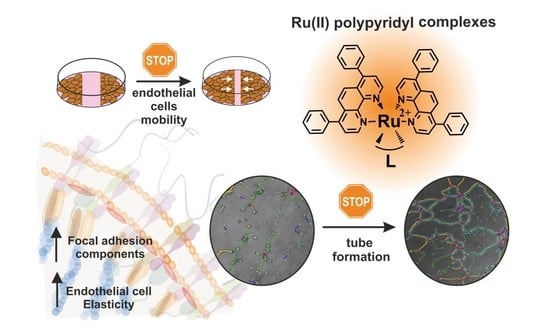
Impact of Polypyridyl Ru Complexes on Angiogenesis—Contribution to Their Antimetastatic Activity
Abstract
:1. Introduction
2. Results and Discussion
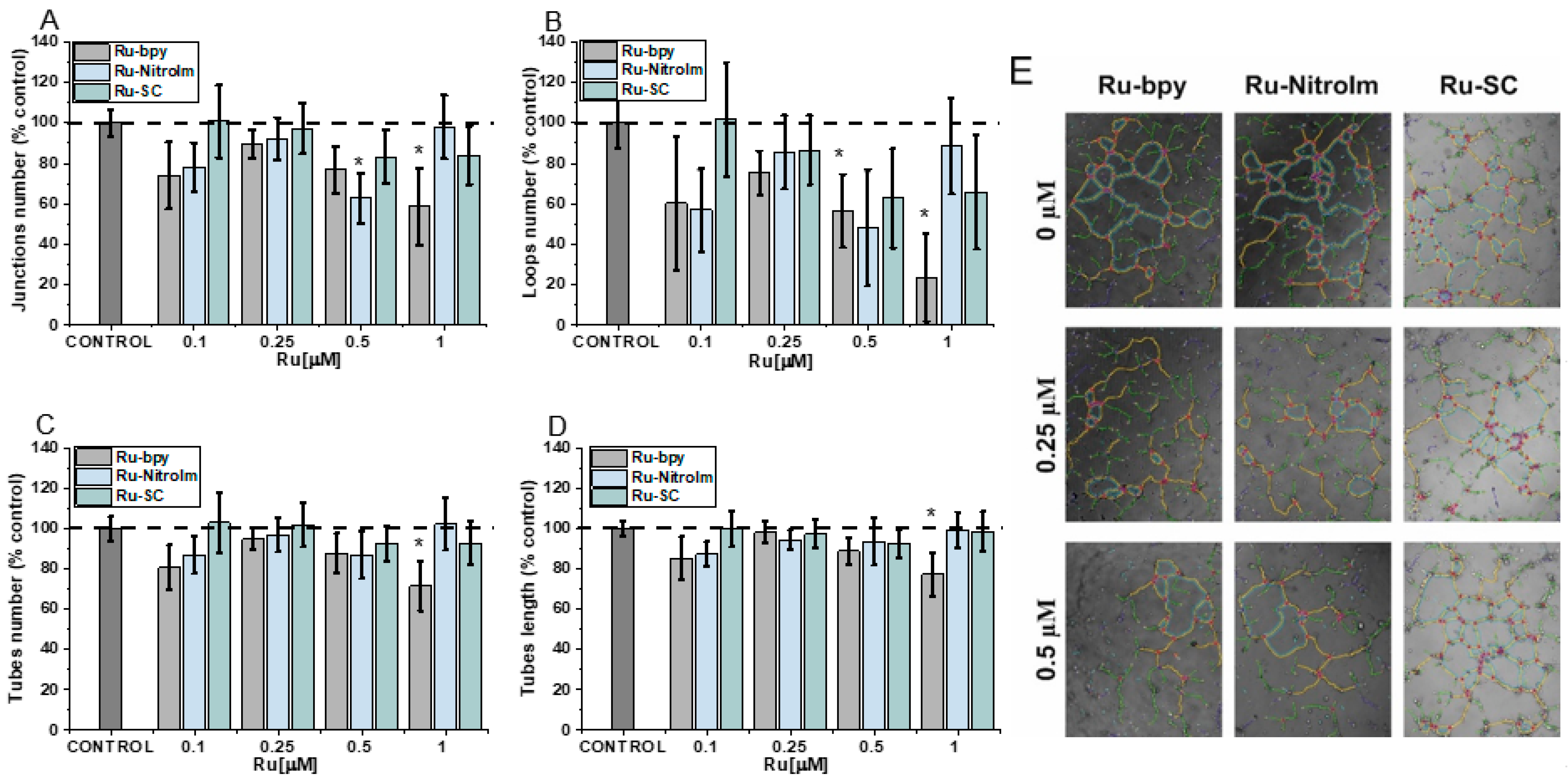
2.1. Negative Impact on Tube Formation
2.2. Inhibitory Effect on Endothelial Cell Migration
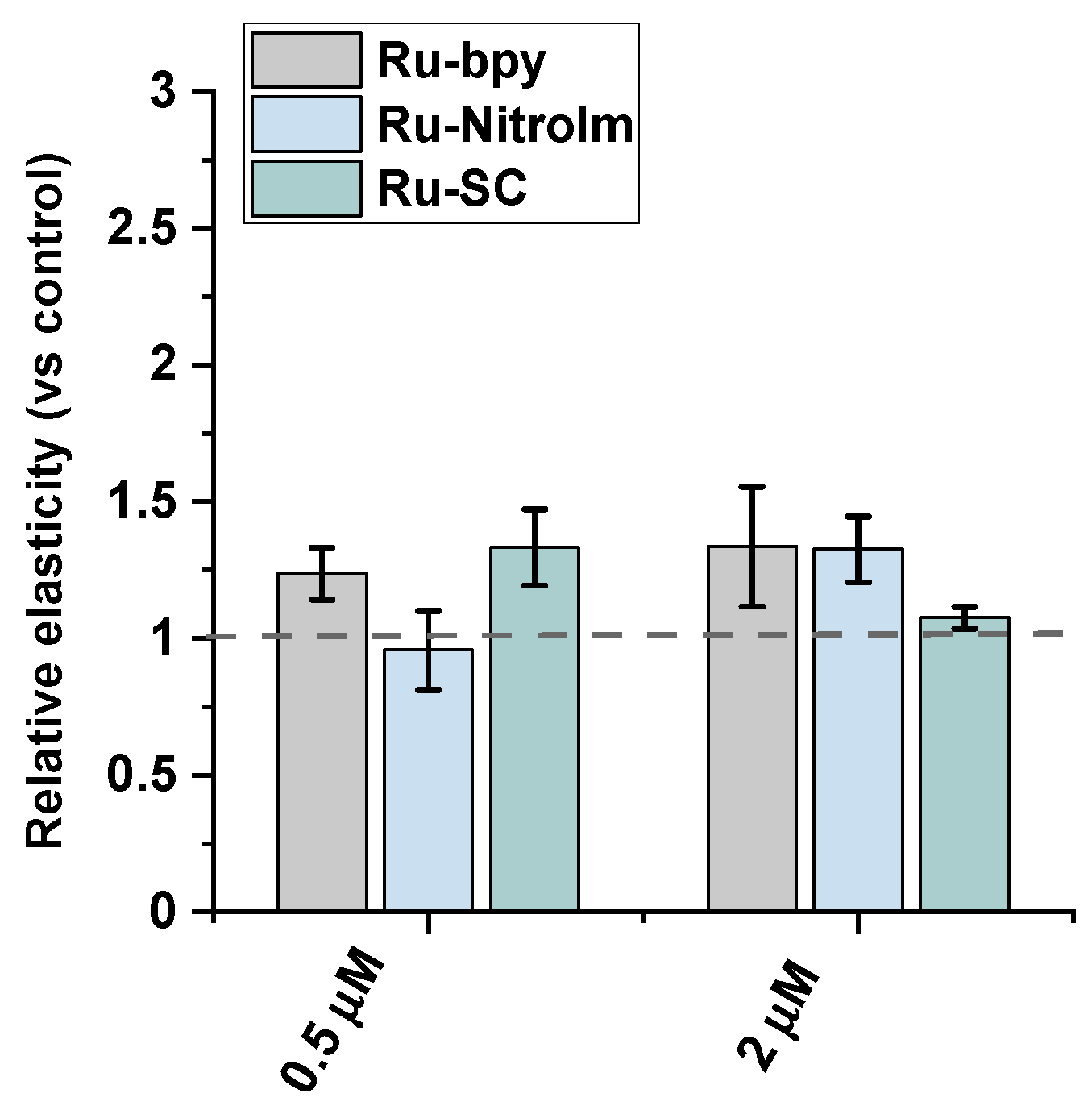
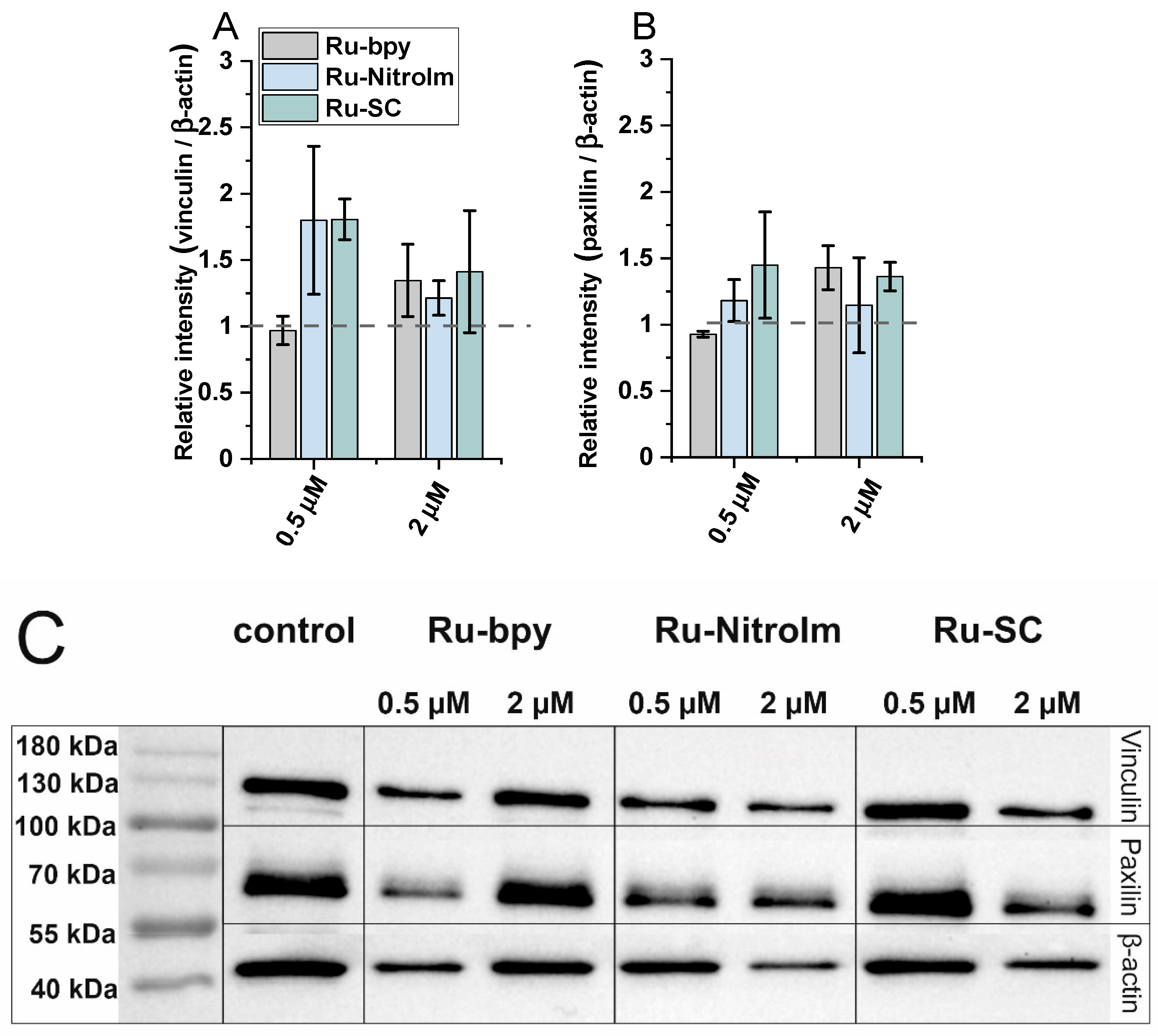
2.3. Impact on Cell Elasticity and Focal Adhesion Components
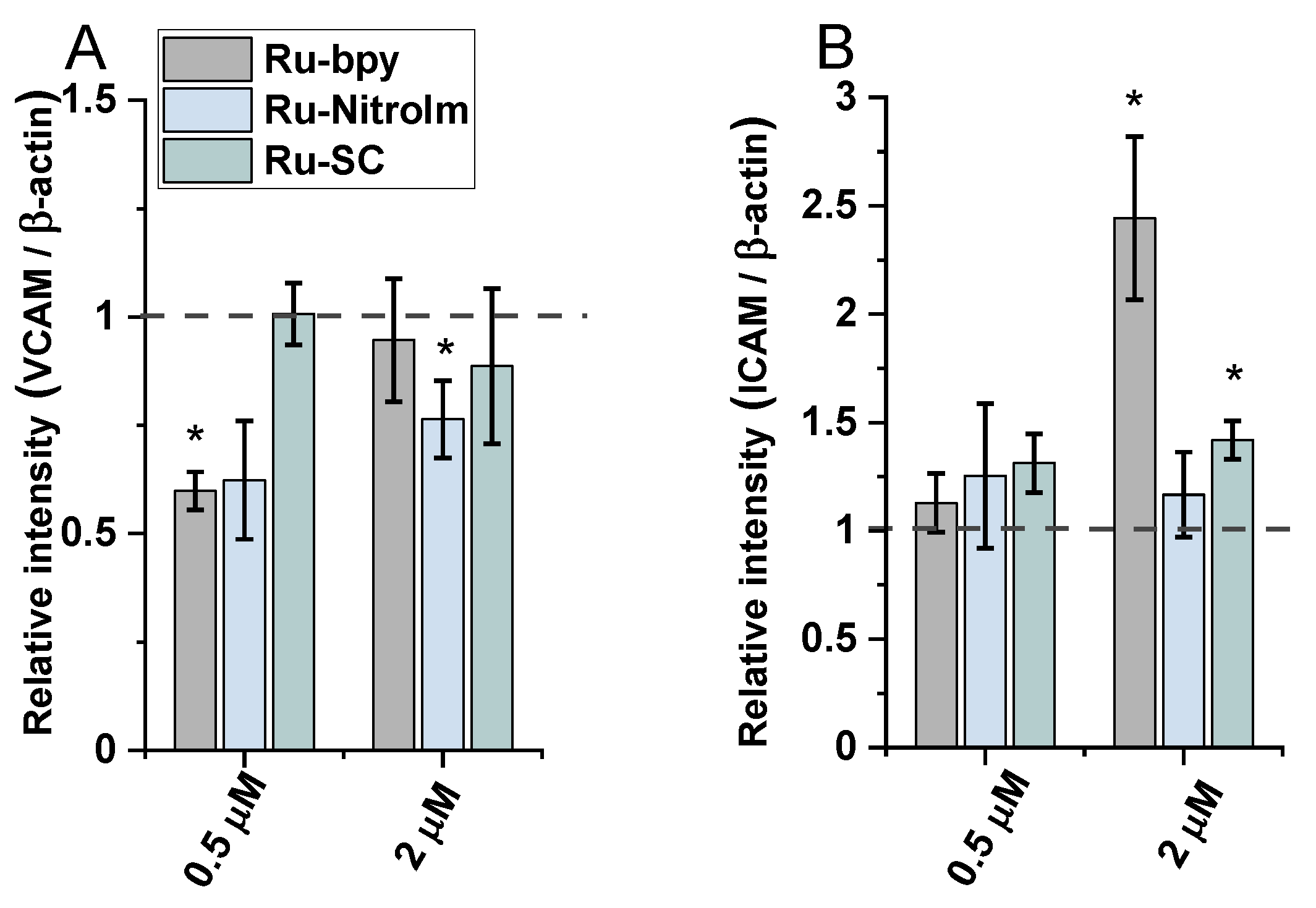
2.4. Effect on Endothelial Cells Response to TNF-α Cytokine
3. Materials and Methods
3.1. Materials
3.2. Cell Culturing and Cytotoxicity Assay
3.3. Tube-Formation Assay
3.4. Wound Healing Assay
3.5. Atomic Force Microscopy—Elasticity Measurements
3.6. Western-Blot Analysis
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poynton, F.E.; Bright, S.A.; Blasco, S.; Williams, D.C.; Kelly, J.M.; Gunnlaugsson, T. The Development of Ruthenium(Ii) Polypyridyl Complexes and Conjugates for in vitro Cellular and in vivo Applications. Chem. Soc. Rev. 2017, 46, 7706–7756. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lai, H.; Xiong, Z.; Chen, B.; Chen, T. Functionalization and Cancer-Targeting Design of Ruthenium Complexes for Precise Cancer Therapy. Chem. Commun. 2019, 55, 9904–9914. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Alonso, M.; Gasser, G. Ruthenium Polypyridyl Complex-Containing Bioconjugates. Coord. Chem. Rev. 2021, 434, 213736. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Jin, S.; Muhammad, N.; Guo, Z. Stimuli-Responsive Therapeutic Metallodrugs. Chem. Rev. 2019, 119, 1138–1192. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, A.; Sava, G. Linking the Future of Anticancer Metal-Complexes to the Therapy of Tumour Metastases. Chem. Soc. Rev. 2015, 44, 8818–8835. [Google Scholar] [CrossRef] [PubMed]
