J.; Du, W.G. Heat shock protein expression enhances heat tolerance of reptile embryos. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141135. [Google Scholar] [CrossRef] [Green Version]
- Sable, A.; Rai, K.M.; Choudhary, A.; Yadav, V.K.; Agarwal, S.K.; Sawant, S.V. Inhibition of Heat Shock proteins HSP90 and HSP70 induce oxidative stress, suppressing cotton fiber development. Sci. Rep. 2018, 8, 3620. [Google Scholar] [CrossRef]
- Rout, P.K.; Kaushik, R.; Ramachandran, N. Differential expression pattern of heat shock protein 70 gene in tissues and heat stress phenotypes in goats during peak heat stress period. Cell Stress Chaperon. 2016, 21, 645–651. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.Y.; Mu, T.; Yang, Z. Methionine protects against hyperthermia-induced cell injury in cultured bovine mammary epithelial cells. Cell Stress Chaperon. 2015, 20, 109–120. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem Cell B 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Jeong, D.K. Stem Cell Research: A novel boulevard towards improved bovine mastitis management. Int. J. Biol. Sci. 2013, 9, 818–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Luo, H.Q.; Sun, L.L.; Xu, M.T.; Yu, J.; Liu, L.L.; Zhang, J.Y.; Wang, Y.Q.; Wang, H.X.; Bao, X.F.; et al. Dihydromyricetin attenuates myocardial hypertrophy induced by transverse aortic constriction via oxidative stress inhibition and SIRT3 pathway enhancement. Int. J. Mol. Sci. 2018, 19, 2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.Z.; Guo, X.T.; Chen, J.W.; Zhao, Y.; Cong, X.; Jiang, Z.L.; Cao, R.F.; Cui, K.; Gao, S.S.; Tian, W.R.; et al. Attenuates heat stress-induced oxidative damage in LLC-PK1 cells by increasing the expression of anti-oxidant enzymes and HSP72. Am. J. Chin. Med. 2014, 42, 1261–1277. [Google Scholar] [CrossRef] [PubMed]
- Su, H.M.; Li, Y.T.; Hu, D.W.; Xie, L.H.; Ke, H.H.; Zheng, X.D.; Chen, W. Procyanidin B2 ameliorates free fatty acids-induced hepatic steatosis through regulating TFEB-mediated lysosomal pathway and redox state. Free Radic. Biol. Med. 2018, 126, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Y.; Zhang, X.Y.; Guan, S.W.; Hua, Z.C. Protective effect of procyanidin B2 against CCl4-induced acute liver injury in mice. Molecules 2015, 20, 12250–12265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.L.; Liu, Z.W.; Wu, W.F.; Lin, S.; Zhang, N.W.; Wang, H.L.; Tan, S.Y.; Lin, P.M.; Chen, X.L.; Wu, L.X.; et al. Effects of FM0807, a novel curcumin derivative, on lipopolysaccharide-induced inflammatory factor release via the ROS/JNK/p53 pathway in RAW264.7 cells. Biosci. Rep. 2018, 38, BSR20180849. [Google Scholar] [CrossRef] [Green Version]
- Habtetsion, T.; Ding, Z.C.; Pi, W.; Li, T.; Lu, C.; Chen, T.; Xi, C.; Spartz, H.; Liu, K.; Hao, Z.; et al. Alteration of tumor metabolism by CD4+ T cells leads to TNF-α-dependent intensification of oxidative stress and tumor cell death. Cell Metab. 2018, 28, 228–242.e6. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Lim, H.J.; Jang, H.J.; Lee, S.W.; Lee, S.J.; Rho, M.C. Portulaca oleracea extracts and their active compounds ameliorate inflammatory bowel diseases in vitro and in vivo by modulating TNF-alpha, IL-6 and IL-1 beta signalling. Food Res. Int. 2018, 106, 335–343. [Google Scholar] [CrossRef]
- Sung, N.Y.; Yang, M.S.; Song, D.S.; Kim, J.K.; Park, J.H.; Song, B.S.; Park, S.H.; Lee, J.W.; Park, H.J.; Kim, J.H. Procyanidin dimer B2-mediated IRAK-M induction negatively regulates TLR4 signaling in macrophages. Biochem. Biophys. Res. Commun. 2013, 438, 122–128. [Google Scholar] [CrossRef]
