Effects of Storage Temperature on Indica-Japonica Hybrid Rice Metabolites, Analyzed Using Liquid Chromatography and Mass Spectrometry
Abstract
1. Introduction
2. Result
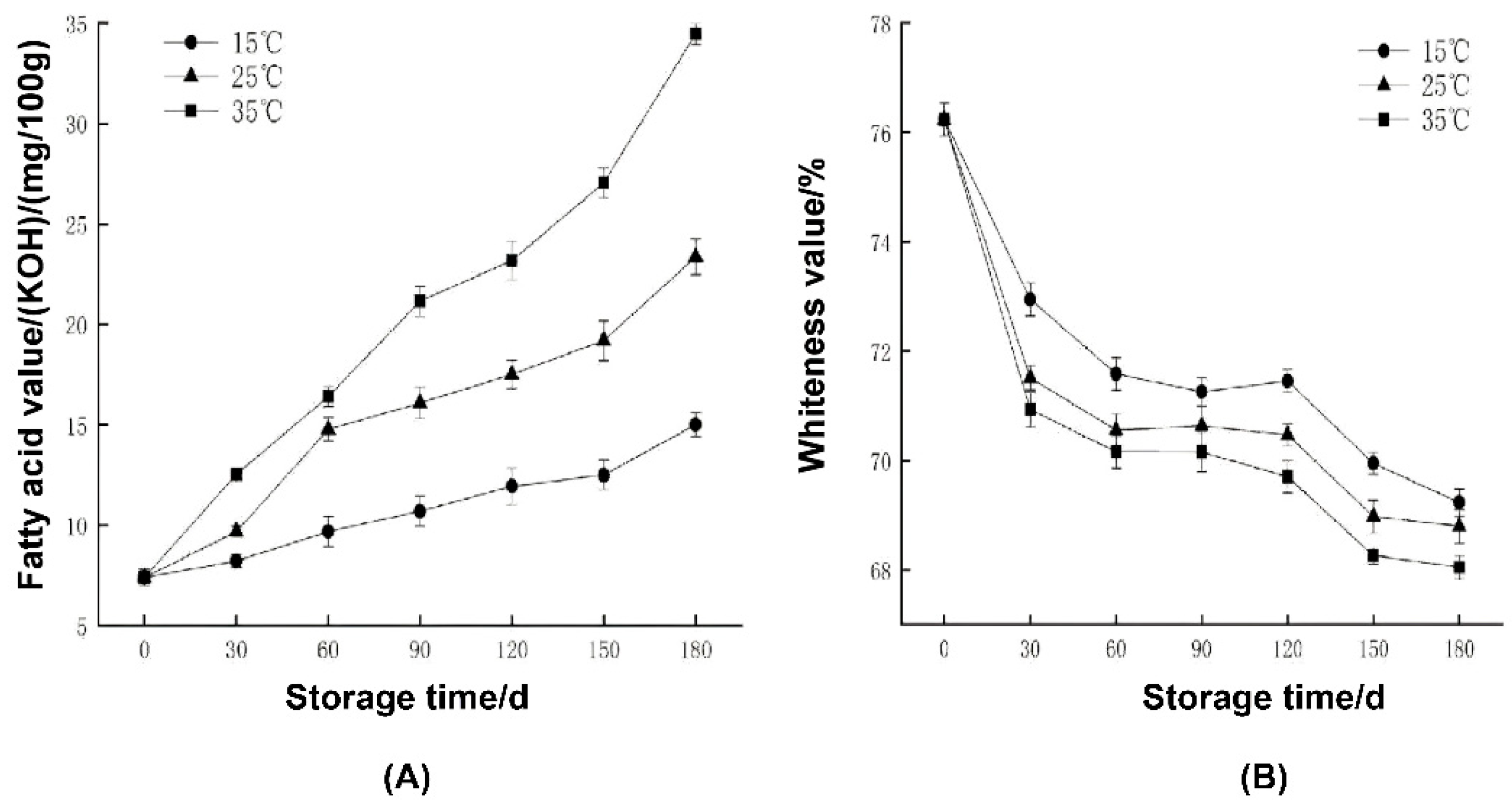
2.1. Measurement Results of Fatty Acid Value and Whiteness Value of Rice
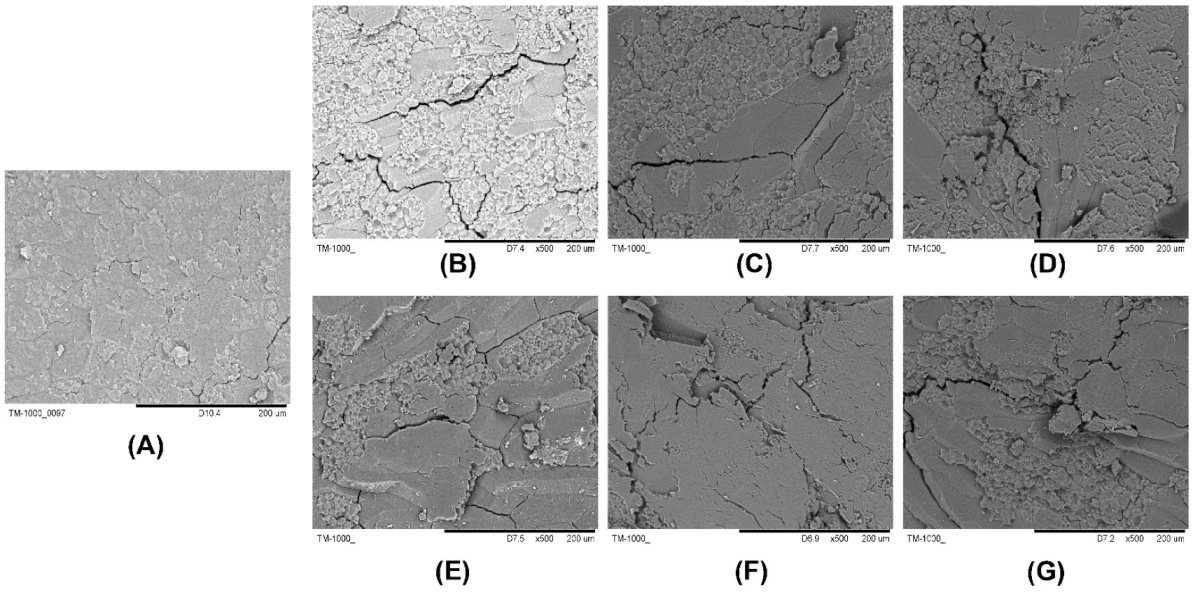
2.2. Electron Microscope Section Observation Results
2.3. Metabolite Characterization of Samples
