MdMKK9-Mediated the Regulation of Anthocyanin Synthesis in Red-Fleshed Apple in Response to Different Nitrogen Signals
Abstract
:1. Introduction
2. Results
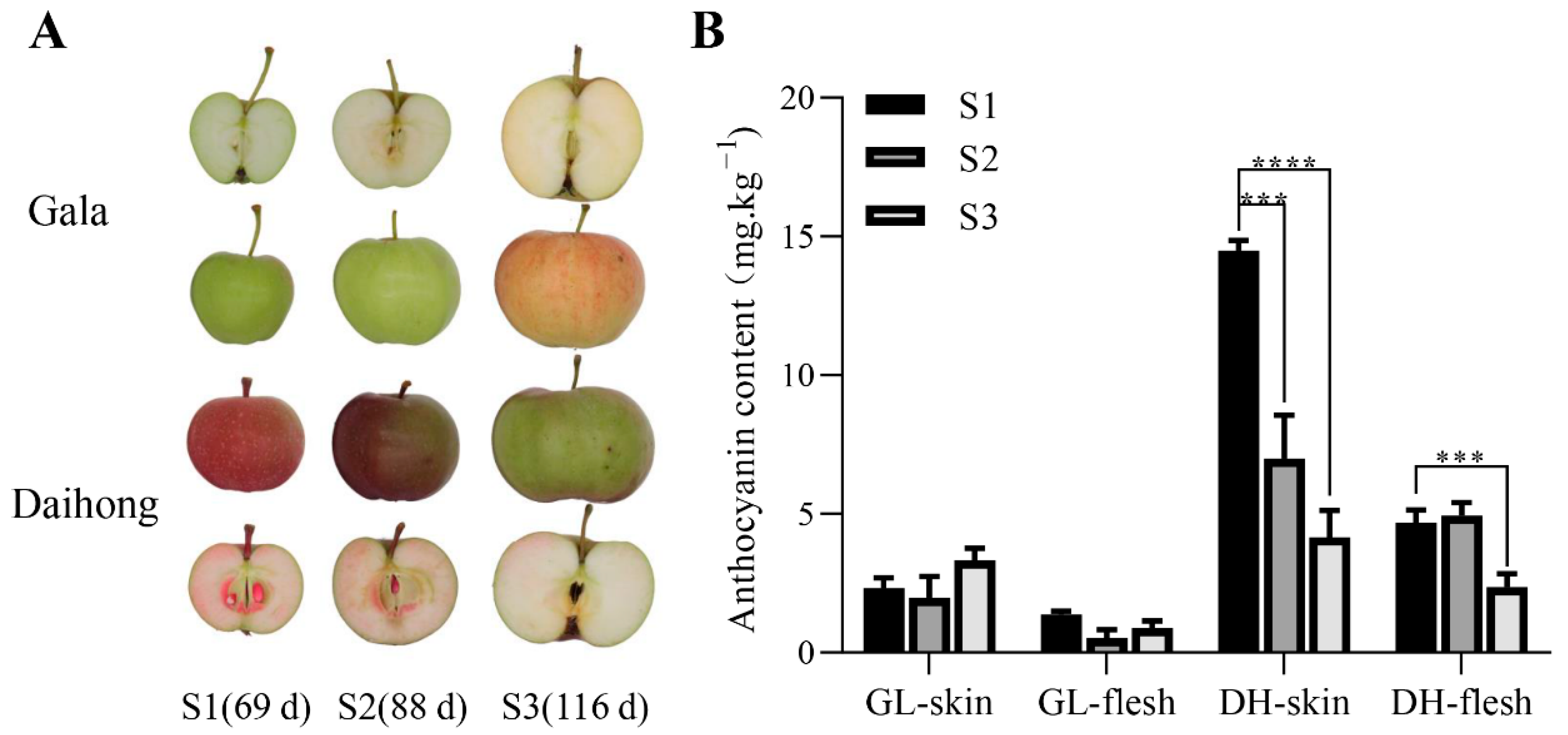
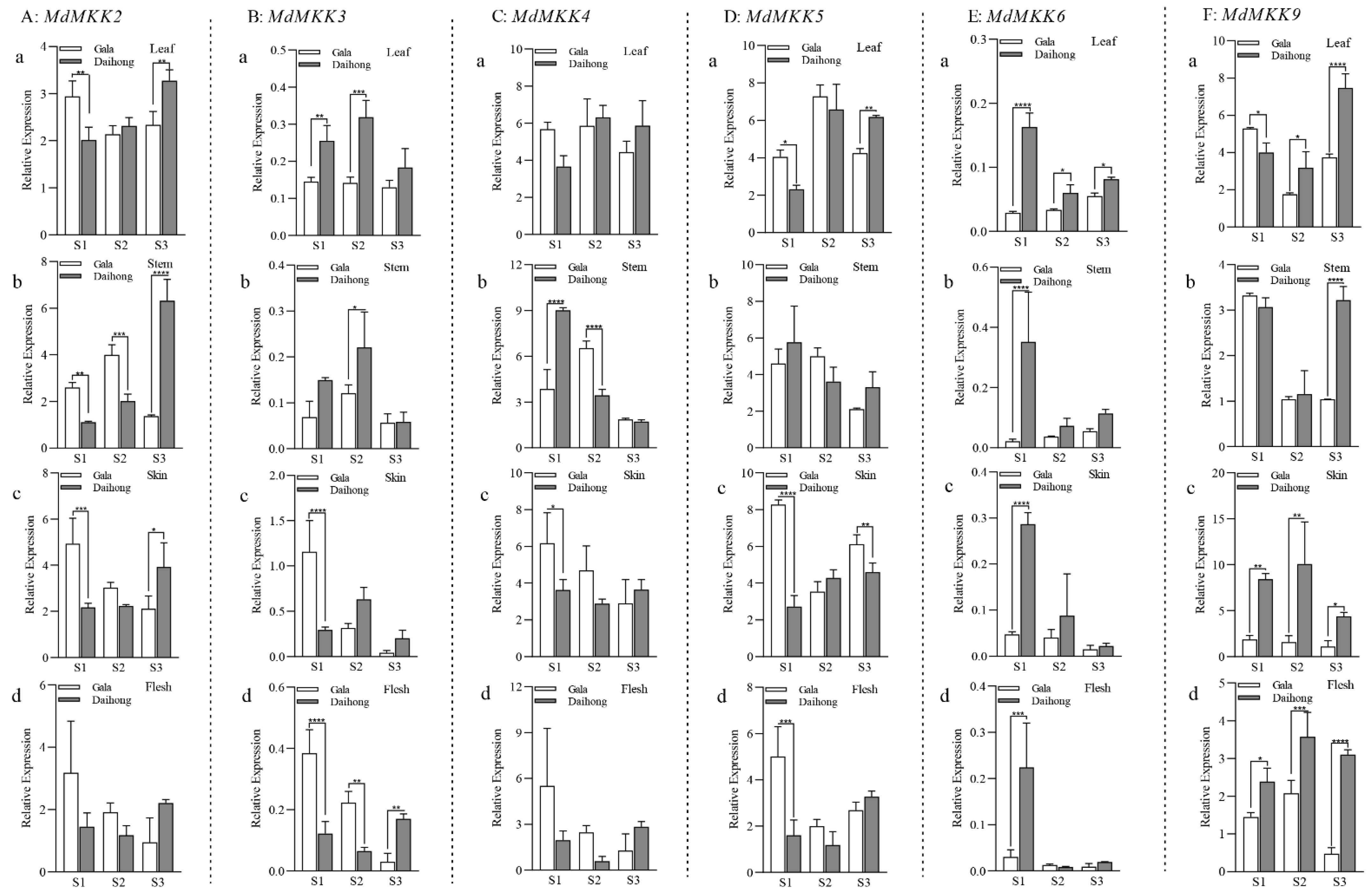
2.1. MdMKK9 Is Directly Related to Changes in the Anthocyanin Content of Red-Fleshed Apples
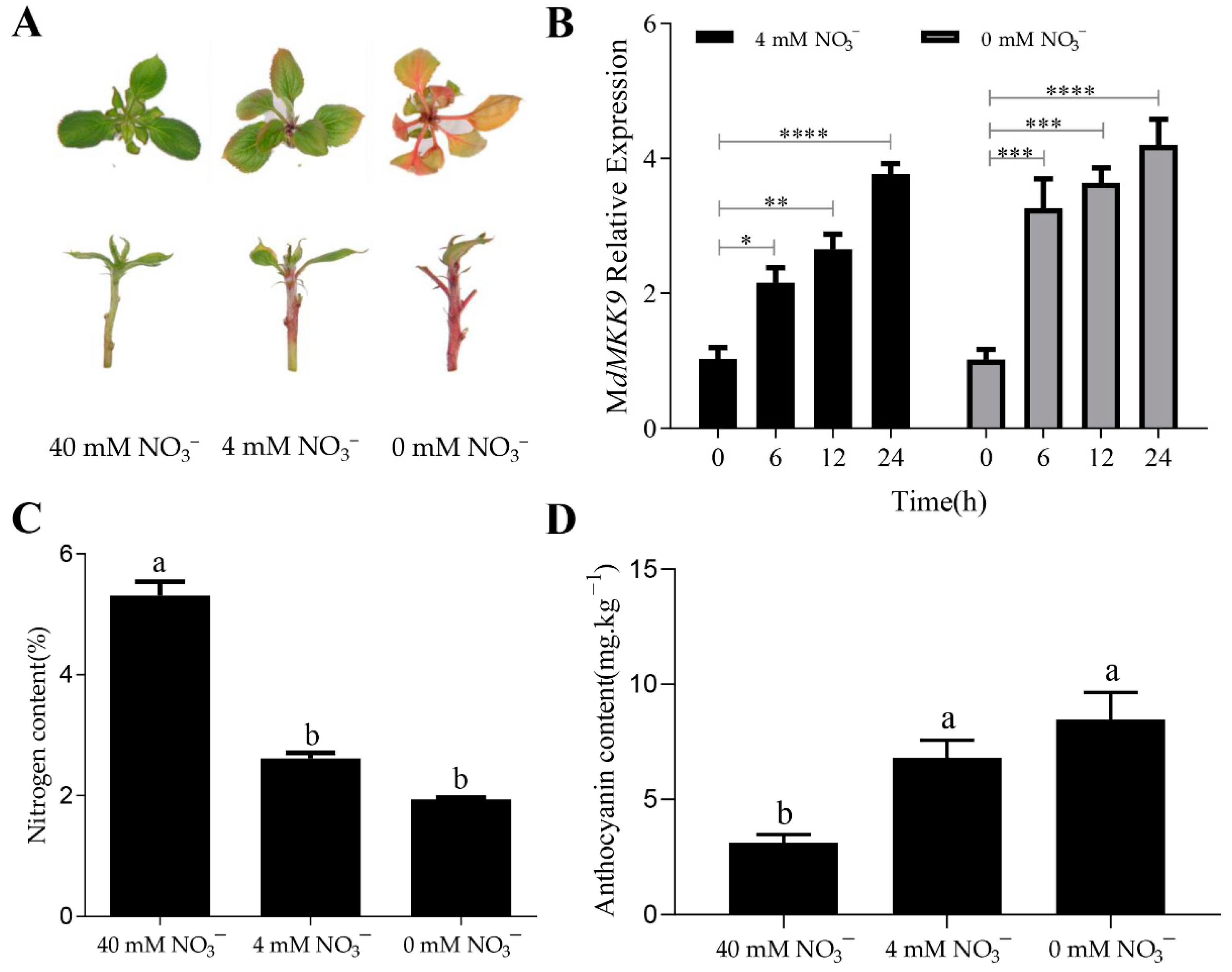
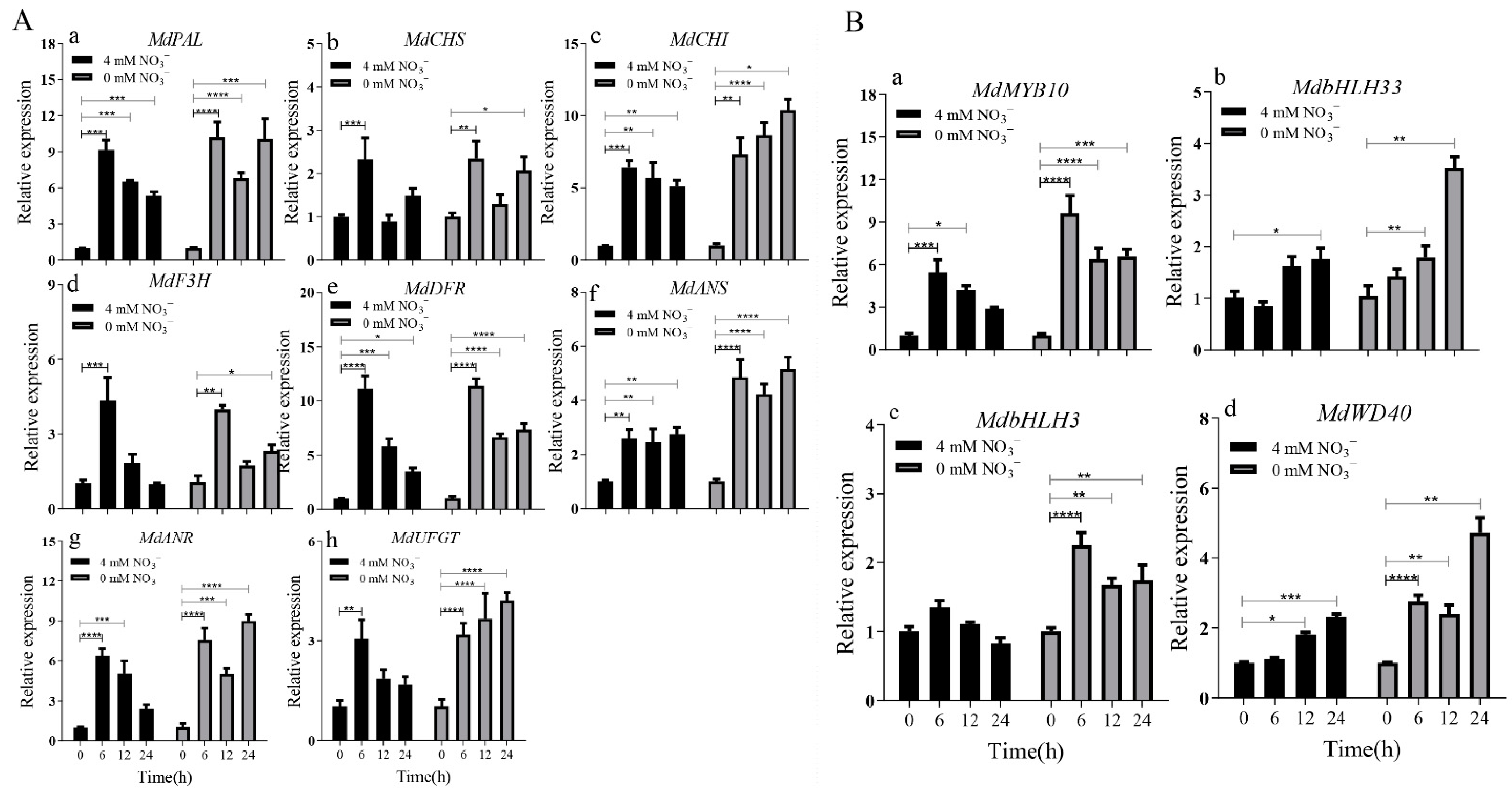
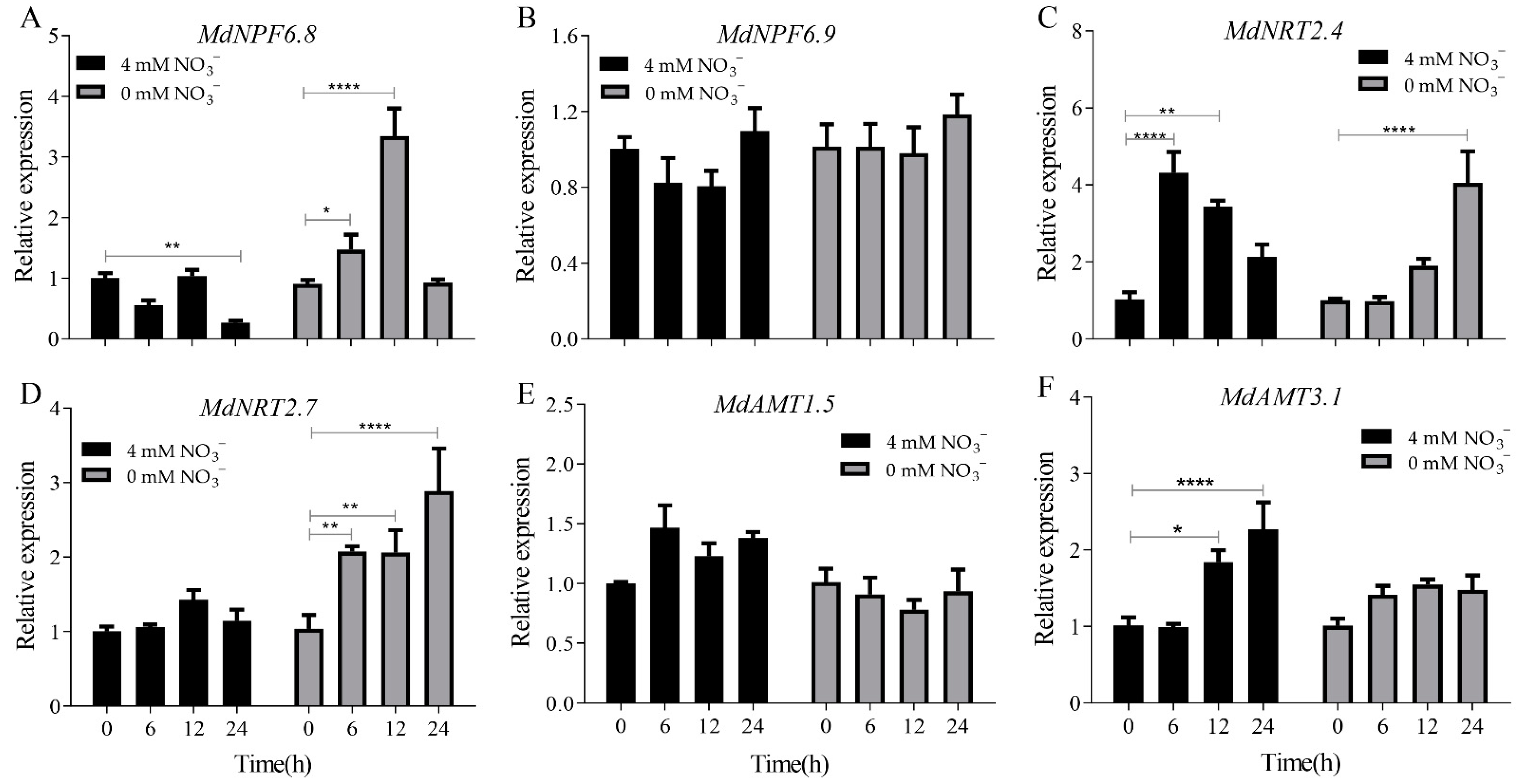
2.2. The Increase in Anthocyanin Content Is Mediated by the Up-Regulation of MdMKK9 in ‘Daihong’ under Low Nitrogen Stress
2.3. Calli with MdMKK9 Deletion No Longer Respond to the Low Nitrogen Signal
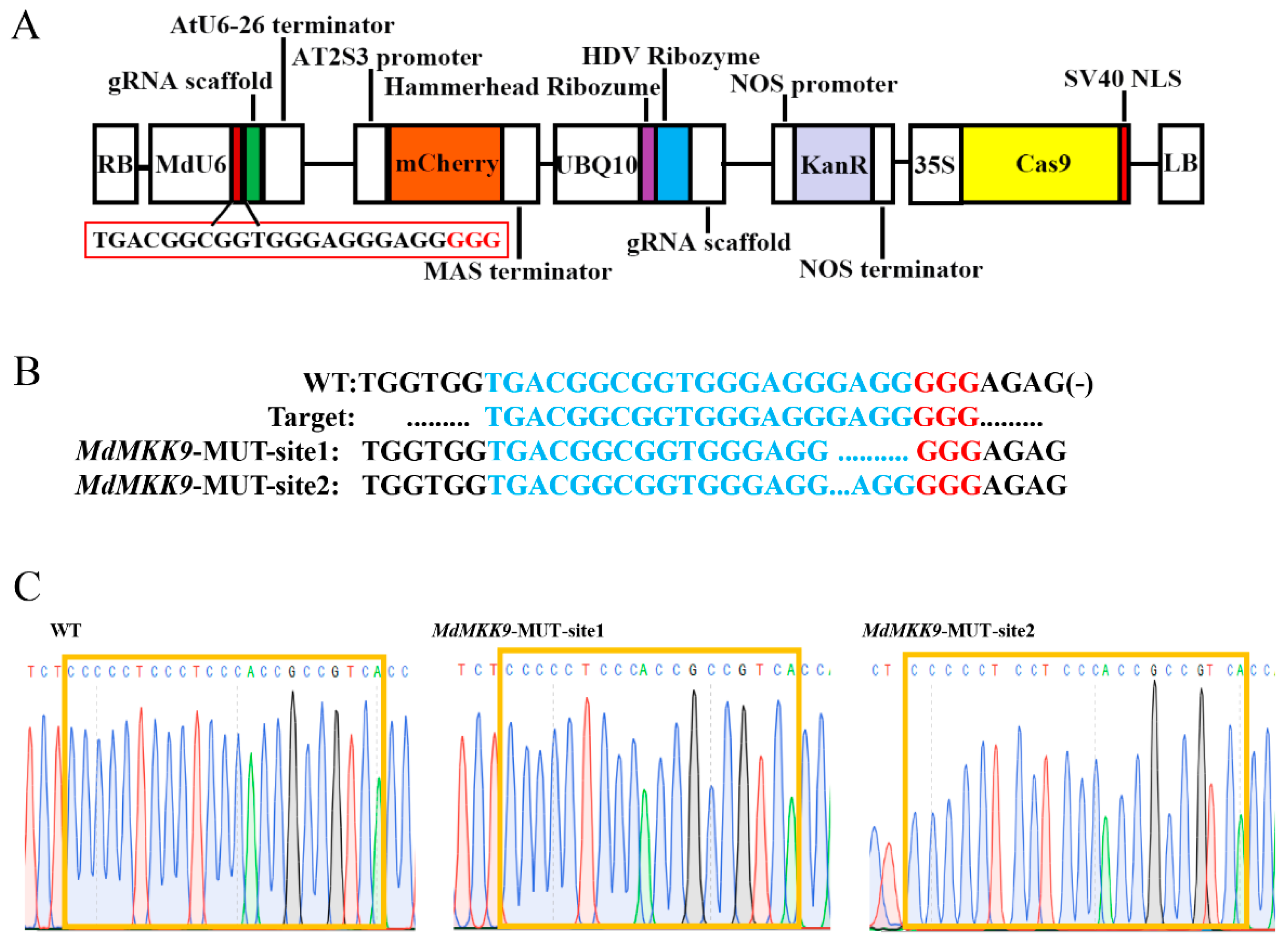
2.3.1. Construction of a CRISPR/Cas9 MdMKK9 Gene Editing Vector and Obtaining MdMKK9 Deletion Mutant Calli
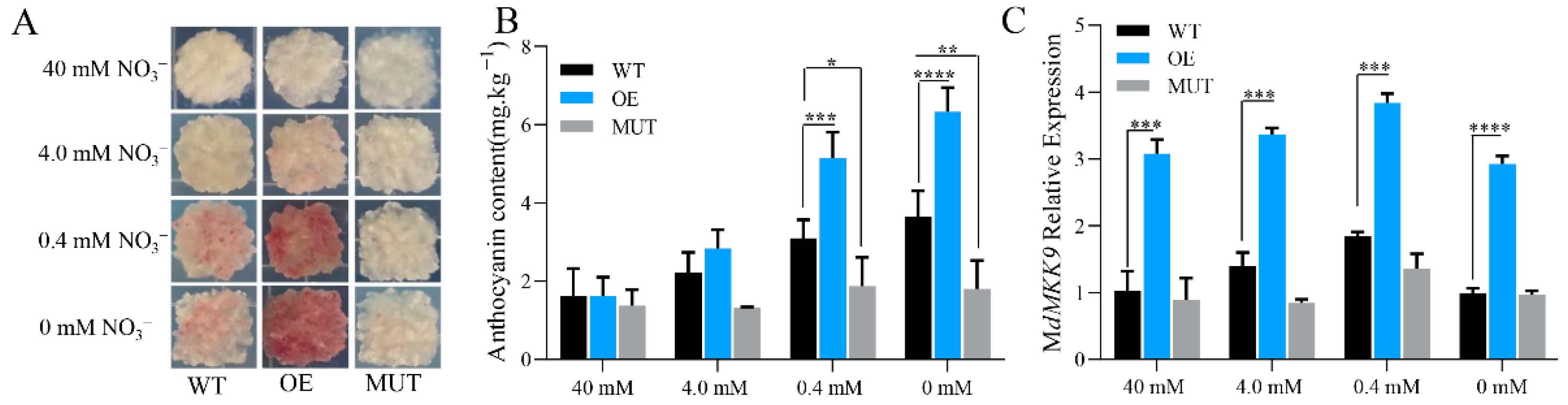
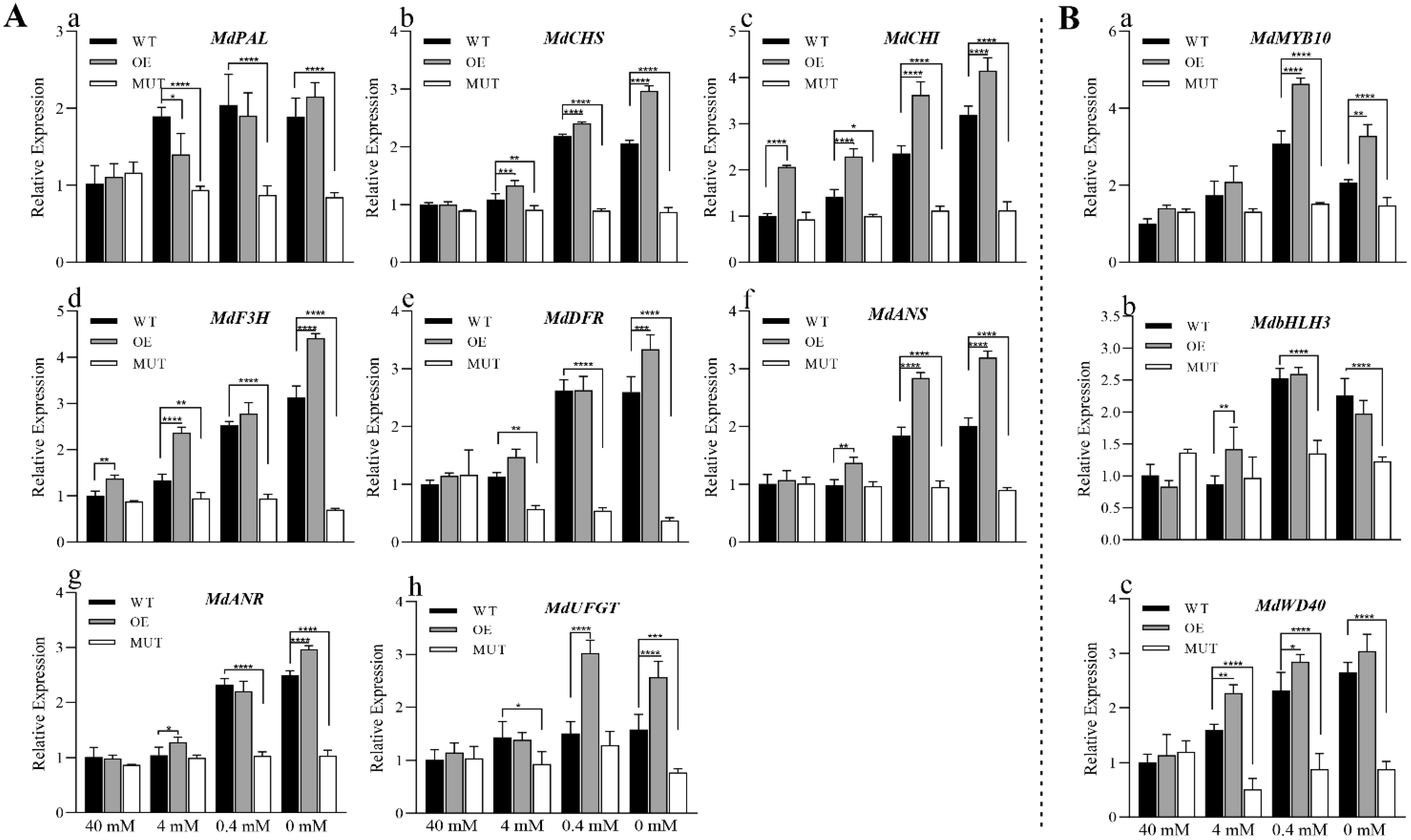
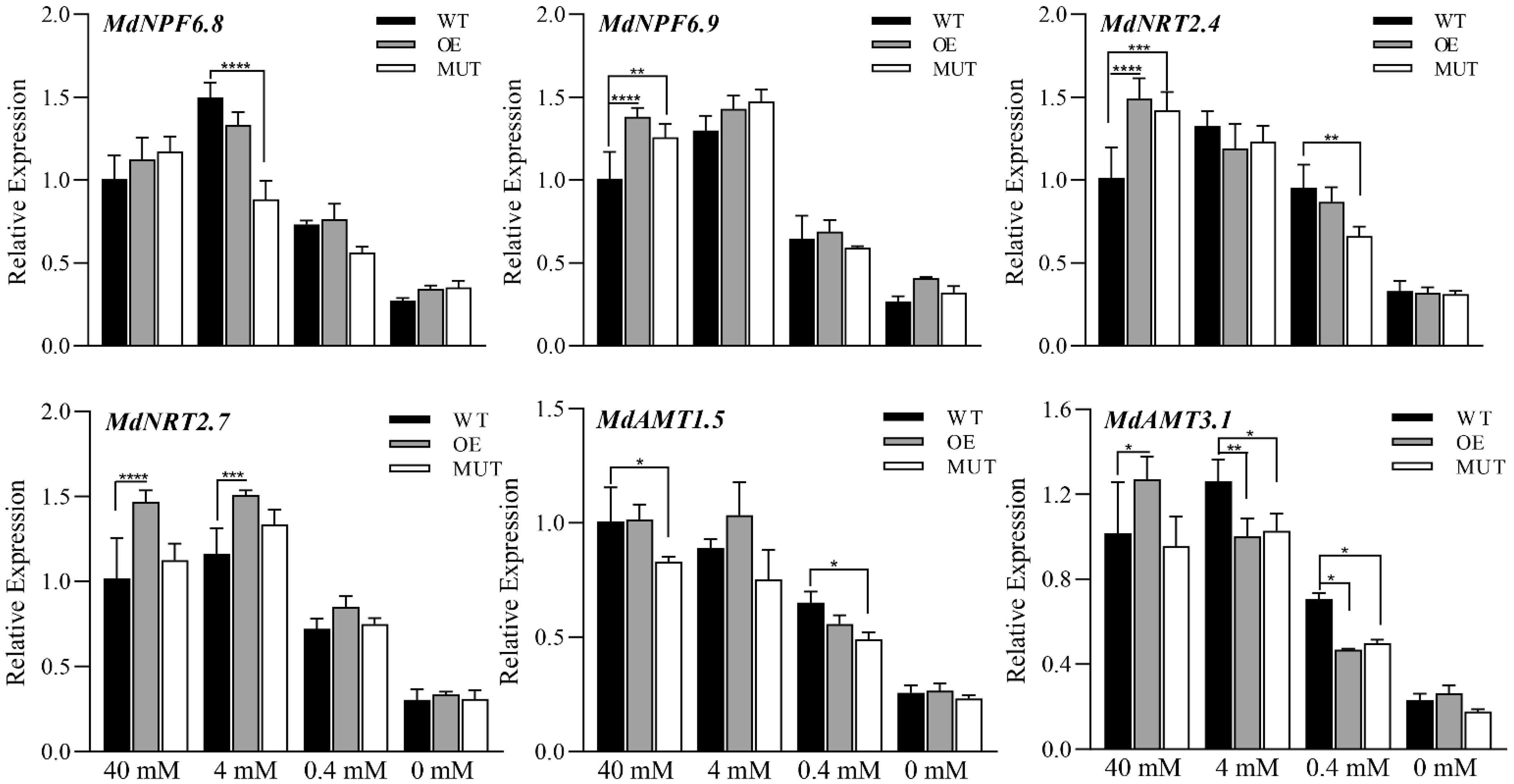
2.3.2. Increase in the Anthocyanin Content of Calli in Transgenic ‘Orin’ under Low Nitrogen Stress Is Mediated by the Up-Regulation of MdMKK9
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Nitrogen Treatments of Tissue Culture Seedlings
4.3. Extraction and Determination of Total Anthocyanin
4.4. Nitrogen Measurement
4.5. RNA Extraction and Gene Expression Analysis
4.6. Construction of the CRISPR/Cas9 MdMKK9 Gene Editing Vector and Overexpression Vector
4.7. Agrobacterium-Mediated Transformation of the Calli of ‘Orin’ Apples
4.8. Nitrogen Treatments of Transgenic Calli
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiang, Y.; Lai, F.N.; He, G.F.; Li, Y.P.; Yang, L.L.; Shen, W.; Huo, H.Q.; Zhu, J.; Dai, H.Y.; Zhang, Y.G. Alleviation of Rosup-induced oxidative stress in porcine granulosa cells by anthocyanins from red-fleshed apples. PLoS ONE 2017, 12, e0184033. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Zhao, R.X.; Liu, W.L.; Sun, X.H.; Bai, S.H.; Xiang, Y.; Dai, H.Y. The anthocyanins component and the influence factors of contents in red-flesh apple ‘Hong-Xun No.1’. Eur. J. Hortic. Sci. 2016, 81, 248–254. [Google Scholar] [CrossRef]
- Espley, R.V.; Brendolise, C.; Chagné, D.; Kutty-Amma, S.; Green, S.; Volz, R.; Putterill, J.; Schouten, H.J.; Gardiner, S.E.; Hellens, R.P.; et al. Multiple repeats of a promoter segment causes transcription factor autoregulation in red apples. Plant Cell 2009, 21, 168–183. [Google Scholar] [CrossRef] [Green Version]
