A Novel Ex Vivo Bone Culture Model for Regulation of Collagen/Apatite Preferential Orientation by Mechanical Loading
Abstract
:1. Introduction
2. Results
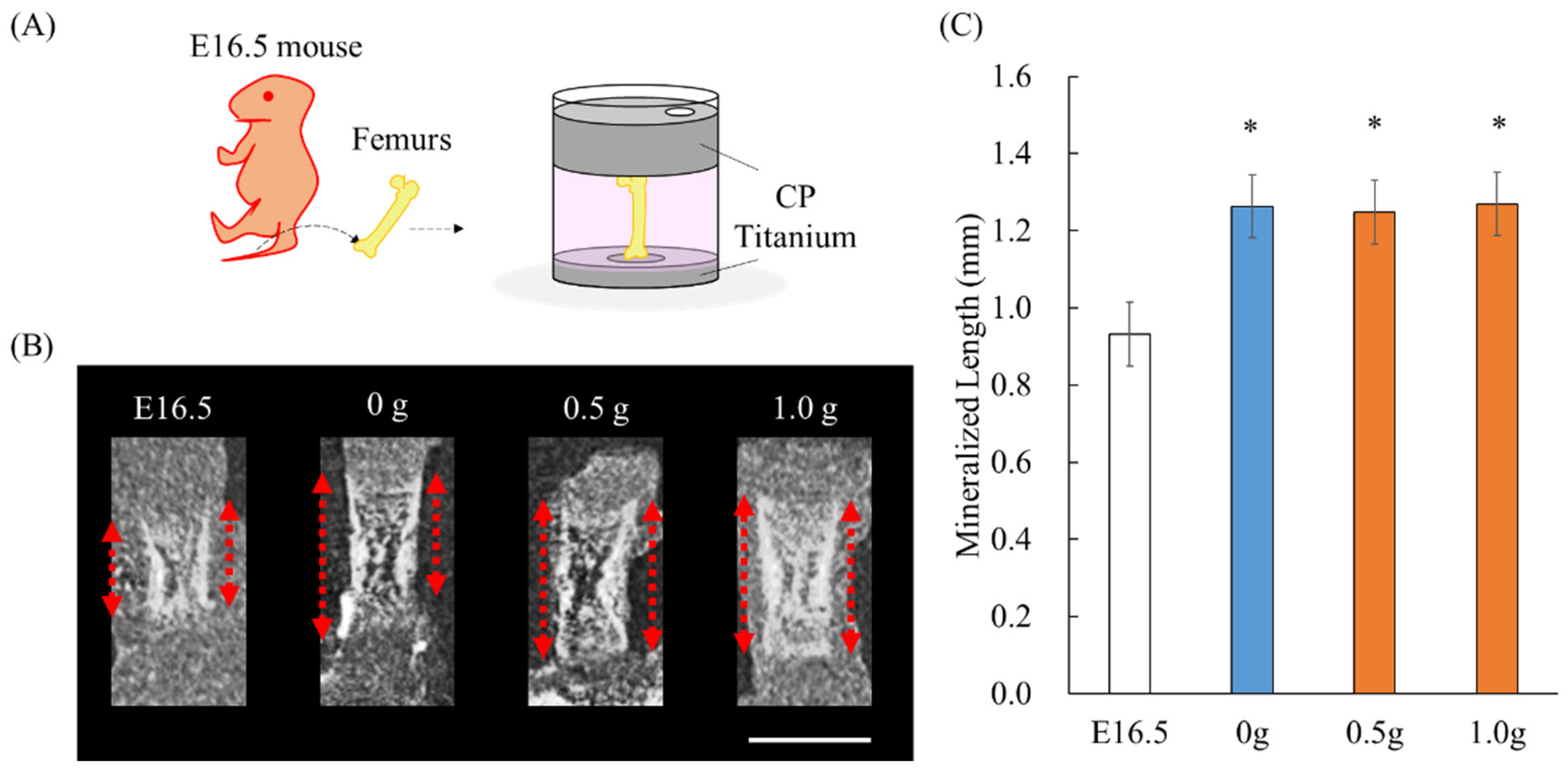
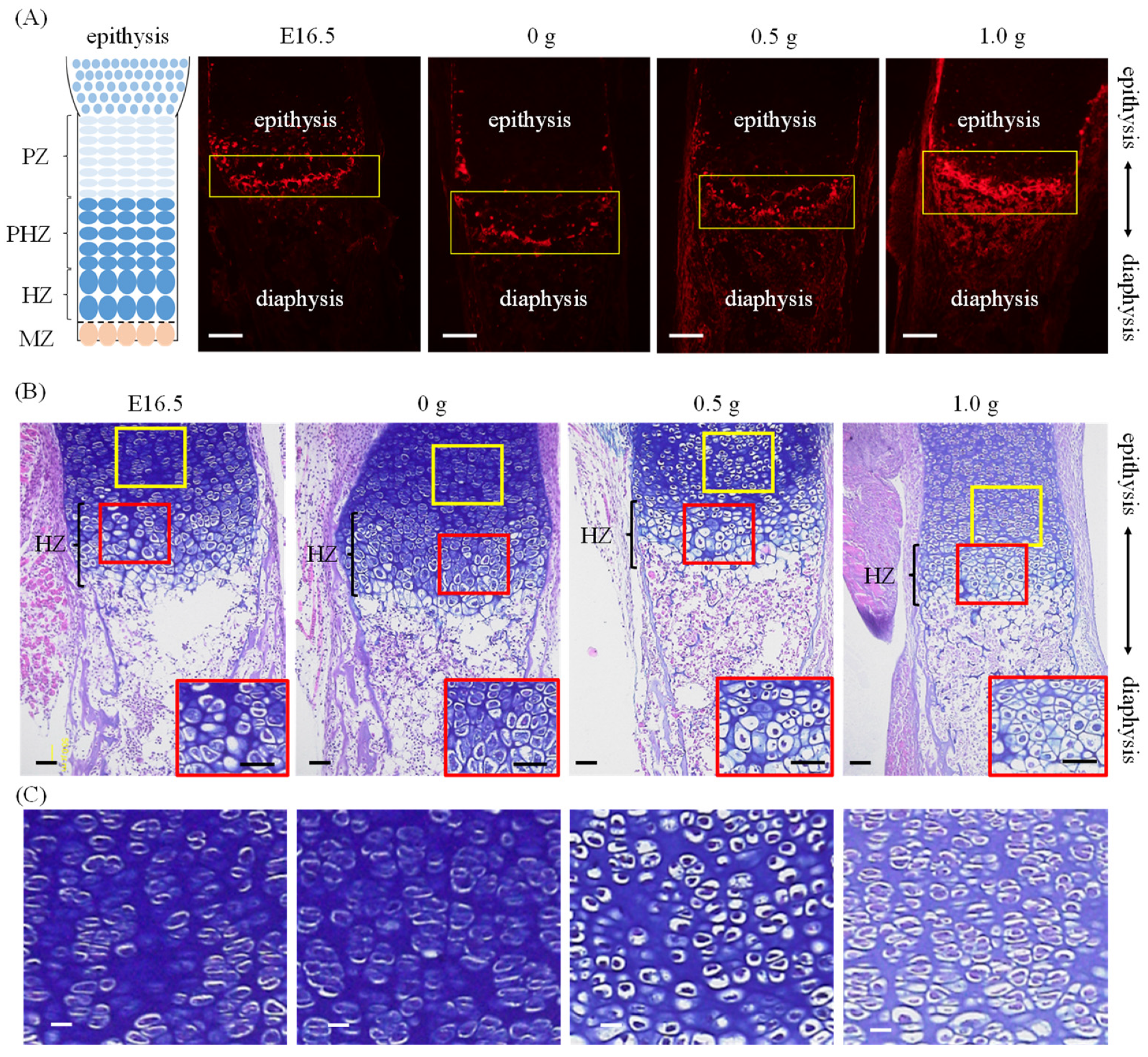
2.1. Establishment of an Ex Vivo Bone Culture Model
2.2. Preferential Apatite Orientation along Femur Long Axis with/without Mechanical Loading
3. Discussion
4. Materials and Methods
4.1. An Ex Vivo Bone Culture Model
4.2. Bone Morphology Analysis
4.3. Analysis of Apatite Orientation
4.4. Histological Analysis
4.5. Immunohistochemistry
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cabinet Office. Annual Report on the Aging Society. 2020. Available online: https://www8.cao.go.jp/kourei/english/annualreport/index-wh.html (accessed on 29 October 2021).