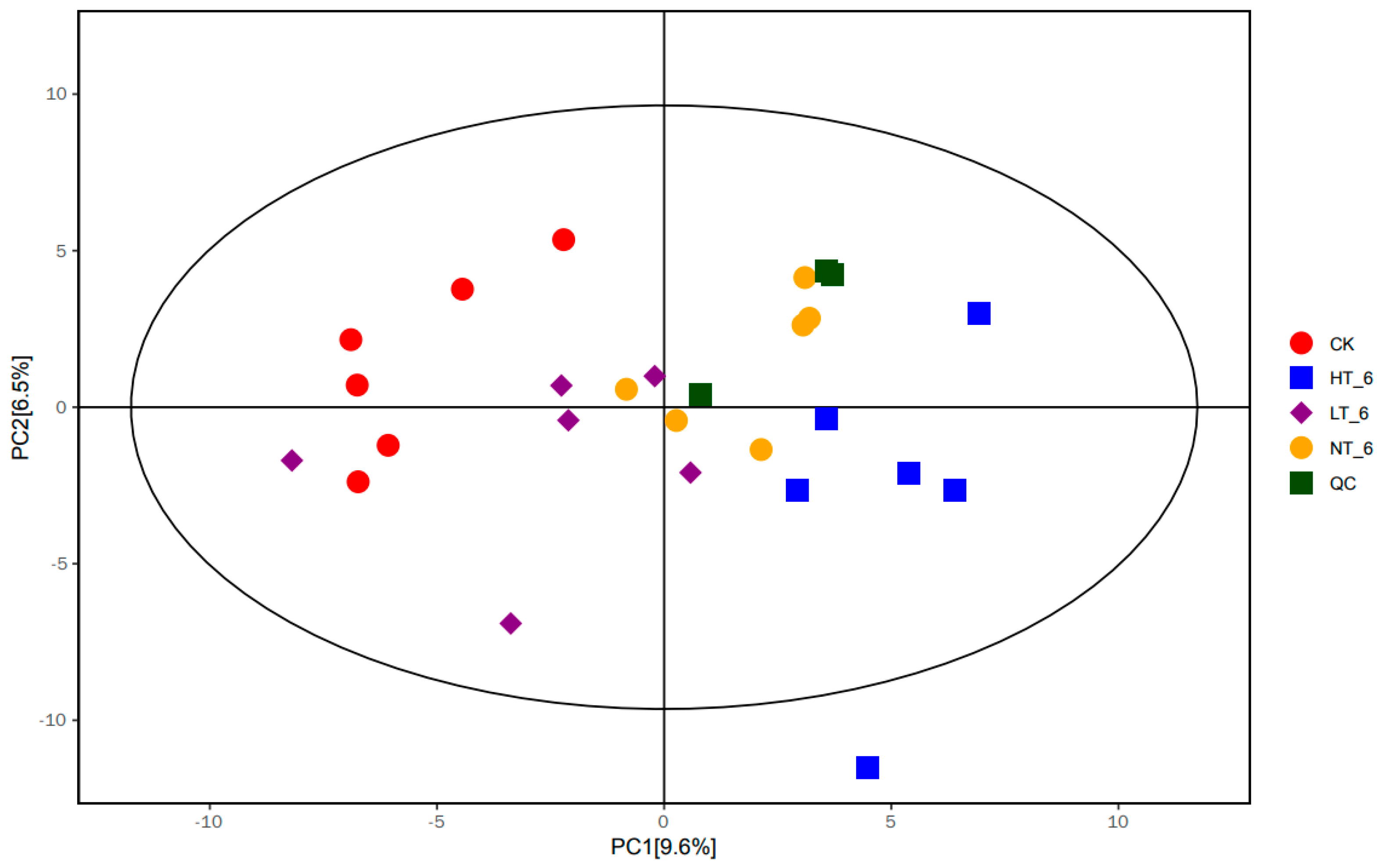
2.4. Principal Component Analysis
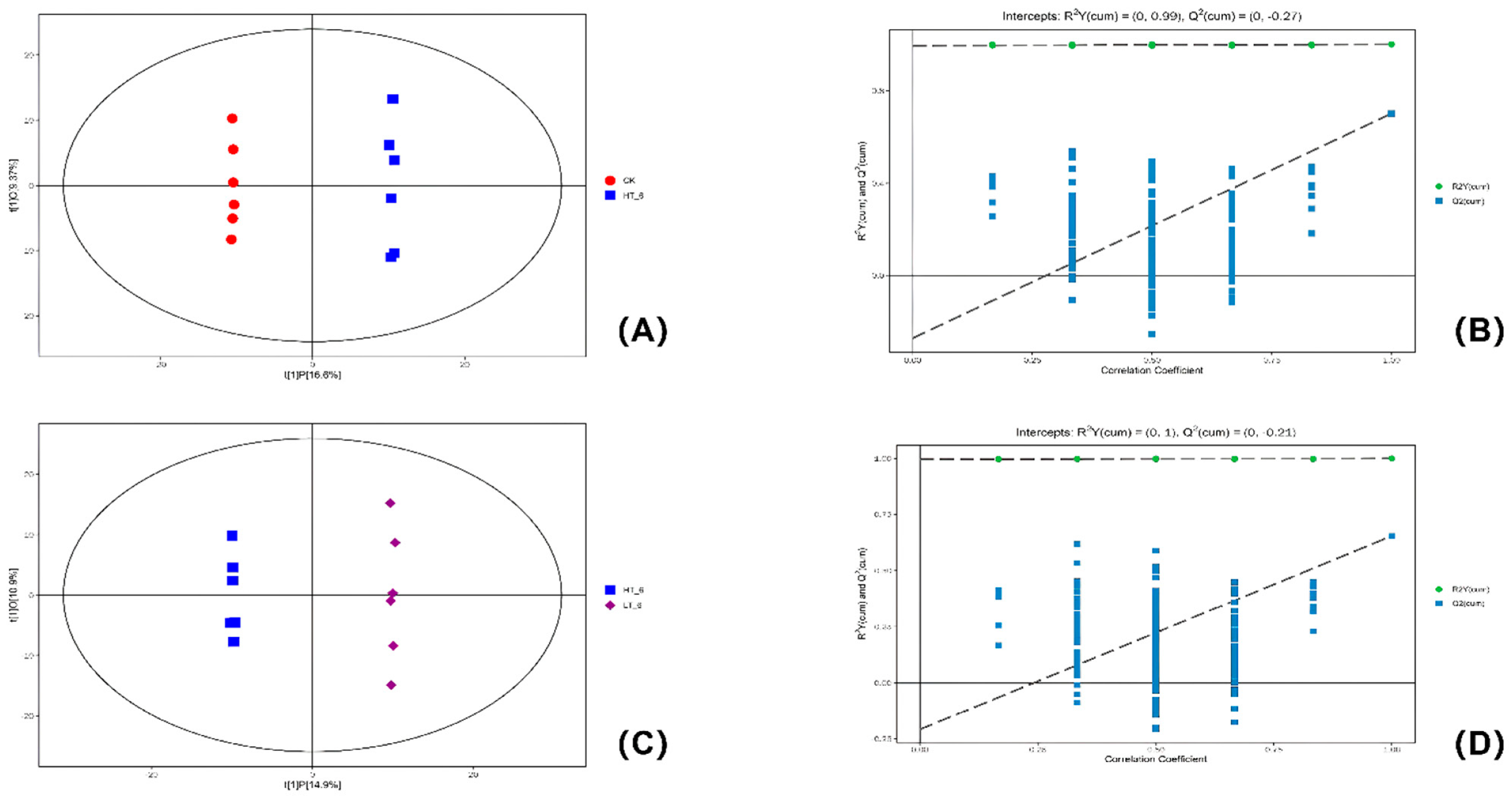
2.5. OPLS-DA Analysis
2.6. Screening and Analysis of Differential Metabolites with Significant Changes in Content
2.7. Effects of Storage on Metabolic Pathways of Rice
3. Discussion
4. Materials and Methods
4.1. Rice Materials and Sampling
4.2. Materials and Reagents
4.3. Metabolites Extraction
4.4. LC-MS Analysis
4.5. Data Analysis
4.6. Determination of Rice Quality
4.6.1. Fatty Acid Value (Petroleum Ether Extraction Method)
4.6.2. Whiteness Value
4.6.3. Observation of Appearance Features in Slices under Electron Microscope
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- USDA. Grain: World Markets and Trade. Available online: https://www.fas.usda.gov/data/grain-world-markets-and-trade (accessed on 10 June 2022).
