Overexpression of UBA5 in Cells Mimics the Phenotype of Cells Lacking UBA5
Abstract
:1. Introduction
2. Results
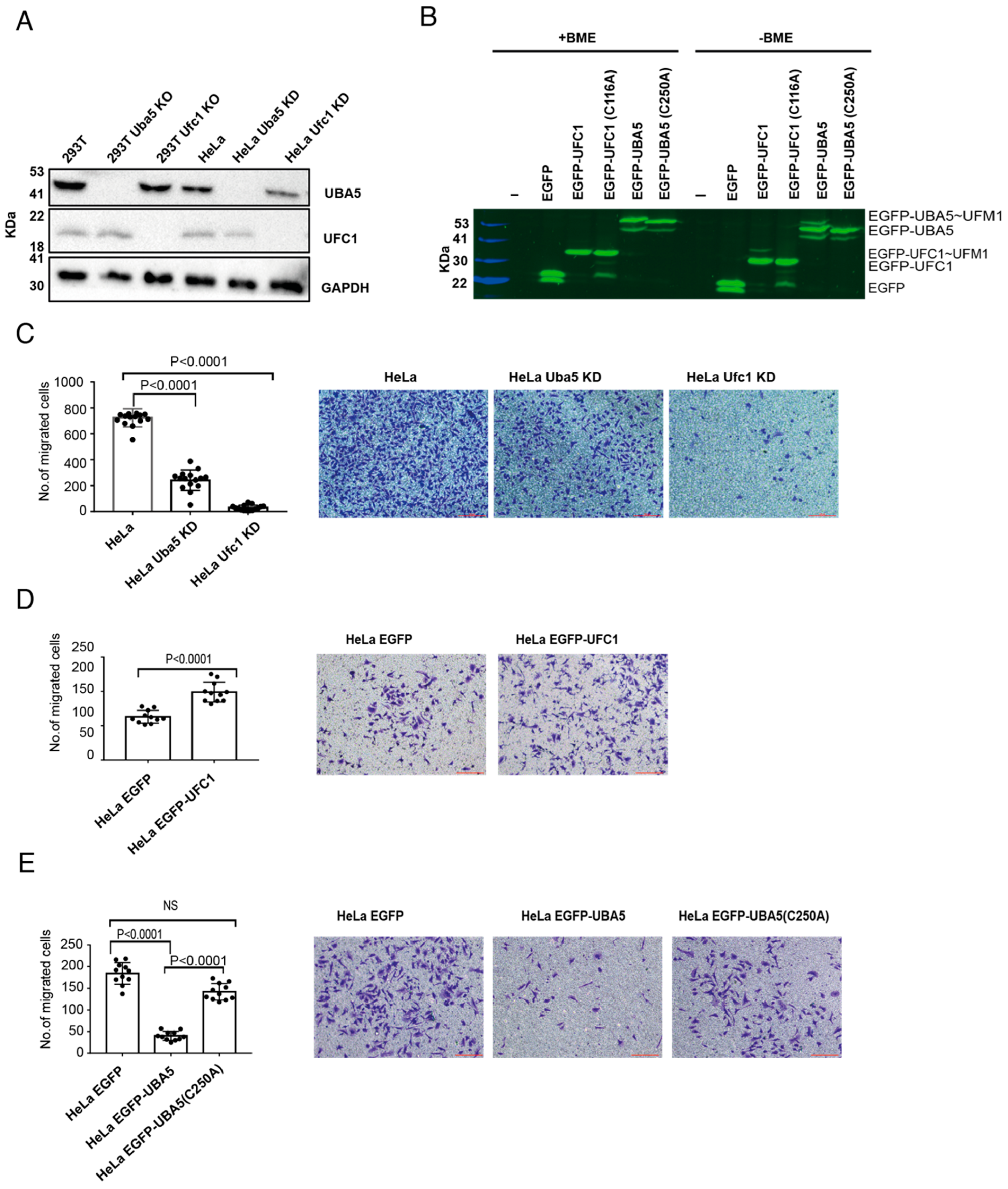
2.1. Alterations in UBA5 Expression Level Affect Cell Migration
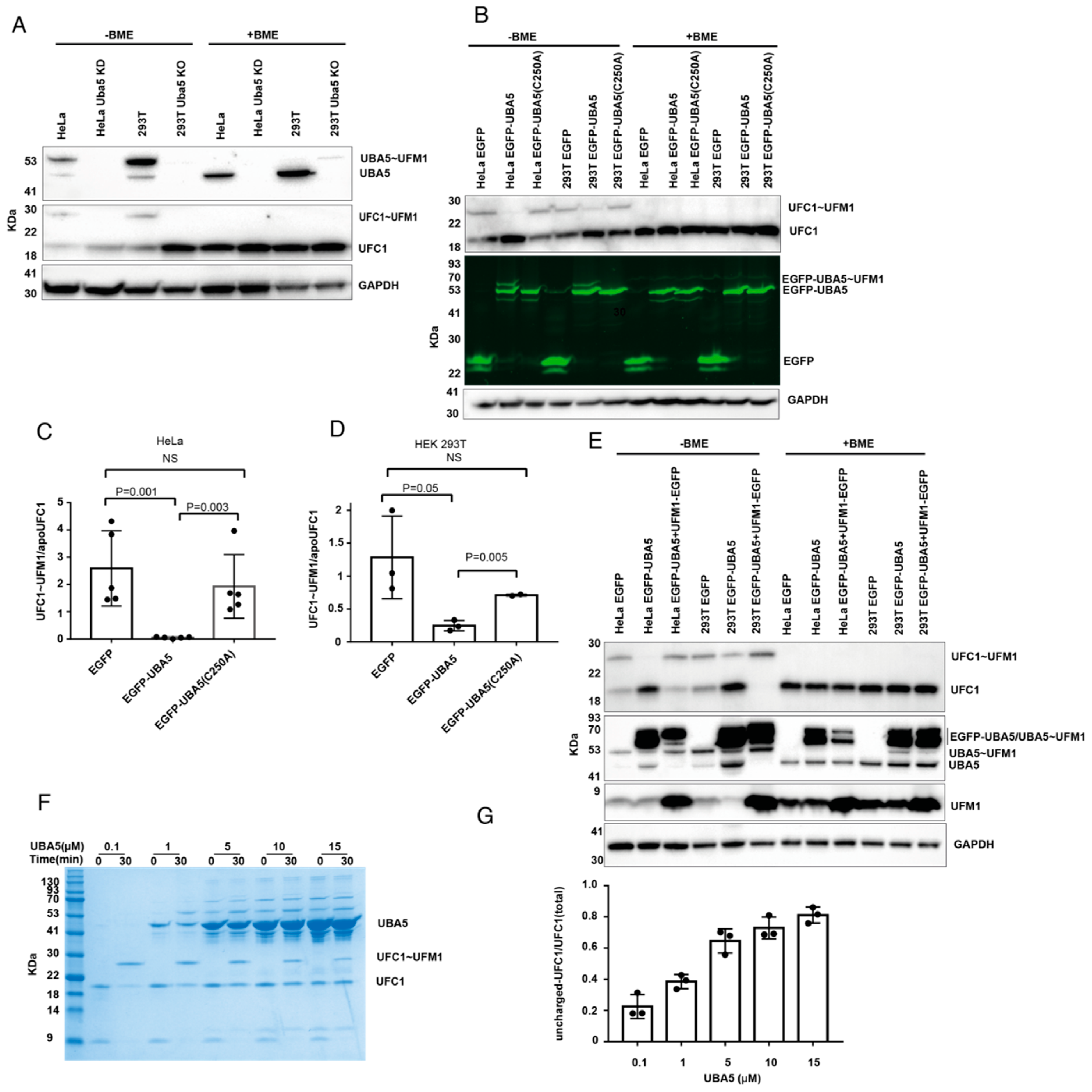
2.2. Overexpression of UBA5 Reduces the Level of Charged UFC1 in the Cell
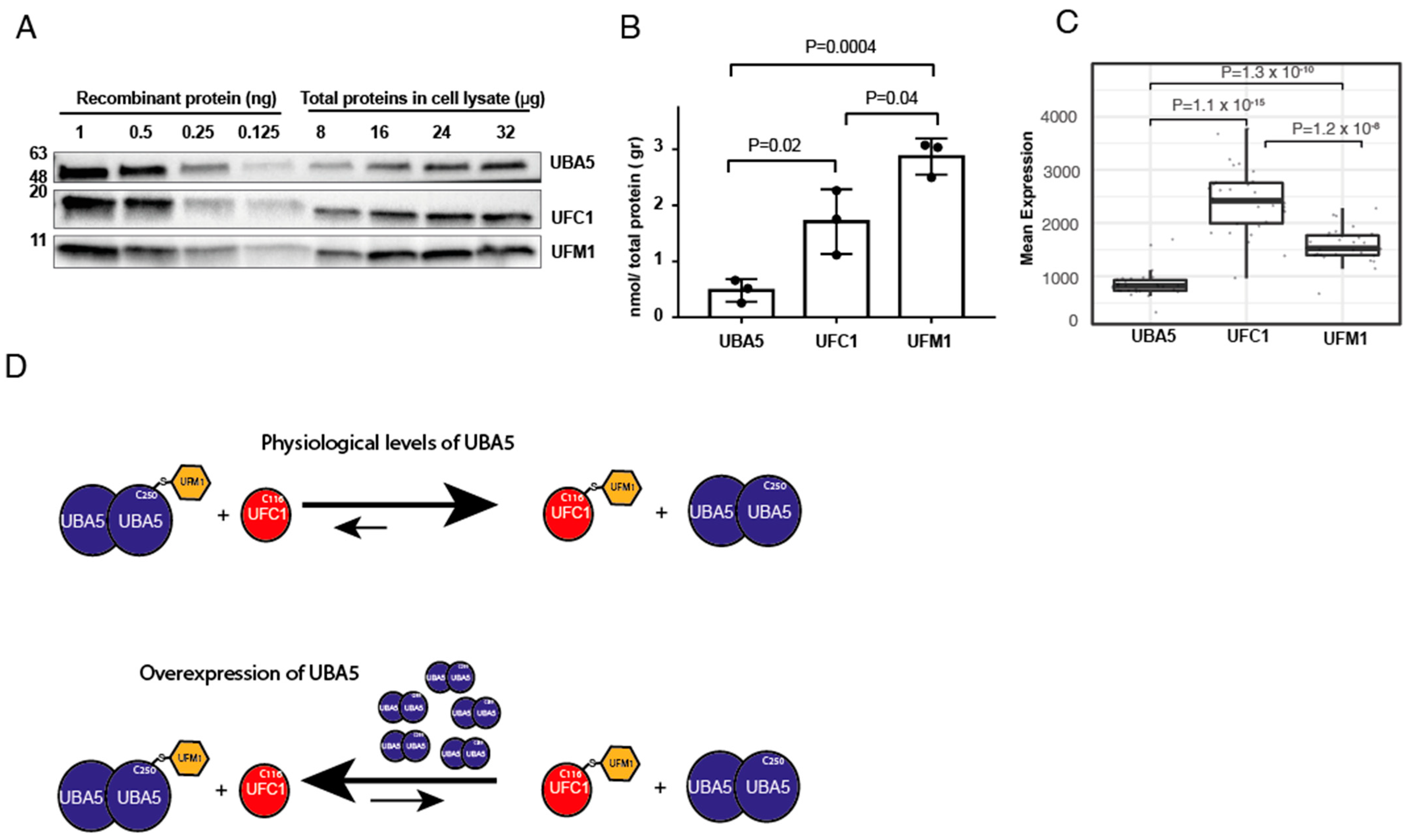
2.3. Cells Express Less UBA5 Than UFM1 or UFC1
3. Discussion
4. Materials and Methods
4.1. Cells Culture and Growth Condition
4.2. Generation of UBA5 and UFC1 Knockdown Cells
4.3. Generation of UBA5 and UFC1 Knockout Cells
4.4. Cloning and Mutagenesis
4.5. Western Blotting
4.6. Fluorescent Gel Imaging
4.7. Trans-Well Migration Assay
4.8. In Vitro Thioester Assay
4.9. UBA5, UFC1 and UFM1 Quantification Assay
4.10. RNAseq Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herrmann, J.; Lerman, L.O.; Lerman, A. Ubiquitin and ubiquitin-like proteins in protein regulation. Circ. Res. 2007, 100, 1276–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochstrasser, M. Origin and function of ubiquitin-like protei ns. Nature 2009, 458, 422–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerscher, O.; Felberbaum, R.; Hochstrasser, M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu. Rev. Cell Dev. Biol. 2006, 22, 159–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komander, D.; Rape, M. The Ubiquitin Code. Annu. Rev. Biochem. 2012, 81, 203–229. [Google Scholar] [CrossRef] [Green Version]
