Nano-Formulation Endows Quorum Quenching Enzyme-Antibiotic Hybrids with Improved Antibacterial and Antibiofilm Activities against Pseudomonas aeruginosa
Abstract
:1. Introduction
2. Results and Discussion
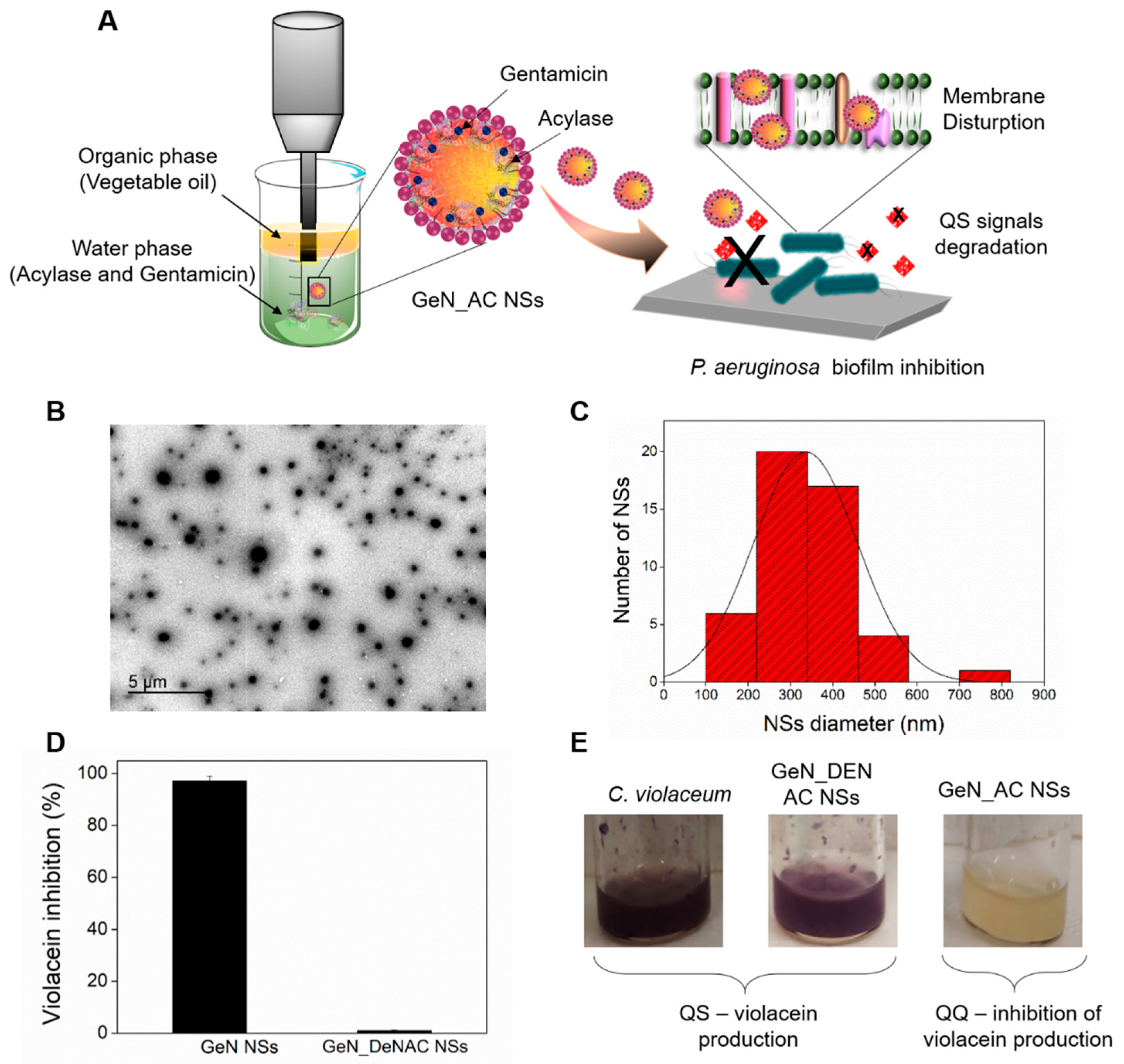
2.1. Hybrid GeN_AC NSs Characterization
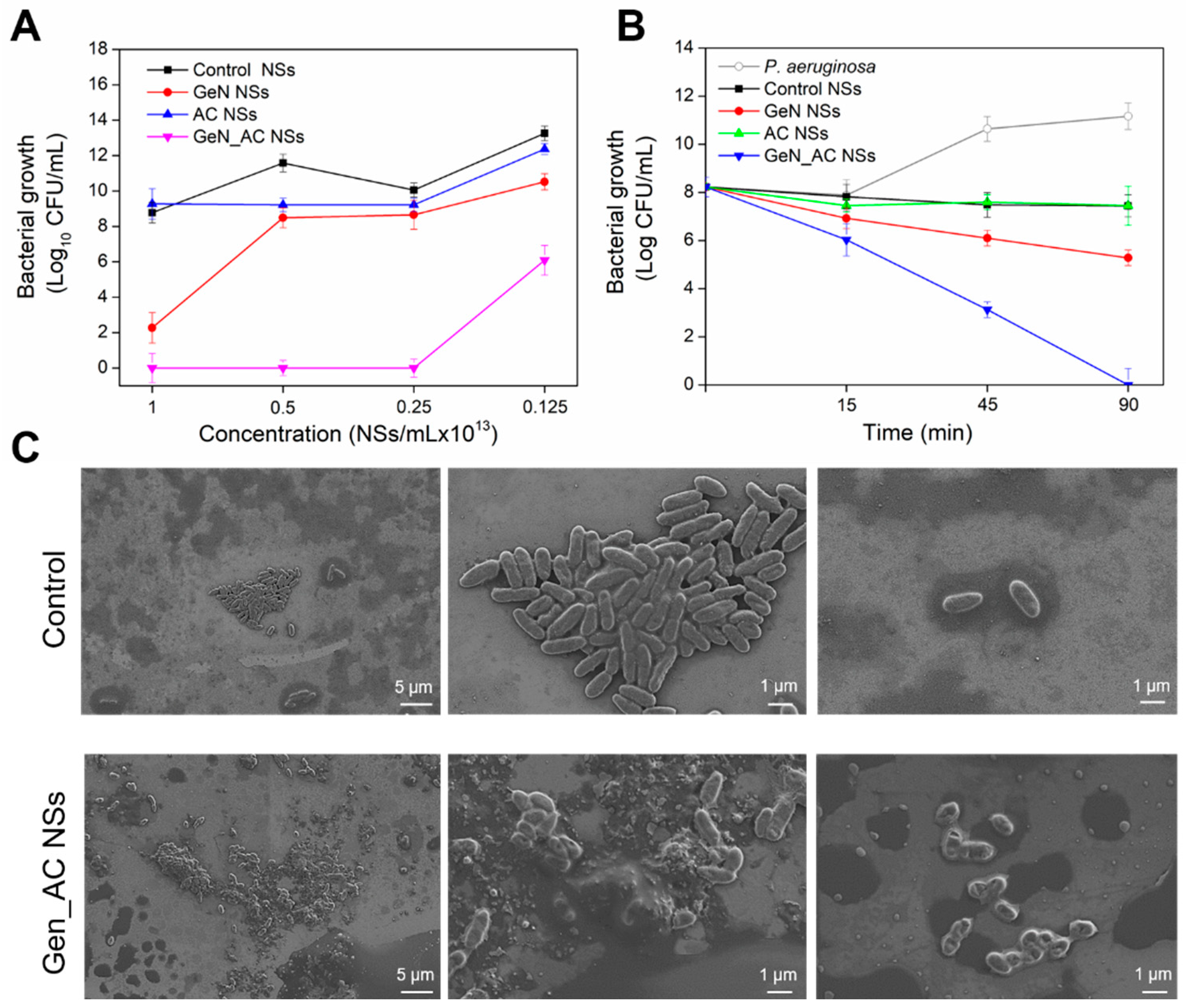
2.2. Antibacterial Activity of GeN_AC NSs against P. aeruginosa
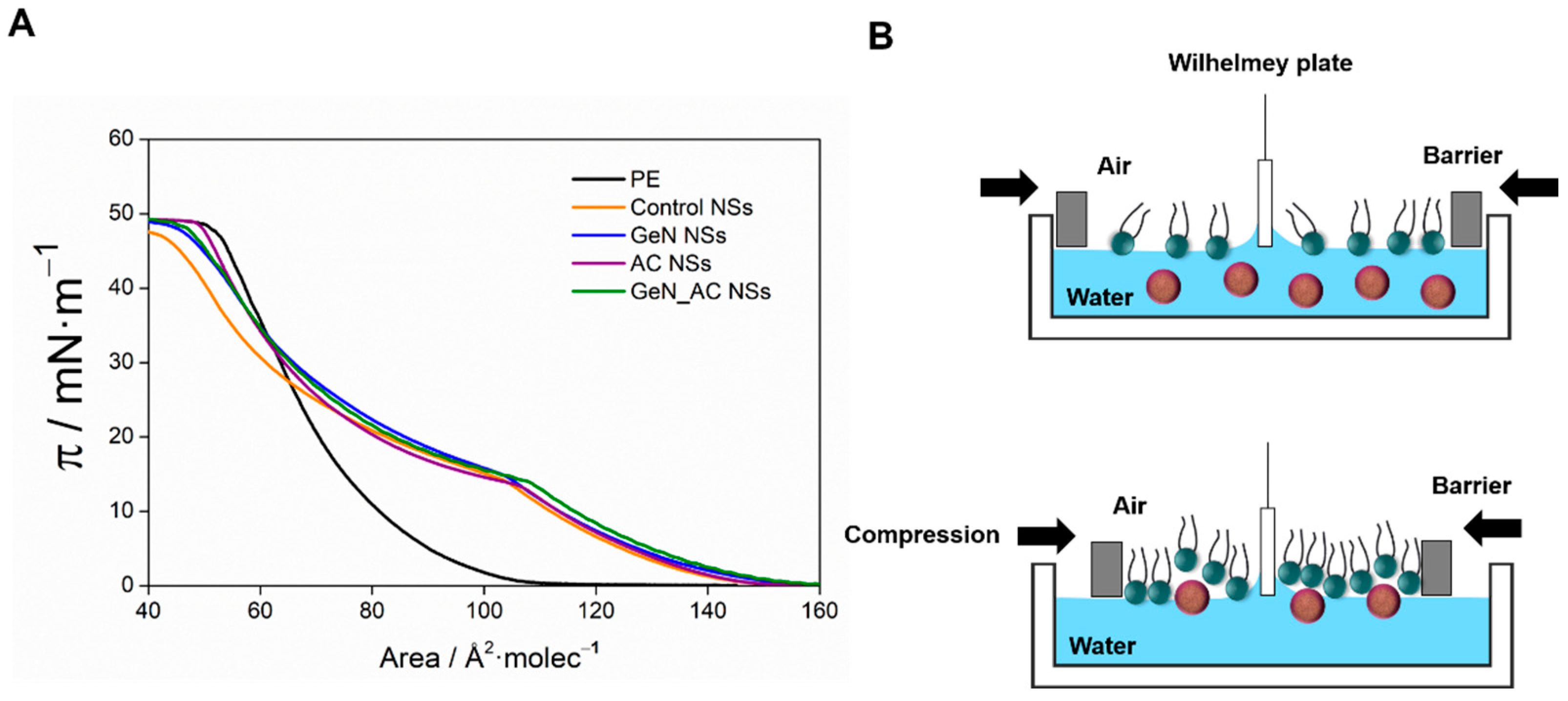
2.3. GeN_AC NSs Interaction with Biomimetic Bacterial Membranes
