Visible-Light-Driven Antimicrobial Activity and Mechanism of Polydopamine-Reduced Graphene Oxide/BiVO4 Composite
Abstract
:1. Introduction
2. Results and Discussion
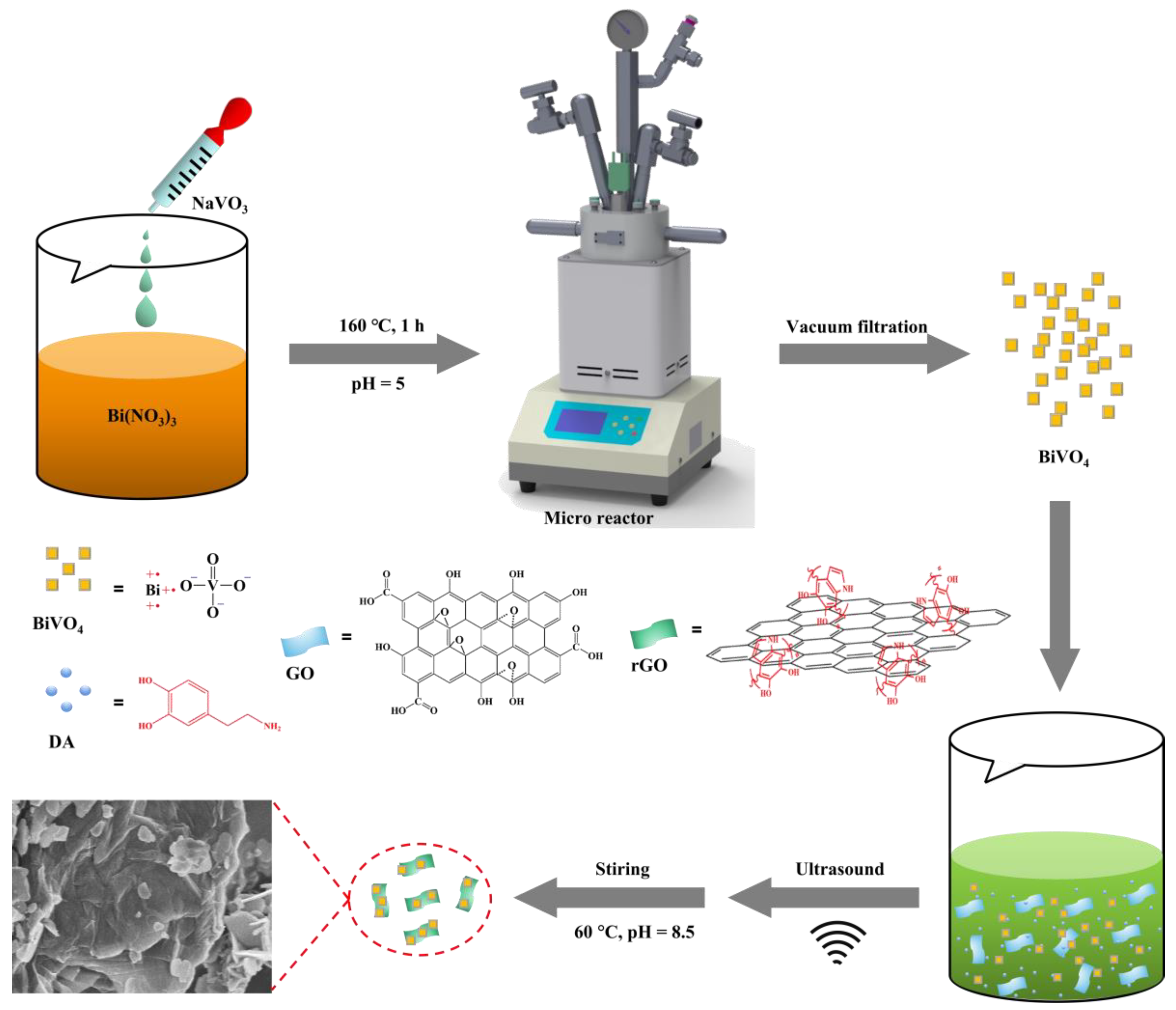
2.1. PDA-rGO/BiVO4
2.2. Photothermal Property
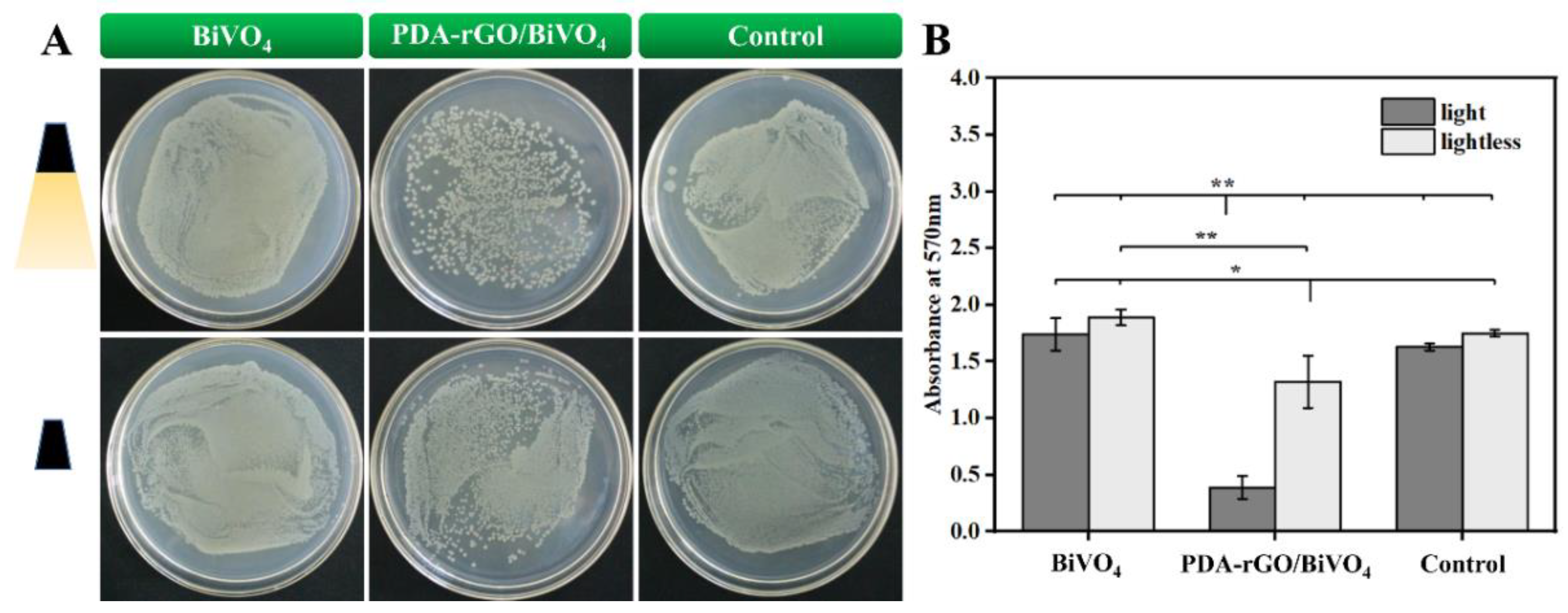
2.3. Antibacterial Activity
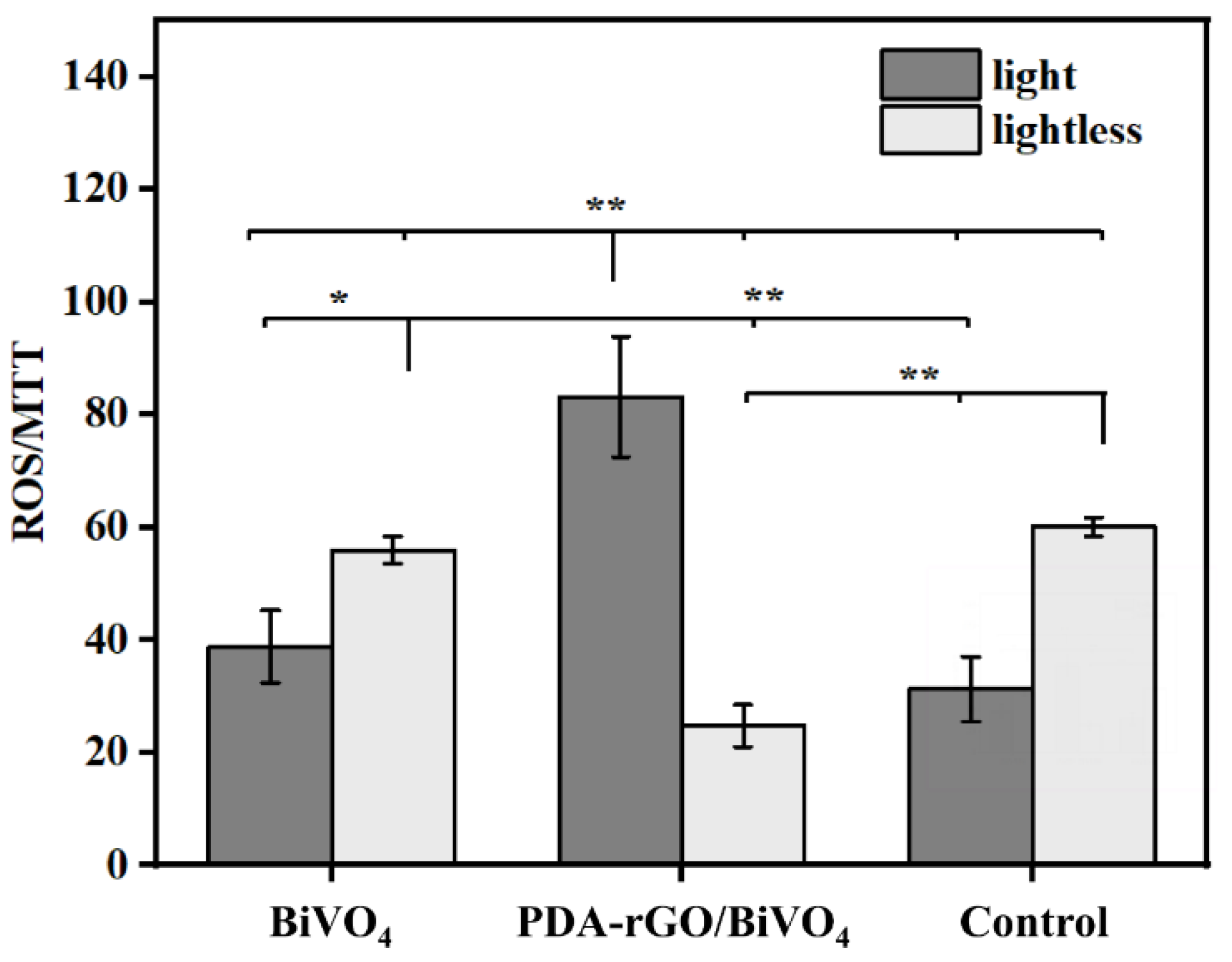
2.4. ROS Production
2.5. Microstructures of Bacteria
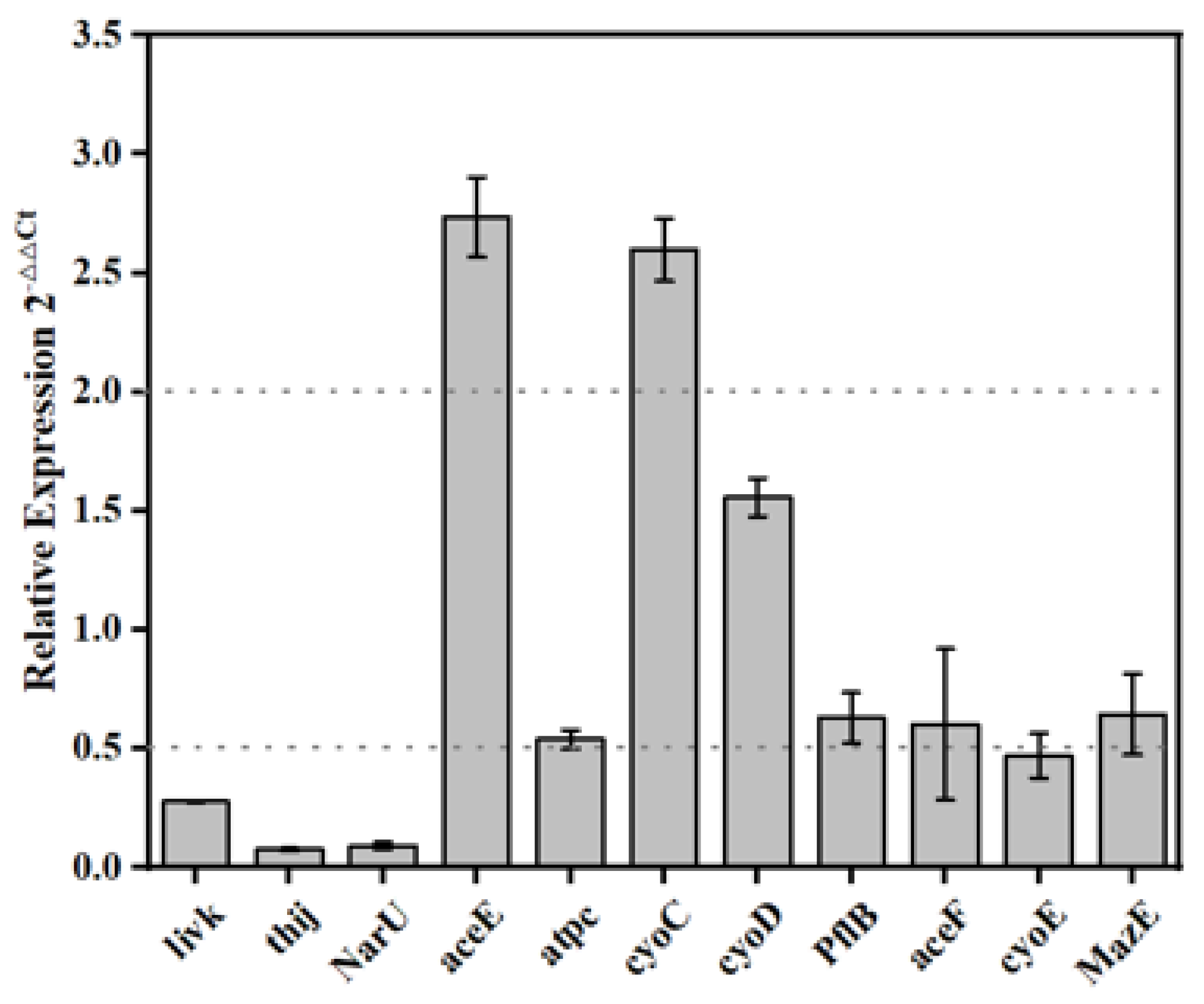
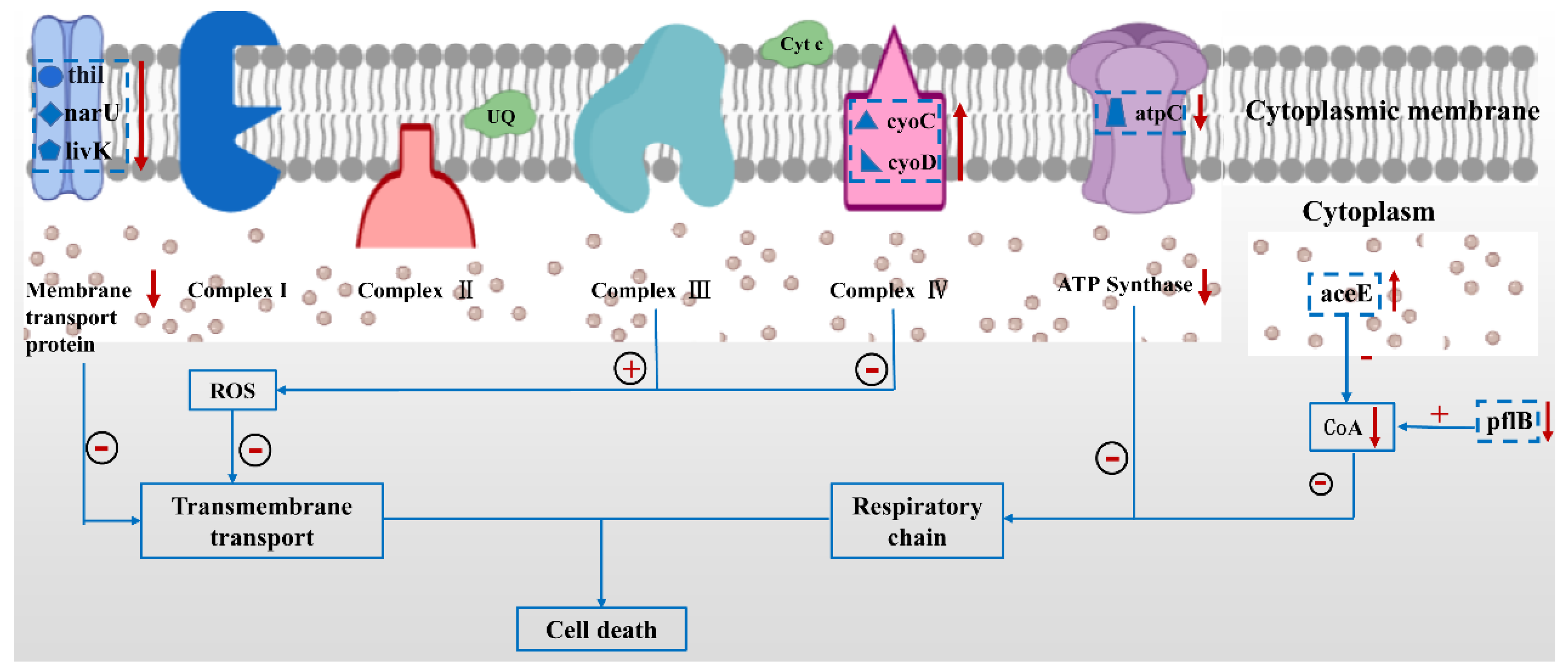
2.6. Genes Expression
3. Materials and Methods
3.1. Materials
3.2. Preparation of BiVO4
3.3. PDA-rGO/BiVO4 Nanocomposite Synthesis
3.4. Characterization
3.5. Antibacterial Ability Assays
3.6. ROS Analysis
3.7. Detection of mRNA Levels of Key Genes
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.F.; Tang, X.N.; Gao, X.; Zhang, B.; Luo, Y.; Yao, X.Y. Antimicrobial property and photocatalytic antibacterial mechanism of the TiO2-doped SiO2 hybrid materials under ultraviolet-light irradiation and visible-light irradiation. Ceram. Int. 2019, 45, 15505–15513. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, C.; Zhao, Y.; Zhou, S.; Hu, X.; Chen, P. Low Ag-doped titanium dioxide nanosheet films with outstanding antimicrobial property. Environ. Sci. Technol. 2010, 44, 8270–8275. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.T.; Manisekaran, R.; Santoyo-Salazar, J.; Schoefs, B.; Velumani, S.; Castaneda, H.; Jantrania, A. Graphene oxide decorated TiO2 and BiVO4 nanocatalysts for enhanced visible-light-driven photocatalytic bacterial inactivation. J. Photochem. Photobiol. A-Chem. 2021, 418, 113374. [Google Scholar]