- Capili, A.D.; Lima, C.D. Taking it step by step: Mechanistic insights from structural studies of ubiquitin/ubiquitin-like protein modification pathways. Curr. Opin. Struct. Biol. 2007, 17, 726–735. [Google Scholar] [CrossRef] [Green Version]
- Cappadocia, L.; Lima, C.D. Ubiquitin-like Protein Conjugation: Structures, Chemistry, and Mechanism. Chem. Rev. 2017, 118, 889–918. [Google Scholar] [CrossRef]
- Pickart, C.M.; Eddins, M.J. Ubiquitin: Structures, functions, mechanisms. Biochim. Biophys. Acta 2004, 1695, 55–72. [Google Scholar] [CrossRef] [Green Version]
- Schulman, B.A.; Harper, J.W. Ubiquitin-like protein activation by E1 enzymes: The apex for downstream signalling pathways. Nat. Rev. Mol. Cell Biol. 2009, 10, 319–331. [Google Scholar] [CrossRef] [Green Version]
- Stewart, M.D.; Ritterhoff, T.; Klevit, R.E.; Brzovic, P.S. E2 enzymes: More than just middle men. Cell Res. 2016, 26, 423–440. [Google Scholar] [CrossRef] [Green Version]
- Berndsen, C.E.; Wolberger, C. New insights into ubiquitin E3 ligase mechanism. Nat. Struct. Mol. Biol. 2014, 21, 301–307. [Google Scholar] [CrossRef]
- Qin, B.; Yu, J.; Nowsheen, S.; Wang, M.; Tu, X.; Liu, T.; Li, H.; Wang, L.; Lou, Z. UFL1 promotes histone H4 ufmylation and ATM activation. Nat. Commun. 2019, 10, 1242. [Google Scholar] [CrossRef] [PubMed]