2.4. Antibiofilm Activity of GeN_AC NSs
2.5. Real-Time Monitoring of the GeN_AC NSs Interaction with Sessile P. aeruginosa Cells
2.6. Biocompatibility of GeN_AC NSs
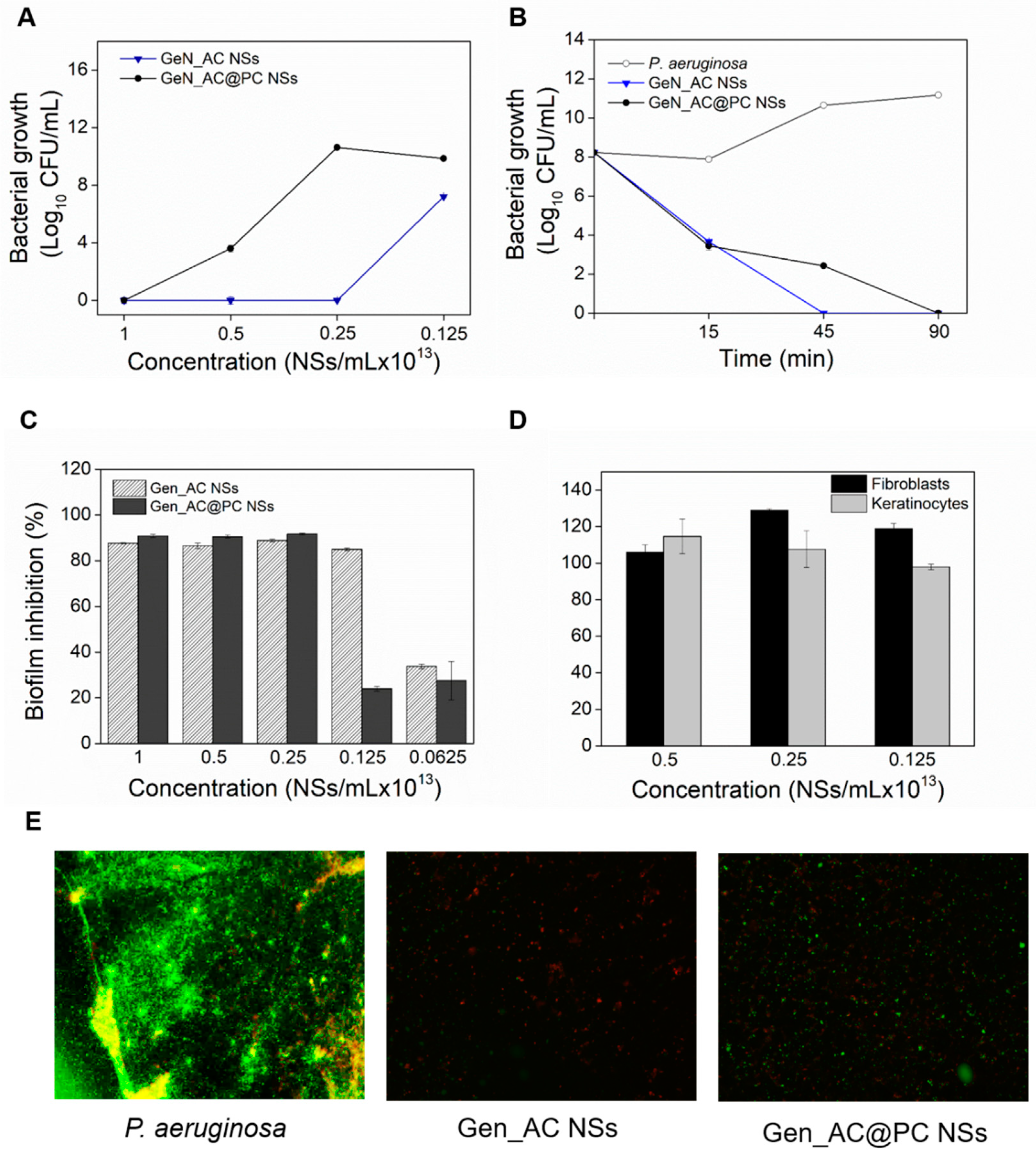
2.7. Influence of Protein Corona Effect on the Functional Properties of GeN_AC NSs
3. Materials and Methods
3.1. Materials, Reagents and Bacteria
3.2. Ultrasound-Assisted Preparation of GeN_AC NSs
3.3. GeN_AC NSs Characterization
3.4. Catalytic Activity of Acylase
3.5. Minimum Bactericidal Concentration Determination
3.6. Time-Kill Kinetics
3.7. Interaction with Biomimetic Membranes
3.8. Antibiofilm Activity of GeN_AC NSs
3.8.1. Crystal Violet Assay for Total Biofilm Mass Quantification
3.8.2. LIVE/DEAD® BacLight™ Bacterial Viability for Microscopic Evaluation of the Biofilms
3.8.3. Real Time Monitoring of GeN_AC NSs Effect on Sessile P. aeruginosa Cells
3.9. Biocompatibility Assessment
3.10. Protein Corona Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haque, M.; Sartelli, M.; Mckimm, J.; Abu Bakar, M. Health Care-Associated Infections-an Overview. Infect. Drug Resist. 2018, 11, 2321–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Rev. Antimicrob. Resist. 2016, 7, 1–84. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control Antimicrobial. Antimicrobial Resistance. 2021, pp. 17–19. Available online: https://www.ecdc.europa.eu/en/antimicrobial-Resistance (accessed on 31 January 2022).
