Human Mesenchymal Stromal Cells Do Not Cause Radioprotection of Head-and-Neck Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Results
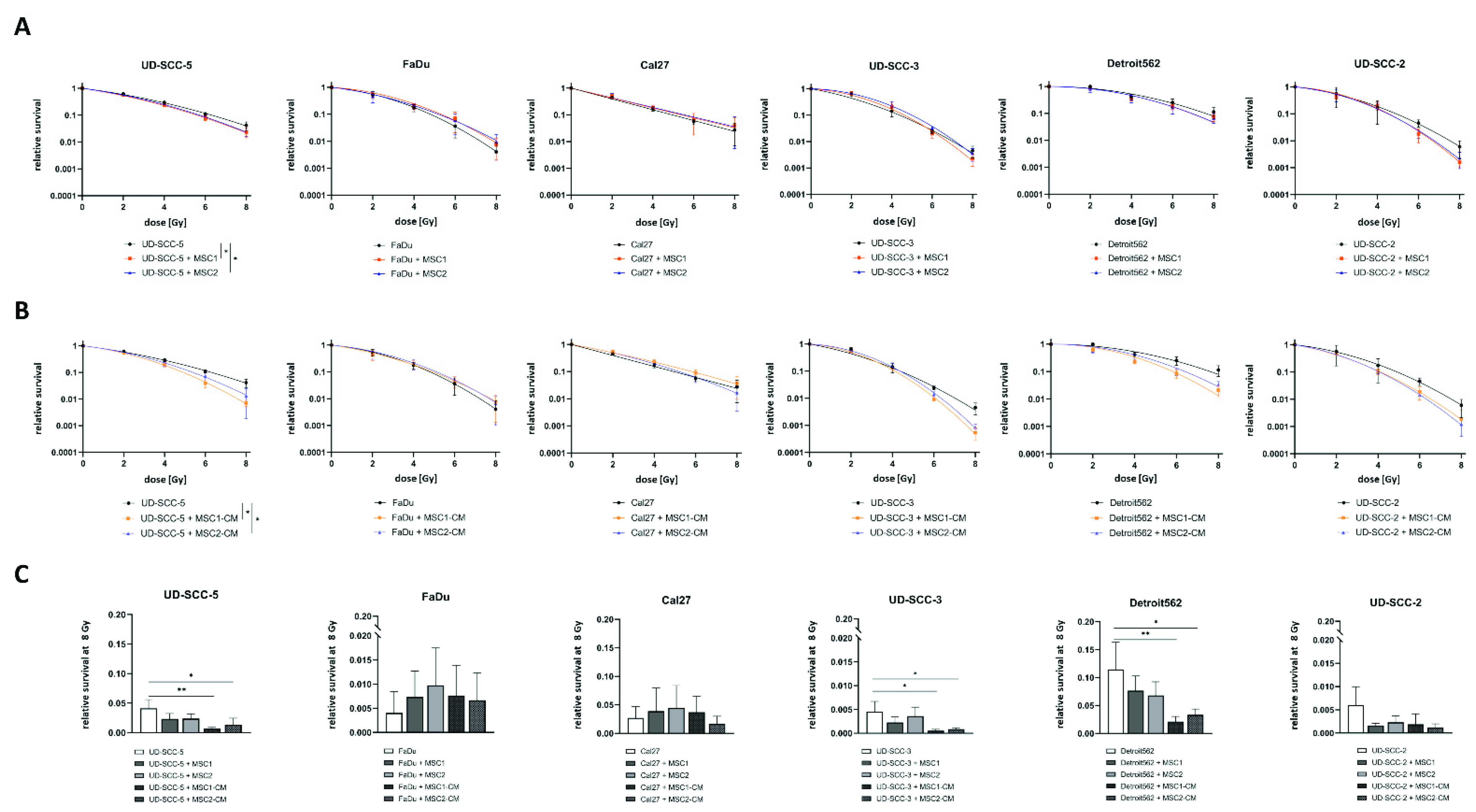
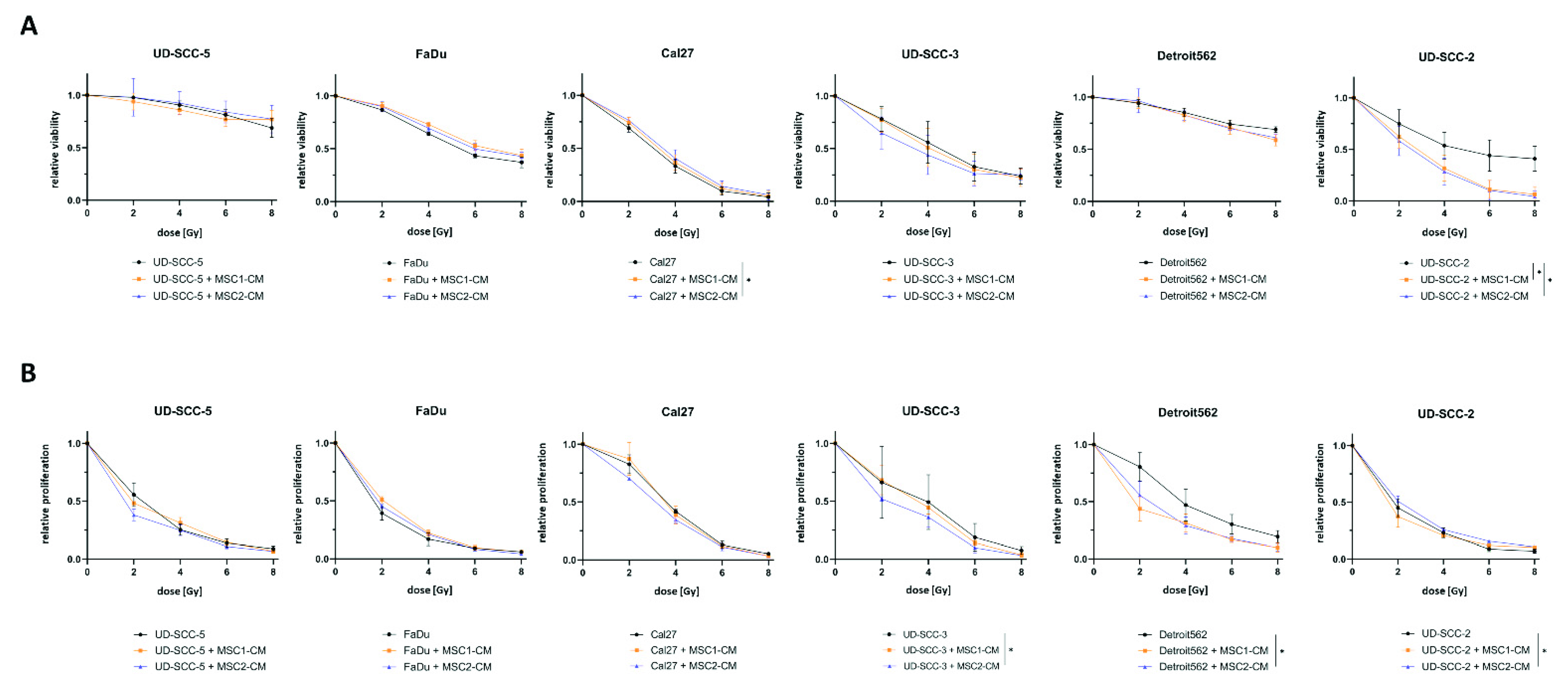
2.1. Neither Direct nor Indirect MSC Co-Culture Result in Increased Radioresistance of HNSCCs
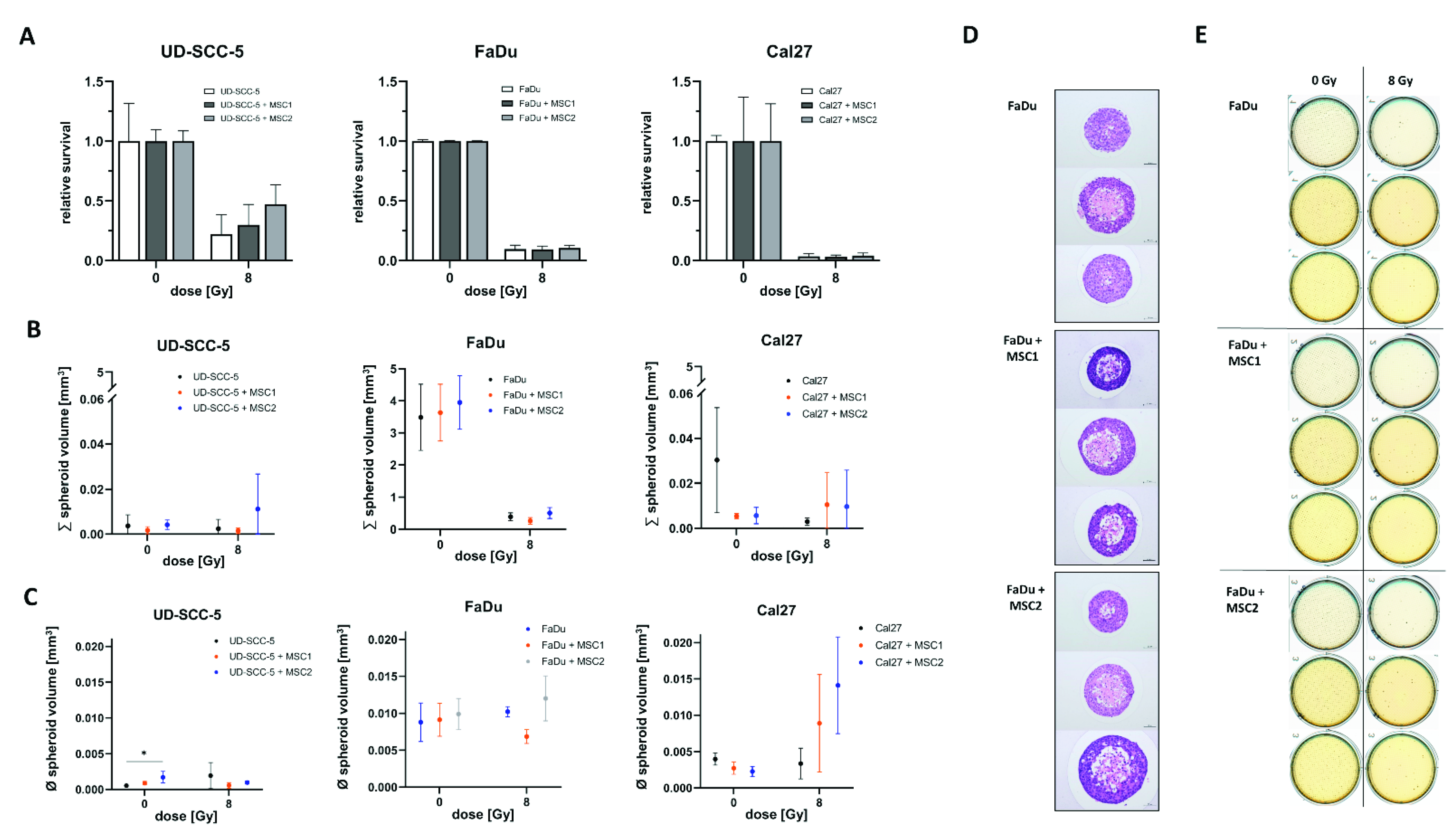
2.2. Direct Three-Dimensional MSC-HNSCC Co-Culture Reveals No Radioprotective Effects of MSCs
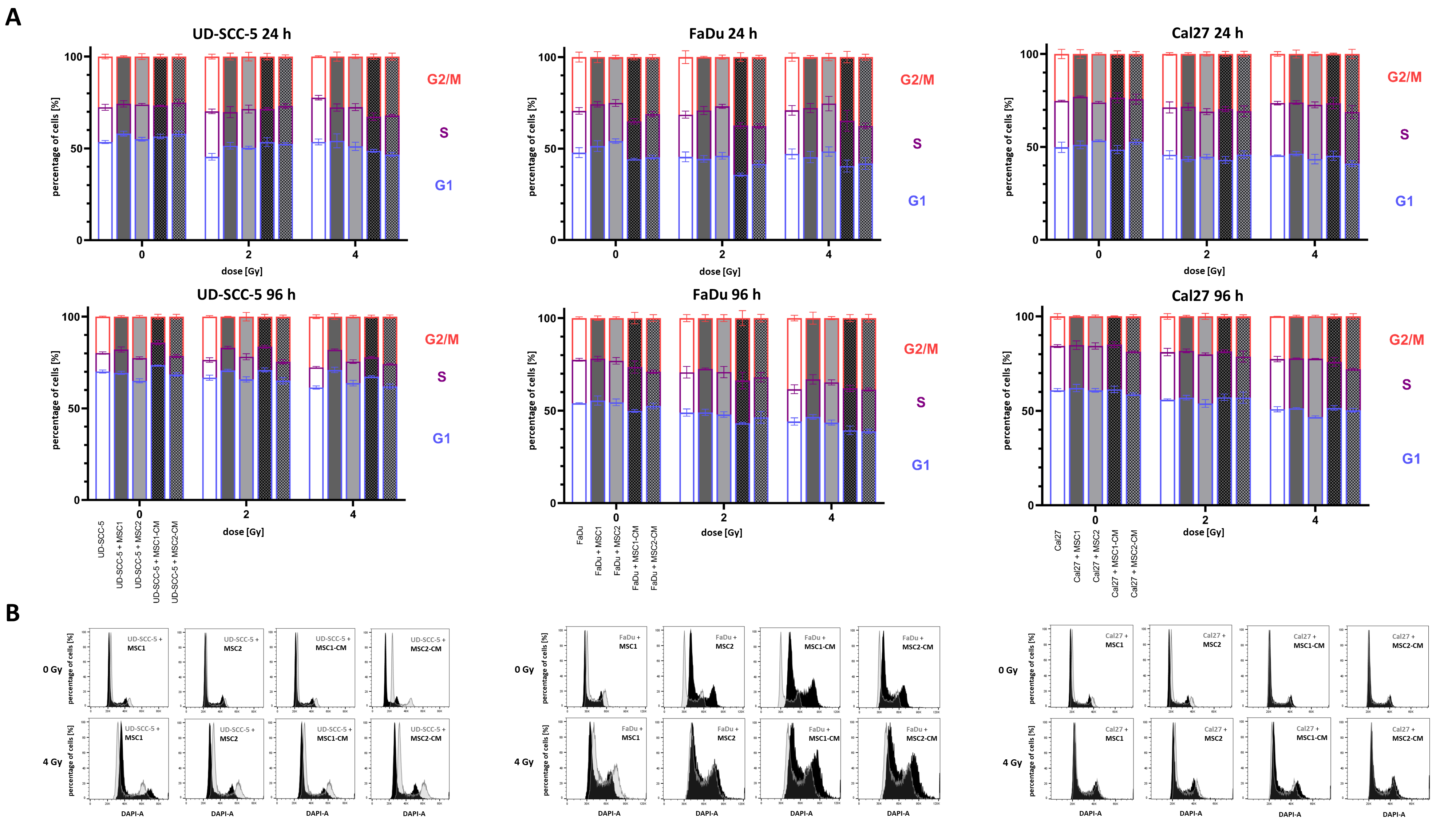
2.3. Irradiation-Induced G2/M Cell Cycle Arrest in HNSCCs Is Not Abrogated by MSCs
2.4. MSCs Do Not Suppress HNSCC Apoptosis Rates after Radiation Treatment
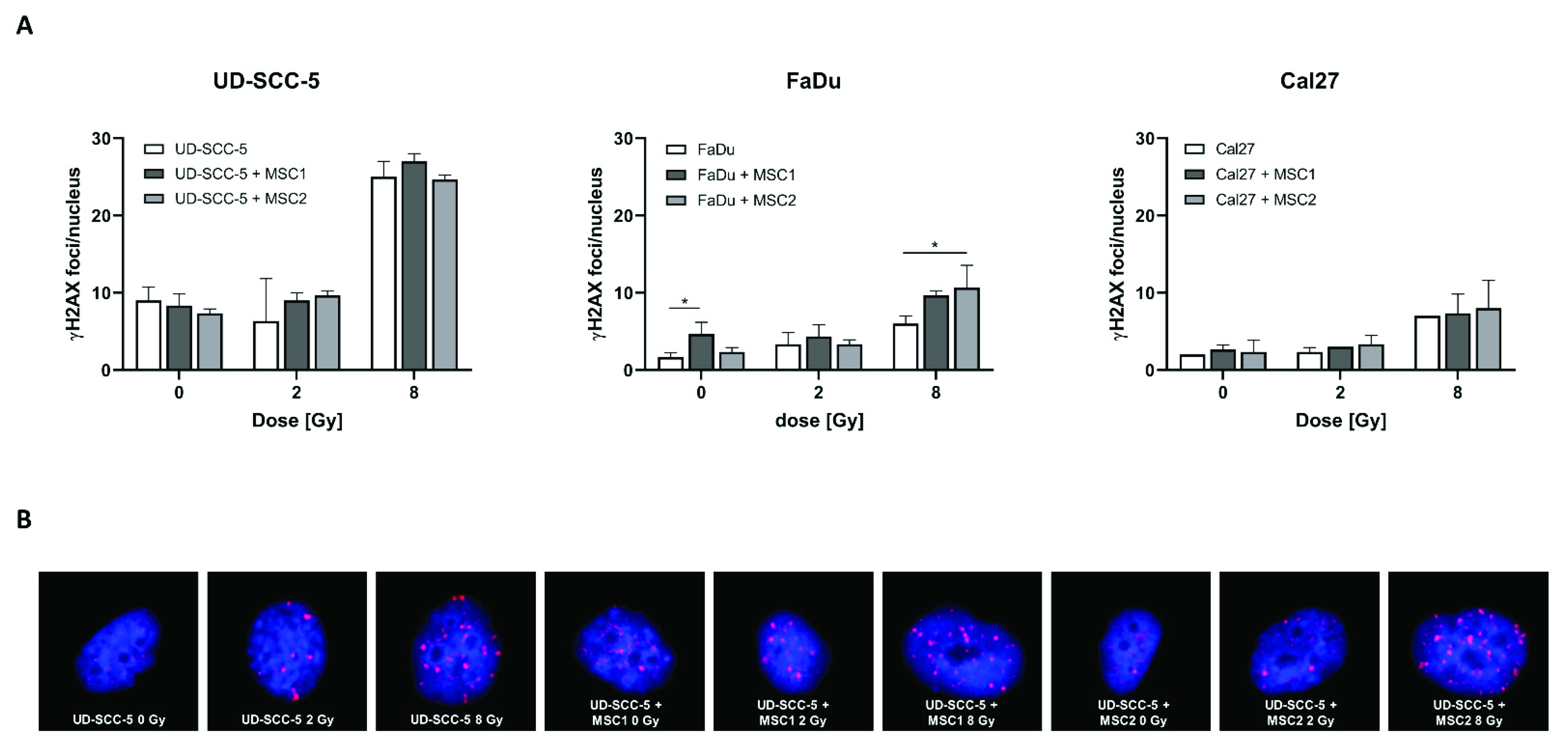
2.5. Levels of Residual DNA Double-Strand Breaks after Irradiation Are Similar between HNSCC Mono-Cultures and HNSCC-MSC Co-Cultures
2.6. CTGF Secretion Is Increased in HNSCC-MSC Co-Cultures Compared to HNSCC Mono-Cultures but Comparable to MSC Mono-Cultures
3. Discussion
4. Materials and Methods
4.1. Cells and Cell Culture
4.2. Radiation Treatments
4.3. Clonogenic Survival
4.4. Viability
4.5. Proliferation
4.6. Three-Dimensional Survival Assays
4.7. Cell Cycle and Apoptosis Measurements
4.8. DNA Double-Strand Break Repair
4.9. Enzyme-Linked Immunosorbent Assays
4.10. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [Green Version]
- Cooper, J.S.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Campbell, B.H.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2004, 350, 1937–1944. [Google Scholar] [CrossRef] [Green Version]
- Bernier, J.; Domenge, C.; Ozsahin, M.; Matuszewska, K.; Lefèbvre, J.-L.; Greiner, R.H.; Giralt, J.; Maingon, P.; Rolland, F.; Bolla, M.; et al. Postoperative Irradiation with or without Concomitant Chemotherapy for Locally Advanced Head and Neck Cancer. N. Engl. J. Med. 2004, 350, 1945–1952. [Google Scholar] [CrossRef] [Green Version]
- Adelstein, D.J.; Li, Y.; Adams, G.L.; Wagner, H., Jr.; Kish, J.A.; Ensley, J.F.; Schuller, D.E.; Forastiere, A.A. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J. Clin. Oncol. 2003, 21, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Nutting, C.M.; Morden, J.P.; Harrington, K.J.; Urbano, T.G.; Bhide, S.A.; Clark, C.; Miles, E.A.; Miah, A.B.; Newbold, K.; Tanay, M.; et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011, 12, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Aarup-Kristensen, S.; Hansen, C.R.; Forner, L.; Brink, C.; Eriksen, J.G.; Johansen, J. Osteoradionecrosis of the mandible after radiotherapy for head and neck cancer: Risk factors and dose-volume correlations. Acta Oncol. 2019, 58, 1373–1377. [Google Scholar] [CrossRef]