- Walczak, C.P.; Leto, D.E.; Zhang, L.; Riepe, C.; Muller, R.Y.; DaRosa, P.A.; Ingolia, N.T.; Elias, J.E.; Kopito, R.R. Ribosomal protein RPL26 is the principal target of UFMylation. Proc. Natl. Acad. Sci. USA 2019, 116, 1299–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Xu, Y.; Rogers, H.; Saidi, L.; Noguchi, C.T.; Li, H.; Yewdell, J.W.; Guydosh, N.R.; Ye, Y. UFMylation of RPL26 links translocation-associated quality control to endoplasmic reticulum protein homeostasis. Cell Res. 2020, 30, 5–20. [Google Scholar] [CrossRef]
- Wang, Z.; Gong, Y.; Peng, B.; Shi, R.; Fan, D.; Zhao, H.; Zhu, M.; Zhang, H.; Lou, Z.; Zhou, J.; et al. MRE11 UFMylation promotes ATM activation. Nucleic Acids Res. 2019, 47, 4124–4135. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Kumar, M.; Wiener, R. Decrypting UFMylation: How Proteins Are Modified with UFM1. Biomolecules 2020, 10, 1442. [Google Scholar] [CrossRef] [PubMed]
- Witting, K.F.; Mulder, M.P.C. Highly Specialized Ubiquitin-Like Modifications: Shedding Light into the UFM1 Enigma. Biomolecules 2021, 11, 255. [Google Scholar] [CrossRef]
- Komatsu, M.; Chiba, T.; Tatsumi, K.; Iemura, S.; Tanida, I.; Okazaki, N.; Ueno, T.; Kominami, E.; Natsume, T.; Tanaka, K. A novel protein-conjugating system for Ufm1, a ubiquitin-fold modifier. EMBO J. 2004, 23, 1977–1986. [Google Scholar] [CrossRef] [Green Version]
- Tatsumi, K.; Sou, Y.S.; Tada, N.; Nakamura, E.; Iemura, S.; Natsume, T.; Kang, S.H.; Chung, C.H.; Kasahara, M.; Kominami, E.; et al. A novel type of E3 ligase for the Ufm1 conjugation system. J. Biol. Chem. 2010, 285, 5417–5427. [Google Scholar] [CrossRef] [Green Version]