- Rupasinghe, H.P.V.; Huber, G.M.; Embree, C.; Forsline, P.L. Red-fleshed apple as a source for functional beverages. Can. J. Biochem. 2010, 90, 95–100. [Google Scholar] [CrossRef]
- Sun, X.H.; Bai, S.H.; Hou, H.M.; Sun, X.; Zhu, J.; Dai, H.Y.; Zhang, Y.G. A New Red-fleshed Apple Cultivar ‘Daihong’. Acta Hortic. Sin. 2019, 46, 2729–2730. [Google Scholar]
- Feng, X.X.; An, Y.Y.; Zheng, J.; Sun, M.; Wang, L.J. Proteomics and SSH analyses of ALA-promoted fruit coloration and evidence for the involvement of a MADS-Box gene, MdMADS1. Front. Plant Sci. 2016, 7, 1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soubeyrand, E.; Basteau, C.; Hilbert, G.; Leeuwen, C.V.; Delrot, S.; Gomès, E. Nitrogen supply affects anthocyanin biosynthetic and regulatory genes in grapevine cv. Cabernet-Sauvignon berries. Phytochemistry 2014, 103, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.-A.; Duan, S.C.; Jeong, H.-Y.; Lee, C.H.; Kang, I.-K.; Eom, S.H. Pigmentation and Flavonoid Metabolite Diversity in Immature ‘Fuji’ Apple Fruits in Response to Lights and Methyl Jasmonate. Int. J. Mol. Sci. 2022, 23, 1722. [Google Scholar] [CrossRef]
- An, J.P.; Wang, X.F.; Li, Y.Y.; Song, L.Q.; Zhao, L.L.; You, C.X.; Hao, Y.J. EIN3-LIKE1, MYB1, and ETHYLENE RESPONSE FACTOR3 act in a regulatory loop that synergistically modulates ethylene biosynthesis and anthocyanin accumulation. Plant Physiol. 2018, 178, 808–823. [Google Scholar] [CrossRef]
- Wang, X.F.; An, J.P.; Liu, X.; Li, Y.Y.; Su, L.; You, C.X.; Hao, Y.J. The nitrate-responsive protein MdBT2 regulates anthocyanin biosynthesis by interacting with the MdMYB1 transcription factor. Plant Physiol. 2018, 178, 890–906. [Google Scholar] [CrossRef] [Green Version]
- Feng, F.J.; Li, M.J.; Ma, F.W.; Cheng, L.L. Phenylpropanoid metabolites and expression of key genes involved in anthocyanin biosynthesis in the shaded peel of apple fruit in response to sun exposure. Plant Physiol. Biochem. 2013, 69, 54–61. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Meng, J.F.; Ning, P.F.; Xu, T.F.; Zhang, Z.W. Effect of rain-shelter cultivation of Vitis vinifera cv. Cabernet Gernischet on the phenolic profile of berry skins and the incidence of grape diseases. Molecules 2013, 18, 381–397. [Google Scholar] [CrossRef]
