Monodisperse Gold Nanoparticles: A Review on Synthesis and Their Application in Modern Medicine
Abstract
1. Introduction
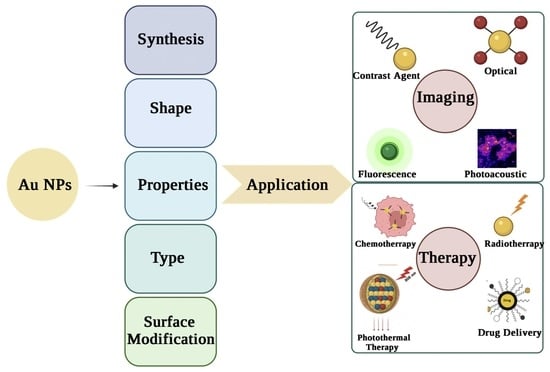
2. Monodisperse AuNPs
3. Synthesis of Monodisperse AuNPs
3.1. Turkevich Method
3.2. The Brust Method
3.3. Seed-Mediated Growth
3.4. Biological Synthesis
3.5. Sonochemical
3.6. Advantages and Limitations of the Methods
4. Type of AuNPs
5. Shapes of AuNPs
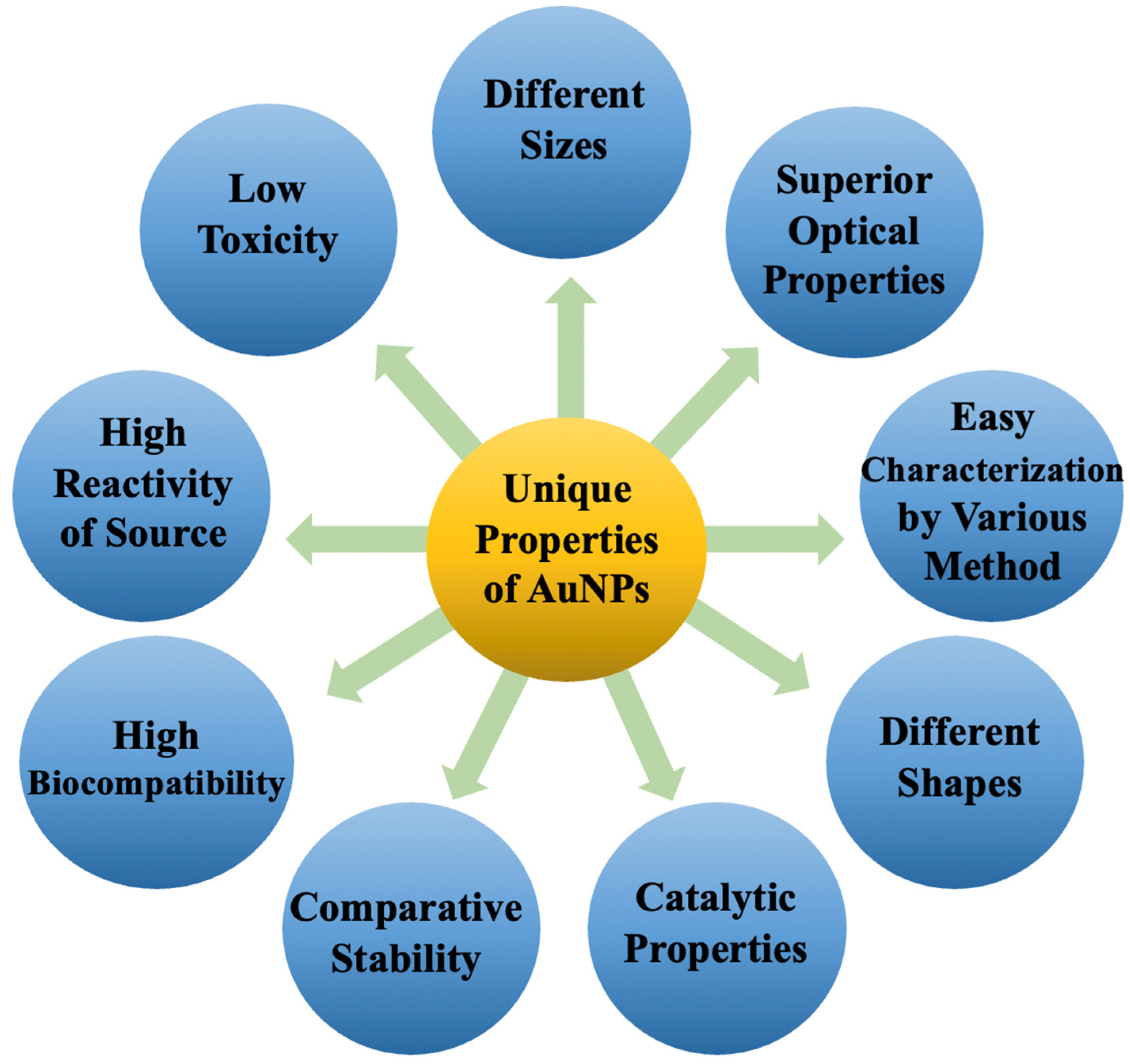
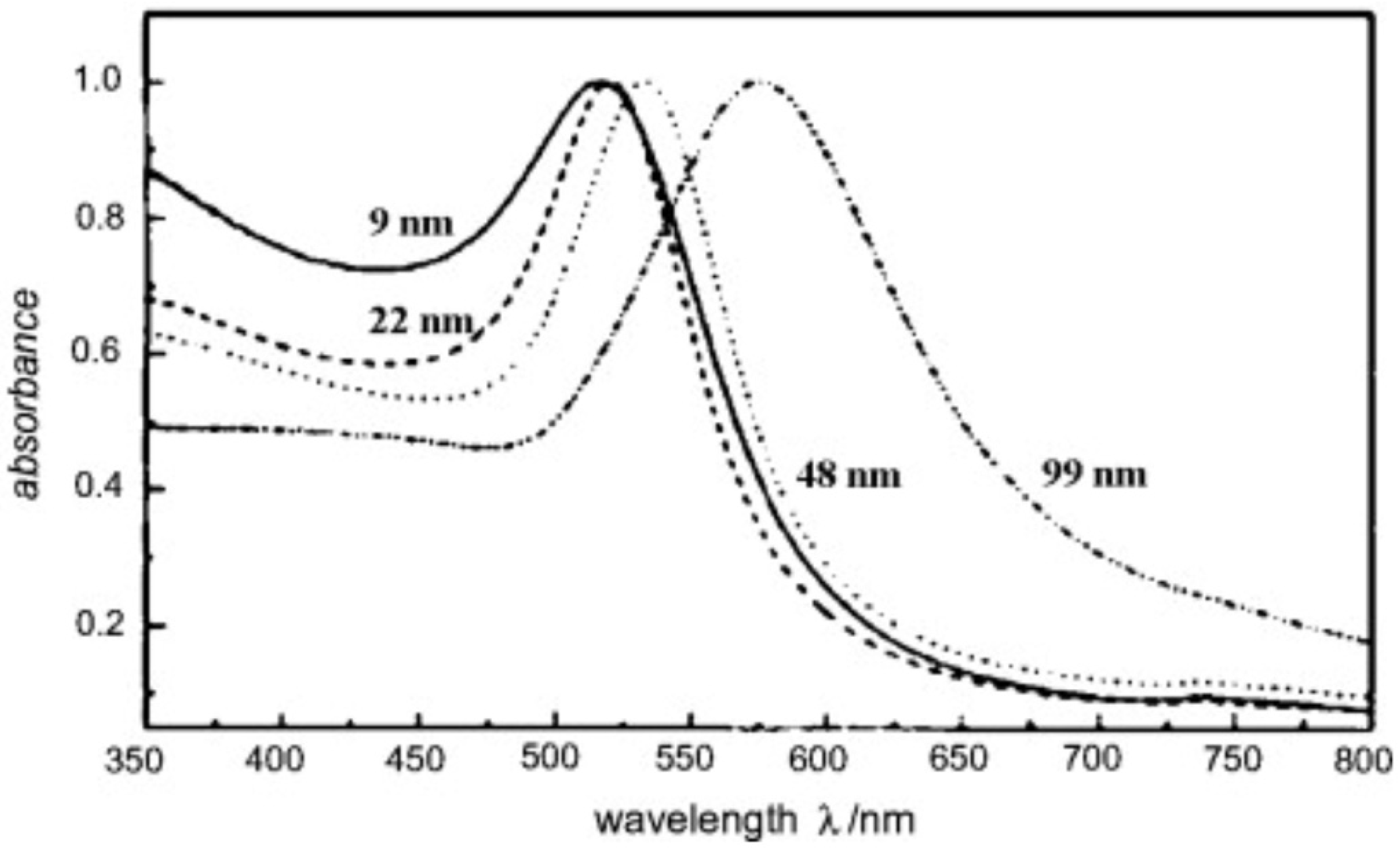
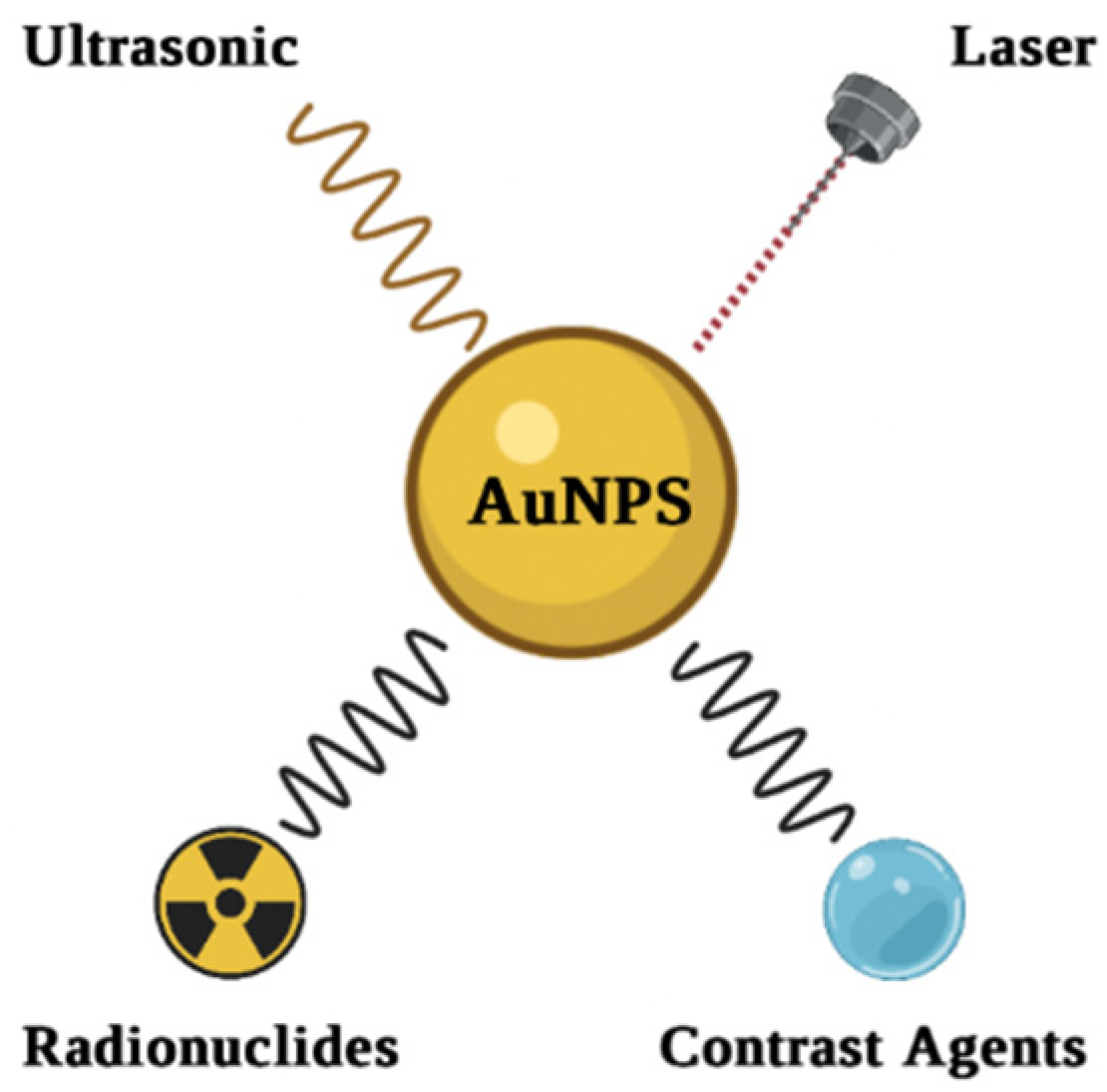
6. Properties of AuNPs
7. Surface Modification
8. Imaging Applications of AuNPs
8.1. Optical Imaging
8.2. Photoacoustic Imaging
8.3. Fluorescence Imaging
8.4. MRI
8.5. CT
8.6. PET
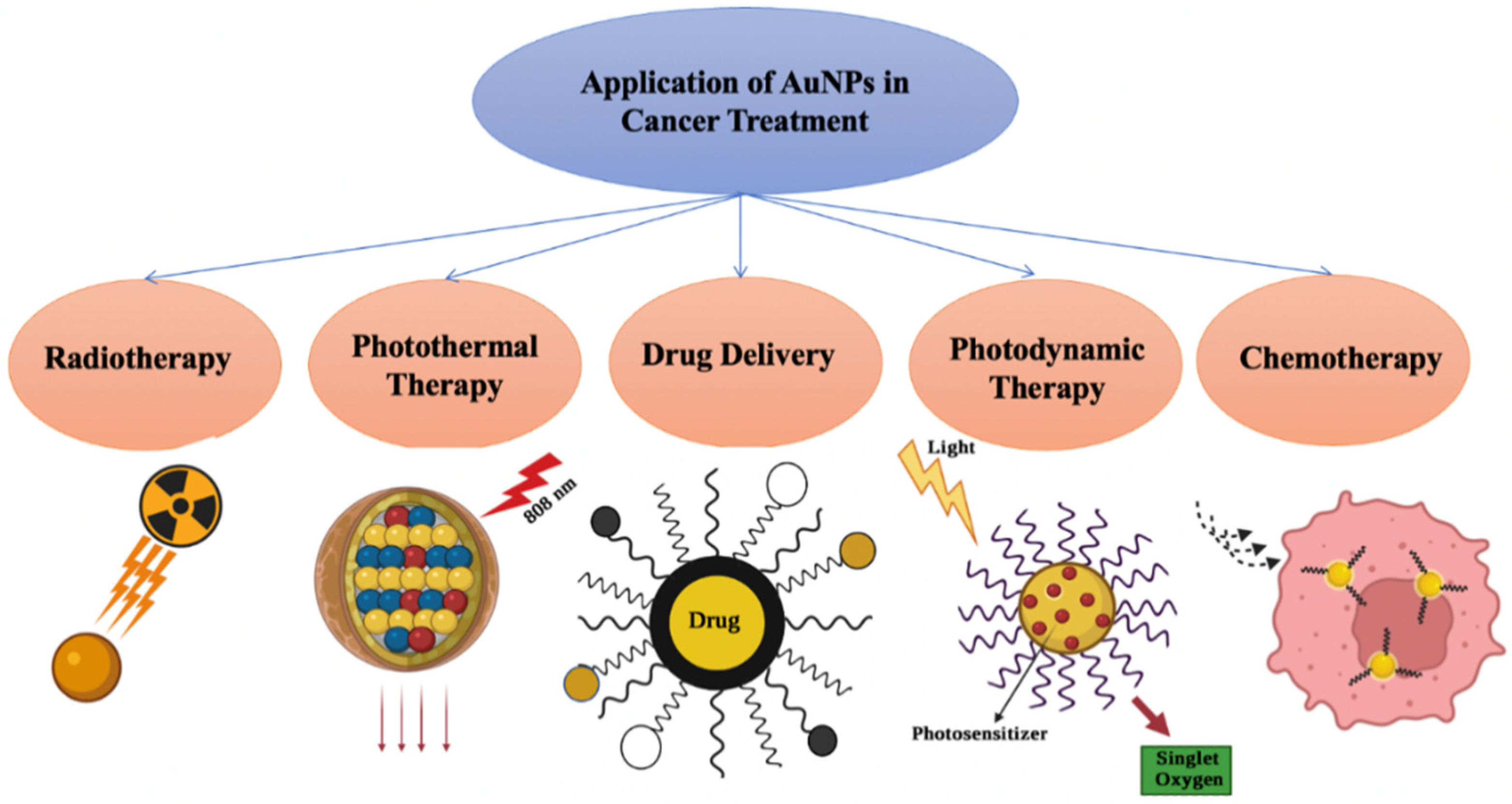
9. Therapy
9.1. Photothermal Therapy (PTT)
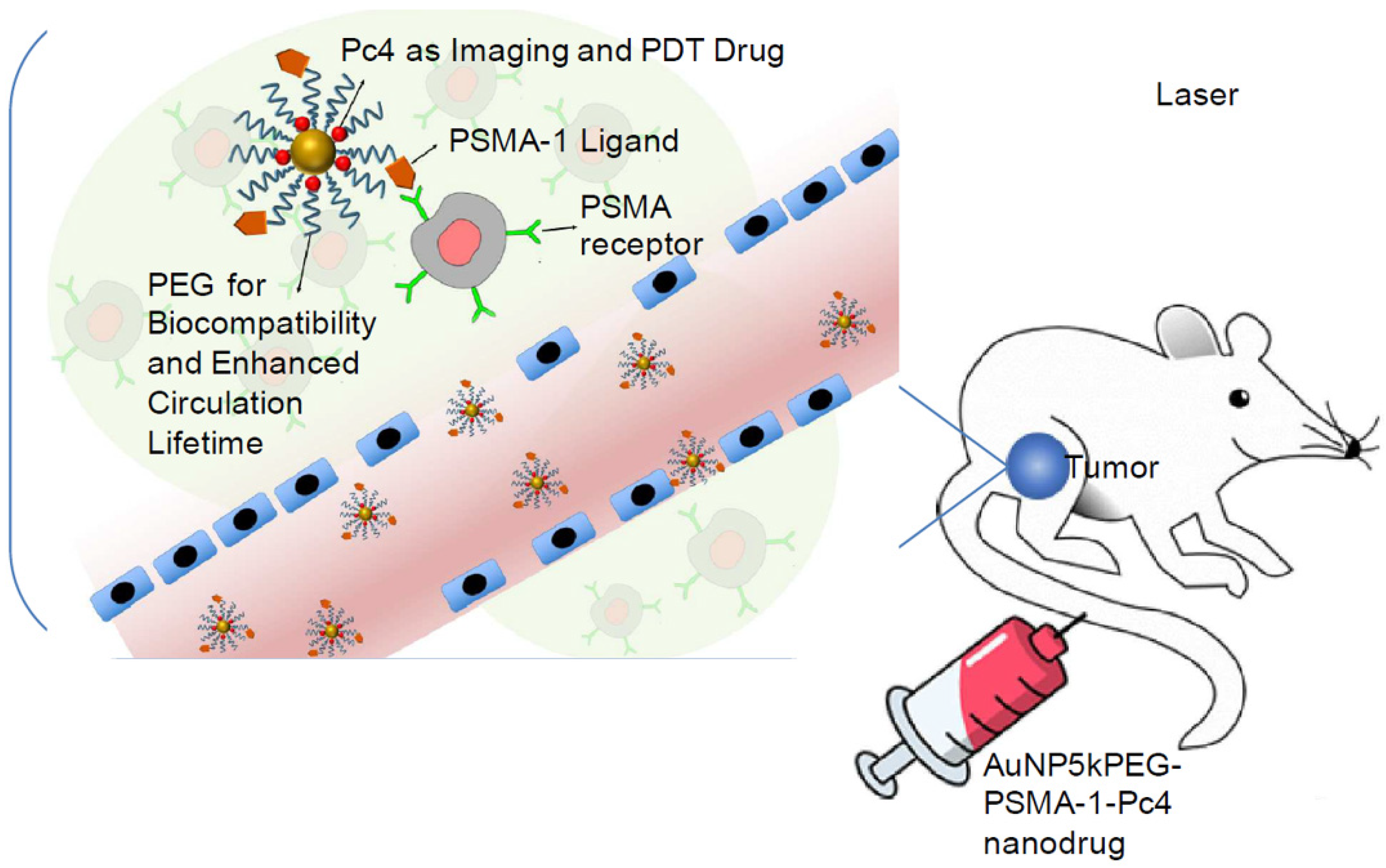
9.2. Photodynamic Therapy (PDT)
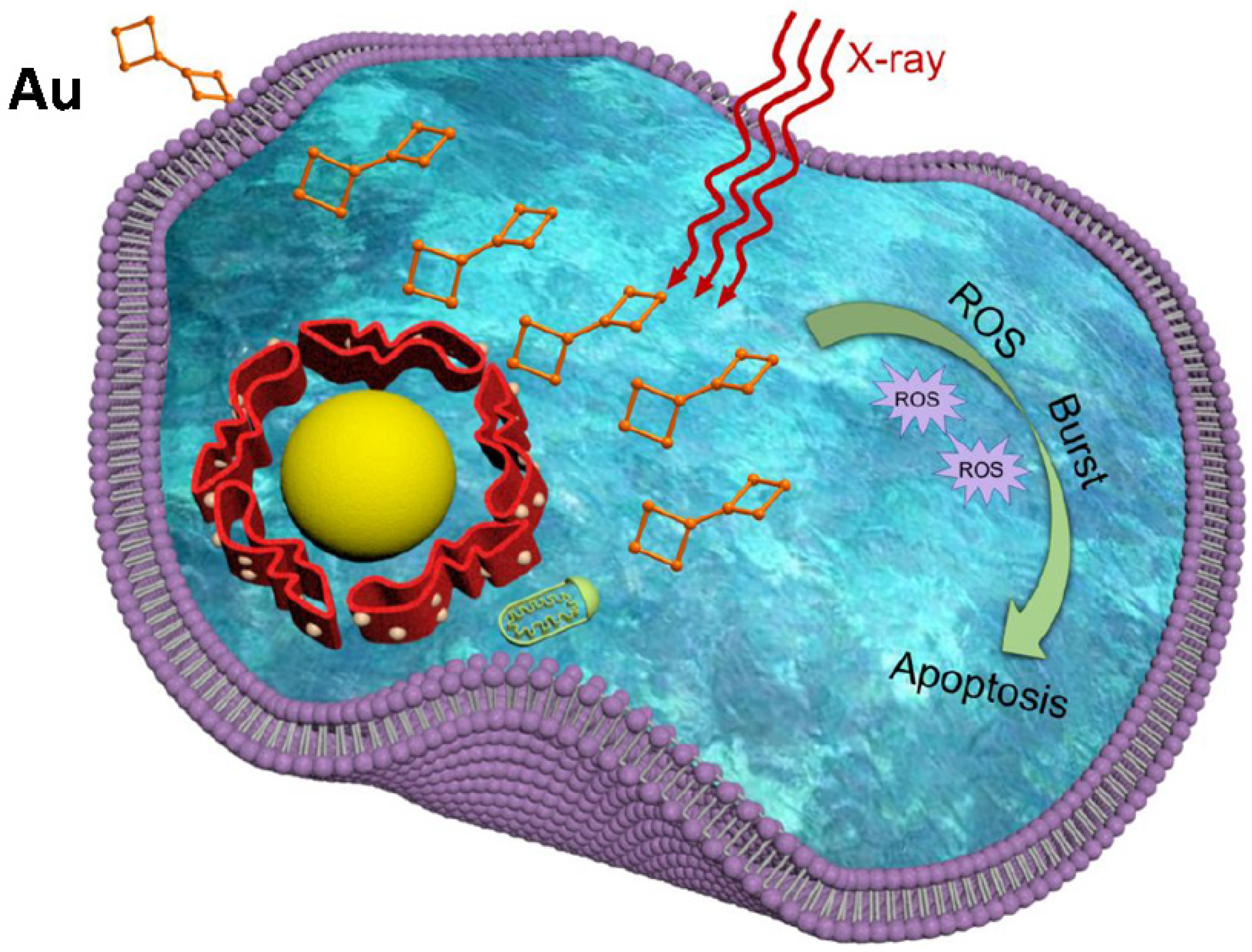
9.3. Radiotherapy
9.4. Chemotherapy
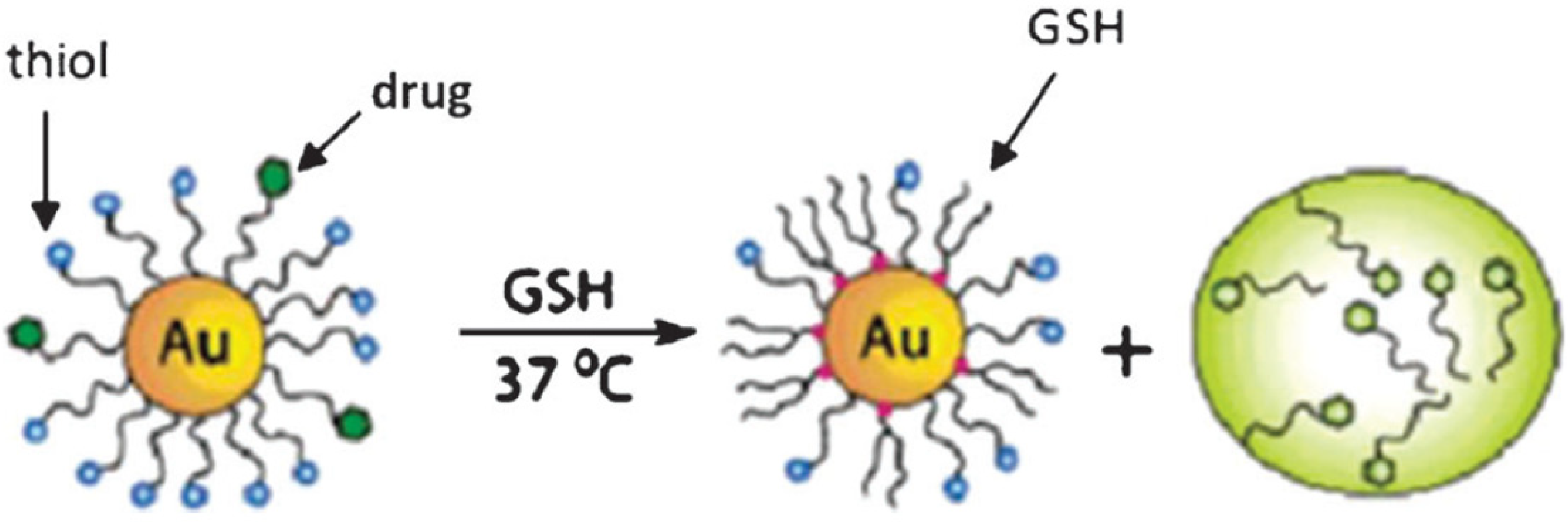
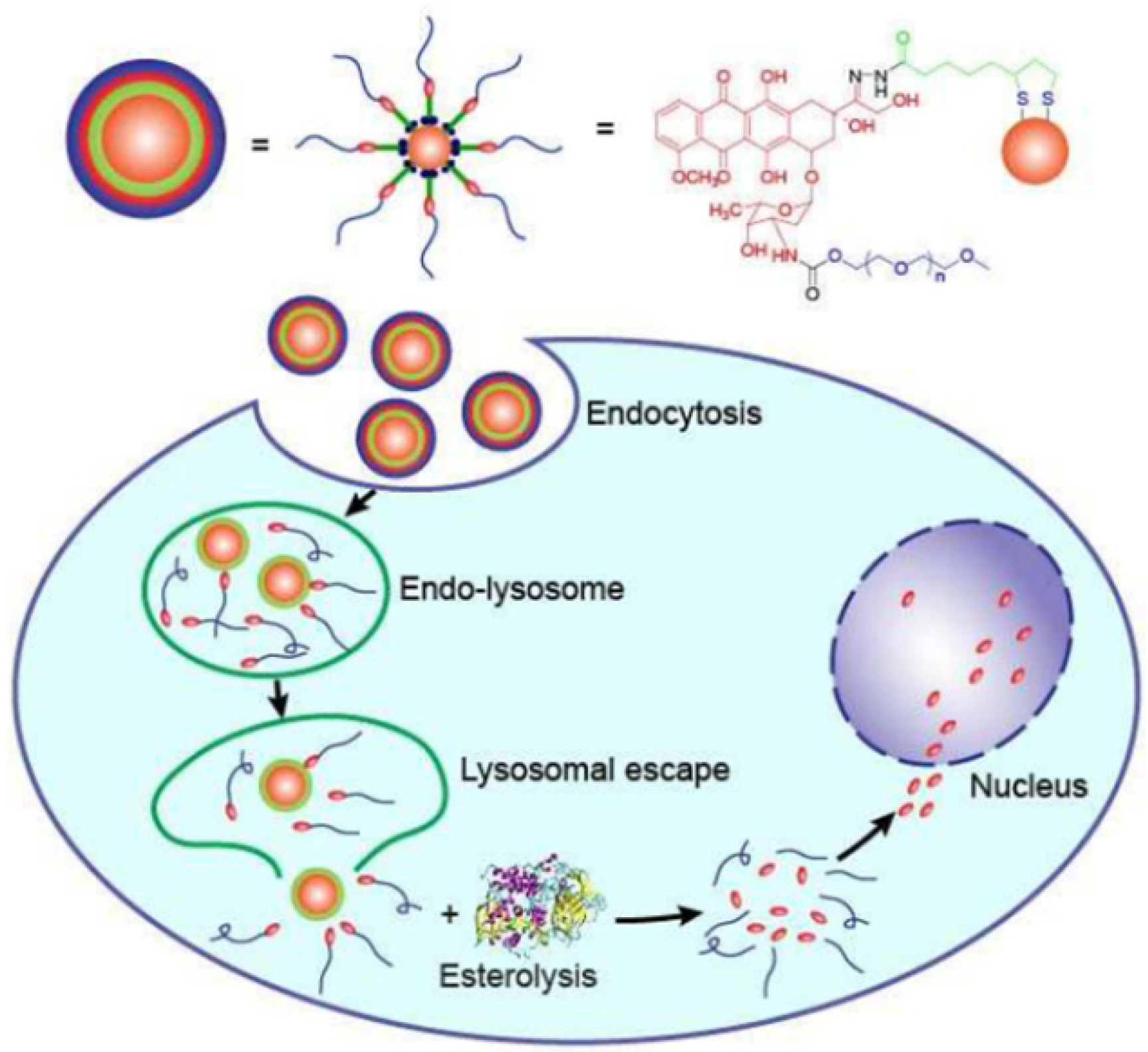
9.5. Drug Delivery
10. Current Limitations
11. Challenges and Future Perspectives
12. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| AuNPs | Gold nanoparticles |
| NPs | nanoparticles |
| SPR | surface plasmon resonance |
| NIR | near-infrared |
| CT | computed tomography |
| PAI | photoacoustic imaging |
| MRI | magnetic resonance imaging |
| FWHM | full width half maximum |
| SB | sodium borohydride |
| SC | sodium citrate |
| NBP | nanobipyramid |
| NRS | nanorod shell |
| TEM | Transmission electron microscopy |
| SEM | Scanning electron microscope |
| SERS | Surface-Enhanced Raman Resonance |
| FA | folic acid |
| MBA | 4-mercaptobenzoic |
| BSA | bovine serum albumin |
| RBSA | reduced bovine serum albumin |
| ALP | alkaline phosphatase |
| SIO2 | Silicon dioxide |
| Ag | silver |
| DOX | doxorubicin |
| PSS | poly sodium 4-styrenesulfonate |
| UV | Ultraviolet |
| HAuCl4 | gold acid |
| HRTEM | high resolution Transmission electron microscopy |
| SAED | selected area electron diffraction |
| NaBH4 | Sodium borohydride |
| OCT | optical coherence tomography |
| DNA | Deoxyribonucleic acid |
| CTAB | hexadecyl trimethyl ammonium bromide |
| siRNA | small interfering Ribonucleic acid |
| HER | Herceptin |
| HER2 | human epidermal growth factor receptor 2 |
| DFM | dimethylformamide |
| FI | fluorescence imaging |
| EPR | enhanced permeability and retention |
| GSH | glutathione |
| CAL-27 | human tongue squamous cell carcinoma |
| PSMA | prostate specific membrane antigen |
| Gd | gadolinium |
| T2-relaxation | spin–spin relaxation |
| NMR | nuclear magnetic resonance |
| DTPA | diethylenetriamine pentaacetate |
| LA | lactobionic acid |
| EDC | 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| NHS | n-hydroxysuccinimide |
| NBP | nanobipyramid |
| PET | positron emission tomography |
| 64Cu | copper-64 |
| RGD | tripeptide arg-gly asp |
| PPT | photothermal therapy |
| PDT | photodynamic therapy |
| Fe3O4 | iron oxide |
| MCF 7 | breast cancer cell line |
| 4T1 | breast cancer cell line |
| ROS | reactive oxygen species |
| ErbB2 | receptor tyrosine kinase 2 |
| DM1 | emtansine |
| PTX | paclitaxel |
| CPT | camptothecin |
| EGFR | epidermal growth factor receptor |
| RES | permeability and retention; |
| COOH | carboxylic group |
| NIH3T3 | fibroblast cell line |
| MG63 | hypotriploid human cell line |
| A542 | epithelial cell |
References
- WHO. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 6 June 2022).
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Gao, T.; Hong, H.; Sun, J. Applications of gold nanoparticles in cancer nanotechnology. Nanotechnol. Sci. Appl. 2008, 1, 17–32. [Google Scholar] [CrossRef]
- Mirkin, C.A.; Letsinger, R.L.; Mucic, R.C.; Storhoff, J.J. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 1996, 382, 607–609. [Google Scholar] [CrossRef]
- Ali Dheyab, M.; Abdul Aziz, A.; Jameel, M.S.; Moradi Khaniabadi, P.; Oglat, A.A. Rapid sonochemically-assisted synthesis of highly stable gold nanoparticles as computed tomography contrast agents. Appl. Sci. 2020, 10, 7020. [Google Scholar] [CrossRef]
- Inose, T.; Kitamura, N.; Takano-Kasuya, M.; Tokunaga, M.; Une, N.; Kato, C.; Tayama, M.; Kobayashi, Y.; Yamauchi, N.; Nagao, D. Development of X-ray contrast agents using single nanometer-sized gold nanoparticles and lactoferrin complex and their application in vascular imaging. Colloids Surf. B Biointerfaces 2021, 203, 111732. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wang, X.; Burda, C.; Basilion, J.P. Recent development of gold nanoparticles as contrast agents for cancer diagnosis. Cancers 2021, 13, 1825. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhao, X.; Liu, Y.; Chen, B.; Ding, X.; Zhao, N.; Xu, F.J. Controlled synthesis and surface engineering of janus chitosan-gold nanoparticles for photoacoustic imaging-guided synergistic gene/photothermal therapy. Small 2021, 17, 2006004. [Google Scholar] [CrossRef] [PubMed]
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S.; Noqta, O.A.; Mehrdel, B. Synthesis and coating methods of biocompatible iron oxide/gold nanoparticle and nanocomposite for biomedical applications. Chin. J. Phys. 2020, 64, 305–325. [Google Scholar] [CrossRef]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold nanoparticles for photothermal cancer therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Ma, X.; Ma, H.; Zheng, K.; Liu, J.; Hou, S.; Meng, J.; Wang, P.C.; Wu, X.; Liang, X.-J. Surface chemistry-mediated penetration and gold nanorod thermotherapy in multicellular tumor spheroids. Nanoscale 2013, 5, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Gong, N.; Chen, S.; Jin, S.; Zhang, J.; Wang, P.C.; Liang, X.-J. Effects of the physicochemical properties of gold nanostructures on cellular internalization. Regen. Biomater. 2015, 2, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Zhang, X.; Liang, X.-J. Gold nanoparticles: Emerging paradigm for targeted drug delivery system. Biotechnol. Adv. 2013, 31, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Tsunoyama, H.; Sakurai, H.; Negishi, Y.; Tsukuda, T. Size-specific catalytic activity of polymer-stabilized gold nanoclusters for aerobic alcohol oxidation in water. J. Am. Chem. Soc. 2005, 127, 9374–9375. [Google Scholar] [CrossRef]
- Nasaruddin, R.R.; Chen, T.; Yan, N.; Xie, J. Roles of thiolate ligands in the synthesis, properties and catalytic application of gold nanoclusters. Coord. Chem. Rev. 2018, 368, 60–79. [Google Scholar] [CrossRef]
- Shcherbakov, V.; Denisov, S.A.; Mostafavi, M. The mechanism of organic radical oxidation catalysed by gold nanoparticles. Phys. Chem. Chem. Phys. 2021, 23, 26494–26500. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial gold nanoclusters. ACS Nano 2017, 11, 6904–6910. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Xu, J.; Zheng, J. Renal clearable luminescent gold nanoparticles: From the bench to the clinic. Angew. Chem. Int. Ed. 2019, 58, 4112–4128. [Google Scholar] [CrossRef] [PubMed]