- Rho, J.Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Meakin, L.B.; Price, J.S.; Lanyon, L.E. The contribution of experimental in vivo models to understanding the mechanisms of adaptation to mechanical loading in bone. Front. Endocrinol. 2014, 5, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sample, S.J.; Collins, R.J.; Wilson, A.P.; Racette, M.A.; Behan, M.; Markel, M.D.; Kalscheur, V.L.; Hao, Z.; Muir, P. Systemic effects of ulna loading in male rats during functional adaptation. J. Bone Miner. Res. 2010, 25, 2016–2028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosley, J.R.; March, B.M.; Lynch, J.; Lanyon, L.E. Strain magnitude related changes in whole bone architecture in growing rats. Bone 1997, 20, 191–198. [Google Scholar] [CrossRef]
- Sugiyama, T.; Meakin, L.B.; Browne, W.J.; Galea, G.L.; Price, J.S.; Lanyon, L.E. Bones’ adaptive response to mechanical loading is essentially linear between the low strains associated with disuse and the high strains associated with the lamellar/woven bone transition. J. Bone Miner. Res. 2012, 27, 1784–1793. [Google Scholar] [CrossRef] [Green Version]
- Nakano, T.; Kaibara, K.; Tabata, Y.; Nagata, N.; Enomoto, S.; Marukawa, E.; Umakoshi, Y. Preferential orientation of biological apatite crystallites in typical calcified tissues analyzed by micro-beam X-ray diffractometer system. Bone 2002, 31, 479–487. [Google Scholar] [CrossRef]
- Ishimoto, T.; Nakano, T.; Umakoshi, Y.; Yamamoto, M.; Tabata, Y. Degree of biological apatite c-axis orientation rather than bone mineral density controls mechanical function in bone regenerated using recombinant bone morphogenetic protein-2. J. Bone Miner. Res. 2013, 28, 1170–1179. [Google Scholar] [CrossRef]
- Katz, J.L. Anisotropy of Young’s modulus of bone. Nature 1980, 283, 106–107. [Google Scholar] [CrossRef]
- Li, S.; Demirci, E.; Silberschmid, V.V. Variability and anisotropy of mechanical behavior of cortical bone in tension and compression. J. Mech. Behav. Biomed. Mater. 2013, 21, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Shinno, T.; Ishimoto, T.; Saito, M.; Uemura, R.; Arino, M.; Marumo, K.; Nakano, T.; Hayashi, M. Comprehensive analyses of how tubule occlusion and advanced glycation end-products diminish strength of aged dentin. Sci. Rep. 2016, 6, 19849. [Google Scholar] [CrossRef] [Green Version]
- Nakano, T.; Kaibara, K.; Ishimoto, T.; Tabata, Y.; Umakoshi, Y. Biological apatite (BAp) crystallographic orientation and texture as a new index for assessing the microstructure and function of bone regenerated by tissue engineering. Bone 2012, 51, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Manjubala, I.; Schell, H.; Epari, D.R.; Roschger, P.; Duda, G.N.; Franzl, P. Size and habit of mineral particles in bone and mineralized callus during bone healing in sheep. J. Bone Miner. Res. 2010, 25, 2029–2038. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, T.; Nakano, T.; Yamamoto, M.; Tabata, Y. Biomechanical evaluation of regenerated long bone by nanoindentation. J. Mater. Sci. Mater. Med. 2011, 22, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, T.; Yamada, K.; Takahashi, H.; Takahata, M.; Ito, M.; Hanawa, T.; Nakano, T. Trabecular health of vertebrae based on anisotropy in trabecular architecture and collagen/apatite micro-arrangement after implantation of intervertebral fusion cages in the sheep spine. Bone 2018, 108, 25–33. [Google Scholar] [CrossRef]
- Noyama, Y.; Miura, T.; Ishimoto, T.; Itaya, T.; Niinomi, M.; Nakano, T. Bone loss and reduced bone quality of the human femur after total hip arthroplasty under stress-shielding effects by titanium-based implant. Mater. Trans. 2012, 53, 565–570. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ishimoto, T.; Nakano, T. Unloading-induced degradation of the anisotropic arrangement of collagen/apatite in rat femurs. Calcif. Tissue Int. 2017, 100, 87–94. [Google Scholar] [CrossRef]