- Laura, J. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar]
- Ji, X.H.; Wang, Y.T.; Zhang, R.; Wu, S.J.; An, M.M.; Li, M.; Wang, C.Z.; Chen, X.L.; Zhang, Y.M.; Chen, X.S. Effect of auxin cytokinin and nitrogen on anthocyanin biosynthesis in calli cultures of red-fleshed apple (Malus sieversiif. niedzwetzkyana). Plant Cell Tissue Organ Cult. 2015, 120, 325–337. [Google Scholar] [CrossRef]
- Castaings, L.; Marchive, C.; Meyer, C.; Krapp, A. Nitrogen signaling in Arabidopsis: How to obtain insights into a complex signaling network. J. Exp. Bot. 2011, 62, 1391–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittgenstein, N.J.V.; Le, C.H.; Hawkins, B.J.; Ehlting, J. Evolutionary classification of ammonium, nitrate, and peptide transporters in land plants. BMC Evol. Biol. 2014, 14, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, H.B.; He, J.J.; Cai, Q.Q.; Zhao, W.G.; Fu, H.; Hua, Y.P.; Li, M.T.; Huang, J.Y. The expression characteristics of NPF genes and their response to vernalization and nitrogen deficiency in rapeseed. Int. J. Mol. Sci. 2021, 22, 4944. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, C.H.; Dong, Q.L.; Huang, D.; Li, C.Y.; Li, P.M.; Ma, F.W. Genome-wide identification and analysis of apple NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER family (NPF) genes reveals MdNPF6.5 confers high capacity for ni-trogen uptake under low-nitrogen conditions. Int. J. Mol. Sci. 2018, 19, 2761. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.L.; Li, M.J.; Yun, S.; Sun, T.T.; Li, C.Y. Ammonium uptake increases in response to PEG-induced drought stress in Malus hupehensis Rehd. Environ. Exp. Bot. 2018, 151, 32–42. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.F.; Li, H.L.; An, X.H.; Song, L.Q.; You, C.X.; Zhao, L.L.; Tian, Y.; Wang, X.F. MdMYB10 affects nitrogen uptake and reallocation by regulating the nitrate transporter MdNRT2.4-1 in red-fleshed apple. Horticul. Res. 2022, 9, uhac016. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, C.H.; Huang, D.; Dong, Q.L.; Li, P.M.; Ma, F.W. High-efficient utilization and uptake of N contribute to higher NUE of ‘Qinguan’ apple under drought and N-deficient conditions compared with ‘Honeycrisp’. Tree Physiol. 