- Bacik, J.P.; Walker, J.R.; Ali, M.; Schimmer, A.D.; Dhe-Paganon, S. Crystal structure of the human ubiquitin-activating enzyme 5 (UBA5) bound to ATP: Mechanistic insights into a minimalistic E1 enzyme. J. Biol. Chem. 2010, 285, 20273–20280. [Google Scholar] [CrossRef] [Green Version]
- Padala, P.; Oweis, W.; Mashahreh, B.; Soudah, N.; Cohen-Kfir, E.; Todd, E.A.; Berndsen, C.E.; Wiener, R. Novel insights into the interaction of UBA5 with UFM1 via a UFM1-interacting sequence. Sci. Rep. 2017, 7, 508. [Google Scholar] [CrossRef] [Green Version]
- Habisov, S.; Huber, J.; Ichimura, Y.; Akutsu, M.; Rogova, N.; Loehr, F.; McEwan, D.G.; Johansen, T.; Dikic, I.; Doetsch, V.; et al. Structural and Functional Analysis of a Novel Interaction Motif within UFM1-activating Enzyme 5 (UBA5) Required for Binding to Ubiquitin-like Proteins and Ufmylation. J. Biol. Chem. 2016, 291, 9025–9041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashahreh, B.; Hassouna, F.; Soudah, N.; Cohen-Kfir, E.; Strulovich, R.; Haitin, Y.; Wiener, R. Trans-binding of UFM1 to UBA5 stimulates UBA5 homodimerization and ATP binding. FASEB J. 2018, 32, 2794–2802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oweis, W.; Padala, P.; Hassouna, F.; Cohen-Kfir, E.; Gibbs, D.R.; Todd, E.A.; Berndsen, C.E.; Wiener, R. Trans-Binding Mechanism of Ubiquitin-like Protein Activation Revealed by a UBA5-UFM1 Complex. Cell Rep. 2016, 16, 3113–3120. [Google Scholar] [CrossRef] [Green Version]
