Biomimetic Modification of Water-Borne Polymer Coating with Carnauba Wax for Controlled Release of Urea
Abstract
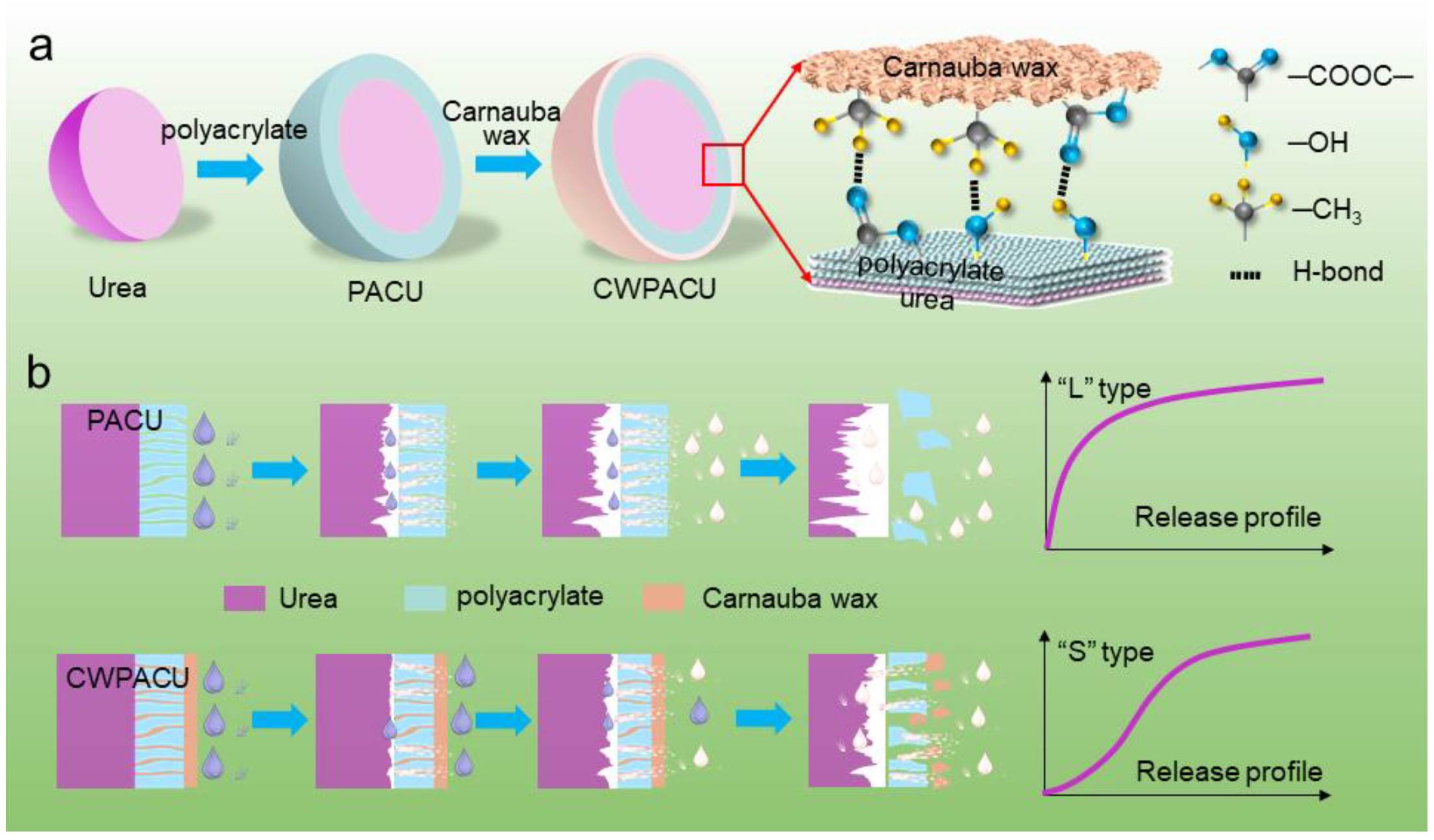
:1. Introduction
2. Results
2.1. Optimization of Carnauba Wax Modefication
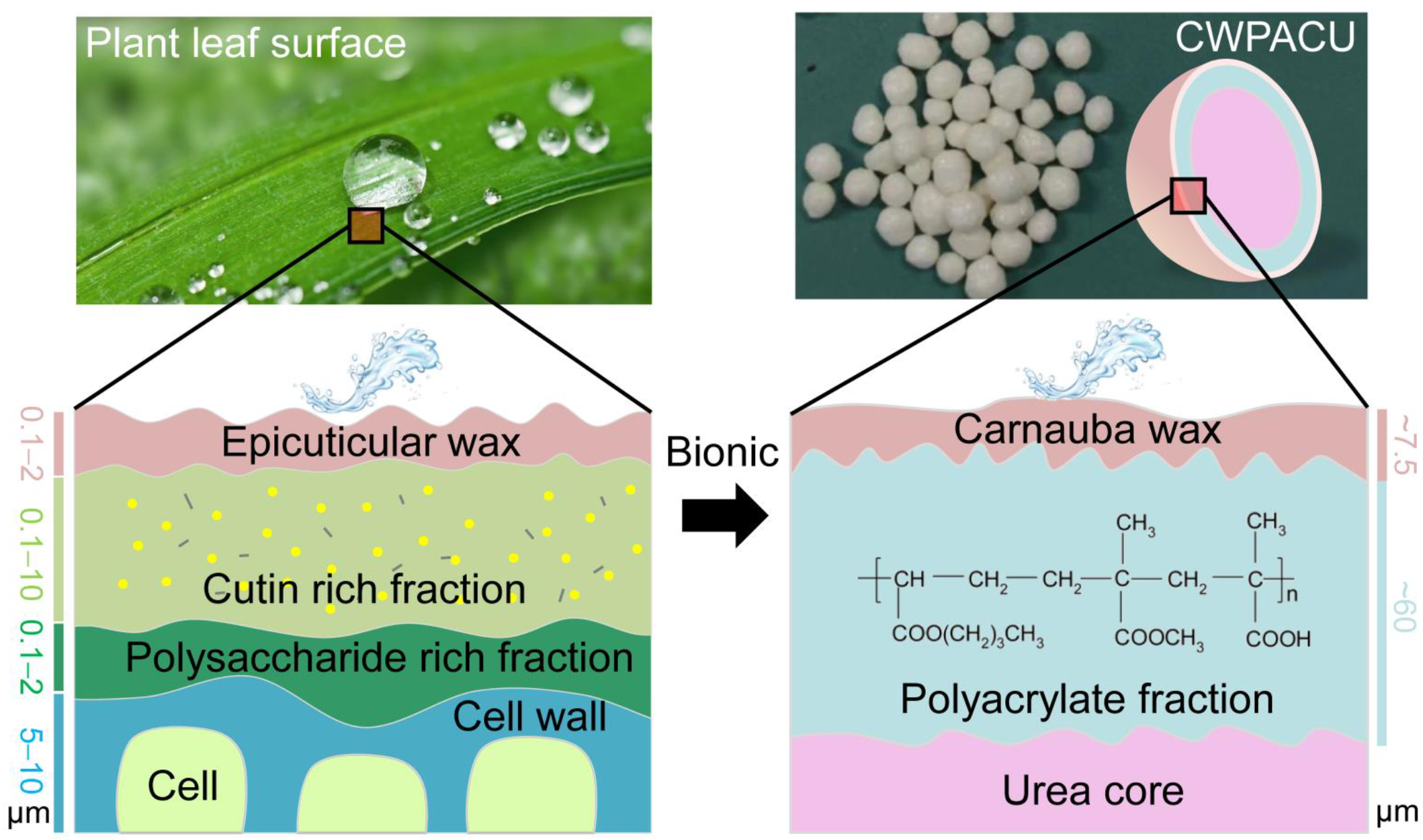
2.2. Morphological Structure
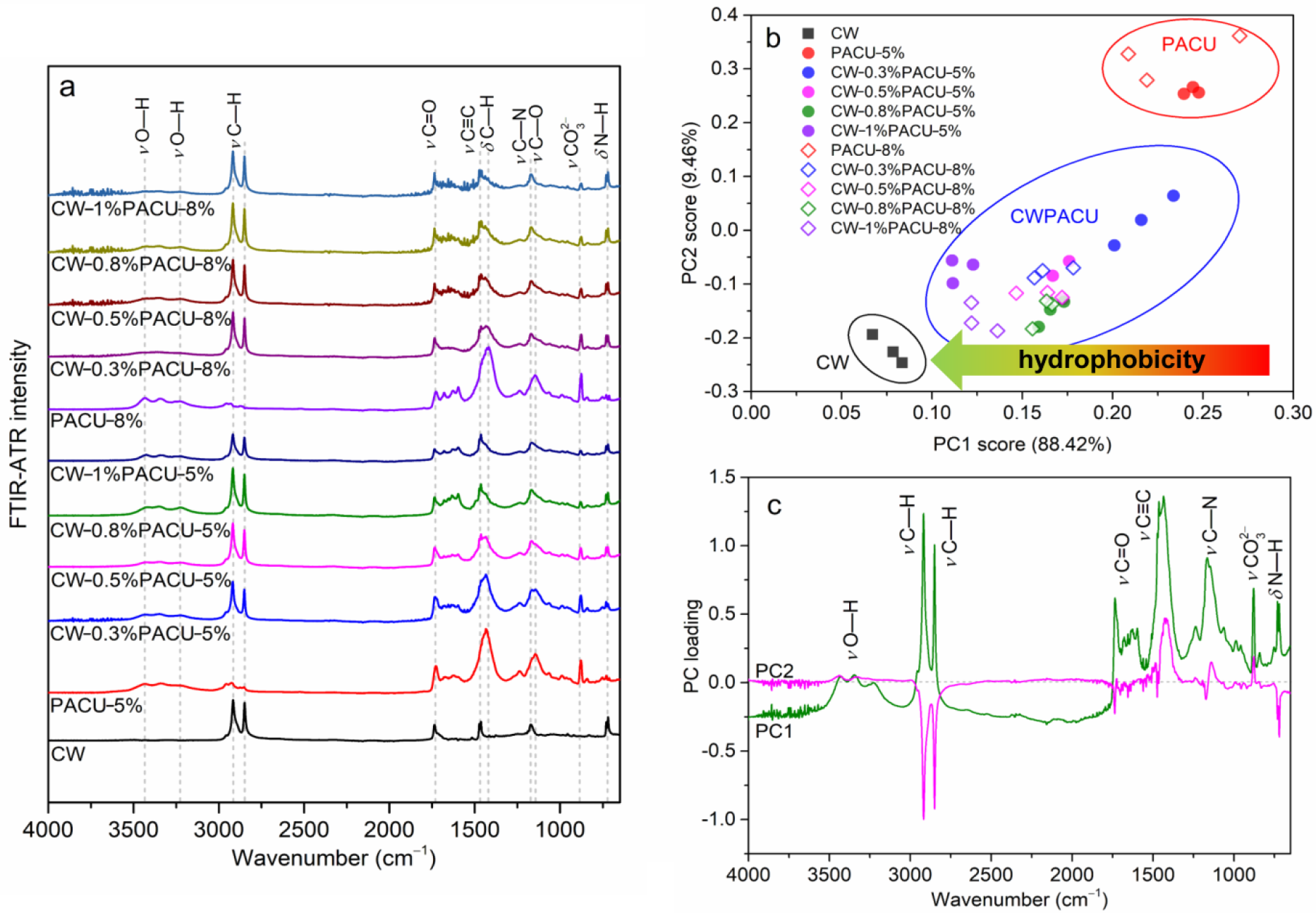
2.3. FTIR Characterization
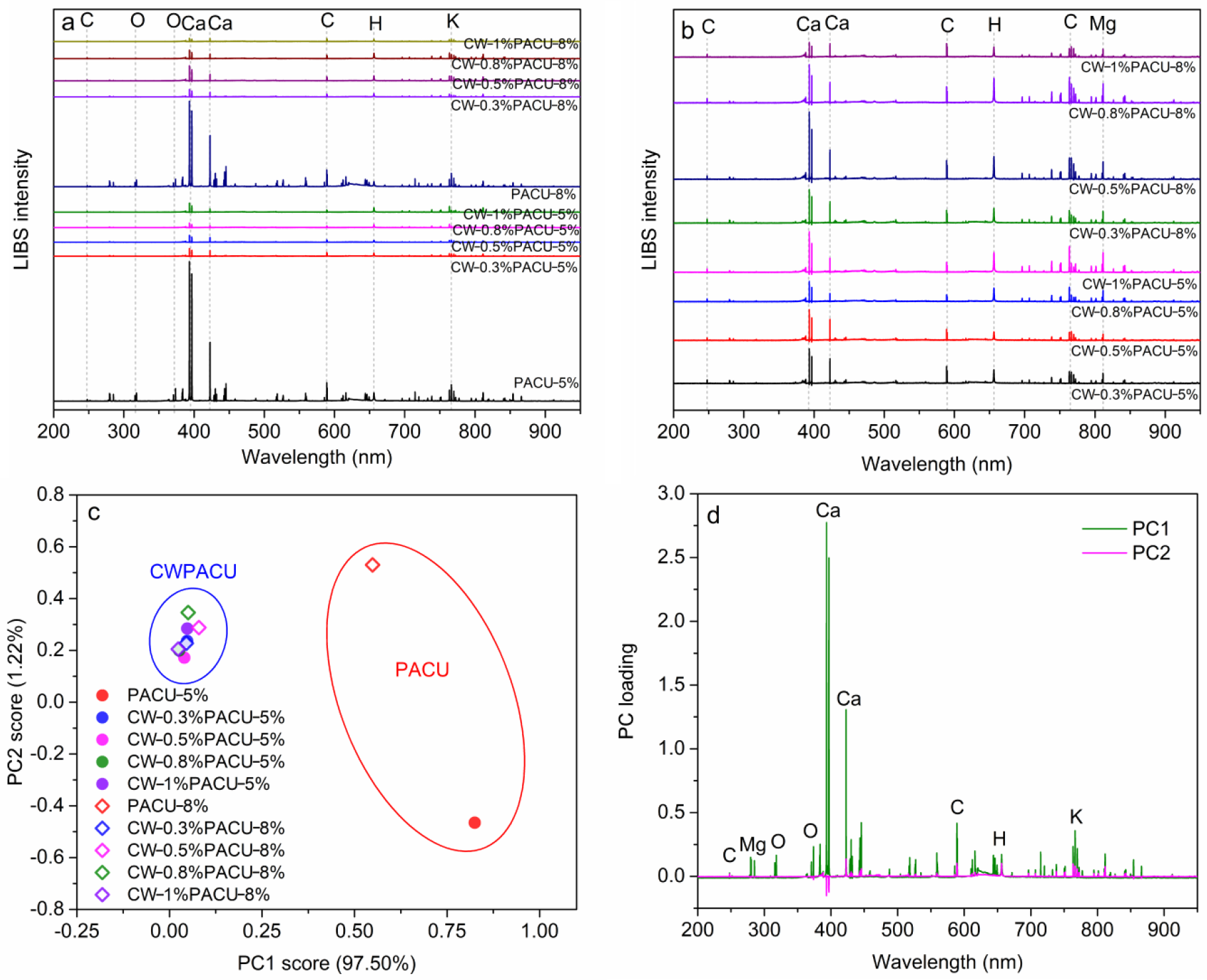
2.4. LIBS Characterization
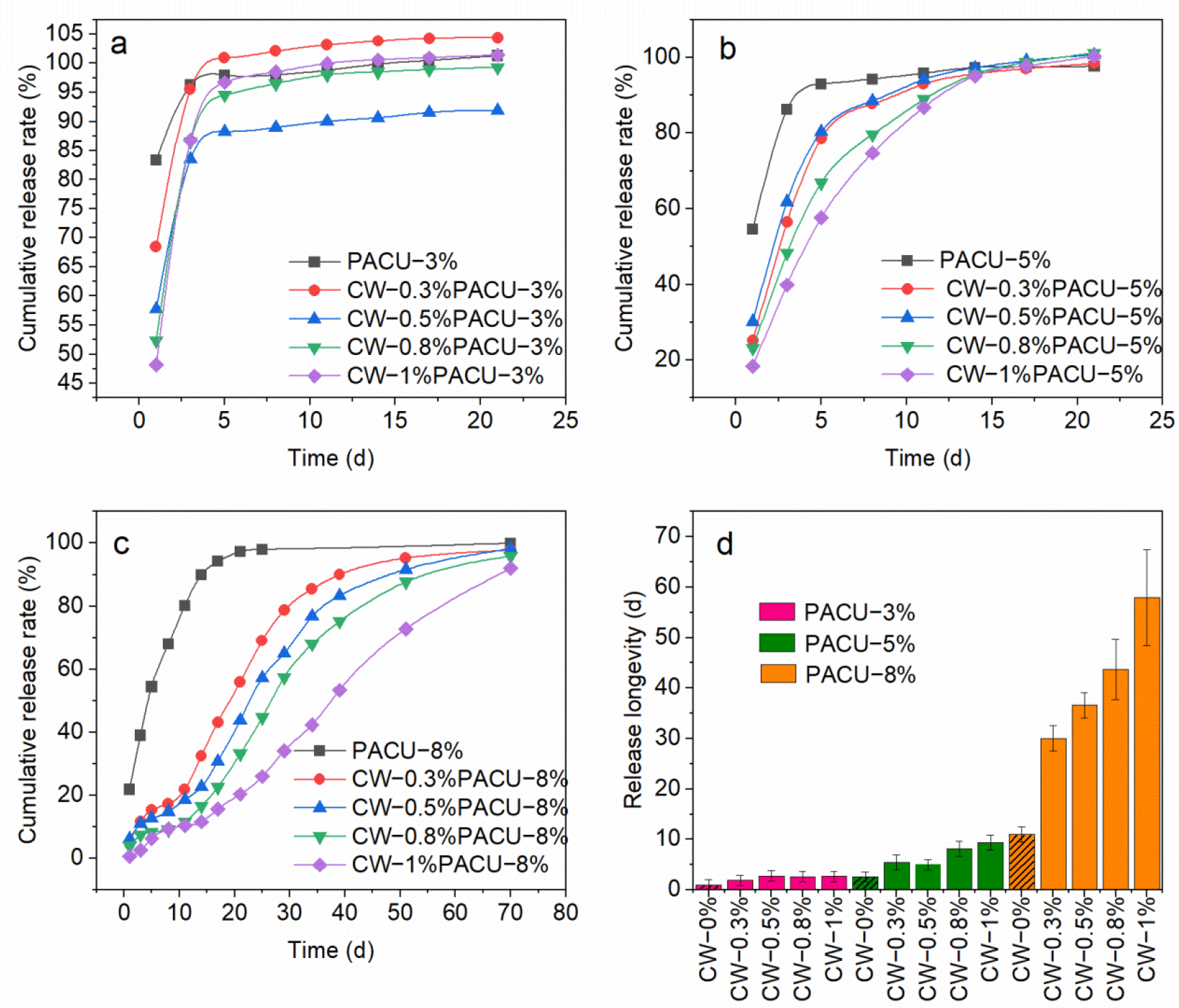
2.5. Release Performance
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Polyacrylate Emulsion
4.3. Preparation of Polyacrylate Coated Urea
4.4. Preparation of CW Modified PACU
4.5. Characterization of the Coating Films
4.6. Spectral Preprocessing and Data Analysis
4.7. Nutrient Release Profile
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ahmad, N. Controlled release fertilizer: A review on developments, applications and potential in agriculture. J. Control. Release 2021, 339, 321–334. [Google Scholar] [CrossRef]
- Shaviv, A. Advances in controlled-release fertilizers. Adv. Agron. 2001, 71, 1–49. [Google Scholar]
- Liu, L.; Shen, T.L.; Yang, Y.C.; Gao, B.; Li, Y.C.; Xie, J.Z.; Tang, Y.F.; Zhang, S.G.; Wang, Z.H.; Chen, J.Q. Bio-based large tablet controlled-release urea: Synthesis, characterization, and controlled-released mechanisms. J. Agric. Food Chem. 2018, 66, 11265–11272. [Google Scholar] [CrossRef] [Green Version]