2019, 39, 1880–1895. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Jin, Y.B.; Tan, K.X.; Zheng, J.Z.; Gao, T.T.; Zhang, Z.J.; Zhao, Y.J.; Ma, F.W.; Li, C. MdTyDc overexpression improves alkalinity tolerance in Malus domestica. Front. Plant Sci. 2021, 12, 625890. [Google Scholar] [CrossRef] [PubMed]
- Dugassa, N.F.; Solveig, M.O.; Behzad, H.; Cathrine, L. Nitrogen depletion and small R3-MYB transcription factors affecting anthocyanin accumulation in Arabidopsis leaves. Phytochemistry 2014, 98, 34–40. [Google Scholar]
- Su, N.N.; Wu, Q.; Cui, J. Increased Sucrose in the Hypocotyls of radish sprouts contributes to nitrogen deficiency-induced anthocyanin accumulation. Front. Plant Sci. 2016, 7, 1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.Q.; Liu, Z.J.; Liu, J.P.; Lin, S.; Wang, J.F.; Lin, W.X.; Xu, W.F. GA-DELLA pathway is involved in regulation of nitrogen deficiency-induced anthocyanin accumulation. Plant Cell Rep. 2017, 36, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Jia, X.; Huo, L.Q.; Che, R.M.; Gong, X.Q.; Wang, P.; Ma, F.W. MdATG18a overexpression improves tolerance to ni-trogen deficiency and regulates anthocyanin accumulation through increased autophagy in transgenic apple. Plant Cell Environ. 2018, 41, 469–480. [Google Scholar] [CrossRef]
- Fiil, B.K.; Petersen, K.; Petersen, M.; Mundy, J. Gene regulation by MAP kinase cascades. Curr. Opin. Plant Biol. 2008, 12, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.C.; Xu, R.R.; Luo, X.C.; Jiang, Z.S.; Shu, H.R. Genome-wide identification and expression analysis of MAPK and MAPKK gene family in Malus domestica. Gene 2013, 531, 377–387. [Google Scholar] [CrossRef]
- Liu, J.Y.; Yang, H.B.; Bao, F.; Ao, K.; Zhang, X.Y.; Zhang, Y.L.; Yang, S.H. IBR5 modulates temperature-dependent R protein CHS3-mediated defense responses in Arabidopsis. PLoS Genet. 2015, 11, e1005584. [Google Scholar] [CrossRef]
- Zhang, M.M.; Su, J.B.; Zhang, Y.; Xu, J.; Zhang, S.Q. Conveying endogenous and exogenous signals: MAPK cascades in plant growth and defense. Curr. Opin. Plant Biol. 2018, 45, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, Y.S.; Dong, C.H.; Zhang, Y.G.; Bai, S.H. MdMAPKKK1 regulates apple resistance to Botryosphaeria dothidea by interacting with MdBSK1. Int. J. Mol. Sci. 2022, 23, 4415. [Google Scholar] [CrossRef] [PubMed]