- Brindell, M.; Gurgul, I.; Janczy-Cempa, E.; Gajda-Morszewski, P.; Mazuryk, O. Moving Ru Polypyridyl Complexes beyond Cytotoxic Activity towards Metastasis Inhibition. J. Inorg. Biochem. 2022, 226, 111652. [Google Scholar] [CrossRef] [PubMed]
- Alessio, E. Thirty Years of the Drug Candidate NAMI-A and the Myths in the Field of Ruthenium Anticancer Compounds: A Personal Perspective. Eur. J. Inorg. Chem. 2017, 2017, 1549–1560. [Google Scholar] [CrossRef]
- Pintus, G.; Tadolini, B.; Posadino, A.M.; Sanna, B.; Debidda, M.; Bennardini, F.; Sava, G.; Ventura, C. Inhibition of the MEK/ERK Signaling Pathway by the Novel Antimetastatic Agent NAMI-A down Regulates c-Myc Gene Expression and Endothelial Cell Proliferation. Eur. J. Biochem. 2002, 269, 5861–5870. [Google Scholar] [CrossRef]
- Oszajca, M.; Collet, G.; Stochel, G.; Kieda, C.; Brindell, M. Hypoxia-Selective Inhibition of Angiogenesis Development by NAMI-A Analogues. BioMetals 2016, 29, 1035–1046. [Google Scholar] [CrossRef] [Green Version]
- Mazuryk, O.; Suzenet, F.; Kieda, C.; Brindell, M. The Biological Effect of the Nitroimidazole Derivative of a Polypyridyl Ruthenium Complex on Cancer and Endothelial Cells. Metallomics 2015, 7, 553–566. [Google Scholar] [CrossRef]