- Rühle, A.; Haehl, E.; Kalckreuth, T.; Stoian, R.; Spohn, S.K.B.; Sprave, T.; Zamboglou, C.; Gkika, E.; Knopf, A.; Grosu, A.-L.; et al. Surviving Elderly Patients with Head-and-Neck Squamous Cell Carcinoma—What Is the Long-Term Quality of Life after Curative Radiotherapy? Cancers 2021, 13, 1275. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tan, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Rühle, A.; Grosu, A.-L.; Nicolay, N.H. De-Escalation Strategies of (Chemo)Radiation for Head-and-Neck Squamous Cell Cancers—HPV and Beyond. Cancers 2021, 13, 2204. [Google Scholar] [CrossRef]
- Braakhuis, B.J.; Leemans, C.R.; Visser, O. Incidence and survival trends of head and neck squamous cell carcinoma in the Netherlands between 1989 and 2011. Oral Oncol. 2014, 50, 670–675. [Google Scholar] [CrossRef] [Green Version]
- Svahn, M.F.; Munk, C.; Nielsen, T.S.S.; von Buchwald, C.; Frederiksen, K.; Kjaer, S.K. Trends in all-cause five-year mortality after head and neck cancers diagnosed over a period of 33 years. Focus on estimated degree of association with human papillomavirus. Acta Oncol. 2016, 55, 1084–1090. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.; Haagen, J.; Noack, R.; Siegemund, A.; Gabriel, P.; Dorr, W. Effects of bone marrow or mesenchymal stem cell transplantation on oral mucositis (mouse) induced by fractionated irradiation. Strahlenther. Onkol. 2014, 190, 399–404. [Google Scholar] [CrossRef]
- Gronhoj, C.; Jensen, D.H.; Vester-Glowinski, P.; Jensen, S.B.; Bardow, A.; Oliveri, R.S.; Fog, L.M.; Specht, L.; Thomsen, C.; Darkner, S.; et al. Safety and Efficacy of Mesenchymal Stem Cells for Radiation-Induced Xerostomia: A Randomized, Placebo-Controlled Phase 1/2 Trial (MESRIX). Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 581–592. [Google Scholar] [CrossRef]
- Gundestrup, A.K.; Lynggaard, C.D.; Forner, L.; Heino, T.J.; Jakobsen, K.K.; Fischer-Nielsen, A.; Grønhøj, C.; von Buchwald, C. Mesenchymal Stem Cell Therapy for Osteoradionecrosis of the Mandible: A Systematic Review of Preclinical and Human Studies. Stem Cell Rev. Rep. 2020, 16, 1208–1221. [Google Scholar] [CrossRef]
- Zanoni, M.; Cortesi, M.; Zamagni, A.; Tesei, A. The Role of Mesenchymal Stem Cells in Radiation-Induced Lung Fibrosis. Int. J. Mol. Sci. 2019, 20, 3876. [Google Scholar] [CrossRef] [Green Version]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Deriglasova, U.F.; Kulagina, N.N.; Panasuk, A.F.; Rudakowa, S.F.; Luria, E.A.; Ruadkow, I.A. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp. Hematol. 1974, 2, 83–92. [Google Scholar]