- Alzwiy, I.A.; Morris, P.C. A mutation in the Arabidopsis MAP kinase kinase 9 gene results in enhanced seedling stress tolerance. Plant Sci. 2007, 173, 302–308. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Yang, J.; Ma, C.L.; Zhang, Y.; Ge, T.; Qi, Z.; Kang, Y. Arabidopsis root hair defective3 is involved in nitrogen starvation-induced anthocyanin accumulation. J. Integr. Plant Boil. 2015, 57, 708–721. [Google Scholar] [CrossRef]
- Luo, J.; Wang, X.; Feng, L.; Li, Y.; He, J.X. The mitogen-activated protein kinase kinase 9 (MKK9) modulates nitrogen acquisition and anthocyanin accumulation under nitrogen-limiting condition in Arabidopsis. Biochem. Biophys. Res. Commu. 2017, 487, 539–544. [Google Scholar] [CrossRef]
- Liu, Z.B.; Li, Y.; Cao, H.W.; Ren, D.T. Comparative phospho-proteomics analysis of salt-responsive phosphoproteins regulated by the MKK9-MPK6 cascade in Arabidopsis. Plant Sci. 2015, 241, 138–150. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.M.; Yang, L.; Nan, W.B.; Ruan, M.J.; Bi, Y.R. Involvement of active MKK9-MAPK3/MAPK6 in increasing respiration in salt-treated Arabidopsis callus. Protoplasma 2020, 257, 965–977. [Google Scholar] [CrossRef]
- Zhou, C.J.; Cai, Z.H.; Guo, Y.F.; Gan, S.S. An Arabidopsis mitogen-activated protein kinase cascade, MKK9-MPK6, plays a role in leaf senescence. Plant Physiol. 2009, 150, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Wang, C.C.; Zhu, F.; Li, Y. Mild osmotic stress promotes 4-methoxy indolyl-3-methyl glucosinolate biosynthesis mediated by the MKK9–MPK3/MPK6 cascade in Arabidopsis. Plant Cell Rep. 2017, 36, 543–555. [Google Scholar] [CrossRef]
- Neilsen, G.H.; Neilsen, D.; Herbert, L. Nitrogen fertigation concentration and timing of application affect nitrogen nutrition, yield, firmness, and color of apples grown at high density. HortScience 2009, 44, 1425–1431. [Google Scholar] [CrossRef] [Green Version]
- Fallahi, E.; Colt, W.M.; Fallahi, B. Optimum ranges of leaf nitrogen for yield, fruit quality, and photosynthesis in ‘BC-2 Fuji’ apple. J. Am. Pomol. Soc. 2001, 55, 68–75. [Google Scholar]