- Qu, Z.; Liu, P.; Yang, X.; Wang, F.; Zhang, W.; Fei, C. Microstructure and characteristic of BiVO4 prepared under different pH values: Photocatalytic efficiency and antibacterial activity. Materials 2016, 9, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.Y.; Li, X.; Liang, J.; Yuan, X.Z.; Jiang, L.B.; Yu, H.B.; Sun, H.B.; Zhu, Z.Q.; Ye, S.J.; Tang, N.; et al. Fabrication and regulation of vacancy-mediated bismuth oxyhalide towards photocatalytic application: Development status and tendency. Coord. Chem. Rev. 2021, 443, 214033. [Google Scholar] [CrossRef]
- Yan, Y.; Ni, T.; Du, J.; Li, L.; Fu, S.; Li, K.; Zhou, J. Green synthesis of balsam pear-shaped BiVO4/BiPO4 nanocomposite for degradation of organic dye and antibiotic metronidazole. Dalton Trans. 2018, 47, 6089–6101. [Google Scholar] [CrossRef]
- Jafari, N.; Ebrahimpour, K.; Abdolahnejad, A.; Karimi, M.; Ebrahimi, A. Efficient degradation of microcystin-LR by BiVO4/TiO2 photocatalytic nanocomposite under visible light. J. Environ. Health Sci. Eng. 2020, 17, 1171–1183. [Google Scholar] [CrossRef]
- Trinh, D.T.T.; Channei, D.; Nakaruk, A.; Khanitchaidecha, W. New insight into the photocatalytic degradation of organic pollutant over BiVO4/SiO2/GO nanocomposite. Sci. Rep. 2021, 11, 4620. [Google Scholar] [CrossRef]
- Lee, G.J.; Lee, X.Y.; Lyu, C.; Liu, N.; Andandan, S.; Wu, J.J. Sonochemical synthesis of copper-doped BiVO4/g-C3N4 nanocomposite materials for photocatalytic degradation of bisphenol a under simulated sunlight irradiation. Nanomaterials 2020, 10, 498. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Wang, C.; Shen, T.; Song, H.; Li, D.; Zhao, R.; Wang, X. Engineering the dimensional interface of BiVO4-2D reduced graphene oxide (RGO) nanocomposite for enhanced visible light photocatalytic performance. Nanomaterials 2019, 9, 907. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Long, Y.; Zhang, D. Novel bifunctional V2O5/BiVO4 nanocomposite materials with enhanced antibacterial activity. J. Taiwan Inst. Chem. Eng. 2016, 68, 387–395. [Google Scholar] [CrossRef]
- Tayebi, M.; Tayyebi, A.; Lee, B.K.; Lee, C.H.; Lim, D.H. The effect of silver doping on photoelectrochemical (PEC) properties of bismuth vanadate for hydrogen production. Sol. Energy Mater. Sol. Cells 2019, 200, 109943. [Google Scholar] [CrossRef]
- Zhao, M.; Shan, T.; Wu, Q.; Gu, L. The antibacterial effect of graphene oxide on Streptococcus mutans. J. Nanosci. Nanotechnol. 2020, 20, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial properties of graphene-based nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Uma; Singh, S.; Verma, A.; Khanuja, M. Visible light induced bactericidal and photocatalytic activity of hydrothermally synthesized BiVO4 nano-octahedrals. J. Photochem. Photobiol. B-Biol. 2016, 162, 266–272. [Google Scholar] [CrossRef]
- El-Yazeed, W.S.A.; El-Hakam, S.A.; Salah, A.A.; Ibrahim, A.A. Fabrication and characterization of reduced graphene-BiVO4 nanocomposites for enhancing visible light photocatalytic and antibacterial activity. J. Photochem. Photobiol. A Chem. 2021, 417, 113362. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, S.; Song, Y.; Yan, X.; Guan, W.; Liu, X.; Shi, W. Microwave-assisted in situ synthesis of reduced graphene oxide-BiVO4 composite photocatalysts and their enhanced photocatalytic performance for the degradation of ciprofloxacin. J. Hazard. Mater. 2013, 250, 106–114. [Google Scholar] [CrossRef]