- Higbee-Dempsey, E.M.; Amirshaghaghi, A.; Case, M.J.; Bouché, M.; Kim, J.; Cormode, D.P.; Tsourkas, A. Biodegradable gold nanoclusters with improved excretion due to pH-triggered hydrophobic-to-hydrophilic transition. J. Am. Chem. Soc. 2020, 142, 7783–7794. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.; Schneider, M. Advances in biomedical and pharmaceutical applications of protein-stabilized gold nanoclusters. J. Mater. Chem. B 2020, 8, 8952–8971. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Wan, A. Luminescent gold nanoclusters for in vivo tumor imaging. Analyst 2020, 145, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Ma, H.; Liu, J.; Huo, S.; Kumar, A.; Wei, T.; Zhang, X.; Jin, S.; Gan, Y.; Wang, P.C. Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano 2012, 6, 4483–4493. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ma, H.; Zhang, X.; Huang, K.; Jin, S.; Liu, J.; Wei, T.; Cao, W.; Zou, G.; Liang, X.-J. Gold nanoparticles functionalized with therapeutic and targeted peptides for cancer treatment. Biomaterials 2012, 33, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.C.; Jo, E.; Kwon, Y.W.; Lee, B.; Ryu, J.H.; Lee, E.J.; Kim, K.; Lee, J. Superparamagnetic gold nanoparticles synthesized on protein particle scaffolds for cancer theragnosis. Adv. Mater. 2017, 29, 1701146. [Google Scholar] [CrossRef] [PubMed]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Long, M.; Qin, Y.; Sun, X.; Zheng, J. Luminescent gold nanoparticles with efficient renal clearance. Angew. Chem. Int. Ed. 2011, 50, 3168–3172. [Google Scholar] [CrossRef] [PubMed]
- Loynachan, C.N.; Soleimany, A.P.; Dudani, J.S.; Lin, Y.; Najer, A.; Bekdemir, A.; Chen, Q.; Bhatia, S.N.; Stevens, M.M. Renal clearable catalytic gold nanoclusters for in vivo disease monitoring. Nat. Nanotechnol. 2019, 14, 883–890. [Google Scholar] [CrossRef]
- Bouché, M.; Hsu, J.C.; Dong, Y.C.; Kim, J.; Taing, K.; Cormode, D.P. Recent advances in molecular imaging with gold nanoparticles. Bioconj. Chem. 2019, 31, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.; Nazari, Z. A review on gold nanoparticles aggregation and its applications. J. Chem. Rev. 2020, 2, 228–242. [Google Scholar]
- Halawa, M.; Lai, J.; Xu, G. Gold nanoclusters: Synthetic strategies and recent advances in fluorescent sensing. Mater. Today Nano 2018, 3, 9–27. [Google Scholar] [CrossRef]
- Wang, P.; Qi, X.; Zhang, X.; Wang, T.; Li, Y.; Zhang, K.; Zhao, S.; Zhou, J.; Fu, Y. Solvent: A key in digestive ripening for monodisperse au nanoparticles. Nanoscale Res. Lett. 2017, 12, 25. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, J.; Zhang, A.; Huang, Z.; Chen, Y.; Zhang, Q.; Cui, D. Monodisperse Au@ Ag core-shell nanoprobes with ultrasensitive SERS-activity for rapid identification and Raman imaging of living cancer cells. Talanta 2019, 198, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Singh, R. Nanotechnology based therapeutic application in cancer diagnosis and therapy. 3 Biotech 2019, 9, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.P.; Prado, A.R.; Keijok, W.J.; Ribeiro, M.R.; Pontes, M.J.; Nogueira, B.V.; Guimaraes, M.C. A helpful method for controlled synthesis of monodisperse gold nanoparticles through response surface modeling. Arab. J. Chem. 2020, 13, 216–226. [Google Scholar] [CrossRef]
- Karimi, S.; Moshaii, A.; Nikkhah, M. Controlled synthesis of colloidal monodisperse gold nanoparticles in a wide range of sizes; investigating the effect of reducing agent. Mater. Res. Express 2019, 6, 1150f2. [Google Scholar] [CrossRef]
- Xu, S.; Ouyang, W.; Xie, P.; Lin, Y.; Qiu, B.; Lin, Z.; Chen, G.; Guo, L. Highly uniform gold nanobipyramids for ultrasensitive colorimetric detection of influenza virus. Anal. Chem. 2017, 89, 1617–1623. [Google Scholar] [CrossRef]
- Wang, H.; Rao, H.; Luo, M.; Xue, X.; Xue, Z.; Lu, X. Noble metal nanoparticles growth-based colorimetric strategies: From monocolorimetric to multicolorimetric sensors. Coord. Chem. Rev. 2019, 398, 113003. [Google Scholar] [CrossRef]
- Liu, S.; Han, M. Synthesis, functionalization, and bioconjugation of monodisperse, Silica-Coated gold nanoparticles: Robust bioprobes. Adv. Funct. Mater. 2005, 15, 961–967. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional gold nanoparticles: A novel nanomaterial for various medical applications and biological activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef]
- Ramalingam, V. Multifunctionality of gold nanoparticles: Plausible and convincing properties. Adv. Colloid Interface Sci. 2019, 271, 101989. [Google Scholar] [CrossRef]
- Venkatesan, R.; Pichaimani, A.; Hari, K.; Balasubramanian, P.K.; Kulandaivel, J.; Premkumar, K. Doxorubicin conjugated gold nanorods: A sustained drug delivery carrier for improved anticancer therapy. J. Mater. Chem. B 2013, 1, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Halder, A.; Das, S.; Ojha, D.; Chattopadhyay, D.; Mukherjee, A. Highly monodispersed gold nanoparticles synthesis and inhibition of herpes simplex virus infections. Mater. Sci. Eng. C 2018, 89, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.; Bürgi, T. Bottom-up organisation of metallic nanoparticles. In Amorphous Nanophotonics; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–37. [Google Scholar]
- Shah, M.; Badwaik, V.; Kherde, Y.; Waghwani, H.K.; Modi, T.; Aguilar, Z.P.; Rodgers, H.; Hamilton, W.; Marutharaj, T.; Webb, C. Gold nanoparticles: Various methods of synthesis and antibacterial applications. Front. Biosci. 2014, 19, 1320–1344. [Google Scholar] [CrossRef] [PubMed]
- Ojea-Jiménez, I.; Romero, F.M.; Bastús, N.G.; Puntes, V. Small gold nanoparticles synthesized with sodium citrate and heavy water: Insights into the reaction mechanism. J. Phys. Chem. C 2010, 114, 1800–1804. [Google Scholar] [CrossRef]
- Mieszawska, A.J.; Mulder, W.J.; Fayad, Z.A.; Cormode, D.P. Multifunctional gold nanoparticles for diagnosis and therapy of disease. Mol. Pharm. 2013, 10, 831–847. [Google Scholar] [CrossRef] [PubMed]
- Herizchi, R.; Abbasi, E.; Milani, M.; Akbarzadeh, A. Current methods for synthesis of gold nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 596–602. [Google Scholar] [CrossRef]
- Piella, J.; Bastus, N.G.; Puntes, V. Size-controlled synthesis of sub-10-nanometer citrate-stabilized gold nanoparticles and related optical properties. Chem. Mater. 2016, 28, 1066–1075. [Google Scholar] [CrossRef]
- Carbo-Argibay, E.; Rodriguez-Gonzalez, B. Controlled growth of colloidal gold nanoparticles: Single-crystalline versus multiply-twinned particles. Isr. J. Chem. 2016, 56, 214–226. [Google Scholar] [CrossRef]
- Niu, J.; Zhu, T.; Liu, Z. One-step seed-mediated growth of 30–150 nm quasispherical gold nanoparticles with 2-mercaptosuccinic acid as a new reducing agent. Nanotechnology 2007, 18, 325607. [Google Scholar] [CrossRef]
- Khaniabadi, P.M.; Ahmed, N.M.; Dheyab, M.A.; Aziz, A.A.; Almessiere, M. Structure, morphology and absorption characteristics of gold nanoparticles produced via PLAL method: Role of low energy X-ray dosage. Surf. Interfaces. 2021, 24, 101139. [Google Scholar] [CrossRef]
- Gerasimov, G.Y. Radiation methods in nanotechnology. J. Eng. Phys. Thermophys. 2011, 84, 947–963. [Google Scholar] [CrossRef]
- Fazolin, G.N.; Varca, G.H.; Kadlubowski, S.; Sowinski, S.; Lugão, A.B. The effects of radiation and experimental conditions over papain nanoparticle formation. Radiat. Phys. Chem. 2020, 169, 107984. [Google Scholar] [CrossRef]
- Cui, Z.; Coletta, C.; Bahry, T.; Marignier, J.-L.; Guigner, J.-M.; Gervais, M.; Baiz, S.; Goubard, F.; Remita, S. A novel radiation chemistry-based methodology for the synthesis of PEDOT/Ag nanocomposites. Mater. Chem. Front. 2017, 1, 879–892. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Okada, I.; Fukuoka, T.; Sakurai, I.; Utsumi, Y. Synthesis of metallic nanoparticles through X-ray radiolysis using synchrotron radiation. Jpn. J. Appl. Phys. 2016, 55, 055502. [Google Scholar] [CrossRef]