- Munnik, T.; Meijer, H.J.G. Osmotic stress activates distinct lipid and MAPK signaling pathways in plants. FEBS Lett. 2001, 498, 172–178. [Google Scholar] [CrossRef] [Green Version]
- Asai, T.; Tena, G.; Plotnikova, J.; Willmann, M.R.; Chiu, W.L.; Gomez-Gomez, L.; Boller, T.; Ausubel, F.M.; Sheen, J. MAP kinase signaling cascade in Arabidopsis innate immunity. Nature 2002, 415, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.C.S.; Petersen, M.; Mundy, J. Mitogen-activated protein kinase signaling in plants. Annu. Rev. Plant Biol. 2010, 61, 621–649. [Google Scholar]
- Colcombet, J.; Hirt, H. Arabidopsis MAPKs: A complex signaling network involved in multiple biological processes. Biochem. J. 2008, 413, 217–226. [Google Scholar] [CrossRef]
- Zhang, H.L.; Mao, K.; Liu, Z.; Xie, X.B.; Feng, X.M.; Hao, Y.J. In silico analysis and expression confirmation of the regulation of fruit coloration by transcriptional factor MdMYB1 in Delicious apple. Acta. Hortic. Sin. 2010, 36, 1581–1588. [Google Scholar]
- Chopin, F.; Orsel, M.; Dorbe, M.F.; Chardon, F.; Truong, H.N.; Mille, A.J.; Krapp, A.; Daniel-Vedele, F. The Arabidopsis ATNRT2.7 nitrate transporter controls nitrate content in seeds. Plant Cell 2007, 19, 1590–1602. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.J.; Gao, N.; Ma, Q.J.; Zhang, J.C.; Wang, X.; Lu, J.; Hao, Y.J.; Wang, X.F.; You, C.X. The MdABI5 transcription factor interacts with the MdNRT1.5/MdNPF7.3 promoter to fine-tune nitrate transport from roots to shoots in apple. Hortic.Res. 2021, 8, 236. [Google Scholar] [CrossRef]
- Ueda, Y.; Kiba, T.; Yanagisawa, S. Nitrate-inducible NIGT1 proteins modulate phosphate uptake and starvation signalling via transcriptional regulation of SPX genes. Plant J. 2020, 102, 448–466. [Google Scholar] [CrossRef]
- Han, P.L.; Dong, Y.H.; Gu, K.D.; Yu, J.Q.; Hu, D.G.; Hao, Y.J. The apple U-box E3 ubiquitin ligase MdPUB29 contributes to activate plant immune response to the fungal pathogen Botryosphaeria dothidea. Planta 2019, 249, 1177–1188. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Qi, C.H.; Jiang, H.; Zhong, M.S.; Zhao, Q.; You, C.X.; Li, Y.Y.; Hao, Y.J. MdWRKY46-enhanced apple resistance to Botryosphaeria dothidea by activating the expression of MdPBS3.1 in the salicylic acid signaling pathway. Mol. Plant Microbe Interact. 