- Soudah, N.; Padala, P.; Hassouna, F.; Kumar, M.; Mashahreh, B.; Lebedev, A.A.; Isupov, M.N.; Cohen-Kfir, E.; Wiener, R. An N-Terminal Extension to UBA5 Adenylation Domain Boosts UFM1 Activation: Isoform-Specific Differences in Ubiquitin-like Protein Activation. J. Mol. Biol. 2019, 431, 463–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, M.; Padala, P.; Fahoum, J.; Hassouna, F.; Tsaban, T.; Zoltsman, G.; Banerjee, S.; Cohen-Kfir, E.; Dessau, M.; Rosenzweig, R.; et al. Structural basis for UFM1 transfer from UBA5 to UFC1. Nat. Commun. 2021, 12, 5708. [Google Scholar] [CrossRef] [PubMed]
- Wesch, N.; Lohr, F.; Rogova, N.; Dotsch, V.; Rogov, V.V. A Concerted Action of UBA5 C-Terminal Unstructured Regions Is Important for Transfer of Activated UFM1 to UFC1. Int. J. Mol. Sci. 2021, 22, 7390. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.X.; Xie, X.S.; Weng, X.F.; Qiu, S.L.; Yoon, C.H.; Lian, N.Z.; Xie, J.W.; Wang, J.B.; Lu, J.; Chen, Q.Y.; et al. UFM1 suppresses invasive activities of gastric cancer cells by attenuating the expres7sion of PDK1 through PI3K/AKT signaling. J. Exp. Clin. Cancer Res. 2019, 38, 1–16. [Google Scholar] [CrossRef]
- Xi, P.; Ding, D.; Zhou, J.; Wang, M.; Cong, Y.S. DDRGK1 regulates NF-kappaB activity by modulating IkappaBalpha stability. PLoS ONE 2013, 8, e64231. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.C. Boyden chamber assay. Methods Mol. Biol. 2005, 294, 15–22. [Google Scholar]