- Treguer, M.; De Cointet, C.; Remita, H.; Khatouri, J.; Mostafavi, M.; Amblard, J.; Belloni, J.; De Keyzer, R. Dose rate effects on radiolytic synthesis of gold−silver bimetallic clusters in solution. J. Phys. Chem. B. 1998, 102, 4310–4321. [Google Scholar] [CrossRef]
- Queiroz, R.; Varca, G.; Kadlubowski, S.; Ulanski, P.; Lugão, A. Radiation-synthesized protein-based drug carriers: Size-controlled BSA nanoparticles. Int. J. Biol. Macromol. 2016, 85, 82–91. [Google Scholar] [CrossRef]
- Anh, N.T.; Van Phu, D.; Duy, N.N.; Du, B.D.; Hien, N.Q. Synthesis of alginate stabilized gold nanoparticles by γ-irradiation with controllable size using different Au3+ concentration and seed particles enlargement. Radiat. Phys. Chem. 2010, 79, 405–408. [Google Scholar] [CrossRef]
- Fuentes-García, J.; Santoyo-Salzar, J.; Rangel-Cortes, E.; Goya, G.; Cardozo-Mata, V.; Pescador-Rojas, J. Effect of ultrasonic irradiation power on sonochemical synthesis of gold nanoparticles. Ultrason. Sonochem. 2021, 70, 105274. [Google Scholar] [CrossRef]
- Handley, D. Colloidal Gold: Principles, Methods and Applications; Hayat, M.A., Ed.; Elsevier: Amsterdam, The Netherlands, 1989; pp. 14–32. [Google Scholar]
- Kumar, S.; Gandhi, K.; Kumar, R. Modeling of formation of gold nanoparticles by citrate method. Ind. Eng. Chem. Res. 2007, 46, 3128–3136. [Google Scholar] [CrossRef]
- Niidome, Y.; Nishioka, K.; Kawasaki, H.; Yamada, S. Rapid synthesis of gold nanorods by the combination of chemical reduction and photoirradiation processes; morphological changes depending on the growing processes. Chem. Commun. 2003, 18, 2376–2377. [Google Scholar] [CrossRef]
- Wangoo, N.; Bhasin, K.; Mehta, S.; Suri, C.R. Synthesis and capping of water-dispersed gold nanoparticles by an amino acid: Bioconjugation and binding studies. J. Colloid Interface Sci. 2008, 323, 247–254. [Google Scholar] [CrossRef]
- Yonezawa, T.; Kunitake, T. Practical preparation of anionic mercapto ligand-stabilized gold nanoparticles and their immobilization. Colloids Surf. A 1999, 149, 193–199. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid system. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Giersig, M.; Mulvaney, P. Preparation of ordered colloid monolayers by electrophoretic deposition. Langmuir 1993, 9, 3408–3413. [Google Scholar] [CrossRef]
- Faraday, M.X. The Bakerian Lecture—Experimental relations of gold (and other metals) to light. Philos. Trans. R. Soc. Lond. 1857, 147, 145–181. [Google Scholar]
- Chen, Y.; Gu, X.; Nie, C.-G.; Jiang, Z.-Y.; Xie, Z.-X.; Lin, C.-J. Shape controlled growth of gold nanoparticles by a solution synthesis. Chem. Commun. 2005, 33, 4181–4183. [Google Scholar] [CrossRef]
- Noruzi, M.; Zare, D.; Khoshnevisan, K.; Davoodi, D. Rapid green synthesis of gold nanoparticles using Rosa hybrida petal extract at room temperature. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 1461–1465. [Google Scholar] [CrossRef]
- Korbekandi, H.; Iravani, S.; Abbasi, S. Production of nanoparticles using organisms. Crit. Rev. Biotechnol. 2009, 29, 279–306. [Google Scholar] [CrossRef]
- Jameel, M.S.; Aziz, A.A.; Dheyab, M.A. Impacts of various solvents in ultrasonic irradiation and green synthesis of platinum nanoparticle. Inorg. Chem. Commun. 2021, 128, 108565. [Google Scholar] [CrossRef]
- Jameel, M.S.; Aziz, A.A.; Dheyab, M.A. Comparative analysis of platinum nanoparticles synthesized using sonochemical-assisted and conventional green methods. Nano-Struct. Nano-Objects 2020, 23, 100484. [Google Scholar] [CrossRef]
- Hinman, J.J.; Suslick, K.S. Nanostructured materials synthesis using ultrasound. Sonochemistry 2017, 375, 59–94. [Google Scholar]
- Suslick, K.S.; Flannigan, D.J. Inside a collapsing bubble: Sonoluminescence and the conditions during cavitation. Annu. Rev. Phys. Chem. 2008, 59, 659–683. [Google Scholar] [CrossRef]
- Bang, J.H.; Suslick, K.S. Applications of ultrasound to the synthesis of nanostructured materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef]
- Rahimi, M.; Movahedirad, S.; Shahhosseini, S. CFD study of the flow pattern in an ultrasonic horn reactor: Introducing a realistic vibrating boundary condition. Ultrason. Sonochem. 2017, 35, 359–374. [Google Scholar] [CrossRef]
- Vabbina, P.K.; Karabiyik, M.; Al-Amin, C.; Pala, N.; Das, S.; Choi, W.; Saxena, T.; Shur, M. Controlled synthesis of single-crystalline ZnO nanoflakes on arbitrary substrates at ambient conditions. Part. Part. Syst. Charact. 2014, 31, 190–194. [Google Scholar] [CrossRef]
- Fu, J.; Daanen, N.N.; Rugen, E.E.; Chen, D.P.; Skrabalak, S.E. Simple reactor for ultrasonic spray synthesis of nanostructured materials. Chem. Mater. 2017, 29, 62–68. [Google Scholar] [CrossRef]
- Hu, X.; Takada, N.; Machmudah, S.; Wahyudiono; Kanda, H.; Goto, M. Ultrasonic-Enhanced Fabrication of Metal Nanoparticles by Laser Ablation in Liquid. Ind. Eng. Chem. Res. 2020, 59, 7512–7519. [Google Scholar] [CrossRef]
- Dong, J.; Carpinone, P.L.; Pyrgiotakis, G.; Demokritou, P.; Moudgil, B.M. Synthesis of precision gold nanoparticles using Turkevich method. KONA Powder Part. J. 2020, 37, 224–232. [Google Scholar]
- Ward, C.J.; Tronndorf, R.; Eustes, A.S.; Auad, M.L.; Davis, E.W. Seed-mediated growth of gold nanorods: Limits of length to diameter ratio control. J. Nanomater. 2014, 2014, 7. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Hung, Y.-C.; Liau, I.; Huang, G.S. Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res. Lett. 2009, 4, 858–864. [Google Scholar]
- Teimuri-Mofrad, R.; Hadi, R.; Tahmasebi, B.; Farhoudian, S.; Mehravar, M.; Nasiri, R. Green synthesis of gold nanoparticles using plant extract: Mini-review. Nanochem. Res. 2017, 2, 8–19. [Google Scholar]
- Bogireddy, N.; Pal, U.; Gomez, L.M.; Agarwal, V. Size controlled green synthesis of gold nanoparticles using Coffea arabica seed extract and their catalytic performance in 4-nitrophenol reduction. RSC Adv. 2018, 8, 24819–24826. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal communication: When i’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef]
- Jimenez-Ruiz, A.; Perez-Tejeda, P.; Grueso, E.; Castillo, P.M.; Prado-Gotor, R. Nonfunctionalized gold nanoparticles: Synthetic routes and synthesis condition dependence. Chem.–A Eur. J. 2015, 21, 9596–9609. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Khaniabadi, P.M.; Jameel, M.S.; Ahmed, N.M.; Ali, A.T. Distinct advantages of using sonochemical over laser ablation methods for a rapid-high quality gold nanoparticles production. Mater. Res. Express 2021, 8, 015009. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S.; Khaniabadi, P.M.; Mehrdel, B. Sonochemical-assisted synthesis of highly stable gold nanoparticles catalyst for decoloration of methylene blue dye. Inorg. Chem. Commun. 2021, 127, 108551. [Google Scholar] [CrossRef]
- Sun, L.; Joh, D.Y.; Al-Zaki, A.; Stangl, M.; Murty, S.; Davis, J.J.; Baumann, B.C.; Alonso-Basanta, M.; Kao, G.D.; Tsourkas, A. Theranostic application of mixed gold and superparamagnetic iron oxide nanoparticle micelles in glioblastoma multiforme. J. Biomed. Nanotechnol. 2016, 12, 347–356. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S. Synthesis and optimization of the sonochemical method for functionalizing gold shell on Fe3O4 core nanoparticles using response surface methodology. Surf. Interfaces. 2020, 21, 100647. [Google Scholar] [CrossRef]
- Mehrdel, B.; Othman, N.; Aziz, A.A.; Khaniabadi, P.M.; Jameel, M.S.; Dheyab, M.A.; Amiri, I. Identifying metal nanoparticle size effect on sensing common human plasma protein by counting the sensitivity of optical absorption spectra damping. Plasmonics 2020, 15, 123–133. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Maturi, M.; Locatelli, E.; Monaco, I.; Franchini, M.C. Current concepts in nanostructured contrast media development for in vivo photoacoustic imaging. Biomater. Sci. 2019, 7, 1746–1775. [Google Scholar] [CrossRef]