- Cao, W.; Zheng, W.; Chen, T. Ruthenium Polypyridyl Complex Inhibits Growth and Metastasis of Breast Cancer Cells by Suppressing FAK Signaling with Enhancement of TRAIL-Induced Apoptosis. Sci. Rep. 2015, 5, 9157. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, L.; Yu, G.; Zhang, S.; Li, Y.; Wu, Q.; Huang, X.; Mei, W. Nucleus-Enriched Ruthenium Polypyridine Complex Acts as a Potent Inhibitor to Suppress Triple-Negative Breast Cancer Metastasis In Vivo. Comput. Struct. Biotechnol. J. 2019, 17, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Gurgul, I.; Mazuryk, O.; Łomzik, M.; Gros, P.C.; Rutkowska-Zbik, D.; Brindell, M. Unexplored Features of Ru(Ii) Polypyridyl Complexes—Towards Combined Cytotoxic and Antimetastatic Activity. Metallomics 2020, 12, 784–793. [Google Scholar] [CrossRef]
- Gurgul, I.; Janczy-Cempa, E.; Mazuryk, O.; Lekka, M.; Łomzik, M.; Suzenet, F.; Gros, P.C. Małgorzata Brindell Inhibition of Metastasis by Polypyridyl Ru(II) Complexes through Modification of Cancer Cell Adhesion—In Vitro Functional and Molecular Studies. J. Med. Chem. 2022. [Google Scholar]
- Li, D.-M.; Feng, Y.-M. Signaling Mechanism of Cell Adhesion Molecules in Breast Cancer Metastasis: Potential Therapeutic Targets. Breast Cancer Res. Treat. 2011, 128, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Pepper, M.S. Role of the Matrix Metalloproteinase and Plasminogen Activator–Plasmin Systems in Angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1104–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, X.; Sun, W.; Wang, Y.; Liu, X.; Wang, A.; Liu, L.; Han, S.; Sun, Y.; Zhang, J.; Guo, L.; et al. Cervical Cancer-Derived Exosomal MiR-663b Promotes Angiogenesis by Inhibiting Vinculin Expression in Vascular Endothelial Cells. Cancer Cell Int. 2021, 21, 684. [Google Scholar] [CrossRef]