- Bieback, K.; Kern, S.; Kluter, H.; Eichler, H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells 2004, 22, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Chapel, A.; Francois, S.; Douay, L.; Benderitter, M.; Voswinkel, J. New insights for pelvic radiation disease treatment: Multipotent stromal cell is a promise mainstay treatment for the restoration of abdominopelvic severe chronic damages induced by radiotherapy. World J. Stem Cells 2013, 5, 106–111. [Google Scholar] [CrossRef]
- Maria, O.M.; Shalaby, M.; Syme, A.; Eliopoulos, N.; Muanza, T. Adipose mesenchymal stromal cells minimize and repair radiation-induced oral mucositis. Cytotherapy 2016, 18, 1129–1145. [Google Scholar] [CrossRef] [Green Version]
- Nicolay, N.H.; Lopez Perez, R.; Debus, J.; Huber, P.E. Mesenchymal stem cells—A new hope for radiotherapy-induced tissue damage? Cancer Lett. 2015, 366, 133–140. [Google Scholar] [CrossRef]
- Ruhle, A.; Lopez Perez, R.; Zou, B.; Grosu, A.L.; Huber, P.E.; Nicolay, N.H. The Therapeutic Potential of Mesenchymal Stromal Cells in the Treatment of Chemotherapy-Induced Tissue Damage. Stem Cell Rev. 2019, 15, 356–373. [Google Scholar] [CrossRef]
- Rühle, A.; Perez, R.L.; Glowa, C.; Weber, K.J.; Ho, A.D.; Debus, J.; Saffrich, R.; Huber, P.E.; Nicolay, N.H. Cisplatin radiosensitizes radioresistant human mesenchymal stem cells. Oncotarget 2017, 8, 87809–87820. [Google Scholar] [CrossRef] [Green Version]
- Francois, S.; Bensidhoum, M.; Mouiseddine, M.; Mazurier, C.; Allenet, B.; Semont, A.; Frick, J.; Sache, A.; Bouchet, S.; Thierry, D.; et al. Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: A study of their quantitative distribution after irradiation damage. Stem Cells 2006, 24, 1020–1029. [Google Scholar] [CrossRef]
- Nicolay, N.H.; Lopez Perez, R.; Saffrich, R.; Huber, P.E. Radio-resistant mesenchymal stem cells: Mechanisms of resistance and potential implications for the clinic. Oncotarget 2015, 6, 19366–19380. [Google Scholar] [CrossRef] [Green Version]
- Schoefinius, J.S.; Brunswig-Spickenheier, B.; Speiseder, T.; Krebs, S.; Just, U.; Lange, C. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Provide Long-Term Survival after Total Body Irradiation without Additional Hematopoietic Stem Cell Support. Stem Cells 2017, 35, 2379–2389. [Google Scholar] [CrossRef] [Green Version]
- Norozi, F.; Ahmadzadeh, A.; Shahrabi, S.; Vosoughi, T.; Saki, N. Mesenchymal stem cells as a double-edged sword in suppression or progression of solid tumor cells. Tumour Biol. 2016, 37, 11679–11689. [Google Scholar] [CrossRef]
- Böhrnsen, F.; Fricke, M.; Sander, C.; Leha, A.; Schliephake, H.; Kramer, F.J. Interactions of human MSC with head and neck squamous cell carcinoma cell line PCI-13 reduce markers of epithelia-mesenchymal transition. Clin. Oral Investig. 2015, 19, 1121–1128. [Google Scholar] [CrossRef]