- Fageria, N.K.; Baligar, V.C. Methodology for evaluation of lowland rice genotypes for nitrogen use efficiency. J. Plant Nutr. 2003, 26, 1315–1333. [Google Scholar] [CrossRef]
- Normile, D. Reinventing rice to feed the world. Science 2008, 321, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Tang, Q.; Zou, Y. Current status and challenges of rice production in China. Plant Prod. Sci. 2009, 12, 3–8. [Google Scholar] [CrossRef]
- Yin, M.; Liu, S.; Zheng, X.; Chu, G.; Xu, C.; Zhang, X.; Wang, D.; Chen, S. Solar radiation-use characteristics of indica/japonica hybrid rice (Oryza sativa L.) in the late season in southeast China. Crop J. 2021, 9, 427–439. [Google Scholar] [CrossRef]
- Zhou, Z.; Robards, K.; Helliwell, S.; Blanchard, C. Composition and functional properties of rice. Int. J. Food Sci. Technol. 2002, 37, 849–868. [Google Scholar] [CrossRef]
- Yoshida, H.; Tomiyama, Y.; Mizushina, Y. Lipid components, fatty acids and triacylglycerol molecular species of black and red rices. Food Chem. 2010, 123, 210–215. [Google Scholar] [CrossRef]
- Kim, N.H.; Kwak, J.; Baik, J.Y.; Yoon, M.R.; Lee, J.S.; Yoon, S.W.; Kim, I.H. Changes in lipid substances in rice during grain development. Phytochemistry 2015, 116, 170–179. [Google Scholar] [CrossRef]
- Heinemann, R.J.B.; Fagundes, P.D.L.; Pinto, E.A.; Penteado, M.D.V.C.; Lanfer-Marquez, U.M. Comparative study of nutrient composition of commercial brown, parboiled and milled rice from Brazil. J. Food Compos. Anal. 2005, 18, 287–296. [Google Scholar] [CrossRef]
- Chen, H.H.; Hung, C.L.; Lin, S.Y.; Liou, G.J. Effect of low-pressure plasma exposure on the storage characteristics of brown rice. Food Bioprocess Technol. 2015, 8, 471–477. [Google Scholar] [CrossRef]
- Saikrishna, A.; Dutta, S.; Subramanian, V.; Moses, J.A.; Anandharamakrishnan, C. Ageing of rice: A review. J. Cereal Sci. 2018, 81, 161–170. [Google Scholar] [CrossRef]
- Sun, Y.V.; Hu, Y.J. Integrative analysis of multi-omics data for discovery and functional studies of complex human diseases. Adv. Genet. 2016, 93, 147–190. [Google Scholar] [PubMed]
- Link, H.; Fuhrer, T.; Gerosa, L.; Zamboni, N.; Sauer, U. Real-time metabolome profiling of the metabolic switch between starvation and growth. Nat. Methods 2015, 12, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Feng, Y.; Zhang, S.; Fu, T.; Sheng, Y.; Zhang, Y.; Jiang, Y.; Yu, M.; Zhang, L.; Zhang, D. Effects of storage on brown rice (Oryza sativa L.) metabolites, analyzed using gas chromatography and mass spectrometry. Food Sci. Nutr. 2020, 8, 2882–2894. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Jiang, Z.; Wei, M. Research on quality changing of stored rice in high temperature. Agric. Sci. Technol. Equip. 2015, 8, 50–53. [Google Scholar]