- Dong, Y.C.; Hajfathalian, M.; Maidment, P.S.; Hsu, J.C.; Naha, P.C.; Si-Mohamed, S.; Breuilly, M.; Kim, J.; Chhour, P.; Douek, P. Effect of gold nanoparticle size on their properties as contrast agents for computed tomography. Sci. Rep. 2019, 9, 14912. [Google Scholar] [CrossRef]
- Knights, O.B.; Ye, S.; Ingram, N.; Freear, S.; McLaughlan, J.R. Optimising gold nanorods for photoacoustic imaging in vitro. Nanoscale Adv. 2019, 1, 1472–1481. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Zhao, Y.; Yoon, S.J.; Gambhir, S.S.; Emelianov, S. Miniature gold nanorods for photoacoustic molecular imaging in the second near-infrared optical window. Nat. Nanotechnol. 2019, 14, 465–472. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Khaniabadi, P.M.; Jameel, M.S. Potential of a sonochemical approach to generate MRI-PPT theranostic agents for breast cancer. Photodiagn. Photodyn. Ther. 2021, 33, 102177. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S.; Noqta, O.A.; Khaniabadi, P.M.; Mehrdel, B. Excellent relaxivity and X-ray attenuation combo properties of Fe3O4@ Au CSNPs produced via Rapid sonochemical synthesis for MRI and CT imaging. Mater. Today Commun. 2020, 25, 101368. [Google Scholar] [CrossRef]
- Millstone, J.E.; Wei, W.; Jones, M.R.; Yoo, H.; Mirkin, C.A. Iodide ions control seed-mediated growth of anisotropic gold nanoparticles. Nano Lett. 2008, 8, 2526–2529. [Google Scholar] [CrossRef]
- Si, P.; Yuan, E.; Liba, O.; Winetraub, Y.; Yousefi, S.; SoRelle, E.D.; Yecies, D.W.; Dutta, R.; de la Zerda, A. Gold nanoprisms as optical coherence tomography contrast agents in the second near-infrared window for enhanced angiography in live animals. ACS Nano 2018, 12, 11986–11994. [Google Scholar] [CrossRef]
- Tang, D.; Gao, W.; Yuan, Y.; Guo, L.; Mei, X. Novel biocompatible Au nanostars@ PEG nanoparticles for in vivo CT imaging and renal clearance properties. Nanoscale Res. Lett. 2017, 12, 1–8. [Google Scholar] [CrossRef]
- Neuschmelting, V.; Harmsen, S.; Beziere, N.; Lockau, H.; Hsu, H.T.; Huang, R.; Razansky, D.; Ntziachristos, V.; Kircher, M.F. Dual-modality surface-enhanced resonance Raman scattering and multispectral optoacoustic tomography nanoparticle approach for brain tumor delineation. Small 2018, 14, 1800740. [Google Scholar] [CrossRef]
- Wang, P.; Wang, X.; Wang, L.; Hou, X.; Liu, W.; Chen, C. Interaction of gold nanoparticles with proteins and cells. Sci. Technol. Adv. Mater. 2015, 16, 034610. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Maity, M.; Das, S.; Maiti, N.C. Stability and binding interaction of bilirubin on a gold nano-surface: Steady state fluorescence and FT-IR investigation. Phys. Chem. Chem. Phys. 2014, 16, 20013–20022. [Google Scholar] [CrossRef]
- Cheng, S.; Hideshima, S.; Kuroiwa, S.; Nakanishi, T.; Osaka, T. Label-free detection of tumor markers using field effect transistor (FET)-based biosensors for lung cancer diagnosis. Sens. Actuators B Chem. 2015, 212, 329–334. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M.; Stener, M.; Guo, Y.; Chen, S.; Crespo, P.; García, M.A.; Hernando, A.; Pengo, P.; Pasquato, L. Physico-chemical characteristics of gold nanoparticles. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2014; Volume 66, pp. 81–152. [Google Scholar]
- DeLong, R.K.; Reynolds, C.M.; Malcolm, Y.; Schaeffer, A.; Severs, T.; Wanekaya, A. Functionalized gold nanoparticles for the binding, stabilization, and delivery of therapeutic DNA, RNA, and other biological macromolecules. Nanotechnol. Sci. Appl. 2010, 3, 53. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Locatelli, E. Synthesis and Surface Modification of Silver and Gold Nanoparticles. Nanomedicine Applications against Glioblastoma Multiforme. Ph.D. Thesis, University of Bologna, Bologna, Italy, 14 April 2014. [Google Scholar]
- Zhang, Z.; Wang, J.; Chen, C. Gold nanorods based platforms for light-mediated theranostics. Theranostics 2013, 3, 223. [Google Scholar] [CrossRef]
- Cobley, C.M.; Chen, J.; Cho, E.C.; Wang, L.V.; Xia, Y. Gold nanostructures: A class of multifunctional materials for biomedical applications. Chem. Soc. Rev. 2011, 40, 44–56. [Google Scholar] [CrossRef]
- Jiang, Y.; Huo, S.; Mizuhara, T.; Das, R.; Lee, Y.-W.; Hou, S.; Moyano, D.F.; Duncan, B.; Liang, X.-J.; Rotello, V.M. The interplay of size and surface functionality on the cellular uptake of sub-10 nm gold nanoparticles. ACS Nano 2015, 9, 9986–9993. [Google Scholar] [CrossRef]
- Alea-Reyes, M.E.; Gonzalez, A.; Calpena, A.C.; Ramos-López, D.; de Lapuente, J.; Pérez-García, L. Gemini pyridinium amphiphiles for the synthesis and stabilization of gold nanoparticles for drug delivery. J. Colloid Interface Sci. 2017, 502, 172–183. [Google Scholar] [CrossRef]
- Xia, Y.; Ma, X.; Gao, J.; Chen, G.; Li, Z.; Wu, X.; Yu, Z.; Xing, J.; Sun, L.; Ruan, H. A Flexible Caterpillar-Like Gold Nanoparticle Assemblies with Ultrasmall Nanogaps for Enhanced Dual-Modal Imaging and Photothermal Therapy. Small 2018, 14, 1800094. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Martin, C.T.; Rotello, V.M. Stability of gold nanoparticle-bound DNA toward biological, physical, and chemical agents. Chem. Biol. Drug Des. 2006, 67, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Chompoosor, A.; Yeh, Y.-C.; Agasti, S.S.; Solfiell, D.J.; Rotello, V.M. Dendronized gold nanoparticles for siRNA delivery. Small 2012, 8, 3253. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi-Gahrouei, D.; Khaniabadi, P.M.; Shahbazi-Gahrouei, S.; Khorasani, A.; Mahmoudi, F. A literature review on multimodality molecular imaging nanoprobes for cancer detection. Pol. J. Med. Phys. Eng. 2019, 25, 57–68. [Google Scholar] [CrossRef]
- Shahbazi-Gahrouei, D.; Khaniabadi, P.M.; Khaniabadi, B.M.; Shahbazi-Gahrouei, S. Medical imaging modalities using nanoprobes for cancer diagnosis: A literature review on recent findings. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2019, 24. [Google Scholar] [CrossRef]
- Raghvendra, R.; Kanthadeivi, A.; Sathesh, K.; Aarrthy, M. Diagnostics and therapeutic application of gold nanoparticles. Medicine 2014, 2, 4. [Google Scholar]
- Ravanshad, R.; Karimi Zadeh, A.; Amani, A.M.; Mousavi, S.M.; Hashemi, S.A.; Savar Dashtaki, A.; Mirzaei, E.; Zare, B. Application of nanoparticles in cancer detection by Raman scattering based techniques. Nano Rev. Exp. 2018, 9, 1373551. [Google Scholar] [CrossRef]
- Rostami, A.; Sazgarnia, A. Gold nanoparticles as cancer theranostic agents. Nanomed. J. 2019, 6, 147–160. [Google Scholar]
- Gobin, A.M.; Lee, M.H.; Halas, N.J.; James, W.D.; Drezek, R.A.; West, J.L. Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Lett. 2007, 7, 1929–1934. [Google Scholar] [CrossRef]