- Wilhelm, C.; Scherzad, A.; Bregenzer, M.; Meyer, T.; Gehrke, T.; Kleinsasser, N.; Hagen, R.; Hackenberg, S. Interaction of head and neck squamous cell carcinoma cells and mesenchymal stem cells under hypoxia and normoxia. Oncol. Lett. 2020, 20, 229. [Google Scholar] [CrossRef]
- Liu, C.; Billet, S.; Choudhury, D.; Cheng, R.; Haldar, S.; Fernandez, A.; Biondi, S.; Liu, Z.; Zhou, H.; Bhowmick, N.A. Bone marrow mesenchymal stem cells interact with head and neck squamous cell carcinoma cells to promote cancer progression and drug resistance. Neoplasia 2021, 23, 118–128. [Google Scholar] [CrossRef]
- He, N.; Kong, Y.; Lei, X.; Liu, Y.; Wang, J.; Xu, C.; Wang, Y.; Du, L.; Ji, K.; Wang, Q.; et al. MSCs inhibit tumor progression and enhance radiosensitivity of breast cancer cells by down-regulating Stat3 signaling pathway. Cell Death Dis. 2018, 9, 1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoghi, H.; Neisi, N.; Saki, G.; Tahmasebi Birgani, M.J.; Danyaei, A. The Radio-Sensitivity Effects of Human Umbilical Cord Wharton’s Jelly Mesenchymal Stem Cell’s Conditional Medium on MDA-MB-231 Cells. Jentashapir J. Cell. Mol. Biol. 2020, 11, e110320. [Google Scholar] [CrossRef]
- Farias, V.d.A.; O’Valle, F.; Lerma, B.A.; Almodóvar, C.R.d.; López-Peñalver, J.J.; Nieto, A.; Santos, A.; Fernández, B.I.; Guerra-Librero, A.; Ruiz-Ruiz, M.C.; et al. Human mesenchymal stem cells enhance the systemic effects of radiotherapy. Oncotarget 2015, 6, 31164–31180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Araujo Farias, V.; O’Valle, F.; Serrano-Saenz, S.; Anderson, P.; Andrés, E.; López-Peñalver, J.; Tovar, I.; Nieto, A.; Santos, A.; Martín, F.; et al. Exosomes derived from mesenchymal stem cells enhance radiotherapy-induced cell death in tumor and metastatic tumor foci. Mol. Cancer 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- François, S.; Usunier, B.; Forgue-Lafitte, M.-E.; L’Homme, B.; Benderitter, M.; Douay, L.; Gorin, N.-C.; Larsen, A.K.; Chapel, A. Mesenchymal Stem Cell Administration Attenuates Colon Cancer Progression by Modulating the Immune Component within the Colorectal Tumor Microenvironment. Stem Cells Transl. Med. 2019, 8, 285–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahar, T.; Rozovski, U.; Hess, K.R.; Hossain, A.; Gumin, J.; Gao, F.; Fuller, G.N.; Goodman, L.; Sulman, E.P.; Lang, F.F. Percentage of mesenchymal stem cells in high-grade glioma tumor samples correlates with patient survival. Neuro-Oncol. 2017, 19, 660–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolay, N.H.; Strack, M.; Heiland, D.H.; Schnell, O.; Grosu, A.-L.; Ruehle, A. Human bone-marrow derived mesenchymal stromal cells increase glioblastoma radioresistance. Brain Tumor Res. Treat. 2022, 10, S43. [Google Scholar]