- Fang, G.; Wang, X.; Zhao, Y. A measuring method of rice whitness. J. Harbin Univ. Sci. Technol. 2008, 4, 14–16+20. [Google Scholar]
- Saccenti, E.; Hoefsloot, H.C.; Smilde, A.K.; Westerhuis, J.A.; Hendriks, M.M. Reflections on univariate and multivariate analysis of metabolomics data. Metabolomics 2014, 10, 361–374. [Google Scholar] [CrossRef]
- Kolde, R.; Kolde, M.R. Package ‘pheatmap’. R Package Version 1.0.8.
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Gross, I.; Durner, J. In search of enzymes with a role in 3′,5′-cyclic guanosine monophosphate metabolism in plants. Front. Plant Sci. 2016, 7, 576. [Google Scholar] [CrossRef]
- Denninger, J.W.; Marletta, M.A. Guanylate cyclase and the NO/cGMP signaling pathway. BBA-Bioenergetics 1999, 1411, 334–350. [Google Scholar] [CrossRef]
- Römling, U.; Galperin, M.Y.; Gomelsky, M. Cyclic di-GMP: The first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. R. 2013, 77, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Klessig, D.F.; Durner, J.; Noad, R.; Navarre, D.A.; Wendehenne, D.; Kumar, D.; Zhou, J.; Shah, J.; Zhang, S.; Kachroo, P.; et al. Nitric oxide and salicylic acid signaling in plant defense. Proc. Natl. Acad. Sci. USA 2000, 97, 8849–8855. [Google Scholar] [CrossRef] [PubMed]
- Pasqualini, S.; Meier, S.; Gehring, C.; Madeo, L.; Fornaciari, M.; Romano, B.; Ederli, L. Ozone and nitric oxide induce cGMP-dependent and -independent transcription of defence genes in tobacco. New Phytol. 2009, 181, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ma, X.; Gong, M.; Shi, L.; Lincoln, T.; Wang, S. Glucose down-regulation of cGMP-dependent protein kinase I expression in vascular smooth muscle cells involves NAD(P)H oxidase-derived reactive oxygen species. Free Radic. Biol. Med. 2007, 42, 852–863. [Google Scholar] [CrossRef]
- Xu, J.; Morris, L.; Thapa, A.; Ma, H.; Michalakis, S.; Biel, M.; Baehr, W.; Peshenko, I.; Dizhoor, A.; Ding, X.Q. cGMP accumulation causes photoreceptor degeneration in CNG channel deficiency: Evidence of cGMP cytotoxicity independently of enhanced CNG channel function. J. Neurosci. 2013, 33, 14939–14948. [Google Scholar] [CrossRef]
- Qi, Z.; Verma, R.; Gehring, C.; Yamaguchi, Y.; Zhao, Y.; Ryan, C.A.; Berkowitz, G.A. Ca2+ signaling by plant Arabidopsis thaliana Pep peptides depends on AtPepR1, a receptor with guanylyl cyclase activity, and cGMP-activated Ca2+ channels. Proc. Natl. Acad. Sci. USA 2010, 107, 21193–21198. [Google Scholar] [CrossRef]
- Pagnussat, G.C.; Lanteri, M.L.; Lamattina, L. Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol. 2003, 132, 1241–1248. [Google Scholar] [CrossRef]
- Xuan, J.; Xiao, W.J. Visible-light photoredox catalysis. Angew. Chem. Int. Ed. 2012, 51, 6828–6838. [Google Scholar] [CrossRef]