- Li, J.Y.; Zheng, X.Y.; Ma, X.Y.; Xu, X.Y.; Du, Y.; Lv, Q.J.; Li, X.R.; Wu, Y.; Sun, H.X.; Yu, L.J.; et al. Melatonin protects against chromium(VI)-induced cardiac injury via activating the AMPK/Nrf2 pathway. J. Inorg. Biochem. 2019, 197, 110698. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, B.Y.; Lu, J.J.; Li, S.Y.; Baiyun, R.Q.; Lv, Y.Y.; Lu, Q.; Zhang, Z.G. Dietary luteolin protects against HgCl2-induced renal injury via activation of Nrf2-mediated signaling in rat. J. Inorg. Biochem. 2018, 179, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Wakabayash, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. 2007, 47, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.L.; Wang, K.; Liu, H.Y.; Hu, F.L.; Zhao, F.Q.; Liu, J.X. Protection of bovine mammary epithelial cells from hydrogen peroxide-induced oxidative cell damage by resveratrol. Oxid. Med. Cell. Longev. 2016, 2016, 2572175. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.L.; Wang, K.; Liu, L.; Liu, H.Y.; Zhao, F.Q.; Liu, J.X. Nuclear factor-like factor 2-antioxidant response element signaling activation by tert-butylhydroquinone attenuates acute heat stress in bovine mammary epithelial cells. J. Dairy Sci. 2016, 99, 9094–9103. [Google Scholar] [CrossRef]
- Yang, M.; Kuang, M.; Wang, G.; Ali, I.; Tang, Y.; Yang, C.; Li, Y.; Li, L. Choline attenuates heat stress-induced oxidative injury and apoptosis in bovine mammary epithelial cells by modulating PERK/Nrf-2 signaling pathway. Mol. Immunol. 2021, 135, 388–397. [Google Scholar] [CrossRef]
- Qin, Y.; Cao, L.R.; Hu, L.L. Sirtuin 6 mitigated LPS-induced human umbilical vein endothelial cells inflammatory responses through modulating nuclear factor erythroid 2-related factor 2. J. Cell. Biochem. 2019, 120, 11305–11317. [Google Scholar] [CrossRef]
- Rodríguez-Ramiro, I.; Ramos, S.; Bravo, L.; Goya, L.; Martín, M.Á. Procyanidin B2 induces Nrf2 translocation and glutathione S-transferase P1 expression via ERKs and p38-MAPK pathways and protect human colonic cells against oxidative stress. Eur. J. Nutr. 2011, 51, 881–892. [Google Scholar] [CrossRef] [Green Version]

| Gene | GenBank ID | Primer Sequences (5′ to 3′) | Product Size |
|---|---|---|---|
| HSP70 | NM_203322.3 | Forward: CAAGATCAGCGAGGCGGACAAG Reverse: ACACCTGCTCCAGCTCCTTCC | 128 bp |
| TNF-α | NM_173966.3 | Forward: CTGGCGGAGGAGGTGCTCTC Reverse: GGAGGAAGGAGAAGAGGCTGAGG | 85 bp |
| IL-1β | NM_174093.1 | Forward: ATGAAGAGCTGCATCCAACACCTG Reverse: ACCGACACCACCTGCCTGAAG | 110 bp |
| Nrf2 | NM_001011678.2 | Forward: TCAGCCAGCACAACACATACCATC Reverse: ACGGGAATGTCTCTGCCAAAAGC | 128 bp |
| HO-1 | NM_001014912.1 | Forward: CCGCTACCTGGGAGACCTGTC Reverse: ACTTGGTGGCACTGGCGATATTG | 128 bp |
| Txnrd1 | NM_174625.5 | Forward: CGTGCCTTACATCTATGCCATCGG Reverse: TGGAGCCACCATACAGCCTCTG | 110 bp |
| GAPDH | NM_001034034.2 | Forward: TGCCCGTTCGACAGATAGCC Reverse: GCGACGATGTCCACTTTGCC | 148 bp |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Hao, W.; Yang, L.; Li, T.; Zhao, C.; Yan, P.; Wei, S. Procyanidin B2 Alleviates Heat-Induced Oxidative Stress through the Nrf2 Pathway in Bovine Mammary Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 7769. https://doi.org/10.3390/ijms23147769
Wang H, Hao W, Yang L, Li T, Zhao C, Yan P, Wei S. Procyanidin B2 Alleviates Heat-Induced Oxidative Stress through the Nrf2 Pathway in Bovine Mammary Epithelial Cells. International Journal of Molecular Sciences. 2022; 23(14):7769. https://doi.org/10.3390/ijms23147769
Chicago/Turabian StyleWang, Hongzhuang, Weiguang Hao, Liang Yang, Tingting Li, Chongchong Zhao, Peishi Yan, and Shengjuan Wei. 2022. "Procyanidin B2 Alleviates Heat-Induced Oxidative Stress through the Nrf2 Pathway in Bovine Mammary Epithelial Cells" International Journal of Molecular Sciences 23, no. 14: 7769. https://doi.org/10.3390/ijms23147769
APA StyleWang, H., Hao, W., Yang, L., Li, T., Zhao, C., Yan, P., & Wei, S. (2022). Procyanidin B2 Alleviates Heat-Induced Oxidative Stress through the Nrf2 Pathway in Bovine Mammary Epithelial Cells. International Journal of Molecular Sciences, 23(14), 7769. https://doi.org/10.3390/ijms23147769