- Eghtedari, M.; Oraevsky, A.; Copland, J.A.; Kotov, N.A.; Conjusteau, A.; Motamedi, M. High sensitivity of in vivo detection of gold nanorods using a laser optoacoustic imaging system. Nano Lett. 2007, 7, 1914–1918. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; So, P.T.; Dasari, R.R.; Lim, D.-K. High resolution live cell Raman imaging using subcellular organelle-targeting SERS-sensitive gold nanoparticles with highly narrow intra-nanogap. Nano Lett. 2015, 15, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Qu, Q.; Zhao, Y.; Luo, Z.; Zhao, Y.; Ng, K.W.; Zhao, Y. Graphene oxide wrapped gold nanoparticles for intracellular Raman imaging and drug delivery. J. Mater. Chem. B 2013, 1, 6495–6500. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.; Lowery, A.; Halas, N.; West, J.; Drezek, R. Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett. 2005, 5, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Bickford, L.; Sun, J.; Fu, K.; Lewinski, N.; Nammalvar, V.; Chang, J.; Drezek, R. Enhanced multi-spectral imaging of live breast cancer cells using immunotargeted gold nanoshells and two-photon excitation microscopy. Nanotechnology 2008, 19, 315102. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Huang, X.; Kang, B.; El-Sayed, M.A. Dark-field light scattering imaging of living cancer cell component from birth through division using bioconjugated gold nanoprobes. J. Biomed. Opt. 2010, 15, 046025. [Google Scholar] [CrossRef]
- Jin, H.-Y.; Li, D.-W.; Zhang, N.; Gu, Z.; Long, Y.-T. Analyzing carbohydrate–protein interaction based on single plasmonic nanoparticle by conventional dark field microscopy. ACS Appl. Mater. Interfaces 2015, 7, 12249–12253. [Google Scholar] [CrossRef]
- Fan, M.; Han, Y.; Gao, S.; Yan, H.; Cao, L.; Li, Z.; Liang, X.-J.; Zhang, J. Ultrasmall gold nanoparticles in cancer diagnosis and therapy. Theranostics 2020, 10, 4944. [Google Scholar] [CrossRef]
- Poon, W.; Heinmiller, A.; Zhang, X.; Nadeau, J.L. Determination of biodistribution of ultrasmall, near-infrared emitting gold nanoparticles by photoacoustic and fluorescence imaging. J. Biomed. Opt. 2015, 20, 066007. [Google Scholar] [CrossRef]
- Aminabad, N.S.; Farshbaf, M.; Akbarzadeh, A. Recent advances of gold nanoparticles in biomedical applications: State of the art. Cell Biochem. Biophys. 2019, 77, 123–137. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, X.; Huang, T.; Song, J.; Wang, Y. A sandwich nanostructure of gold nanoparticle coated reduced GRAPHENE oxide for photoacoustic imaging-guided photothermal therapy in the second NIR window. Front. Bioeng. Biotechnol. 2020, 8, 655. [Google Scholar] [CrossRef] [PubMed]
- Salah, D.; Moghanm, F.S.; Arshad, M.; Alanazi, A.A.; Latif, S.; El-Gammal, M.I.; Shimaa, E.M.; Elsayed, S. Polymer-peptide modified gold nanorods to improve cell conjugation and cell labelling for stem cells photoacoustic imaging. Diagnostics 2021, 11, 1196. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, W.C. Liposomal, nanoparticle, and conjugated formulations of anticancer agents. Clin. Cancer Res. 2005, 11, 8230–8234. [Google Scholar] [CrossRef]
- Peng, C.; Gao, X.; Xu, J.; Du, B.; Ning, X.; Tang, S.; Bachoo, R.M.; Yu, M.; Ge, W.-P.; Zheng, J. Targeting orthotopic gliomas with renal-clearable luminescent gold nanoparticles. Nano Res. 2017, 10, 1366–1376. [Google Scholar] [CrossRef]
- Liu, J.; Yu, M.; Zhou, C.; Yang, S.; Ning, X.; Zheng, J. Passive tumor targeting of renal-clearable luminescent gold nanoparticles: Long tumor retention and fast normal tissue clearance. J. Am. Chem. Soc. 2013, 135, 4978–4981. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Xia, F.; Alfranca, G.; Yan, H.; Zhi, X.; Liu, Y.; Peng, C.; Zhang, C.; de la Fuente, J.M.; Cui, D. Nanoparticles for multi-modality cancer diagnosis: Simple protocol for self-assembly of gold nanoclusters mediated by gadolinium ions. Biomaterials 2017, 120, 103–114. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Xu, L.; Shi, X.; Li, X.; Xu, X.; Sun, H.; Yang, B.; Lin, Q. A galvanic replacement route to prepare strongly fluorescent and highly stable gold nanodots for cellular imaging. Small 2013, 9, 413–420. [Google Scholar] [CrossRef]
- Venkatesh, V.; Shukla, A.; Sivakumar, S.; Verma, S. Purine-stabilized green fluorescent gold nanoclusters for cell nuclei imaging applications. ACS Appl. Mater. Interfaces 2014, 6, 2185–2191. [Google Scholar] [CrossRef]
- Yahia-Ammar, A.; Sierra, D.; Mérola, F.; Hildebrandt, N.; Le Guével, X. Self-assembled gold nanoclusters for bright fluorescence imaging and enhanced drug delivery. ACS Nano 2016, 10, 2591–2599. [Google Scholar] [CrossRef]
- Liu, H.; Hong, G.; Luo, Z.; Chen, J.; Chang, J.; Gong, M.; He, H.; Yang, J.; Yuan, X.; Li, L. Atomic-precision gold clusters for NIR-II imaging. Adv. Mater. 2019, 31, 1901015. [Google Scholar] [CrossRef]
- Li, M.; Zhang, W.; Zhang, Y. Aptamer–gold nanoparticle-signal probe bioconjugates amplify electrochemical signal for the detection of prostate specific antigen. Anal. Methods 2021, 13, 4150–4156. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, S.; Kang, W.; Li, N.; Guo, F.; Chang, H.; Wei, W. Multifunctional gold nanoparticle based selective detection of esophageal squamous cell carcinoma cells using resonance Rayleigh scattering assay. Microchem. J. 2021, 163, 105905. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, R.; Liu, Y.; Chen, X.; Li, K.; Pickwell-Macpherson, E. Gold nanoparticle enhanced detection of EGFR with a terahertz metamaterial biosensor. Biomed. Opt. Express 2021, 12, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Eivazi, M.; Khaniabadi, P.M.; Hejazi, S.H.; Shahbazi-Gahrouei, D. Porphyrin–iron oxide nanoparticle functionalized with trastuzumab (ION–PP–TZ) potential MR imaging probe for breast cancer cells. Appl. Phys. A 2022, 128, 509. [Google Scholar] [CrossRef]
- Khaniabadi, P.M.; Shahbazi-Gahrouei, D.; Majid, A.M.S.A.; Khaniabadi, B.M. Study the Anti-MUC1 antibody-based iron oxide nanoparticles on three-dimension spheroid and breast cancer (MCF-7) cell imaging. Pol. J. Med. Phys. Eng. 2019, 25, 69–77. [Google Scholar] [CrossRef]
- Luo, D.; Johnson, A.; Wang, X.; Li, H.; Erokwu, B.O.; Springer, S.; Lou, J.; Ramamurthy, G.; Flask, C.A.; Burda, C. Targeted radiosensitizers for MR-guided radiation therapy of prostate cancer. Nano Lett. 2020, 20, 7159–7167. [Google Scholar] [CrossRef]
- Alric, C.; Taleb, J.; Le Duc, G.; Mandon, C.; Billotey, C.; Le Meur-Herland, A.; Brochard, T.; Vocanson, F.; Janier, M.; Perriat, P. Gadolinium chelate coated gold nanoparticles as contrast agents for both X-ray computed tomography and magnetic resonance imaging. J. Am. Chem. Soc. 2008, 130, 5908–5915. [Google Scholar] [CrossRef]
- Kim, D.; Yu, M.K.; Lee, T.S.; Park, J.J.; Jeong, Y.Y.; Jon, S. Amphiphilic polymer-coated hybrid nanoparticles as CT/MRI dual contrast agents. Nanotechnology 2011, 22, 155101. [Google Scholar] [CrossRef]
- Meola, A.; Rao, J.; Chaudhary, N.; Sharma, M.; Chang, S.D. Gold nanoparticles for brain tumor imaging: A systematic review. Front. Neurol. 2018, 9, 328. [Google Scholar] [CrossRef]
- Iancu, S.D.; Albu, C.; Chiriac, L.; Moldovan, R.; Stefancu, A.; Moisoiu, V.; Coman, V.; Szabo, L.; Leopold, N.; Bálint, Z. Assessment of gold-coated iron oxide nanoparticles as negative T2 contrast agent in small animal MRI studies. Int. J. Nanomed. 2020, 15, 4811–4824. [Google Scholar] [CrossRef]
- Shahid, M. Water soluble gold nanoparticles based high relaxivity MRI contrast agents. Mater. Res. Express 2020, 6, 1250h1. [Google Scholar] [CrossRef]
- Usman, A.I.; Aziz, A.A.; Khaniabadi, P.M. Sonochemical synthesis of gold nanoparticles via palm oil fronds extracts for cytotoxicity assay. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Cairo, Egypt, 7–9 April 2020; p. 012004. [Google Scholar]
- Na, H.B.; Song, I.C.; Hyeon, T. Inorganic nanoparticles for MRI contrast agents. Adv. Mater. 2009, 21, 2133–2148. [Google Scholar] [CrossRef]
- Meir, R.; Shamalov, K.; Betzer, O.; Motiei, M.; Horovitz-Fried, M.; Yehuda, R.; Popovtzer, A.; Popovtzer, R.; Cohen, C.J. Nanomedicine for cancer immunotherapy: Tracking cancer-specific T-cells in vivo with gold nanoparticles and CT imaging. ACS Nano 2015, 9, 6363–6372. [Google Scholar] [CrossRef] [PubMed]