- Jensen, D.H.; Oliveri, R.S.; Trojahn Kølle, S.F.; Fischer-Nielsen, A.; Specht, L.; Bardow, A.; Buchwald, C. Mesenchymal stem cell therapy for salivary gland dysfunction and xerostomia: A systematic review of preclinical studies. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 335–342. [Google Scholar] [CrossRef]
- Blitzer, G.C.; Rogus-Pulia, N.M.; Mattison, R.J.; Varghese, T.; Ganz, O.; Chappell, R.; Galipeau, J.; McDowell, K.A.; Meyers, R.O.; Glazer, T.A.; et al. Marrow-Derived Autologous Stromal Cells for the Restoration of Salivary Hypofunction (MARSH): Study protocol for a phase 1 dose-escalation trial of patients with xerostomia after radiation therapy for head and neck cancer: MARSH: Marrow-Derived Autologous Stromal Cells for the Restoration of Salivary Hypofunction. Cytotherapy 2022, 24, 534–543. [Google Scholar]
- Schmidt, M.; Piro-Hussong, A.; Siegemund, A.; Gabriel, P.; Dorr, W. Modification of radiation-induced oral mucositis (mouse) by adult stem cell therapy: Single-dose irradiation. Radiat. Environ. Biophys. 2014, 53, 629–634. [Google Scholar] [CrossRef]
- Portas, M.; Mansilla, E.; Drago, H.; Dubner, D.; Radl, A.; Coppola, A.; Di Giorgio, M. Use of Human Cadaveric Mesenchymal Stem Cells for Cell Therapy of a Chronic Radiation-Induced Skin Lesion: A Case Report. Radiat. Prot. Dosim. 2016, 171, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Hormozi Moghaddam, Z.; Mokhtari-Dizaji, M.; Nilforoshzadeh, M.A.; Bakhshandeh, M.; Ghaffari Khaligh, S. Low-intensity ultrasound combined with allogenic adipose-derived mesenchymal stem cells (AdMSCs) in radiation-induced skin injury treatment. Sci. Rep. 2020, 10, 20006. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Wu, W.; Yang, S.; Huang, L.; Chen, J.; Gong, C.; Fu, Z.; Zhang, L.; Tan, J. Bone marrow mesenchymal stem cell implantation for the treatment of radioactivity-induced acute skin damage in rats. Mol. Med. Rep. 2015, 12, 7065–7071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Zhang, Y.; Song, X.; Zhu, J.; Zhu, Q. The Healing Effects of Conditioned Medium Derived from Mesenchymal Stem Cells on Radiation-Induced Skin Wounds in Rats. Cell Transplant. 2019, 28, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Wen, S.; Dooner, M.; Cheng, Y.; Papa, E.; Del Tatto, M.; Pereira, M.; Deng, Y.; Goldberg, L.; Aliotta, J.; Chatterjee, D.; et al. Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia 2016, 30, 2221–2231. [Google Scholar] [CrossRef]
- An, H.-Y.; Shin, H.-S.; Choi, J.-S.; Kim, H.J.; Lim, J.-Y.; Kim, Y.-M. Adipose Mesenchymal Stem Cell Secretome Modulated in Hypoxia for Remodeling of Radiation-Induced Salivary Gland Damage. PLoS ONE 2015, 10, e0141862. [Google Scholar] [CrossRef] [Green Version]