- Griffioen, A.W.; Damen, C.A.; Martinotti, S.; Blijham, G.H.; Groenewegen, G. Endothelial Intercellular Adhesion Molecule I Expression Is Suppressed in Human: The Role of Angiogenic Factors. Cancer Res. 1996, 56, 1111–1117. [Google Scholar]
- El-Kenawi, A.E.; El-Remessy, A.B. Angiogenesis Inhibitors in Cancer Therapy: Mechanistic Perspective on Classification and Treatment Rationales. Br. J. Pharmacol. 2013, 170, 712–729. [Google Scholar] [CrossRef] [Green Version]
- Lopes-Coelho, F.; Martins, F.; Pereira, S.A.; Serpa, J. Anti-Angiogenic Therapy: Current Challenges and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 3765. [Google Scholar] [CrossRef]
- Fallah, A.; Sadeghinia, A.; Kahroba, H.; Samadi, A.; Heidari, H.R.; Bradaran, B.; Zeinali, S.; Molavi, O. Therapeutic Targeting of Angiogenesis Molecular Pathways in Angiogenesis-Dependent Diseases. Biomed. Pharmacother. 2019, 110, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.J.; Sharma, R. Angiogenesis Inhibitors in Cancer-Mechanisms of Action. Exp. Clin. Pharmacol. 2006, 29, 9–12. [Google Scholar]
- Tabruyn, S.P.; Griffioen, A.W. Molecular Pathways of Angiogenesis Inhibition. Biochem. Biophys. Res. Commun. 2007, 355, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajda-Morszewski, P.; Gurgul, I.; Janczy-Cempa, E.; Mazuryk, O.; Łomzik, M.; Brindell, M. Inhibition of Matrix Metalloproteinases and Cancer Cell Detachment by Ru(II) Polypyridyl Complexes Containing 4,7-Diphenyl-1,10-Phenanthroline Ligands-New Candidates for Antimetastatic Agents. Pharmaceuticals 2021, 14, 1014. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, H.; Blobe, G.C.; Theuer, C.P.; Hurwitz, H.I.; Nixon, A.B. Effects of the Combination of TRC105 and Bevacizumab on Endothelial Cell Biology. Investig. New Drugs 2014, 32, 851–859. [Google Scholar] [CrossRef] [Green Version]
- Kodera, Y.; Katanasaka, Y.; Kitamura, Y.; Tsuda, H.; Nishio, K.; Tamura, T.; Koizumi, F. Sunitinib Inhibits Lymphatic Endothelial Cell Functions and Lymph Node Metastasis in a Breast Cancer Model through Inhibition of Vascular Endothelial Growth Factor Receptor 3. Breast Cancer Res. 2011, 13, R66. [Google Scholar] [CrossRef] [Green Version]
- Ziyad, S.; Iruela-Arispe, M.L. Molecular Mechanisms of Tumor Angiogenesis. Genes Cancer 2011, 2, 1085–1096. [Google Scholar] [CrossRef] [Green Version]
- Luo, Q.; Kuang, D.; Zhang, B.; Song, G. Cell Stiffness Determined by Atomic Force Microscopy and Its Correlation with Cell Motility. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 1953–1960. [Google Scholar] [CrossRef]
- Ding, Y.; Xu, G.-K.; Wang, G.-F. On the Determination of Elastic Moduli of Cells by AFM Based Indentation. Sci. Rep. 2017, 7, 45575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lekka, M.; Laidler, P.; Gil, D.; Lekki, J.; Stachura, Z.; Hrynkiewicz, A.Z. Elasticity of Normal and Cancerous Human Bladder Cells Studied by Scanning Force Microscopy. Eur. Biophys. J. 1999, 28, 312–316. [Google Scholar] [CrossRef] [PubMed]