- Neill, S.; Barros, R.; Bright, J.; Desikan, R.; Hancock, J.; Harrison, J.; Morris, P.; Ribeiro, D.; Wilson, I. Nitric oxide, stomatal closure, and abiotic stress. J. Exp. Bot. 2008, 59, 165–176. [Google Scholar] [CrossRef]
- Joudoi, T.; Shichiri, Y.; Kamizono, N.; Akaike, T.; Sawa, T.; Yoshitake, J.; Yamada, N.; Iwai, S. Nitrated cyclic GMP modulates guard cell signaling in Arabidopsis. Plant Cell 2013, 25, 558–571. [Google Scholar] [CrossRef]
- Isner, J.C.; Nühse, T.; Maathuis, F.J. The cyclic nucleotide cGMP is involved in plant hormone signalling and alters phosphorylation of Arabidopsis thaliana root proteins. J. Exp. Bot. 2012, 63, 3199–3205. [Google Scholar] [CrossRef] [PubMed]
- Marondedze, C.; Wong, A.; Thomas, L.; Irving, H.; Gehring, C. Cyclic nucleotide monophosphates in plants and plant signaling. In Non-Canonical Cyclic Nucleotides. Handbook of Experimental Pharmacology; Seifert, R., Ed.; Springer: Cham, Switzerland, 2015; Volume 238, pp. 87–103. [Google Scholar]
- Suita, K.; Kiryu, T.; Sawada, M.; Mitsui, M.; Nakagawa, M.; Kanamaru, K.; Yamagata, H. Cyclic GMP acts as a common regulator for the transcriptional activation of the flavonoid biosynthetic pathway in soybean. Planta 2009, 229, 403–413. [Google Scholar] [CrossRef][Green Version]
- Broquist, H.P. Lysine-pipecolic acid metabolic relationships in microbes and mammals. Annu. Rev. Nutr. 1991, 11, 435–448. [Google Scholar] [CrossRef]
- Zacharius, R.M.; Thompson, J.F.; Steward, F.C. The Detection, Isolation and Identification of L(—)Pipecolic Acid in the Non-protein Fraction of Beans (Phascolus vulgaris). J. Am. Chem. Soc. 1954, 76, 2908–2912. [Google Scholar] [CrossRef]
- Lugon-Moulin, N.; Martin, F.; Krauss, M.R.; Ramey, P.B.; Rossi, L. Cadmium concentration in tobacco (Nicotiana tabacum L.) from different countries and its relationship with other elements. Chemosphere 2006, 63, 1074–1086. [Google Scholar] [CrossRef] [PubMed]
- Pálfi, G.; Dézsi, L. Pipecolic acid as an indicator of abnormal protein metabolism in diseased plants. Plant Soil 1968, 29, 285–291. [Google Scholar] [CrossRef]
- Yatsu, L.; Boynton, D. Pipecolic acid in leaves of strawberry plant as influenced by treatments affecting growth. Science 1959, 130, 864–865. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Guo, H.; Xie, Y.; Zhao, L.; Gu, J.; Zhao, S.; Li, J.; Liu, L. RNAseq analysis reveals pathways and candidate genes associated with salinity tolerance in a spaceflight-induced wheat mutant. Sci. Rep. 2017, 7, 2731. [Google Scholar] [CrossRef]
- Obata, T.; Witt, S.; Lisec, J.; Palacios-Rojas, N.; Florez-Sarasa, I.; Yousfi, S.; Araus, J.L.; Cairns, J.E.; Fernie, A.R. Metabolite profiles of maize leaves in drought, heat, and combined stress field trials reveal the relationship between metabolism and grain yield. Plant Physiol. 2015, 169, 2665–2683. [Google Scholar] [CrossRef]