- Chien, S.H.; Prochnow, L.I.; Cantarella, H. Recent developments of fertilizer production and use to improve nutrient efficiency and minimize environmental impacts. Adv. Agron. 2009, 102, 267–322. [Google Scholar]
- Azeem, B.; KuShaari, K.; Man, Z.B.; Basit, A.; Thanh, T.H. Review on materials & methods to produce controlled release coated urea fertilizer. J. Control. Release 2014, 181, 11–21. [Google Scholar]
- Calabi-Floody, M.; Medina, J.; Rumpel, C.; Condron, L.M.; Hernandez, M.; Dumont, M.; Mora, M.D. Smart fertilizers as a strategy for sustainable agriculture. Adv. Agron. 2018, 147, 119–157. [Google Scholar]
- An, D.; Liu, B.Y.; Yang, L.; Wang, T.J.; Kan, C.Y. Fabrication of graphene oxide/polymer latex composite film coated on KNO3 fertilizer to extend its release duration. Chem. Eng. J. 2017, 311, 318–325. [Google Scholar] [CrossRef]
- Hanafi, M.M.; Eltaib, S.M.; Ahmad, M.B. Physical and chemical characteristics of controlled release compound fertilizer. Eur. Polym. J. 2000, 36, 2081–2088. [Google Scholar] [CrossRef]
- Irfan, S.A.; Razali, R.; KuShaari, K.; Mansor, N.; Azeem, B.; Versypt, A.N.F. A review of mathematical modeling and simulation of controlled-release fertilizers. J. Control. Release 2018, 271, 45–54. [Google Scholar] [CrossRef]