- German, A.E.; Mammoto, T.; Jiang, E.; Ingber, D.E.; Mammoto, A. Paxillin Controls Endothelial Cell Migration and Tumor Angiogenesis by Altering Neuropilin 2 Expression. J. Cell Sci. 2014, 127, 1672–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, D.-H.; Kim, Y.; Kim, M.; Jang, J.; Lee, S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.-B. Association of VCAM-1 Overexpression with Oncogenesis, Tumor Angiogenesis and Metastasis of Gastric Carcinoma. World J. Gastroenterol. 2003, 9, 1409. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, R.; Khaket, T.P.; Dutta, C.; Chakraborty, B.; Mukherjee, T.K. Breast Cancer Metastasis: Putative Therapeutic Role of Vascular Cell Adhesion Molecule-1. Cell. Oncol. 2017, 40, 199–208. [Google Scholar] [CrossRef]
- Scalici, J.M.; Harrer, C.; Allen, A.; Jazaeri, A.; Atkins, K.A.; McLachlan, K.R.; Slack-Davis, J.K. Inhibition of A4β1 Integrin Increases Ovarian Cancer Response to Carboplatin. Gynecol. Oncol. 2014, 132, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Chimiche, S.; Alessio, E.; Mestroni, G.; Nardin, G.; Attia, W.M.; Calligaris, M.; Sava, G.; Zorzet, S. Contribution from the Dipartimento Di Cis-and Trans-Dihalotetrakis(Dimethyl Sulfoxide)Ruthenium(II) Complexes (RuX2(DMSO)4; X = Cl, Br): Synthesis, Structure, and Antitumor Activity. Inorg. Chem. 1988, 27, 4099–4106. [Google Scholar]
- Taftaf, R.; Liu, X.; Singh, S.; Jia, Y.; Dashzeveg, N.K.; Hoffmann, A.D.; El-Shennawy, L.; Ramos, E.K.; Adorno-Cruz, V.; Schuster, E.J.; et al. ICAM1 Initiates CTC Cluster Formation and Trans-Endothelial Migration in Lung Metastasis of Breast Cancer. Nat. Commun. 2021, 12, 4867. [Google Scholar] [CrossRef]