- Sawada, Y.; Akiyama, K.; Sakata, A.; Kuwahara, A.; Otsuki, H.; Sakurai, T.; Saito, K.; Hirai, M.Y. Widely targeted metabolomics based on large-scale MS/MS data for elucidating metabolite accumulation patterns in plants. Plant Cell Physiol. 2009, 50, 37–47. [Google Scholar] [CrossRef]
- Zha, H.; Cai, Y.; Yin, Y.; Wang, Z.; Li, K.; Zhu, Z.J. SWATHtoMRM: Development of high-coverage targeted metabolomics method using SWATH technology for biomarker discovery. Anal. Chem. 2018, 90, 4062–4070. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, S.; Jasbi, P.; Turner, C.; Hrovat, J.; Wei, Y.; Liu, J.; Gu, H. Database-assisted globally optimized targeted mass spectrometry (dGOT-MS): Broad and reliable metabolomics analysis with enhanced identification. Anal. Chem. 2019, 91, 13737–13745. [Google Scholar] [CrossRef]
- De Vos, R.C.; Moco, S.; Lommen, A.; Keurentjes, J.J.; Bino, R.J.; Hall, R.D. Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2007, 2, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Doppler, M.; Kluger, B.; Bueschl, C.; Schneider, C.; Krska, R.; Delcambre, S.; Hiller, K.; Lemmens, M.; Schuhmacher, R. Stable isotope-assisted evaluation of different extraction solvents for untargeted metabolomics of plants. Int. J. Mol. Sci. 2016, 17, 1017. [Google Scholar] [CrossRef]
- Cai, Y.; Weng, K.; Guo, Y.; Peng, J.; Zhu, Z.J. An integrated targeted metabolomic platform for high-throughput metabolite profiling and automated data processing. Metabolomics 2015, 11, 1575–1586. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Want, E.J.; Wilson, I.D.; Gika, H.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Holmes, E.; Nicholson, J.K. Global metabolic profiling procedures for urine using UPLC–MS. Nat. Protoc. 2010, 5, 1005–1018. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- Kuhl, C.; Tautenhahn, R.; Bottcher, C.; Larson, T.R.; Neumann, S. CAMERA: An integrated strategy for compound spectra extraction and annotation of liquid chromatography/mass spectrometry data sets. Anal. Chem. 2012, 84, 283–289. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Tong, X.; Peng, Y.; Ma, P.; Zhang, M.J.; Lu, H.M.; Chen, X.Q.; Liang, Y.Z. Multiscale peak detection in wavelet space. Analyst 2015, 140, 7955–7964. [Google Scholar] [CrossRef] [PubMed]
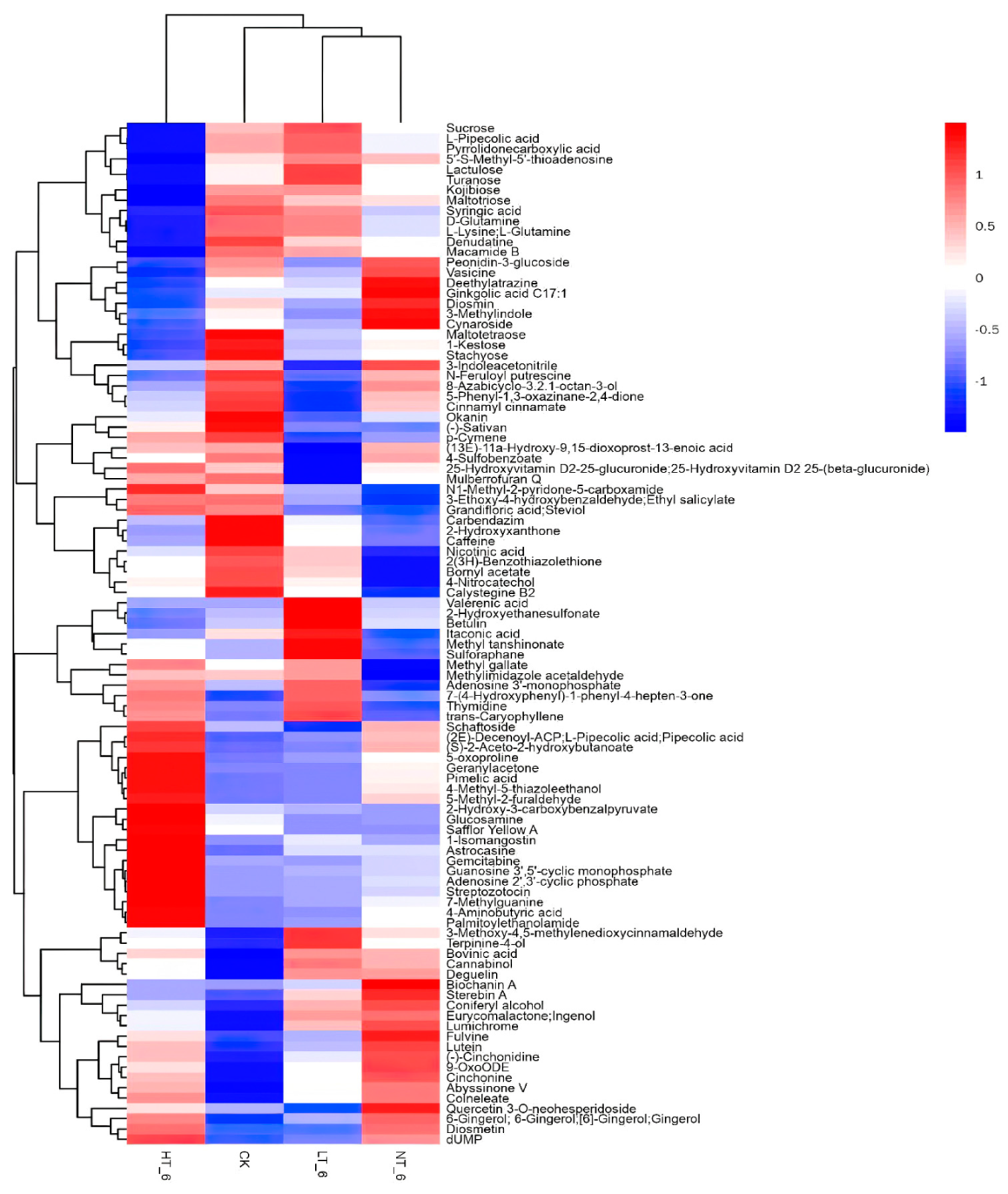
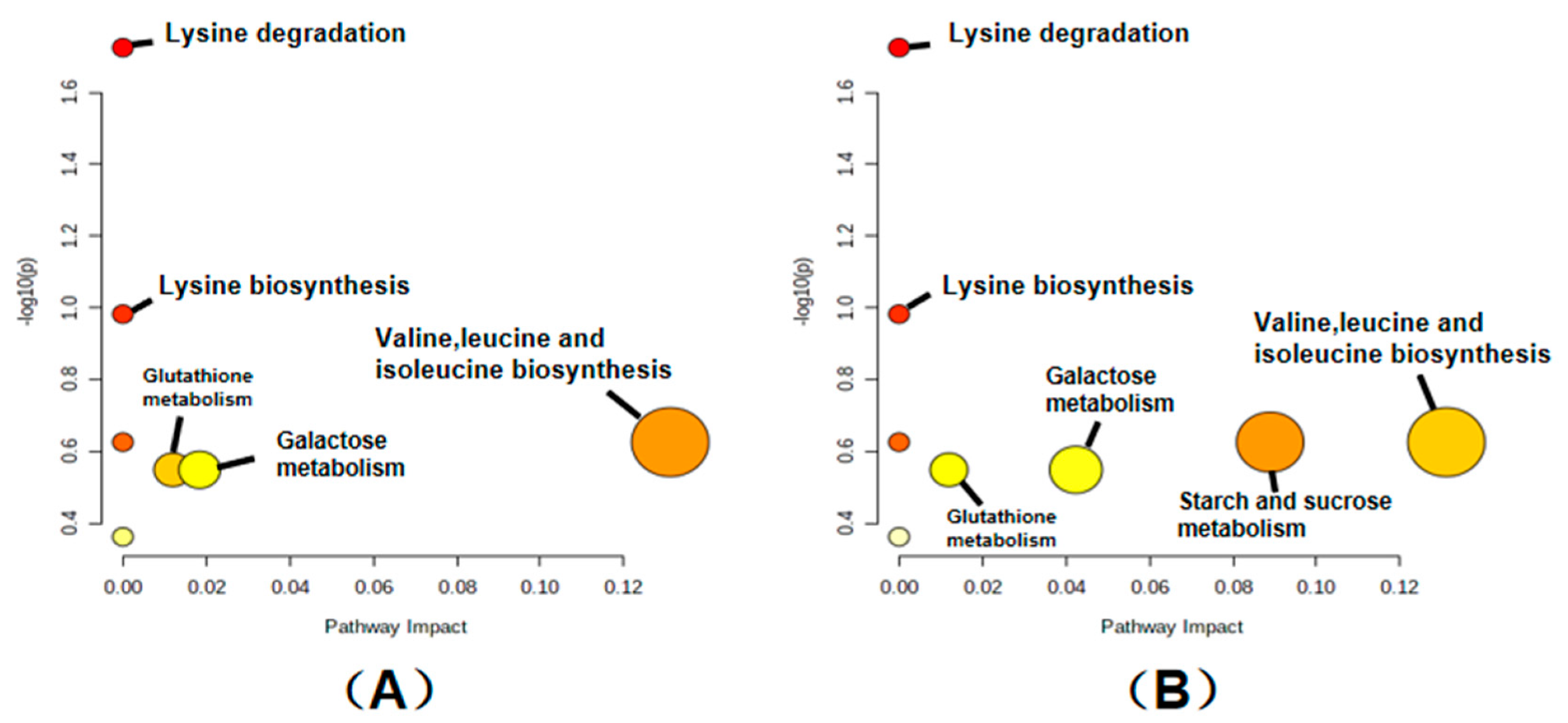
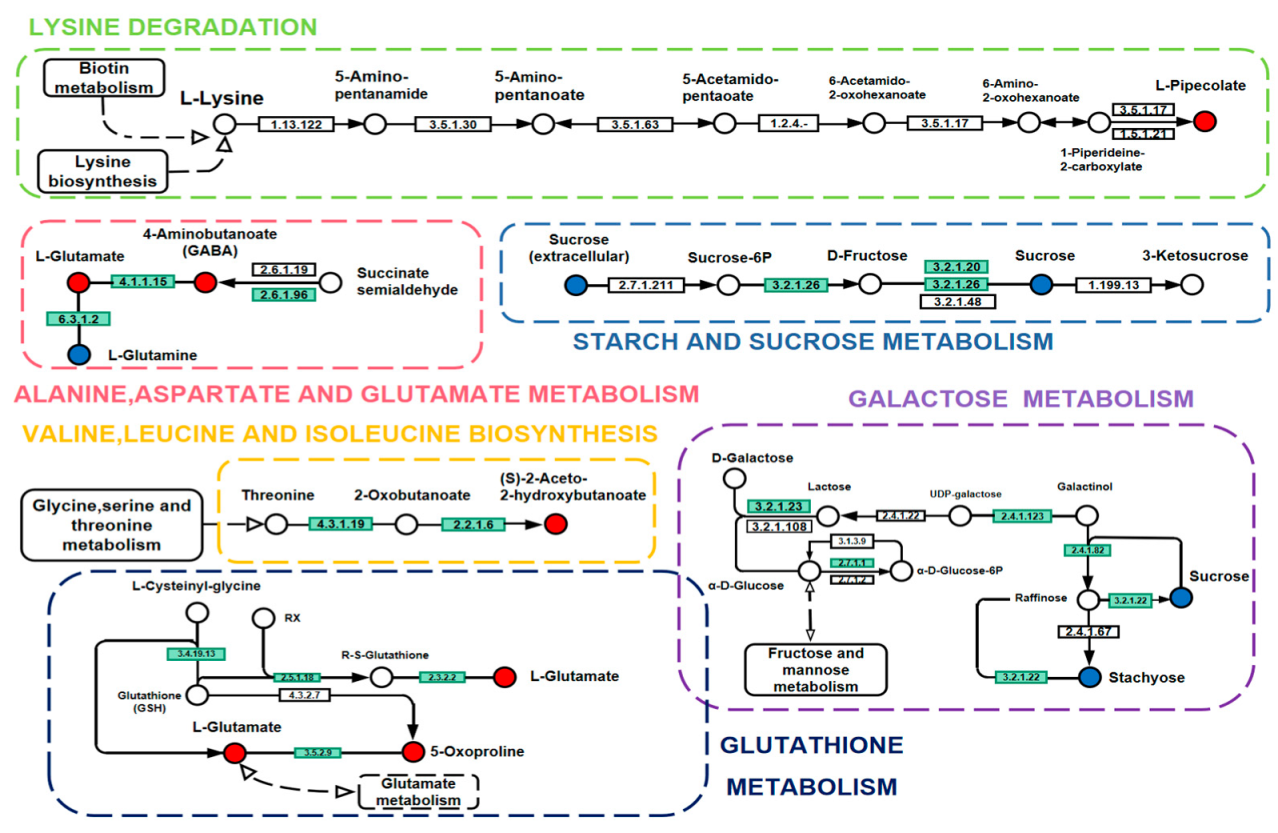
| No. | Compound Name | Rt(min) | KEGG_ID | EXACT_MASS | VIP | p-Value | Fold Change | LOG_ Foldchange |
|---|---|---|---|---|---|---|---|---|
| 1 | Adenosine 2′,3′−cyclic phosphate | 1.86 | C02353 | 329.0525 | 2.192 | 0.0001337 | 30.64 | 4.937 |
| 2 | (S)−2−Aceto−2−hydroxybutanoate | 0.66 | C06006 | 146.0579 | 2.258 | 0.00002167 | 3.301 | 1.723 |