- Beig, B.; Niazi, M.B.K.; Jahan, Z.; Hussain, A.; Zia, M.H.; Mehran, M.T. Coating materials for slow release of nitrogen from urea fertilizer: A review. J. Plant Nutr. 2020, 43, 1510–1533. [Google Scholar] [CrossRef]
- El Assimi, T.; Lakbita, O.; El Meziane, A.; Khouloud, M.; Dahchour, A.; Beniazza, R.; Boulif, R.; Raihane, M.; Lahcini, M. Sustainable coating material based on chitosan-clay composite and paraffin wax for slow-release DAP fertilizer. Int. J. Biol. Macromol. 2020, 161, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Saleh, K.; Steinmetz, D.; Hemati, A. Experimental study and modeling of fluidized bed coating and agglomeration. Powder Technol. 2003, 130, 116–123. [Google Scholar] [CrossRef]
- Shen, Y.; Zhou, J.; Du, C. Development of a polyacrylate/silica nanoparticle hybrid emulsion for delaying nutrient release in coated controlled-release urea. Coatings 2019, 9, 88. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.J.; Du, C.W.; Li, T.; Shen, Y.Z.; Zeng, Y.; Du, J.; Zhou, J.M. Biodegradation of a biochar-modified waterborne polyacrylate membrane coating for controlled-release fertilizer and its effects on soil bacterial community profiles. Environ. Sci. Pollut. Res. 2015, 22, 8672–8682. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Du, C.; Zhou, J. Aqueous polyacrylate/poly(silicone-co-acrylate) emulsion coated fertilizers for slow nutrient-release application. J. Appl. Polym. Sci. 2014, 131, 40369. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, C.; Zhou, J.M.; Du, C.W. Application of waterborne acrylic emulsions in coated controlled release fertilizer using reacted layer technology. Chin. J. Chem. Eng. 2015, 23, 309–314. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Y.W.; Kaneko, M.; Uchikoba, F. Profile characterization and temperature effect on the wettability of microstructured surfaces. J. Surf. Eng. Mater. Adv. Technol. 2018, 8, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Wang, J.N.; Wu, S.Z.; Chen, Q.D.; Zhao, S.A.; Zhang, H.; Sun, H.B.; Jiang, L. Three-level biomimetic rice-leaf surfaces with controllable anisotropic sliding. Adv. Funct. Mater. 2011, 21, 2927–2932. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.H.; Li, Y.S.; Li, H.J.; Zhang, L.J.; Zhai, J.; Song, Y.L.; Liu, B.Q.; Jiang, L.; Zhu, D.B. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.A.; Xi, J.M.; Zhu, Y.; Wang, N.; Xia, F.; Jiang, L. Petal effect: A superhydrophobic state with high adhesive force. Langmuir 2008, 24, 4114–4119. [Google Scholar] [CrossRef] [PubMed]