- Ng, Y.H.; Iwase, A.; Bell, N.J.; Kudo, A.; Amal, R. Semiconductor/reduced graphene oxide nanocomposites derived from photocatalytic reactions. Catal. Today 2011, 164, 353–357. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, X.; Wang, X. BiVO4-graphene catalyst and its high photocatalytic performance under visible light irradiation. Mater. Chem. Phys. 2011, 131, 325–330. [Google Scholar] [CrossRef]
- Liao, J.; He, S.; Guo, S.; Luan, P.; Mo, L.; Li, J. Antibacterial performance of a mussel-inspired polydopamine-treated Ag/graphene nanocomposite material. Materials 2019, 12, 3360. [Google Scholar] [CrossRef] [Green Version]
- Li, B.Y.; Xiong, F.; Yao, B.; Du, Q.; Cao, J.; Qu, J.G.; Feng, W.; Yuan, H.H. Preparation and characterization of antibacterial dopamine-functionalized reduced graphene oxide/PLLA composite nanofibers. RSC Adv. 2020, 10, 18614–18623. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Bi, S.; Jiang, H.; Telegin, F.; Bai, X.; Yang, H.; Cheng, D.; Cai, G.; Wang, X. Core-shell BiVO4@PDA composite photocatalysts on cotton fabrics for highly efficient photodegradation under visible light. Cellulose 2019, 26, 6259–6273. [Google Scholar] [CrossRef]
- Ou, M.; Zhong, Q.; Zhang, S.L.; Nie, H.Y.; Lv, Z.J.; Cai, W. Graphene-decorated 3D BiVO4 superstructure: Highly reactive (040) facets formation and enhanced visible-light-induced photocatalytic oxidation of NO in gas phase. Appl. Catal. B-Environ. 2016, 193, 160–169. [Google Scholar] [CrossRef]