| 3 | Pimelic acid | 0.55 | C02656 | 160.0736 | 2.131 | 0.00008361 | 3.421 | 1.774 |
| 4 | Pipecolic acid | 1.47 | C00408 | 129.0790 | 2.303 | 0.0002440 | 3.809 | 1.930 |
| 5 | 9−OxoODE | 11.98 | C14766 | 294.2195 | 1.504 | 0.0005610 | 3.886 | 1.958 |
| 6 | Palmitoylethanolamide | 12.61 | C16512 | 299.2824 | 2.259 | 0.0008559 | 4.063 | 2.023 |
| 7 | 4−Methyl−5−thiazoleethanol | 2.66 | C04294 | 143.0405 | 2.419 | 0.000003395 | 5.152 | 2.365 |
| 8 | 5−Methyl−2−furaldehyde | 0.76 | C11115 | 110.0368 | 2.329 | 0.00000001875 | 5.440 | 2.444 |
| 9 | 5−Oxoproline | 0.56 | C01879 | 129.0426 | 2.238 | 0.0002517 | 6.411 | 2.681 |
| 10 | 7−Methylguanine | 1.56 | C02242 | 165.0651 | 2.425 | 0.000007288 | 10.393 | 3.378 |
| 11 | Streptozotocin | 0.72 | C07313 | 265.0910 | 2.025 | 0.0002545 | 16.726 | 4.064 |
| 12 | Guanosine 3′,5′−cyclic monophosphate | 2.26 | C00942 | 345.0474 | 2.135 | 0.00002303 | 18.115 | 4.179 |
| 13 | 4−Aminobutyric acid | 0.66 | C00334 | 103.0633 | 2.062 | 0.000000003593 | 21.814 | 4.447 |
| 14 | d−Glutamine | 0.66 | C00819 | 146.0691 | 2.373 | 0.0005950 | 0.2028 | −2.302 |
| 15 | l−Glutamine | 0.64 | C00047; C00064 | 146.1055 | 2.319 | 0.0005209 | 0.2270 | −2.139 |
| 16 | Denudatine | 1.59 | C08680 | 343.2511 | 2.327 | 0.000001120 | 0.3601 | −1.474 |
| 17 | Maltotetraose | 0.64 | C02052 | 666.2219 | 1.865 | 0.0026925 | 0.3738 | −1.420 |
| 18 | Stachyose | 0.73 | C01613 | 666.2219 | 1.865 | 0.0026925 | 0.3738 | −1.420 |
| 19 | Kojibiose | 0.72 | C19632 | 342.1162 | 2.316 | 0.0002408 | 0.4181 | −1.258 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Tian, Y.; Ling, J.; Gong, X.; Sun, J.; Tong, L. Effects of Storage Temperature on Indica-Japonica Hybrid Rice Metabolites, Analyzed Using Liquid Chromatography and Mass Spectrometry. Int. J. Mol. Sci. 2022, 23, 7421. https://doi.org/10.3390/ijms23137421
Zhu L, Tian Y, Ling J, Gong X, Sun J, Tong L. Effects of Storage Temperature on Indica-Japonica Hybrid Rice Metabolites, Analyzed Using Liquid Chromatography and Mass Spectrometry. International Journal of Molecular Sciences. 2022; 23(13):7421. https://doi.org/10.3390/ijms23137421
Chicago/Turabian StyleZhu, Lin, Yu Tian, Jiangang Ling, Xue Gong, Jing Sun, and Litao Tong. 2022. "Effects of Storage Temperature on Indica-Japonica Hybrid Rice Metabolites, Analyzed Using Liquid Chromatography and Mass Spectrometry" International Journal of Molecular Sciences 23, no. 13: 7421. https://doi.org/10.3390/ijms23137421
APA StyleZhu, L., Tian, Y., Ling, J., Gong, X., Sun, J., & Tong, L. (2022). Effects of Storage Temperature on Indica-Japonica Hybrid Rice Metabolites, Analyzed Using Liquid Chromatography and Mass Spectrometry. International Journal of Molecular Sciences, 23(13), 7421. https://doi.org/10.3390/ijms23137421