- World Health Organization. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis; World Health Organization: Geneva, Switzerland, 2017.
- Bowler, P.; Murphy, C.; Wolcott, R. Biofilm Exacerbates Antibiotic Resistance: Is This a Current Oversight in Antimicrobial Stewardship? Antimicrob. Resist. Infect. Control 2020, 9, 9–162. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.R.; Kopecki, Z.; Wignall, A.; Kral, A.; Prestidge, C.A.; Thomas, N. Liquid Crystal Nanoparticle Platform for Increased Efficacy of Cationic Antimicrobials against Biofilm Infections. Nanomed. Nanotechnol. Biol. Med. 2022, 42, 102536. [Google Scholar] [CrossRef]
- Jamal, M.; Andleeb, S. Bacterial Biofilm: Its Composition, Formation and Role in Human Infections. Res. Rev. J. Microbiol. Biotechnol. 2015, 4, 7–11. [Google Scholar]
- Ivanova, A.; Ivanova, K.; Tzanov, T. Inhibition of Quorum-Sensing: A New Paradigm in Controlling Bacterial Virulence and Biofilm Formation. In Biotechnological Applications of Quorum Sensing Inhibitors; Kalia, V.C., Ed.; Springer: Singapore, 2018; ISBN 9789811090257. [Google Scholar]
- Singh, B.N.; Prateeksha; Upreti, D.K.; Singh, B.R.; Defoirdt, T.; Gupta, V.K.; De Souza, A.O.; Singh, H.B.; Barreira, J.C.M.; Ferreira, I.C.F.R.; et al. Bactericidal, Quorum Quenching and Anti-Biofilm Nanofactories: A New Niche for Nanotechnologists. Crit. Rev. Biotechnol. 2017, 37, 525–540. [Google Scholar] [CrossRef]
- Rasko, D.A.; Sperandio, V. Anti-Virulence Strategies to Combat Bacteria-Mediated Disease. Nat. Rev. Drug Discov. 2010, 9, 117–128. [Google Scholar] [CrossRef]
- Ivanova, K.; Fernandes, M.M.; Mendoza, E.; Tzanov, T. Enzyme Multilayer Coatings Inhibit Pseudomonas aeruginosa Biofilm Formation on Urinary Catheters. Appl. Microbiol. Biotechnol. 2015, 99, 4373–4385. [Google Scholar] [CrossRef]
- Ivanova, K.; Fernandes, M.M.; Francesko, A.; Mendoza, E.; Guezguez, J.; Burnet, M.; Tzanov, T. Quorum-Quenching and Matrix-Degrading Enzymes in Multilayer Coatings Synergistically Prevent Bacterial Biofilm Formation on Urinary Catheters. ACS Appl. Mater. Interfaces 2015, 7, 27066–27077. [Google Scholar] [CrossRef]
- Gupta, A.; Mumtaz, S.; Li, C.H.; Hussain, I.; Rotello, V.M. Combatting Antibiotic-Resistant Bacteria Using Nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef]
- Krzyżek, P. Challenges and Limitations of Anti-Quorum Sensing Therapies. Front. Microbiol. 2019, 10, 2473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinoj, G.; Pati, R.; Sonawane, A.; Vaseeharan, B. In Vitro Cytotoxic Effects of Gold Nanoparticles Coated with Functional Acyl Homoserine Lactone Lactonase Protein from Bacillus Licheniformis and Their Antibiofilm Activity against Proteus Species. Antimicrob. Agents Chemother. 2015, 59, 763–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanova, A.; Ivanova, K.; Tied, A.; Heinze, T.; Tzanov, T. Layer-By-Layer Coating of Aminocellulose and Quorum Quenching Acylase on Silver Nanoparticles Synergistically Eradicate Bacteria and Their Biofilms. Adv. Funct. Mater. 2020, 30, 2001284. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, M.M.; Ivanova, K.; Francesko, A.; Rivera, D.; Torrent-Burgués, J.; Gedanken, A.; Mendonza, E.; Tzanov, T. Escherichia coli and Pseudomonas aeruginosa Eradication by Nano-Penicillin G. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2061–2069. [Google Scholar] [CrossRef]