- Vidya, J.; Bosco, A.J.; Haribaaskar, K.; Balamurugan, P. Polyaniline-BiVO4 nanocomposite as an efficient adsorbent for the removal of methyl orange from aqueous solution. Mater. Sci. Semicond. Process 2019, 103, 104645. [Google Scholar] [CrossRef]
- Azad, R.; Bezaatpour, A.; Amiri, M.; Eskandari, H.; Nouhi, S.; Taffa, D.H.; Wark, M.; Boukherroub, R.; Szunerits, S. Excellent photocatalytic reduction of nitroarenes to aminoarenes by BiVO4 nanoparticles grafted on reduced graphene oxide (rGO/BiVO4). Appl. Organomet. Chem. 2019, 33, e5059. [Google Scholar] [CrossRef]
- Packiaraj, R.; Venkatesh, K.S.; Devendran, P.; Bahadur, S.A.; Nallamuthu, N. Structural, morphological and electrochemical studies of nanostructured BiVO4 for supercapacitor application. Mater. Sci. Semicond. Process 2020, 115, 105122. [Google Scholar] [CrossRef]
- Zhang, A.P.; Zhang, J.Z. Hydrothermal processing for obtaining of BiVO4 nanoparticles. Mater. Lett. 2009, 63, 1939–1942. [Google Scholar] [CrossRef]
- Vasileiadis, T.; Dalvise, T.M.; Saak, C.M.; Pochylski, M.; Harvey, S.; Synatschke, C.V.; Gapinski, J.; Fytas, G.; Backus, E.H.G.; Weil, T.; et al. Fast light-driven motion of polydopamine nanomembranes. Nano Lett. 2022, 22, 578–585. [Google Scholar] [CrossRef]
- Zong, P.; Liang, J.; Zhang, P.; Wan, C.; Wang, Y.; Koumoto, K. Graphene-based thermoelectrics. ACS Appl. Energy Mater. 2020, 3, 2224–2239. [Google Scholar] [CrossRef]
- Saleem, A.; Ahmed, T.; Ammar, M.; Zhang, H.L.; Xu, H.B.; Tabassum, R. Direct growth of m-BiVO4@carbon fibers for highly efficient and recyclable photocatalytic and antibacterial applications. J. Photochem. Photobiol. B Biol. 2020, 213, 112070. [Google Scholar] [CrossRef]