- Chhour, P.; Naha, P.C.; O’Neill, S.M.; Litt, H.I.; Reilly, M.P.; Ferrari, V.A.; Cormode, D.P. Labeling monocytes with gold nanoparticles to track their recruitment in atherosclerosis with computed tomography. Biomaterials 2016, 87, 93–103. [Google Scholar] [CrossRef]
- Cao, Y.; He, Y.; Liu, H.; Luo, Y.; Shen, M.; Xia, J.; Shi, X. Targeted CT imaging of human hepatocellular carcinoma using low-generation dendrimer-entrapped gold nanoparticles modified with lactobionic acid. J. Mater. Chem. B 2015, 3, 286–295. [Google Scholar] [CrossRef]
- Hara, D.; Tao, W.; Totiger, T.; Pourmand, A.; Dogan, N.; Ford, J.C.; Shi, J.; Pollack, A. Prostate cancer targeted X-ray fluorescence imaging via gold nanoparticles functionalized with prostate-specific membrane antigen (PSMA). Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 220–232. [Google Scholar] [CrossRef]
- Chen, F.; Goel, S.; Hernandez, R.; Graves, S.A.; Shi, S.; Nickles, R.J.; Cai, W. Dynamic positron emission tomography imaging of renal clearable gold nanoparticles. Small 2016, 12, 2775–2782. [Google Scholar] [CrossRef]
- Zhong, Y.; Meng, F.; Deng, C.; Zhong, Z. Ligand-directed active tumor-targeting polymeric nanoparticles for cancer chemotherapy. Biomacromolecules 2014, 15, 1955–1969. [Google Scholar] [CrossRef]
- Sun, X.; Huang, X.; Yan, X.; Wang, Y.; Guo, J.; Jacobson, O.; Liu, D.; Szajek, L.P.; Zhu, W.; Niu, G. Chelator-free 64Cu-integrated gold nanomaterials for positron emission tomography imaging guided photothermal cancer therapy. ACS Nano 2014, 8, 8438–8446. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Khaniabadi, P.M.; Aziz, A.A.; Jameel, M.S.; Mehrdel, B.; Oglat, A.A.; Khaleel, H.A. Focused role of nanoparticles against COVID-19: Diagnosis and treatment. Photodiagn. Photodyn. Ther. 2021, 34, 102287. [Google Scholar] [CrossRef]
- Zou, L.; Wang, H.; He, B.; Zeng, L.; Tan, T.; Cao, H.; He, X.; Zhang, Z.; Guo, S.; Li, Y. Current approaches of photothermal therapy in treating cancer metastasis with nanotherapeutics. Theranostics 2016, 6, 762. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef] [PubMed]
- Khaniabadi, P.M.; Shahbazi-Gahrouei, D.; Aziz, A.A.; Dheyab, M.A.; Khaniabadi, B.M.; Mehrdel, B.; Jameel, M.S. Trastuzumab conjugated porphyrin-superparamagnetic iron oxide nanoparticle: A potential PTT-MRI bimodal agent for herceptin positive breast cancer. Photodiagn. Photodyn. Ther. 2020, 31, 101896. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Tang, Y.a.; Elmenoufy, A.H.; Xu, H.; Cheng, Z.; Yang, X. Nanocomposite-based photodynamic therapy strategies for deep tumor treatment. Small 2015, 11, 5860–5887. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-L.; Gu, M. Gold-nanoparticle-enhanced cancer photothermal therapy. IEEE J. Sel. Top. Quantum Electron. 2009, 16, 989–996. [Google Scholar]
- Abadeer, N.S.; Murphy, C.J. Recent progress in cancer thermal therapy using gold nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.; Sershen, S.R.; Rivera, B.; Price, R.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef]
- Liu, H.; Liu, T.; Wang, H.; Li, L.; Tan, L.; Fu, C.; Nie, G.; Chen, D.; Tang, F. Impact of PEGylation on the biological effects and light heat conversion efficiency of gold nanoshells on silica nanorattles. Biomaterials 2013, 34, 6967–6975. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, Y.; Hou, S.; Upputuri, P.K.; Wu, D.; Li, J.; Wang, P.; Zhen, X.; Pramanik, M.; Pu, K. Compact plasmonic blackbody for cancer theranosis in the near-infrared II window. Acs Nano 2018, 12, 2643–2651. [Google Scholar] [CrossRef]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef]
- Dheyab, M.; Aziz, A.; Jameel, M.; Khaniabadi, P.; Mehrdel, B.; Khaniabadi, B. Gold-coated iron oxide nanoparticles as a potential photothermal therapy agent to enhance eradication of breast cancer cells. J. Phys. Conf. Ser. 2020, 1497, 012003. [Google Scholar] [CrossRef]
- Cheng, X.; Sun, R.; Yin, L.; Chai, Z.; Shi, H.; Gao, M. Light-triggered assembly of gold nanoparticles for photothermal therapy and photoacoustic imaging of tumors in vivo. Adv. Mater. 2017, 29, 1604894. [Google Scholar] [CrossRef]
- Hwang, S.; Nam, J.; Jung, S.; Song, J.; Doh, H.; Kim, S. Gold nanoparticle-mediated photothermal therapy: Current status and future perspective. Nanomedicine 2014, 9, 2003–2022. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.-Y.; Yang, X.-Q.; An, J.; Zhang, L.; Zhao, K.; Qin, M.-Y.; Fang, B.-Y.; Li, C.; Xuan, Y.; Zhang, X.-S. Multifunctional magnetic-hollow gold nanospheres for bimodal cancer cell imaging and photothermal therapy. Nanotechnology 2015, 26, 315701. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Guo, F.; Wang, J.; Tan, F.; Li, N. Photosensitizer-loaded pH-responsive hollow gold nanospheres for single light-induced photothermal/photodynamic therapy. ACS Appl. Mater. Interfaces 2015, 7, 17592–17597. [Google Scholar] [CrossRef]
- Wu, Y.; Ali, M.R.; Dong, B.; Han, T.; Chen, K.; Chen, J.; Tang, Y.; Fang, N.; Wang, F.; El-Sayed, M.A. Gold nanorod photothermal therapy alters cell junctions and actin network in inhibiting cancer cell collective migration. ACS Nano 2018, 12, 9279–9290. [Google Scholar] [CrossRef]
- Wang, J.; Dong, B.; Chen, B.; Jiang, Z.; Song, H. Selective photothermal therapy for breast cancer with targeting peptide modified gold nanorods. Dalton Trans. 2012, 41, 11134–11144. [Google Scholar] [CrossRef]
- Eshghi, H.; Sazgarnia, A.; Rahimizadeh, M.; Attaran, N.; Bakavoli, M.; Soudmand, S. Protoporphyrin IX–gold nanoparticle conjugates as an efficient photosensitizer in cervical cancer therapy. Photodiagn. Photodyn. Ther. 2013, 10, 304–312. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Kudgus, R.A.; Bhattacharya, R.; Mukherjee, P. Inorganic nanoparticles in cancer therapy. Pharm. Res. 2011, 28, 237–259. [Google Scholar] [CrossRef]
- Cheng, Y.; Samia, A.C.; Meyers, J.D.; Panagopoulos, I.; Fei, B.; Burda, C. Highly efficient drug delivery with gold nanoparticle vectors for in vivo photodynamic therapy of cancer. J. Am. Chem. Soc. 2008, 130, 10643–10647. [Google Scholar] [CrossRef]
- Xia, F.; Hou, W.; Zhang, C.; Zhi, X.; Cheng, J.; Jesús, M.; Song, J.; Cui, D. pH-responsive gold nanoclusters-based nanoprobes for lung cancer targeted near-infrared fluorescence imaging and chemo-photodynamic therapy. Acta Biomater. 2018, 68, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, Y.; Du, H.; Ren, L.; Wang, H. Colloidal plasmonic gold nanoparticles and gold nanorings: Shape-dependent generation of singlet oxygen and their performance in enhanced photodynamic cancer therapy. Int. J. Nanomed. 2018, 13, 2065. [Google Scholar] [CrossRef] [PubMed]
- Meyers, J.D.; Cheng, Y.; Broome, A.M.; Agnes, R.S.; Schluchter, M.D.; Margevicius, S.; Wang, X.; Kenney, M.E.; Burda, C.; Basilion, J.P. Peptide-targeted gold nanoparticles for photodynamic therapy of brain cancer. Part. Part. Syst. Charact. 2015, 32, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Calavia, P.G.; Chambrier, I.; Cook, M.J.; Haines, A.H.; Field, R.A.; Russell, D.A. Targeted photodynamic therapy of breast cancer cells using lactose-phthalocyanine functionalized gold nanoparticles. J. Colloid Interface Sci. 2018, 512, 249–259. [Google Scholar] [CrossRef]
- Mangadlao, J.D.; Wang, X.; McCleese, C.; Escamilla, M.; Ramamurthy, G.; Wang, Z.; Govande, M.; Basilion, J.P.; Burda, C. Prostate-specific membrane antigen targeted gold nanoparticles for theranostics of prostate cancer. ACS Nano 2018, 12, 3714–3725. [Google Scholar] [CrossRef]