2019, 32, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.J.; Bai, S.H.; Wang, N.; Sun, X.H.; Zhang, Y.G.; Zhu, J.; Dong, C.H. CRISPR/Cas9-mediated mutagenesis of MdCNGC2 in apple callus and VIGS-mediated silencing of MdCNGC2 in fruits improve resistance to Botryosphaeria dothidea. Front. Plant Sci. 2020, 11, 575477. [Google Scholar] [CrossRef] [PubMed]
- Li, R.Y.; Vavrik, C.; Danna, C.H. Proxies of CRISPR/Cas9 activity to aid in the identification of mutagenized Arabidopsis plants. G3-Genes Genom. Genet. 2020, 10, 2033–2042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.H.; Zhang, L.; Lu, L.Q.; Liu, J.H.; Yi, H.L.; Wu, J.X. An efficient CRISPR/Cas9 system for simultaneous editing two target sites in Fortunella hindsii. Horticul. Res. 2022, 9, uhac064. [Google Scholar] [CrossRef]
- Li, R.; Liu, C.X.; Zhao, R.R.; Wang, L.; Chen, L.; Yu, W.Q.; Zhang, S.J.; Sheng, J.P.; Shen, L. CRISPR/Cas9-Mediated SlNPR1 mutagenesis reduces tomato plant drought tolerance. BMC Plant Biol. 2019, 19, 38. [Google Scholar] [CrossRef] [Green Version]
- Ueta, R.; Abe, C.; Watanabe, T.; Sugano, S.S.; Ishihara, R.; Ezura, H.; Osakabe, Y.; Osakabe, K. Rapid breeding of parthenocarpic tomato plants using CRISPR/Cas9. Sci. Rep. 2020, 10, 16776. [Google Scholar] [CrossRef]
- Pompili, V.; Costa, L.D.; Piazza, S.; Pindo, M.; Malnoy, M. Reduced fire blight susceptibility in apple cultivars using a high-efficiency CRISPR/Cas9-FLP/FRT-based gene editing system. Plant Biotechnol. J. 2020, 18, 845–858. [Google Scholar] [CrossRef]
- Karanjalker, G.R.; Ravishankar, K.V.; Shivashankara, K.S.; Karanjalker, G.R.; Ravishankar, K.V.; Shivashankara, K.S.; Dinesh, M.R.; Roy, T.K.; Sudhakar Rao, D.V. A Study on the expression of genes involved in carotenoids and anthocyanins during ripening in fruit peel of green, yellow, and red colored mango cultivars. Appl. Biochem. Biotechnol. 2018, 184, 140–154. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Acree, T.E., Decker, E.A., Penner, M.H., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Smith, D.M., Sporns, P., Eds.; John Wiley and Sons Inc.: New York, NY, USA, 2001; pp. F1.2.1–F1.2.13. [Google Scholar]