- Hegab, H.M.; ElMekawy, A.; Zou, L.; Mulcahy, D.; Saint, C.P.; Ginic-Markovic, M. The controversial antibacterial activity of graphene-based materials. Carbon 2016, 105, 362–376. [Google Scholar] [CrossRef]
- Jeevitha, G.; Abhinayaa, R.; Mangalaraj, D.; Ponpandian, N. Tungsten oxide-graphene oxide (WO3-GO) nanocomposite as an efficient photocatalyst, antibacterial and anticancer agent. J. Phys. Chem. Solids 2018, 116, 137–147. [Google Scholar] [CrossRef]
- Jovanovic, S.; Holclajtner-Antunovic, I.; Uskokovic-Markovic, S.; Bajuk-Bogdanovic, D.; Pavlovic, V.; Tosic, D.; Milenkovic, M.; Markovic, B.T. Modification of graphene oxide surfaces with 12-molybdophosphoric acid: Structural and antibacterial study. Mater. Chem. Phys. 2018, 213, 157–167. [Google Scholar] [CrossRef]
- Aditya, A.; Chattopadhyay, S.; Jha, D.; Gautam, H.K.; Maiti, S.; Ganguli, M. Zinc oxide nanoparticles dispersed in ionic liquids show high antimicrobial efficacy to skin-specific bacteria. ACS Appl. Mater. Interfaces 2018, 10, 15401–15411. [Google Scholar] [CrossRef] [PubMed]
- Phanichphant, S.; Nakaruk, A.; Chansaenpak, K.; Channei, D. Evaluating the photocatalytic efficiency of the BiVO4/rGO photocatalyst. Sci. Rep. 2019, 9, 16091. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, Y.; Liu, D.; Ding, H.; Mamba, B.B.; Kuvarega, A.T.; Gui, J. Fabrication of a La-doped BiVO4@CN step-scheme heterojunction for effective tetracycline degradation with dual-enhanced molecular oxygen activation. Sep. Purif. Technol. 2021, 277, 119224. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, W.; Wu, Y.; Zhou, C.; Li, L.; Liu, Z.; Dong, J.; Zhou, K. Antibacterial behaviors of Cu2O particles with controllable morphologies in acrylic coatings. Appl. Surf. Sci. 2019, 465, 279–287. [Google Scholar] [CrossRef]