- Cheng, Y.; Meyers, J.D.; Broome, A.-M.; Kenney, M.E.; Basilion, J.P.; Burda, C. Deep penetration of a PDT drug into tumors by noncovalent drug-gold nanoparticle conjugates. J. Am. Chem. Soc. 2011, 133, 2583–2591. [Google Scholar] [CrossRef]
- Cheng, Y.; Doane, T.L.; Chuang, C.H.; Ziady, A.; Burda, C. Near infrared light-triggered drug generation and release from gold nanoparticle carriers for photodynamic therapy. Small 2014, 10, 1799–1804. [Google Scholar] [CrossRef]
- Penon, O.; Patiño, T.; Barrios, L.; Nogués, C.; Amabilino, D.B.; Wurst, K.; Pérez-García, L. A new porphyrin for the preparation of functionalized water-soluble gold nanoparticles with low intrinsic toxicity. ChemistryOpen 2015, 4, 127. [Google Scholar] [CrossRef]
- Goswami, N.; Luo, Z.; Yuan, X.; Leong, D.T.; Xie, J. Engineering gold-based radiosensitizers for cancer radiotherapy. Mater. Horiz. 2017, 4, 817–831. [Google Scholar] [CrossRef]
- Zhang, X.D.; Chen, J.; Luo, Z.; Wu, D.; Shen, X.; Song, S.S.; Sun, Y.M.; Liu, P.X.; Zhao, J.; Huo, S. Radiosensitizers: Enhanced Tumor Accumulation of Sub-2 nm Gold Nanoclusters for Cancer Radiation Therapy. Adv. Healthc. Mater. 2014, 3, 152. [Google Scholar] [CrossRef]
- Zhang, X.D.; Luo, Z.; Chen, J.; Shen, X.; Song, S.; Sun, Y.; Fan, S.; Fan, F.; Leong, D.T.; Xie, J. Ultrasmall Au10− 12 (SG) 10− 12 nanomolecules for high tumor specificity and cancer radiotherapy. Adv. Mater. 2014, 26, 4565–4568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-D.; Luo, Z.; Chen, J.; Song, S.; Yuan, X.; Shen, X.; Wang, H.; Sun, Y.; Gao, K.; Zhang, L. Ultrasmall glutathione-protected gold nanoclusters as next generation radiotherapy sensitizers with high tumor uptake and high renal clearance. Sci. Rep. 2015, 5, 8669. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.-T.; Yang, G.; Mo, S.-J.; Wang, Z.-Y.; Li, B.-J.; Ma, W.; Guo, Y.-X.; Chen, X.; Zhao, X.; Liu, J.-Q. Atomically precise gold–levonorgestrel nanocluster as a radiosensitizer for enhanced cancer therapy. ACS Nano 2019, 13, 8320–8328. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Jiang, Y.-W.; Ma, N.; Xia, L.-Y.; Cheng, X.; Jia, H.-R.; Liu, P.; Gu, N.; Chen, Z. Glutathione-depleting gold nanoclusters for enhanced cancer radiotherapy through synergistic external and internal regulations. ACS Appl. Mater. Interfaces 2018, 10, 10601–10606. [Google Scholar] [CrossRef] [PubMed]
- Broekgaarden, M.; Bulin, A.-L.; Porret, E.; Musnier, B.; Chovelon, B.; Ravelet, C.; Sancey, L.; Elleaume, H.; Hainaut, P.; Coll, J.-L. Surface functionalization of gold nanoclusters with arginine: A trade-off between microtumor uptake and radiotherapy enhancement. Nanoscale 2020, 12, 6959–6963. [Google Scholar] [CrossRef]
- Luo, D.; Wang, X.; Zeng, S.; Ramamurthy, G.; Burda, C.; Basilion, J.P. Targeted gold nanocluster-enhanced radiotherapy of prostate cancer. Small 2019, 15, 1900968. [Google Scholar] [CrossRef]
- Kefayat, A.; Ghahremani, F.; Motaghi, H.; Amouheidari, A. Ultra-small but ultra-effective: Folic acid-targeted gold nanoclusters for enhancement of intracranial glioma tumors’ radiation therapy efficacy. Nanomed. Nanotechnol. Biol. Med. 2019, 16, 173–184. [Google Scholar] [CrossRef]
- Ghahremani, F.; Shahbazi-Gahrouei, D.; Kefayat, A.; Motaghi, H.; Mehrgardi, M.A.; Javanmard, S.H. AS1411 aptamer conjugated gold nanoclusters as a targeted radiosensitizer for megavoltage radiation therapy of 4T1 breast cancer cells. RSC Adv. 2018, 8, 4249–4258. [Google Scholar] [CrossRef]
- Llevot, A.; Astruc, D. Applications of vectorized gold nanoparticles to the diagnosis and therapy of cancer. Chem. Soc. Rev. 2012, 41, 242–257. [Google Scholar] [CrossRef]
- Pan, L.; Liu, J.; Shi, J. Cancer cell nucleus-targeting nanocomposites for advanced tumor therapeutics. Chem. Soc. Rev. 2018, 47, 6930–6946. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Irudayaraj, J. Nuclear targeting dynamics of gold nanoclusters for enhanced therapy of HER2+ breast cancer. ACS Nano 2011, 5, 9718–9725. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, B.; Ren, X.; Li, S.; Ma, Y.; Cui, S.; Gu, Y. Multifunctional near-infrared-emitting nano-conjugates based on gold clusters for tumor imaging and therapy. Biomaterials 2012, 33, 8461–8476. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wang, H.; Liu, X.; Wu, Y.; Nie, G. iRGD-coupled responsive fluorescent nanogel for targeted drug delivery. Biomaterials 2013, 34, 3523–3533. [Google Scholar] [CrossRef]
- Croissant, J.G.; Zhang, D.; Alsaiari, S.; Lu, J.; Deng, L.; Tamanoi, F.; AlMalik, A.M.; Zink, J.I.; Khashab, N.M. Protein-gold clusters-capped mesoporous silica nanoparticles for high drug loading, autonomous gemcitabine/doxorubicin co-delivery, and in-vivo tumor imaging. J. Control. Release 2016, 229, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Hale, S.J.; Perrins, R.D.; García, C.E.; Pace, A.; Peral, U.; Patel, K.R.; Robinson, A.; Williams, P.; Ding, Y.; Saito, G. DM1 loaded ultrasmall gold nanoparticles display significant efficacy and improved tolerability in murine models of hepatocellular carcinoma. Bioconj. Chem. 2018, 30, 703–713. [Google Scholar] [CrossRef]
- Chen, D.; Li, B.; Cai, S.; Wang, P.; Peng, S.; Sheng, Y.; He, Y.; Gu, Y.; Chen, H. Dual targeting luminescent gold nanoclusters for tumor imaging and deep tissue therapy. Biomaterials 2016, 100, 1–16. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Amatore, C.; Chen, Y.; Jiang, H.; Wang, X.M. Gold nanoclusters and graphene nanocomposites for drug delivery and imaging of cancer cells. Angew. Chem. 2011, 123, 11848–11852. [Google Scholar] [CrossRef]
- Wang, X.; Cai, X.; Hu, J.; Shao, N.; Wang, F.; Zhang, Q.; Xiao, J.; Cheng, Y. Glutathione-triggered “off–on” release of anticancer drugs from dendrimer-encapsulated gold nanoparticles. J. Am. Chem. Soc. 2013, 135, 9805–9810. [Google Scholar] [CrossRef]
- Chen, T.; Xu, S.; Zhao, T.; Zhu, L.; Wei, D.; Li, Y.; Zhang, H.; Zhao, C. Gold nanocluster-conjugated amphiphilic block copolymer for tumor-targeted drug delivery. ACS Appl. Mater. Interfaces 2012, 4, 5766–5774. [Google Scholar] [CrossRef]
- Zhou, F.; Feng, B.; Yu, H.; Wang, D.; Wang, T.; Liu, J.; Meng, Q.; Wang, S.; Zhang, P.; Zhang, Z. Cisplatin prodrug-conjugated gold nanocluster for fluorescence imaging and targeted therapy of the breast cancer. Theranostics 2016, 6, 679. [Google Scholar] [CrossRef]
- Chen, D.; Luo, Z.; Li, N.; Lee, J.Y.; Xie, J.; Lu, J. Amphiphilic polymeric nanocarriers with luminescent gold nanoclusters for concurrent bioimaging and controlled drug release. Adv. Funct. Mater. 2013, 23, 4324–4331. [Google Scholar] [CrossRef]
- Chen, H.; Li, S.; Li, B.; Ren, X.; Li, S.; Mahounga, D.M.; Cui, S.; Gu, Y.; Achilefu, S. Folate-modified gold nanoclusters as near-infrared fluorescent probes for tumor imaging and therapy. Nanoscale 2012, 4, 6050–6064. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, S.; Mukherjee, S.; Das, S.; Bhat, F.A.; Raja Singh, P.; Patra, C.R.; Arunakaran, J. Gold nanoparticles–conjugated quercetin induces apoptosis via inhibition of EGFR/PI3K/Akt–mediated pathway in breast cancer cell lines (MCF-7 and MDA-MB-231). Cell Biochem. Funct. 2017, 35, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Liang, J.-J.; Chen, H.; Geng, D.-D.; Jiao, L.; Yang, J.-Y.; Qian, H.; Zhang, C.; Ding, Y. Performance of doxorubicin-conjugated gold nanoparticles: Regulation of drug location. ACS Appl. Mater. Interfaces 2017, 9, 8569–8580. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Wang, Y.; Liu, Z.; Fu, L.; Hu, L. Enhancement of radiation effect and increase of apoptosis in lung cancer cells by thio-glucose-bound gold nanoparticles at megavoltage radiation energies. J. Nanopart. Res. 2013, 15, 1642. [Google Scholar] [CrossRef]