- Gugliandolo, A.; Fonticoli, L.; Trubiani, O.; Rajan, T.S.; Marconi, G.D.; Bramanti, P.; Mazzon, E.; Pizzicannella, J.; Diomede, F. Oral Bone Tissue Regeneration: Mesenchymal Stem Cells, Secretome, and Biomaterials. Int. J. Mol. Sci. 2021, 22, 5236. [Google Scholar] [CrossRef]
- Liotta, F.; Querci, V.; Mannelli, G.; Santarlasci, V.; Maggi, L.; Capone, M.; Rossi, M.C.; Mazzoni, A.; Cosmi, L.; Romagnani, S.; et al. Mesenchymal stem cells are enriched in head neck squamous cell carcinoma, correlates with tumour size and inhibit T-cell proliferation. Br. J. Cancer 2015, 112, 745–754. [Google Scholar] [CrossRef] [Green Version]
- Xia, L.; Peng, R.; Leng, W.; Jia, R.; Zeng, X.; Yang, X.; Fan, M. TRAIL-expressing gingival-derived mesenchymal stem cells inhibit tumorigenesis of tongue squamous cell carcinoma. J. Dent. Res. 2015, 94, 219–228. [Google Scholar] [CrossRef]
- Chiu, Y.J.; Yang, J.S.; Hsu, H.S.; Tsai, C.H.; Ma, H. Adipose-derived stem cell conditioned medium attenuates cisplatin-triggered apoptosis in tongue squamous cell carcinoma. Oncol. Rep. 2018, 39, 651–658. [Google Scholar] [CrossRef] [Green Version]
- Wessely, A.; Waltera, A.; Reichert, T.E.; Stöckl, S.; Grässel, S.; Bauer, R.J. Induction of ALP and MMP9 activity facilitates invasive behavior in heterogeneous human BMSC and HNSCC 3D spheroids. FASEB J. 2019, 33, 11884–11893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, S.; Narjus-Sterba, M.; Tuomainen, K.; Kaur, S.; Seppänen-Kaijansinkko, R.; Salo, T.; Mannerström, B.; Al-Samadi, A. Adipose-Derived Mesenchymal Stem Cells do not Affect the Invasion and Migration Potential of Oral Squamous Carcinoma Cells. Int. J. Mol. Sci. 2020, 21, 6455. [Google Scholar] [CrossRef] [PubMed]
- Rowan, B.G.; Lacayo, E.A.; Sheng, M.; Anbalagan, M.; Gimble, J.M.; Jones, R.K.; Joseph, W.J.; Friedlander, P.L.; Chiu, E.S. Human Adipose Tissue-Derived Stromal/Stem Cells Promote Migration and Early Metastasis of Head and Neck Cancer Xenografts. Aesthet. Surg. J. 2016, 36, 93–104. [Google Scholar] [CrossRef]
- Danan, D.; Lehman, C.E.; Mendez, R.E.; Langford, B.; Koors, P.D.; Dougherty, M.I.; Peirce, S.M.; Gioeli, D.G.; Jameson, M.J. Effect of Adipose-Derived Stem Cells on Head and Neck Squamous Cell Carcinoma. Otolaryngol. Head Neck Surg. 2018, 158, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.S.; Lee, J.C.; Lee, Y.S.; Lee, B.J.; Wang, S.G. Growth Inhibitory Effect of Palatine Tonsil-derived Mesenchymal Stem Cells on Head and Neck Squamous Cell Carcinoma Cells. Clin. Exp. Otorhinolaryngol. 2012, 5, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, K.; Eggersmann, T.K.; Haider, S.P.; Schwenk-Zieger, S.; Zhou, J.; Gires, O.; Lechner, A.; Canis, M.; Haubner, F. Human Adipose-Derived Stem/Stromal Cells Promote Proliferation and Migration in Head and Neck Cancer Cells. Cancers 2021, 13, 2751. [Google Scholar] [CrossRef]