- Zorba, V.; Stratakis, E.; Barberoglou, M.; Spanakis, E.; Tzanetakis, P.; Anastasiadis, S.H.; Fotakis, C. Biomimetic artificial surfaces quantitatively reproduce the water repellency of a lotus leaf. Adv. Mater. 2008, 20, 4049–4054. [Google Scholar] [CrossRef]
- Koch, K.; Neinhuis, C.; Ensikat, H.J.; Barthlott, W. Self assembly of epicuticular waxes on living plant surfaces imaged by atomic force microscopy (AFM). J. Exp. Bot. 2004, 55, 711–718. [Google Scholar] [CrossRef] [Green Version]
- Yong, J.L.; Chen, F.; Yang, Q.; Huo, J.L.; Hou, X. Superoleophobic surfaces. Chem. Soc. Rev. 2017, 46, 4168–4217. [Google Scholar] [CrossRef] [Green Version]
- Blossey, R. Self-cleaning surfaces—Virtual realities. Nat. Mater. 2003, 2, 301–306. [Google Scholar] [CrossRef]
- Darmanin, T.; Guittard, F. Superhydrophobic and superoleophobic properties in nature. Mater. Today 2015, 18, 273–285. [Google Scholar] [CrossRef]
- Winkler-Moser, J.K.; Anderson, J.; Felker, F.C.; Hwang, H.S. Physical properties of beeswax, sunflower wax, and candelilla wax mixtures and oleogels. J. Am. Oil Chem. Soc. 2019, 96, 1125–1142. [Google Scholar] [CrossRef]
- De Campos, A.; Claro, P.C.; Luchesi, B.R.; Miranda, M.; Souza, F.V.D.; Ferreira, M.D.; Marconcini, J.M. Curaua cellulose sheets dip coated with micro and nano carnauba wax emulsions. Cellulose 2019, 26, 7983–7993. [Google Scholar] [CrossRef]