- De Jong, W.H.; Hagens, W.I.; Krystek, P.; Burger, M.C.; Sips, A.J.; Geertsma, R.E. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 2008, 29, 1912–1919. [Google Scholar] [CrossRef]
- Alam, F.; Naim, M.; Aziz, M.; Yadav, N. Unique roles of nanotechnology in medicine and cancer-II. Indian J. Cancer 2015, 52, 1. [Google Scholar] [CrossRef]
- Gibson, J.D.; Khanal, B.P.; Zubarev, E.R. Paclitaxel-functionalized gold nanoparticles. J. Am. Chem. Soc. 2007, 129, 11653–11661. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Mwakwari, S.C.; Sodji, Q.H.; Oyelere, A.K.; El-Sayed, M.A. Tamoxifen-poly (ethylene glycol)-thiol gold nanoparticle conjugates: Enhanced potency and selective delivery for breast cancer treatment. Bioconj. Chem. 2009, 20, 2247–2253. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Tsai, C.-Y.; Huang, P.-Y.; Chang, M.-Y.; Cheng, P.-C.; Chou, C.-H.; Chen, D.-H.; Wang, C.-R.; Shiau, A.-L.; Wu, C.-L. Methotrexate conjugated to gold nanoparticles inhibits tumor growth in a syngeneic lung tumor model. Mol. Pharm. 2007, 4, 713–722. [Google Scholar] [CrossRef]
- Dhar, S.; Daniel, W.L.; Giljohann, D.A.; Mirkin, C.A.; Lippard, S.J. Polyvalent oligonucleotide gold nanoparticle conjugates as delivery vehicles for platinum (IV) warheads. J. Am. Chem. Soc. 2009, 131, 14652–14653. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.D.; Nativo, P.; Smith, J.-A.; Stirling, D.; Edwards, P.R.; Venugopal, B.; Flint, D.J.; Plumb, J.A.; Graham, D.; Wheate, N.J. Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. J. Am. Chem. Soc. 2010, 132, 4678–4684. [Google Scholar] [CrossRef] [PubMed]
- Ajnai, G.; Chiu, A.; Kan, T.; Cheng, C.-C.; Tsai, T.-H.; Chang, J. Trends of gold nanoparticle-based drug delivery system in cancer therapy. J. Exp. Clin. Med. 2014, 6, 172–178. [Google Scholar] [CrossRef]
- Annadhasan, M.; Kasthuri, J.; Rajendiran, N. Green synthesis of gold nanoparticles under sunlight irradiation and their colorimetric detection of Ni2+ and Co2+ ions. RSC Adv. 2015, 5, 11458–11468. [Google Scholar] [CrossRef]
- Lipka, J.; Semmler-Behnke, M.; Sperling, R.A.; Wenk, A.; Takenaka, S.; Schleh, C.; Kissel, T.; Parak, W.J.; Kreyling, W.G. Biodistribution of PEG-modified gold nanoparticles following intratracheal instillation and intravenous injection. Biomaterials 2010, 31, 6574–6581. [Google Scholar] [CrossRef]
- Truong, N.P.; Whittaker, M.R.; Mak, C.W.; Davis, T.P. The importance of nanoparticle shape in cancer drug delivery. Expert Opin. Drug Deliv. 2015, 12, 129–142. [Google Scholar] [CrossRef]
- Kel, A.E.; Stegmaier, P.; Valeev, T.; Koschmann, J.; Poroikov, V.; Kel-Margoulis, O.V.; Wingender, E. Multi-omics “upstream analysis” of regulatory genomic regions helps identifying targets against methotrexate resistance of colon cancer. EuPA Open Proteom. 2016, 13, 1–13. [Google Scholar] [CrossRef]
- Rizk, N.; Christoforou, N.; Lee, S. Optimization of anti-cancer drugs and a targeting molecule on multifunctional gold nanoparticles. Nanotechnology 2016, 27, 185704. [Google Scholar] [CrossRef]
- Heo, D.N.; Yang, D.H.; Moon, H.-J.; Lee, J.B.; Bae, M.S.; Lee, S.C.; Lee, W.J.; Sun, I.-C.; Kwon, I.K. Gold nanoparticles surface-functionalized with paclitaxel drug and biotin receptor as theranostic agents for cancer therapy. Biomaterials 2012, 33, 856–866. [Google Scholar] [CrossRef]
- Lin, W.; Yao, N.; Qian, L.; Zhang, X.; Chen, Q.; Wang, J.; Zhang, L. pH-responsive unimolecular micelle-gold nanoparticles-drug nanohybrid system for cancer theranostics. Acta Biomater. 2017, 58, 455–465. [Google Scholar] [CrossRef]
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AuNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.V.; Gunsolus, I.L.; Qiu, T.A.; Hurley, K.R.; Nyberg, L.H.; Frew, H.; Johnson, K.P.; Vartanian, A.M.; Jacob, L.M.; Lohse, S.E. Impacts of gold nanoparticle charge and ligand type on surface binding and toxicity to Gram-negative and Gram-positive bacteria. Chem. Sci. 2015, 6, 5186–5196. [Google Scholar] [CrossRef] [PubMed]
- Schaeublin, N.M.; Braydich-Stolle, L.K.; Schrand, A.M.; Miller, J.M.; Hutchison, J.; Schlager, J.J.; Hussain, S.M. Surface charge of gold nanoparticles mediates mechanism of toxicity. Nanoscale 2011, 3, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.J.; MacCuspie, R.I.; Gigault, J.; Gorham, J.M.; Elliott, J.T.; Hackley, V.A. Highly stable positively charged dendron-encapsulated gold nanoparticles. Langmuir 2014, 30, 3883–3893. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Xu, K.; Ji, L.; Tang, B. Effect of gold nanoparticles on glutathione depletion-induced hydrogen peroxide generation and apoptosis in HL7702 cells. Toxicol. Lett. 2011, 205, 86–95. [Google Scholar] [CrossRef]
- Rosli, N.S.b.; Rahman, A.A.; Aziz, A.A.; Shamsuddin, S. Determining the size and concentration dependence of gold nanoparticles in vitro cytotoxicity (IC50) test using WST-1 assay. AIP Conf. Proc. 2015, 1657, 060001. [Google Scholar]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef]

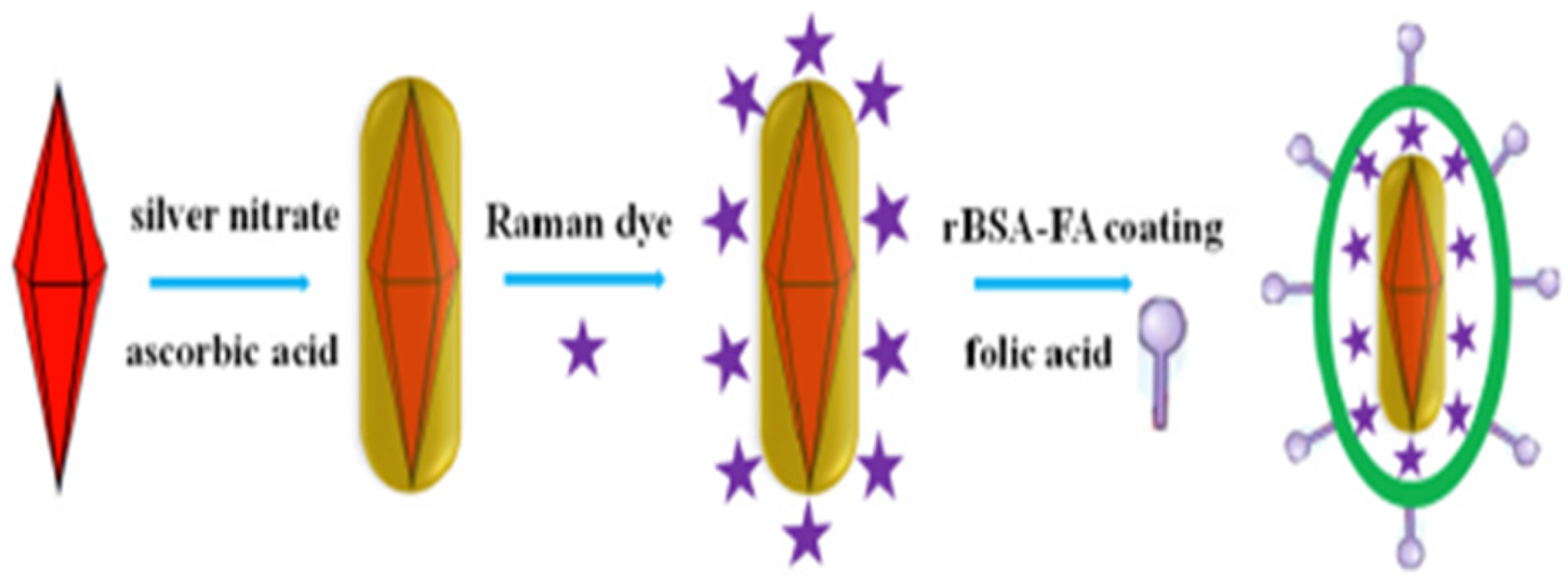


| Nanoparticle | Method | Size (nm) | Shape | Application | Ref |
|---|---|---|---|---|---|
| AuNPs | Turkevich | 3.5 | Spherical | ………. | [49] |
| AuNPs | Seed-mediated | 30–150 | Spherical | ………. | [51] |
| AuNPs | Laser irradiation | 24 | Spherical | ………. | [52] |
| AuNPs | γ-irradiation | 5–40 | Semi-spherical | ………. | [59] |
| AuNRs | Chemical reduction | ………. | rod | ………. | [63] |
| AuNPs | Reduction by glutamic acid | 40 | Spherical | Bioconjugates | [64] |
| AuNPs | Brust | 1–3 | Semi-spherical | ………. | [66] |
| AuNPs | Reduction | 180 | Decahedral | ………. | [69] |
| AuNPs | Green | 10 | Spherical, triangular and hexagonal | ………. | [70] |
| AuNPs | Sonochemical | 22 | Spherical | ………. | [88] |
| AuNPs | Laser ablation | 49 | Spherical | ………. | [88] |
| AuNPs | Sonochemical | 18.5 | Spherical | Computed tomography | [5] |
| AuNPs | Sonochemical | 13.6, 18.6 and 22.3 | Spherical | Catalysis | [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dheyab, M.A.; Aziz, A.A.; Moradi Khaniabadi, P.; Jameel, M.S.; Oladzadabbasabadi, N.; Mohammed, S.A.; Abdullah, R.S.; Mehrdel, B. Monodisperse Gold Nanoparticles: A Review on Synthesis and Their Application in Modern Medicine. Int. J. Mol. Sci. 2022, 23, 7400. https://doi.org/10.3390/ijms23137400
Dheyab MA, Aziz AA, Moradi Khaniabadi P, Jameel MS, Oladzadabbasabadi N, Mohammed SA, Abdullah RS, Mehrdel B. Monodisperse Gold Nanoparticles: A Review on Synthesis and Their Application in Modern Medicine. International Journal of Molecular Sciences. 2022; 23(13):7400. https://doi.org/10.3390/ijms23137400
Chicago/Turabian StyleDheyab, Mohammed Ali, Azlan Abdul Aziz, Pegah Moradi Khaniabadi, Mahmood S. Jameel, Nazila Oladzadabbasabadi, Selwan Abduljabbar Mohammed, Raja Saleh Abdullah, and Baharak Mehrdel. 2022. "Monodisperse Gold Nanoparticles: A Review on Synthesis and Their Application in Modern Medicine" International Journal of Molecular Sciences 23, no. 13: 7400. https://doi.org/10.3390/ijms23137400
APA StyleDheyab, M. A., Aziz, A. A., Moradi Khaniabadi, P., Jameel, M. S., Oladzadabbasabadi, N., Mohammed, S. A., Abdullah, R. S., & Mehrdel, B. (2022). Monodisperse Gold Nanoparticles: A Review on Synthesis and Their Application in Modern Medicine. International Journal of Molecular Sciences, 23(13), 7400. https://doi.org/10.3390/ijms23137400