- Fernandes, M.M.; Ivanova, K.; Hoyo, J.; Pérez-Rafael, S.; Francesko, A.; Tzanov, T. Nanotransformation of Vancomycin Overcomes the Intrinsic Resistance of Gram-Negative Bacteria. ACS Appl. Mater. Interfaces 2017, 9, 15022–15030. [Google Scholar] [CrossRef]
- Ivanova, A.; Ivanova, K.; Hoyo, J.; Heinze, T.; Sanchez-Gomez, S.; Tzanov, T. Layer-By-Layer Decorated Nanoparticles with Tunable Antibacterial and Antibiofilm Properties against Both Gram-Positive and Gram-Negative Bacteria. ACS Appl. Mater. Interfaces 2018, 10, 3314–3323. [Google Scholar] [CrossRef]
- Fernandes, M.M.; Francesko, A.; Torrent-Burgués, A.; Carrión-Fité, F.J.; Heinze, T.; Tzanov, T. Sonochemically Processed Cationic Nanocapsules: Efficient Antimicrobials with Membrane Disturbing Capacity. Biomacromolecules 2014, 15, 1365–1374. [Google Scholar] [CrossRef]
- Fernandes, M.M.; Rivera, D.; Francesko, A.; Šližytė, R.; Mozuraityte, R.; Rommi, K.; Lantto, R.; Tzanov, T. Bio/Sonochemical Conversion of Fish Backbones into Bioactive Nanospheres. Process Biochem. 2015, 50, 1843–1851. [Google Scholar] [CrossRef]
- Koshani, R.; Jafari, S.M. Ultrasound-Assisted Preparation of Different Nanocarriers Loaded with Food Bioactive Ingredients. Adv. Colloid Interface Sci. 2019, 270, 123–146. [Google Scholar] [CrossRef]
- Cortés, H.; Hernández-Parra, H.; Bernal-Chávez, S.A.; Del Prado-Audelo, M.L.; Caballero-Florán, I.H.; Borbolla-Jiménez, F.V.; González-Torres, M.; Magaña, J.J.; Leyva-Gómez, G. Non-Ionic Surfactants for Stabilization of Polymeric Nanoparticles for Biomedical Uses. Materials 2021, 14, 3197. [Google Scholar] [CrossRef]
- Gómez-Gómez, B.; Arregui, L.; Serrano, S.; Santos, A.; Pérez-Corona, T.; Madrid, Y. Selenium and Tellurium-Based Nanoparticles as Interfering Factors in Quorum Sensing-Regulated Processes: Violacein Production and Bacterial Biofilm Formation. Metallomics 2019, 11, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Chen, F.; Chen, Y.C.; Peng, H.; Wang, K.J. The Long-Term Effect of a Nine Amino-Acid Antimicrobial Peptide AS-Hepc3(48-56) Against Pseudomonas aeruginosa With No Detectable Resistance. Front. Cell. Infect. Microbiol. 2021, 11, 752637. [Google Scholar] [CrossRef] [PubMed]
- Jangir, P.K.; Ogunlana, L.; MacLean, R.C. Evolutionary Constraints on the Acquisition of Antimicrobial Peptide Resistance in Bacterial Pathogens. Trends Microbiol. 2021, 29, 1058–1061. [Google Scholar] [CrossRef] [PubMed]
- Hoyo, J.; Torrent-Burgués, J.; Guaus, E. Biomimetic Monolayer Films of Monogalactosyldiacylglycerol Incorporating Ubiquinone. J. Colloid. Interf. Sci. 2012, 384, 189–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoyo, J.; Guaus, E.; Torrent-Burgués, J.; Sanz, F. Biomimetic Monolayer Films of Digalactosyldiacylglycerol Incorporating Plastoquinone. Biochim. Biophys. Acta-Biomembr. 2015, 1848, 1341–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreres, G.; Bassegoda, A.; Hoyo, J.; Torrent-burgue, J.; Tzanov, T. Metal—Enzyme Nanoaggregates Eradicate Both Gram-Positive and Gram-Negative Bacteria and Their Biofilms. ACS Appl. Mater. Interfaces 2018, 10, 40434–40442. [Google Scholar] [CrossRef] [PubMed]