- Harrell, C.R.; Volarevic, A.; Djonov, V.G.; Jovicic, N.; Volarevic, V. Mesenchymal Stem Cell: A Friend or Foe in Anti-Tumor Immunity. Int. J. Mol. Sci. 2021, 22, 12429. [Google Scholar] [CrossRef]
- Eke, I.; Cordes, N. Radiobiology goes 3D: How ECM and cell morphology impact on cell survival after irradiation. Radiother. Oncol. 2011, 99, 271–278. [Google Scholar] [CrossRef]
- Thomsen, A.R.; Aldrian, C.; Bronsert, P.; Thomann, Y.; Nanko, N.; Melin, N.; Rücker, G.; Follo, M.; Grosu, A.L.; Niedermann, G.; et al. A deep conical agarose microwell array for adhesion independent three-dimensional cell culture and dynamic volume measurement. Lab Chip 2017, 18, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Waldschmidt, J.M.; Fruttiger, S.J.; Wider, D.; Jung, J.; Thomsen, A.R.; Hartmann, T.N.; Duyster, J.; Hug, M.J.; Azab, K.A.; Jung, M.; et al. Ex vivo propagation in a novel 3D high-throughput co-culture system for multiple myeloma. J. Cancer Res. Clin. Oncol. 2022, 148, 1045–1055. [Google Scholar] [CrossRef]
- Ahmed, K.M.; Pandita, R.K.; Singh, D.K.; Hunt, C.R.; Pandita, T.K. β1-Integrin Impacts Rad51 Stability and DNA Double-Strand Break Repair by Homologous Recombination. Mol. Cell Biol. 2018, 38, e00672-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branzei, D.; Foiani, M. Regulation of DNA repair throughout the cell cycle. Nat. Rev. Mol. Cell Biol. 2008, 9, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Centurione, L.; Aiello, F.B. DNA Repair and Cytokines: TGF-β, IL-6, and Thrombopoietin as Different Biomarkers of Radioresistance. Front. Oncol. 2016, 6, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bickelhaupt, S.; Erbel, C.; Timke, C.; Wirkner, U.; Dadrich, M.; Flechsig, P.; Tietz, A.; Pföhler, J.; Gross, W.; Peschke, P.; et al. Effects of CTGF Blockade on Attenuation and Reversal of Radiation-Induced Pulmonary Fibrosis. JNCI J. Natl. Cancer Inst. 2017, 109, djw339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.C.; Hsu, W.H.; Wang, C.C.; Chou, C.H.; Kuo, M.Y.; Lin, B.R.; Chen, S.T.; Tai, S.K.; Kuo, M.L.; Yang, M.H. Connective tissue growth factor activates pluripotency genes and mesenchymal-epithelial transition in head and neck cancer cells. Cancer Res. 2013, 73, 4147–4157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikuchi, R.; Kikuchi, Y.; Tsuda, H.; Maekawa, H.; Kozaki, K.; Imoto, I.; Tamai, S.; Shiotani, A.; Iwaya, K.; Sakamoto, M.; et al. Expression and clinical significance of connective tissue growth factor in advanced head and neck squamous cell cancer. Hum. Cell 2014, 27, 121–128. [Google Scholar] [CrossRef]