- Kang, S.H.; Kim, G.R.; Seong, M.; Baek, S.H.; Seol, J.H.; Bang, O.S.; Ovaa, H.; Tatsumi, K.; Komatsu, M.; Tanaka, K.; et al. Two novel ubiquitin-fold modifier 1 (Ufm1)-specific proteases, UfSP1 and UfSP2. J. Biol. Chem. 2007, 282, 5256–5262. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Use R! Springer: New York, NY, USA, 2009; pp. 1–212. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, S.; Banerjee, S.; Kumar, M.; Hayashi, A.; Solaimuthu, B.; Cohen-Kfir, E.; Shaul, Y.D.; Rouvinski, A.; Wiener, R. Overexpression of UBA5 in Cells Mimics the Phenotype of Cells Lacking UBA5. Int. J. Mol. Sci. 2022, 23, 7445. https://doi.org/10.3390/ijms23137445
Kumari S, Banerjee S, Kumar M, Hayashi A, Solaimuthu B, Cohen-Kfir E, Shaul YD, Rouvinski A, Wiener R. Overexpression of UBA5 in Cells Mimics the Phenotype of Cells Lacking UBA5. International Journal of Molecular Sciences. 2022; 23(13):7445. https://doi.org/10.3390/ijms23137445
Chicago/Turabian StyleKumari, Sujata, Sayanika Banerjee, Manoj Kumar, Arata Hayashi, Balakrishnan Solaimuthu, Einav Cohen-Kfir, Yoav D. Shaul, Alexander Rouvinski, and Reuven Wiener. 2022. "Overexpression of UBA5 in Cells Mimics the Phenotype of Cells Lacking UBA5" International Journal of Molecular Sciences 23, no. 13: 7445. https://doi.org/10.3390/ijms23137445
APA StyleKumari, S., Banerjee, S., Kumar, M., Hayashi, A., Solaimuthu, B., Cohen-Kfir, E., Shaul, Y. D., Rouvinski, A., & Wiener, R. (2022). Overexpression of UBA5 in Cells Mimics the Phenotype of Cells Lacking UBA5. International Journal of Molecular Sciences, 23(13), 7445. https://doi.org/10.3390/ijms23137445