- Edwards, W.B.; Miller, R.H.; Derrick, T.R. Femoral strain during walking predicted with muscle forces from static and dynamic optimization. J. Biomech. 2016, 49, 1206–1213. [Google Scholar] [CrossRef]
- Seo, J.W.; Kang, D.W.; Kim, J.Y.; Yang, S.T.; Kim, D.H.; Choi, J.S.; Tack, G.R. Finite element analysis of the femur during stance phase of gait based on musculoskeletal model simulation. Bio-Med. Mater. Eng. 2014, 24, 2485–2493. [Google Scholar] [CrossRef] [Green Version]
- Matsugaki, A.; Fujiwara, N.; Nakano, T. Continuous cyclic stretch induces osteoblast alignment and formation of anisotropic collagen fiber matrix. Acta Biomater. 2013, 9, 7227–7235. [Google Scholar] [CrossRef]
- White, A.; Wallis, G. Endochondral ossification: A delicate balance between growth and mineralization. Curr. Biol. 2001, 11, 589–591. [Google Scholar] [CrossRef] [Green Version]
- Kronenberg, H.M. Developmental regulation of the growth plate. Nature 2003, 423, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Kanczler, J.M.; Smith, E.L.; Roberts, C.A.; Oreffo, R.O.C. A novel approach for studying the temporal modulation of embryonic skeletal development using organotypic bone cultures and microcomputed tomography. Tissue Eng. Part C Methods 2012, 18, 747–760. [Google Scholar] [CrossRef] [Green Version]
- Gerstenfeld, L.C.; Shapiro, F.D. Expression of bone-specific gens by hypertrophic chondrocytes: Implication of the complex functions of the hypertrophic chondrocyte during endochondral bone development. J. Cell. Biochem. 1996, 62, 1–9. [Google Scholar] [CrossRef]
- McKee, M.D.; Glimcher, M.J.; Nanci, A. High-resolution immunolocalization of osteopontin and osteocalcin in bone and cartilage during endochondral ossification in the chick tibia. Anat. Rec. 1992, 234, 479–492. [Google Scholar] [CrossRef]
- Sommer, B.; Bickel, M.; Hofstetter, W.; Wetterwald, A. Expression of matrix proteins during the development of mineralized tissues. Bone 1996, 19, 371–380. [Google Scholar] [CrossRef]
- Stokes, I.A.; Mente, P.L.; Iatridis, J.C.; Farnum, C.E.; Aronsson, D.D. Enlargement of growth plate chondrocytes modulated by sustained mechanical loading. J. Bone Jt. Surg. 2002, 84, 1842–1848. [Google Scholar] [CrossRef]
- Robling, A.G.; Duijvelaar, K.M.; Geevers, J.V.; Ohashi, N.; Turner, C.H. Modulation of appositional and longitudinal bone growth in the rat ulna by applied static and dynamic force. Bone 2001, 29, 105–113. [Google Scholar] [CrossRef]
- Lee, D.; Erickson, A.; Dudley, A.T.; Ryu, S. Mechanical stimulation of growth plate chondrocytes: Previous approaches and future directions. Exp. Mech. 2019, 59, 1261–1274. [Google Scholar] [CrossRef]
- Klein-Nulend, J.; Veldhuijzen, J.P.; Burger, E.H. Increased calcification of growth plate cartilage as a result of compressive force in vitro. Arthritis Rheumatol. 1986, 29, 1002–1009. [Google Scholar] [CrossRef]
- Stern, T.; Aviram, R.; Rot, C.; Galili, T.; Sharir, A.; Achrai, N.K.; Keller, Y.; Shahar, R.; Zelzer, E. Isometric scaling in developing long bones is achieved by an optimal epiphyseal growth balance. PLoS Biol. 2015, 13, e1002212. [Google Scholar] [CrossRef] [Green Version]
- Shiu, Y.T.; Weiss, J.A.; Hoying, J.B.; Iwamoto, M.N.; Joung, I.S.; Quam, C.T. The role of mechanical stresses in angiogenesis. Crit. Rev. Biomed. Eng. 2005, 33, 431–510. [Google Scholar] [CrossRef] [PubMed]
- Grosso, A.; Burger, M.G.; Lunger, A.; Schaefer, D.J.; Banfi, A.; Di Maggio, N. It takes two to tango: Coupling of angiogenesis and osteogenesis for bone regeneration. Front. Bioeng. Biotechnol. 2017, 5, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsugaki, A.; Harada, T.; Kimura, Y.; Sekita, A.; Nakano, T. Dynamic collision behavior between osteoblasts and tumor cells regulates the disordered arrangement of collagen fiber/apatite crystals in metastasized bone. Int. J. Mol. Sci. 2018, 19, 3474. [Google Scholar] [CrossRef] [Green Version]