- Harron, A.F.; Powell, M.J.; Nunez, A.; Moreau, R.A. Analysis of sorghum wax and carnauba wax by reversed phase liquid chromatography mass spectrometry. Ind. Crops Prod. 2017, 98, 116–129. [Google Scholar] [CrossRef]
- Wang, W.; Ran, Y.Y.; Wang, I.J.M. Improved performance of thermally modified wood via impregnation with carnauba wax/organoclay emulsion. Constr. Build. Mater. 2020, 247, 74. [Google Scholar] [CrossRef]
- Singh, R.; Poddar, S.S.; Chivate, A. Sintering of wax for controlling release from pellets. Aaps Pharmscitech 2007, 8, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.L.; Ando, S.; Ishida, Y.; Ohtani, H.; Tsuge, S.; Nakayama, T. Quantitative and discriminative analysis of carnauba waxes by reactive pyrolysis-GC in the presence of organic alkali using a vertical microfurnace pyrolyzer. J. Anal. Appl. Pyrolysis 2001, 58, 525–537. [Google Scholar] [CrossRef]
- Bayer, I.S. Superhydrophobic Coatings from ecofriendly materials and processes: A review. Adv. Mater. Interfaces 2020, 7, 2000095. [Google Scholar] [CrossRef]
- Ahmad, N.N.R.; Fernando, W.J.N.; Uzir, M.H. Parametric evaluation using mechanistic model for release rate of phosphate ions from chitosan-coated phosphorus fertilizer pellets. Biosyst. Eng. 2015, 129, 78–86. [Google Scholar] [CrossRef]