- Cai, Q.; Gao, Y.; Gao, T.; Lan, S.; Simalou, O.; Zhou, X.; Zhang, Y.; Harnoode, C.; Gao, G.; Dong, A. Insight into biological effects of zinc oxide nanoflowers on bacteria: Why morphology matters. ACS Appl. Mater. Interfaces 2016, 8, 10109–10120. [Google Scholar] [CrossRef]
- Liang, X.L.; Liang, Z.M.; Wang, S.; Chen, X.H.; Ruan, Y.; Zhang, Q.Y.; Zhang, H.Y. An analysis of the mechanism underlying photocatalytic disinfection based on integrated metabolic networks and transcriptional data. J. Environ. Sci. 2020, 92, 28–37. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Suo, X.; Liu, X.; Liu, B.; Yuan, M.; Wang, G.; Liang, C.; Shi, H. Cellular response of Escherichia coli to photocatalysis: Flagellar assembly variation and beyond. ACS Nano 2019, 13, 2004–2014. [Google Scholar]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Qu, J.G.; Qian, J.Q.; Wu, M.T.; Mao, Q.H.; Li, M. Hydrothermal synthesis of cotton-based BiVO4/Ag composite for photocatalytic degradation of CI Reactive Black 5. RSC Adv. 2020, 10, 39295–39303. [Google Scholar] [CrossRef] [PubMed]



| Gene Name | Description | Forward (5′→3′) | Reversed (5′→3′) |
|---|---|---|---|
| thil | tRNA uridine 4-sulfurtransferase | GGCTGTACGTGCTATAAAAG | GCGCAAAAGATGAAAACCAG |
| narU | nitrate/nitrite transporter | GACATAAACGGAAAGAGCCG | AAAAGCCAAACAAGGGAGCG |
| aceE | pyruvate dehydrogenase E1 component | CGTCAAGGGCTGGCATAGAT | GGTCGTCTGACTCAGGAGCA |
| pflB | pyruvate formate-lyase | ATGCTCCCACTGCTCAGGAA | GCTTAATGAAAAGTTAGCCACA |