- Hoyo, J.; Ivanova, K.; Torrent-Burgues, J.; Tzanov, T. Interaction of Silver-Lignin Nanoparticles With Mammalian Mimetic Membranes. Front. Bioeng. Biotechnol. 2020, 8, 439. [Google Scholar] [CrossRef]
- Ivanova, K.; Ivanova, A.; Ramon, E.; Hoyo, J.; Sanchez-Gomez, S.; Tzanov, T. Antibody-Enabled Antimicrobial Nanocapsules for Selective Elimination of Staphylococcus aureus. ACS Appl. Mater. Interfaces 2020, 12, 35918–35927. [Google Scholar] [CrossRef]
- Subramaniyan, S.B.; Vijayakumar, S.; Megarajan, S.; Kamlekar, R.K.; Anbazhagan, V. Remarkable Effect of Jacalin in Diminishing the Protein Corona Interference in the Antibacterial Activity of Pectin-Capped Copper Sulfide Nanoparticles. ACS Omega 2019, 4, 14049–14056. [Google Scholar] [CrossRef] [Green Version]
- Tekie, F.S.M.; Hajiramezanali, M.; Geramifar, P.; Raoufi, M.; Dinarvand, R.; Soleimani, M.; Atyabi, F. Controlling Evolution of Protein Corona: A Prosperous Approach to Improve Chitosan-Based Nanoparticle Biodistribution and Half-Life. Sci. Rep. 2020, 10, 9664. [Google Scholar] [CrossRef]
- Isaia, H.A.; Pinilla, C.M.B.; Brandelli, A. Evidence That Protein Corona Reduces the Release of Antimicrobial Peptides from Polymeric Nanocapsules in Milk. Food Res. Int. 2021, 140, 110074. [Google Scholar] [CrossRef] [PubMed]
- Wagenlehner, F.M.E.; Naber, K.G. Treatment of Bacterial Urinary Tract Infections: Presence and Future. Eur. Urol. 2006, 49, 235–244. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, K.; Ivanova, A.; Hoyo, J.; Pérez-Rafael, S.; Tzanov, T. Nano-Formulation Endows Quorum Quenching Enzyme-Antibiotic Hybrids with Improved Antibacterial and Antibiofilm Activities against Pseudomonas aeruginosa. Int. J. Mol. Sci. 2022, 23, 7632. https://doi.org/10.3390/ijms23147632
Ivanova K, Ivanova A, Hoyo J, Pérez-Rafael S, Tzanov T. Nano-Formulation Endows Quorum Quenching Enzyme-Antibiotic Hybrids with Improved Antibacterial and Antibiofilm Activities against Pseudomonas aeruginosa. International Journal of Molecular Sciences. 2022; 23(14):7632. https://doi.org/10.3390/ijms23147632
Chicago/Turabian StyleIvanova, Kristina, Aleksandra Ivanova, Javier Hoyo, Silvia Pérez-Rafael, and Tzanko Tzanov. 2022. "Nano-Formulation Endows Quorum Quenching Enzyme-Antibiotic Hybrids with Improved Antibacterial and Antibiofilm Activities against Pseudomonas aeruginosa" International Journal of Molecular Sciences 23, no. 14: 7632. https://doi.org/10.3390/ijms23147632
APA StyleIvanova, K., Ivanova, A., Hoyo, J., Pérez-Rafael, S., & Tzanov, T. (2022). Nano-Formulation Endows Quorum Quenching Enzyme-Antibiotic Hybrids with Improved Antibacterial and Antibiofilm Activities against Pseudomonas aeruginosa. International Journal of Molecular Sciences, 23(14), 7632. https://doi.org/10.3390/ijms23147632