- Zhang, S.G.; Yang, Y.C.; Gao, B.; Li, Y.C.; Liu, Z.G. Superhydrophobic controlled-release fertilizers coated with bio-based polymers with organosilicon and nano-silica modifications. J. Mater. Chem. A 2017, 5, 19943–19953. [Google Scholar] [CrossRef]
- Salem, K.S.; Naithani, V.; Jameel, H.; Lucia, L.; Pal, L. Lignocellulosic Fibers from Renewable Resources Using Green Chemistry for a Circular Economy. Glob. Chall. 2021, 5, 2000065. [Google Scholar] [CrossRef]
- Salem, K.S.; Starkey, H.R.; Pal, L.; Lucia, L.; Jameel, H. The Topochemistry of Cellulose Nanofibrils as a Function of Mechanical Generation Energy. ACS Sustain. Chem. Eng. 2020, 8, 1471–1478. [Google Scholar] [CrossRef]
- Du, C.; Zhou, J. Application of infrared photoacoustic spectroscopy in soil analysis. Appl. Spectrosc. Rev. 2011, 46, 405–422. [Google Scholar] [CrossRef]
- Li, J.L.; Tian, J.H.; Gao, Y.T.; Qin, R.R.; Pi, H.M.; Li, M.J.; Yang, P. All-natural superhydrophobic coating for packaging and blood-repelling materials. Chem. Eng. J. 2021, 410, 128347. [Google Scholar] [CrossRef]
- Hobza, P.; Havlas, Z. Blue-shifting hydrogen bonds. Chem. Rev. 2000, 100, 4253–4264. [Google Scholar] [CrossRef]
- Kramida, A. NIST Atomic Spectra Database (version 5.9), National Institute of Standards and Technology, Gaithersburg, MD, 2021. Available online: https://www.nist.gov/pml/atomic-spectra-database (accessed on 13 June 2022).