- Shen, Y.W.; Zhou, Y.D.; Chen, H.Z.; Luan, X.; Zhang, W.D. Targeting CTGF in Cancer: An Emerging Therapeutic Opportunity. Trends Cancer 2021, 7, 511–524. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Li, H.-Y.; Zhao, X.-P.; Jiao, J.-Y.; Tang, D.-X.; Yan, L.-J.; Wan, Q.; Pan, C.-B. Mesenchymal stem cell-derived CCN2 promotes the proliferation, migration and invasion of human tongue squamous cell carcinoma cells. Cancer Sci. 2017, 108, 897–909. [Google Scholar] [CrossRef] [Green Version]
- Rühle, A.; Ping, D.; Lopez Perez, R.; Strack, M.; Brons, S.; Yijia, Q.; Debus, J.; Wuchter, P.; Grosu, A.-L.; Huber, P.E.; et al. Human mesenchymal stromal cells maintain their stem cell traits after high-LET particle irradiation—Potential implications for particle radiotherapy and manned space missions. Cancer Lett. 2022, 524, 172–181. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Hauser, U.; Balz, V.; Carey, T.E.; Grénman, R.; Van Lierop, A.; Scheckenbach, K.; Bier, H. Reliable detection of p53 aberrations in squamous cell carcinomas of the head and neck requires transcript analysis of the entire coding region. Head Neck 2002, 24, 868–873. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rühle, A.; Lies, M.; Strack, M.; Perez, R.L.; Bieber, B.; Thomsen, A.R.; Bronsert, P.; Huber, P.E.; Hess, J.; Knopf, A.; et al. Human Mesenchymal Stromal Cells Do Not Cause Radioprotection of Head-and-Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 7689. https://doi.org/10.3390/ijms23147689
Rühle A, Lies M, Strack M, Perez RL, Bieber B, Thomsen AR, Bronsert P, Huber PE, Hess J, Knopf A, et al. Human Mesenchymal Stromal Cells Do Not Cause Radioprotection of Head-and-Neck Squamous Cell Carcinoma. International Journal of Molecular Sciences. 2022; 23(14):7689. https://doi.org/10.3390/ijms23147689
Chicago/Turabian StyleRühle, Alexander, Marie Lies, Maren Strack, Ramon Lopez Perez, Birgit Bieber, Andreas R. Thomsen, Peter Bronsert, Peter E. Huber, Jochen Hess, Andreas Knopf, and et al. 2022. "Human Mesenchymal Stromal Cells Do Not Cause Radioprotection of Head-and-Neck Squamous Cell Carcinoma" International Journal of Molecular Sciences 23, no. 14: 7689. https://doi.org/10.3390/ijms23147689
APA StyleRühle, A., Lies, M., Strack, M., Perez, R. L., Bieber, B., Thomsen, A. R., Bronsert, P., Huber, P. E., Hess, J., Knopf, A., Wuchter, P., Grosu, A.-L., & Nicolay, N. H. (2022). Human Mesenchymal Stromal Cells Do Not Cause Radioprotection of Head-and-Neck Squamous Cell Carcinoma. International Journal of Molecular Sciences, 23(14), 7689. https://doi.org/10.3390/ijms23147689