- Matsugaki, A.; Kimura, Y.; Watanabe, R.; Nakamura, F.; Takehana, R.; Nakano, T. Impaired alignment of bone matrix microstructure associated with disorganized osteoblast arrangement in malignant melanoma metastasis. Biomolecules 2021, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Ozasa, R.; Ishimoto, T.; Miyabe, S.; Hashimoto, J.; Hirao, M.; Yoshikawa, H.; Nakano, T. Osteoporosis changes collagen/apatite orientation and Young’s modulus in vertebral cortical bone of rat. Calcif. Tissue Int. 2019, 104, 449–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weibaum, S.; Cowin, S.C.; Zeng, Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stress. J. Biomech. 1994, 27, 339–360. [Google Scholar] [CrossRef]
- Klein-Nulend, J.; Bakker, A.D.; Bacabac, R.G.; Vatsa, A.; Weinbaum, S. Mechanosensation and transduction in osteocytes. Bone 2013, 54, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Vatsa, A.; Mizuno, D.; Smit, T.M.; Schmidt, C.F.; Mackintosh, F.C.; Klein-Nulend, J. Bio imaging of intracellular NO production in single bone cells after mechanical stimulation. J. Bone Miner. Res. 2006, 21, 1722–1728. [Google Scholar] [CrossRef]
- Vatsa, A.; Semeins, C.M.; Smit, T.H.; Klein-Nulend, J. Paxillin localization in osteocytes—Is it determined by the direction of loading? Biochem. Biophys. Res. Commun. 2008, 377, 1019–1024. [Google Scholar] [CrossRef]
- Vatsa, A.; Breuls, R.G.; Semeins, C.M.; Salmon, P.L.; Smit, T.H.; Klein-Nulend, J. Osteocytes morphology in fibula and calvaria—Is there a role for mechanosensing? Bone 2008, 43, 452–458. [Google Scholar] [CrossRef]
- Ishimoto, T.; Kawahara, K.; Matsugaki, A.; Kamioka, H.; Nakano, T. Quantitative evaluation of osteocyte morphology and bone anisotropic extracellular matrix in rat femur. Calcif. Tissue Int. 2021, 109, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Lanyon, L.E.; Rubin, C.T. Static vs dynamic loads as an influence on bone remodeling. J. Biomech. 1984, 17, 897–905. [Google Scholar] [CrossRef]
- Turner, C.H. Three rules for bone adaptation to mechanical stimuli. Bone 1998, 23, 399–407. [Google Scholar] [CrossRef]
- Matsuzaka, T.; Matsugaki, A.; Nakano, T. Control of osteoblast arrangement by osteocyte mechanoresponse through prostaglandin E2 signaling under oscillatory fluid flow stimuli. Biomaterials 2021, 279, 121203. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, R.; Matsugaki, A.; Ishimoto, T.; Ozasa, R.; Matsumoto, T.; Nakano, T. A Novel Ex Vivo Bone Culture Model for Regulation of Collagen/Apatite Preferential Orientation by Mechanical Loading. Int. J. Mol. Sci. 2022, 23, 7423. https://doi.org/10.3390/ijms23137423
Watanabe R, Matsugaki A, Ishimoto T, Ozasa R, Matsumoto T, Nakano T. A Novel Ex Vivo Bone Culture Model for Regulation of Collagen/Apatite Preferential Orientation by Mechanical Loading. International Journal of Molecular Sciences. 2022; 23(13):7423. https://doi.org/10.3390/ijms23137423
Chicago/Turabian StyleWatanabe, Ryota, Aira Matsugaki, Takuya Ishimoto, Ryosuke Ozasa, Takuya Matsumoto, and Takayoshi Nakano. 2022. "A Novel Ex Vivo Bone Culture Model for Regulation of Collagen/Apatite Preferential Orientation by Mechanical Loading" International Journal of Molecular Sciences 23, no. 13: 7423. https://doi.org/10.3390/ijms23137423
APA StyleWatanabe, R., Matsugaki, A., Ishimoto, T., Ozasa, R., Matsumoto, T., & Nakano, T. (2022). A Novel Ex Vivo Bone Culture Model for Regulation of Collagen/Apatite Preferential Orientation by Mechanical Loading. International Journal of Molecular Sciences, 23(13), 7423. https://doi.org/10.3390/ijms23137423