- Ghislin, S.; Obino, D.; Middendorp, S.; Boggetto, N.; Alcaide-Loridan, C.; Deshayes, F. LFA-1 and ICAM-1 Expression Induced during Melanoma-Endothelial Cell Co-Culture Favors the Transendothelial Migration of Melanoma Cell Lines in Vitro. BMC Cancer 2012, 12, 455. [Google Scholar] [CrossRef]
- Zhang, P.; Goodrich, C.; Fu, C.; Dong, C. Melanoma Upregulates ICAM-1 Expression on Endothelial Cells through Engagement of Tumor CD44 with Endothelial E-selectin and Activation of a PKCα–P38-SP-1 Pathway. FASEB J. 2014, 28, 4591–4609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazuryk, O.; Magiera, K.; Rys, B.; Suzenet, F.; Kieda, C.; Brindell, M. Multifaceted Interplay between Lipophilicity, Protein Interaction and Luminescence Parameters of Non-Intercalative Ruthenium(II) Polypyridyl Complexes Controlling Cellular Imaging and Cytotoxic Properties. J. Biol. Inorg. Chem. 2014, 19, 1305–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Łomzik, M.; Mazuryk, O.; Rutkowska-Zbik, D.; Stochel, G.; Gros, P.C.; Brindell, M. New Ruthenium Compounds Bearing Semicarbazone 2-Formylopyridine Moiety: Playing with Auxiliary Ligands for Tuning the Mechanism of Biological Activity. J. Inorg. Biochem. 2017, 175, 80–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazuryk, O.; Maciuszek, M.; Stochel, G.; Suzenet, F.; Brindell, M. 2-Nitroimidazole-Ruthenium Polypyridyl Complex as a New Conjugate for Cancer Treatment and Visualization. J. Inorg. Biochem. 2014, 134, 83–91. [Google Scholar] [CrossRef]
- Carpentier, G. Contribution: Angiogenesis Analyzer. ImageJ News 2012, 5. [Google Scholar]
- Carpentier, G.; Berndt, S.; Ferratge, S.; Rasband, W.; Cuendet, M.; Uzan, G.; Albanese, P. Angiogenesis Analyzer for ImageJ—A comparative morphometric analysis of “Endothelial Tube Formation Assay” and “Fibrin Bead Assay”. Sci. Rep. 2020, 10, 11568. [Google Scholar] [CrossRef]