- Williams, P.; Huang, Z.L.; Wang, B.W.; Pace, R.D. Identification of anthocyanins in muscadine grapes with HPLC-ESI-MS. LWT-Food Sci. Technol. 2009, 42, 819–824. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Stemmer, M.; Thumberger, T.; Keyer, M.D.S.; Wittbrodt, J.; Mateo, J.L. CCTop: An intuitive, flexible and reliable CRISPR/Cas9 target prediction tool. PLoS ONE 2015, 10, e0124633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.H.; Chen, J.L.; Dai, X.H.; Zhang, D.; Zhao, Y.D. An effective strategy for reliably isolating heritable and Cas9-free Arabidopsis mutants generated by CRISPR/Cas9-mediated genome editing. Plant Physiol. 2016, 171, 1794–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, J.P.; Liu, X.; Song, L.Q.; You, C.X.; Wang, X.F.; Hao, Y.J. Apple RING finger E3 ubiquitin MdMIEL1 negatively regulates salt and oxidative stress tolerance. J. Plant Biol. 2017, 60, 137–145. [Google Scholar] [CrossRef]
- Lv, L.L.; Liu, Y.S.; Bai, S.H.; Turakulov, K.S.; Dong, C.H.; Zhang, Y.G. A TIR-NBS-LRR gene MdTNL1 regulates resistance to glomerella leaf spot in apple. Int. J. Mol. Sci. 2022, 23, 6323. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Li, X.; Wang, Y.; Xu, J.; Jiang, S.; Zhang, Y. MdMKK9-Mediated the Regulation of Anthocyanin Synthesis in Red-Fleshed Apple in Response to Different Nitrogen Signals. Int. J. Mol. Sci. 2022, 23, 7755. https://doi.org/10.3390/ijms23147755
Sun X, Li X, Wang Y, Xu J, Jiang S, Zhang Y. MdMKK9-Mediated the Regulation of Anthocyanin Synthesis in Red-Fleshed Apple in Response to Different Nitrogen Signals. International Journal of Molecular Sciences. 2022; 23(14):7755. https://doi.org/10.3390/ijms23147755
Chicago/Turabian StyleSun, Xiaohong, Xinxin Li, Yanbo Wang, Jihua Xu, Shenghui Jiang, and Yugang Zhang. 2022. "MdMKK9-Mediated the Regulation of Anthocyanin Synthesis in Red-Fleshed Apple in Response to Different Nitrogen Signals" International Journal of Molecular Sciences 23, no. 14: 7755. https://doi.org/10.3390/ijms23147755
APA StyleSun, X., Li, X., Wang, Y., Xu, J., Jiang, S., & Zhang, Y. (2022). MdMKK9-Mediated the Regulation of Anthocyanin Synthesis in Red-Fleshed Apple in Response to Different Nitrogen Signals. International Journal of Molecular Sciences, 23(14), 7755. https://doi.org/10.3390/ijms23147755