- Lawrence, J.F.; Iyengar, J.R.; Page, B.D.; Conacher, H.B.S. Characterization of commercial waxes by high-temperature gas-chromatography. J. Chromatogr. 1982, 236, 403–419. [Google Scholar] [CrossRef]
- Shaviv, A.; Mikkelsen, R.L. Controlled-release fertilizers to increase efficiency of nutrient use and minimize environmental degradation—A review. Fertil. Res. 1993, 35, 1–12. [Google Scholar] [CrossRef]
- Yang, Y.C.; Zhang, M.; Li, Y.C.; Fan, X.H.; Geng, Y.Q. Improving the quality of polymer-coated urea with recycled plastic, proper additives, and large tablets. J. Agric. Food Chem. 2012, 60, 11229–11237. [Google Scholar] [CrossRef] [PubMed]
- Vogg, G.; Fischer, S.; Leide, E.; Emmanuel, J.; Jetter, R.; Levy, A.A.; Riederer, M. Tomato fruit cuticular waxes and their effects on transpiration barrier properties: Functional characterization of a mutant deficient in a very-long-chain fatty acid b-ketoacyl-CoA synthase. J. Exp. Bot. 2004, 55, 1401–1410. [Google Scholar] [CrossRef] [Green Version]
- Lv, G.; Du, C.; Ma, F.; Shen, Y.; Zhou, J. Rapid and nondestructive detection of pesticide residues by depth-profiling Fourier transform infrared photoacoustic spectroscopy. ACS Omega 2018, 3, 3548–3553. [Google Scholar] [CrossRef] [Green Version]
- Lv, G.; Du, C.; Ma, F.; Shen, Y.; Zhou, J. In situ detection of rice leaf cuticle responses to nitrogen supplies by depth-profiling Fourier transform photoacoustic spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117759. [Google Scholar] [CrossRef]
- Shaviv, A.; Raban, S.; Zaidel, E. Modeling controlled nutrient release from a population of polymer coated fertilizers: Statistically based model for diffusion release. Environ. Sci. Technol. 2003, 37, 2257–2261. [Google Scholar] [CrossRef]
- Shen, Y.; Du, C.W.; Zhou, J.M.; Ma, F. Characterization of the release of urea from coated fertilizer by Fourier transform infrared spectroscopy with attenuated total reflectance. Anal. Lett. 2015, 48, 2380–2390. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, C.; Xu, X.; Ma, F.; Zhou, J.; Du, C. Biomimetic Modification of Water-Borne Polymer Coating with Carnauba Wax for Controlled Release of Urea. Int. J. Mol. Sci. 2022, 23, 7422. https://doi.org/10.3390/ijms23137422
Ge C, Xu X, Ma F, Zhou J, Du C. Biomimetic Modification of Water-Borne Polymer Coating with Carnauba Wax for Controlled Release of Urea. International Journal of Molecular Sciences. 2022; 23(13):7422. https://doi.org/10.3390/ijms23137422
Chicago/Turabian StyleGe, Cong, Xuebin Xu, Fei Ma, Jianmin Zhou, and Changwen Du. 2022. "Biomimetic Modification of Water-Borne Polymer Coating with Carnauba Wax for Controlled Release of Urea" International Journal of Molecular Sciences 23, no. 13: 7422. https://doi.org/10.3390/ijms23137422
APA StyleGe, C., Xu, X., Ma, F., Zhou, J., & Du, C. (2022). Biomimetic Modification of Water-Borne Polymer Coating with Carnauba Wax for Controlled Release of Urea. International Journal of Molecular Sciences, 23(13), 7422. https://doi.org/10.3390/ijms23137422