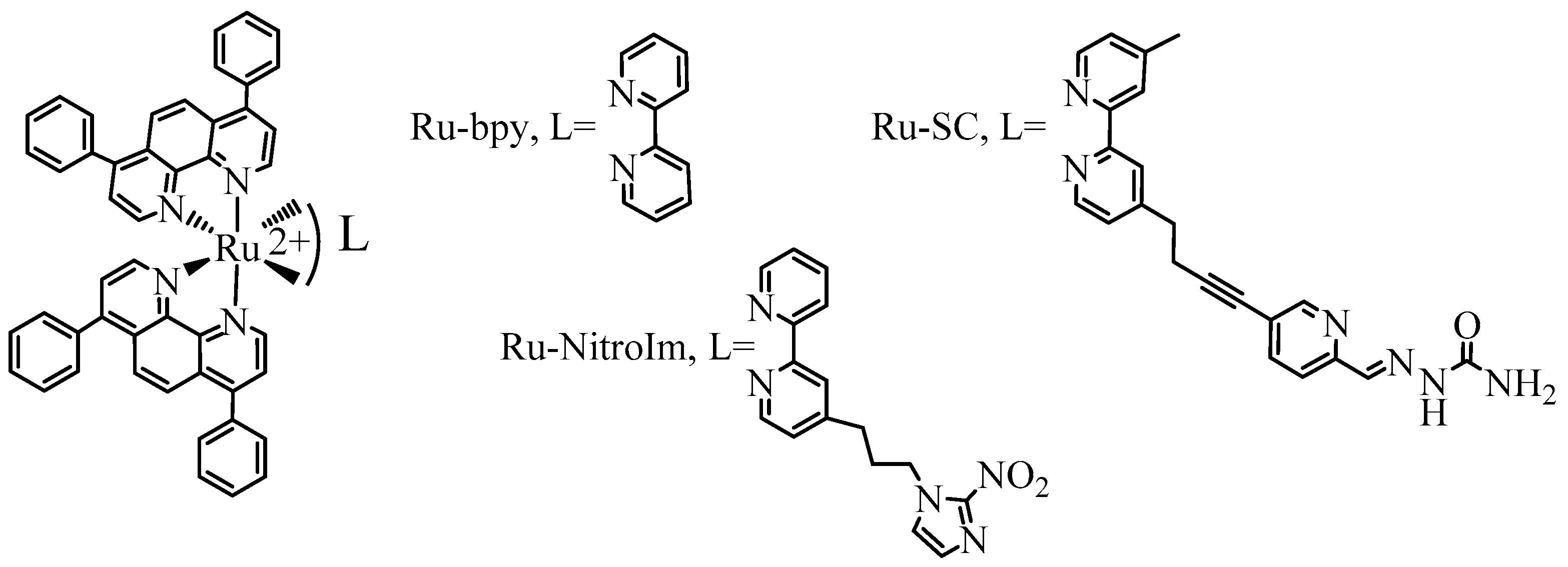

| Compound | IC50 [µM] |
|---|---|
| Ru-bpy | 4.6 ± 1.0 |
| Ru-NitroIm | 6.9 ± 1.3 |
| Ru-SC | 5.1 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurgul, I.; Mazuryk, O.; Stachyra, K.; Olszanecki, R.; Lekka, M.; Łomzik, M.; Suzenet, F.; Gros, P.C.; Brindell, M. Impact of Polypyridyl Ru Complexes on Angiogenesis—Contribution to Their Antimetastatic Activity. Int. J. Mol. Sci. 2022, 23, 7708. https://doi.org/10.3390/ijms23147708
Gurgul I, Mazuryk O, Stachyra K, Olszanecki R, Lekka M, Łomzik M, Suzenet F, Gros PC, Brindell M. Impact of Polypyridyl Ru Complexes on Angiogenesis—Contribution to Their Antimetastatic Activity. International Journal of Molecular Sciences. 2022; 23(14):7708. https://doi.org/10.3390/ijms23147708
Chicago/Turabian StyleGurgul, Ilona, Olga Mazuryk, Kamila Stachyra, Rafał Olszanecki, Małgorzata Lekka, Michał Łomzik, Franck Suzenet, Philippe C. Gros, and Małgorzata Brindell. 2022. "Impact of Polypyridyl Ru Complexes on Angiogenesis—Contribution to Their Antimetastatic Activity" International Journal of Molecular Sciences 23, no. 14: 7708. https://doi.org/10.3390/ijms23147708
APA StyleGurgul, I., Mazuryk, O., Stachyra, K., Olszanecki, R., Lekka, M., Łomzik, M., Suzenet, F., Gros, P. C., & Brindell, M. (2022). Impact of Polypyridyl Ru Complexes on Angiogenesis—Contribution to Their Antimetastatic Activity. International Journal of Molecular Sciences, 23(14), 7708. https://doi.org/10.3390/ijms23147708