| cyoD | cytochrome bo3 ubiquinol oxidase subunit 4 | GCAAACAGAAGTGGCACGAT | CTGGCAATGGCAGTGGTACA |
| cyoC | cytochrome bo3 ubiquinol oxidase subunit 3 | CTACCACGTTTAGCGGGCAG | GGCTTCCTGTCAGCGTTCTT |
| atpC | ATP synthase F1 complex subunit epsilon | GTACCTTCGCTTTGCATTCC | TATCTATCTGTCTGGCGGCATT |
| livK | L-leucine/L-phenylalanine ABC transporter periplasmic binding protein | GTAGGTGCCGTCGGTTCACT | CGTGATTGGGCCGCTGAACT |
| 16S rRNA (reference) | 16S ribosomal RNA | CCAACACGGGAACTCCGCAC | CTGGACGAAGACTGACGCTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Gao, X.; Qu, J.; Xiong, F.; Xuan, H.; Jin, Y.; Yuan, H. Visible-Light-Driven Antimicrobial Activity and Mechanism of Polydopamine-Reduced Graphene Oxide/BiVO4 Composite. Int. J. Mol. Sci. 2022, 23, 7712. https://doi.org/10.3390/ijms23147712
Li B, Gao X, Qu J, Xiong F, Xuan H, Jin Y, Yuan H. Visible-Light-Driven Antimicrobial Activity and Mechanism of Polydopamine-Reduced Graphene Oxide/BiVO4 Composite. International Journal of Molecular Sciences. 2022; 23(14):7712. https://doi.org/10.3390/ijms23147712
Chicago/Turabian StyleLi, Biyun, Xiaoxiao Gao, Jiangang Qu, Feng Xiong, Hongyun Xuan, Yan Jin, and Huihua Yuan. 2022. "Visible-Light-Driven Antimicrobial Activity and Mechanism of Polydopamine-Reduced Graphene Oxide/BiVO4 Composite" International Journal of Molecular Sciences 23, no. 14: 7712. https://doi.org/10.3390/ijms23147712
APA StyleLi, B., Gao, X., Qu, J., Xiong, F., Xuan, H., Jin, Y., & Yuan, H. (2022). Visible-Light-Driven Antimicrobial Activity and Mechanism of Polydopamine-Reduced Graphene Oxide/BiVO4 Composite. International Journal of Molecular Sciences, 23(14), 7712. https://doi.org/10.3390/ijms23147712






