H2+CO2 Synergistic Plasma Positioning Carboxyl Defects in g-C3N4 with Engineered Electronic Structure and Active Sites for Efficient Photocatalytic H2 Evolution
Abstract
:1. Introduction
2. Results and Discussion
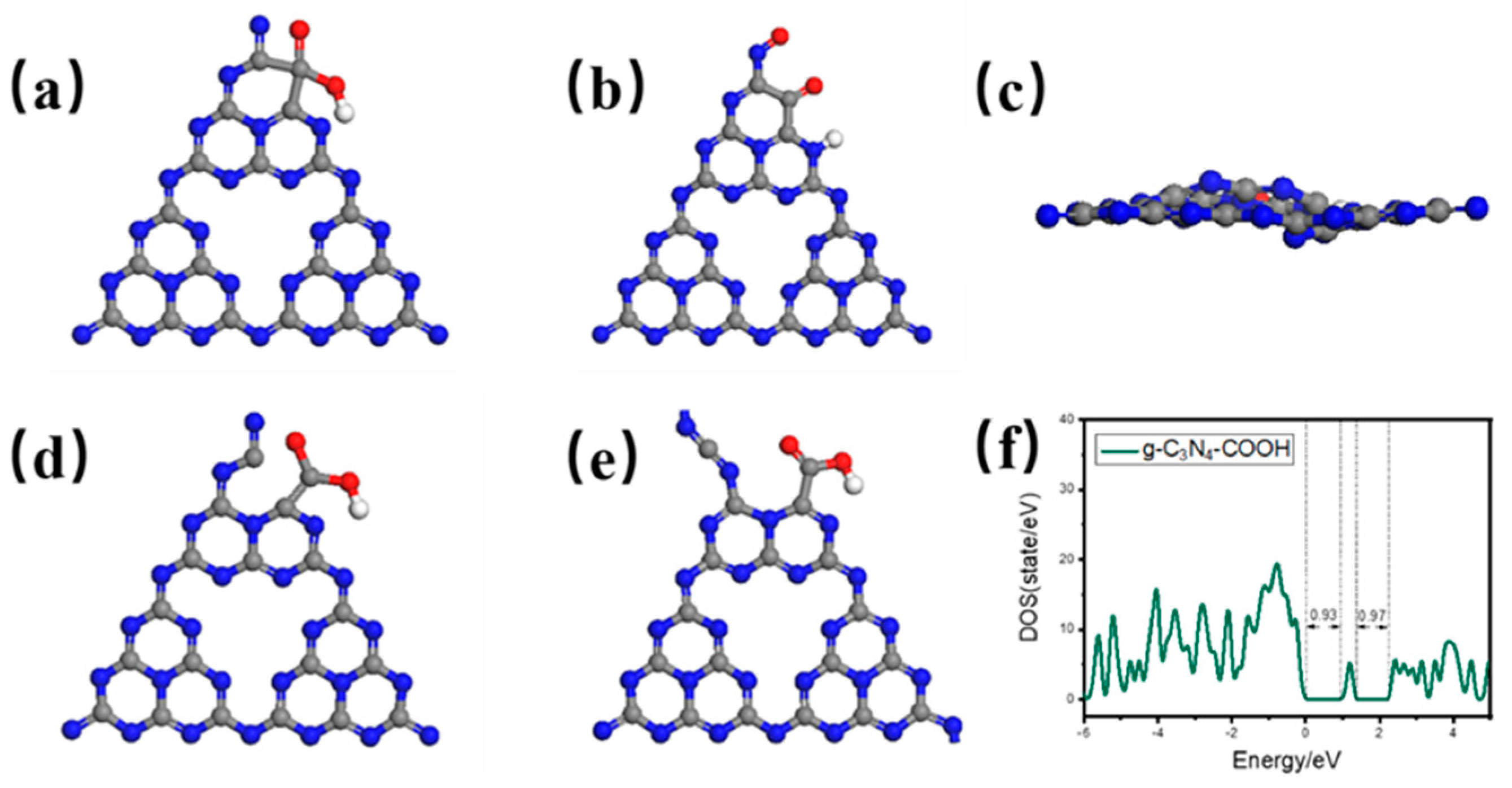
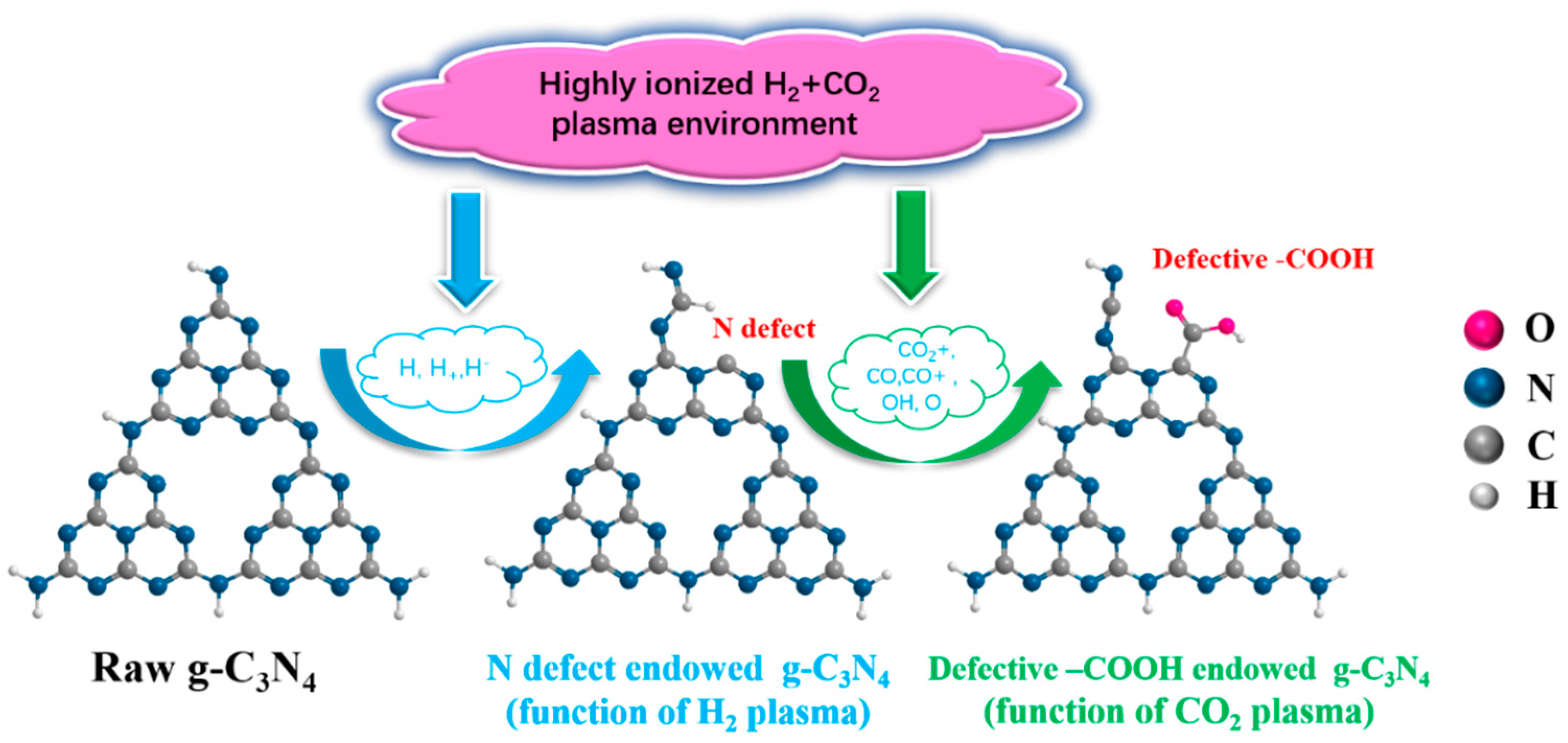
2.1. DFT Calculation of Carboxyl-Defective g-C3N4
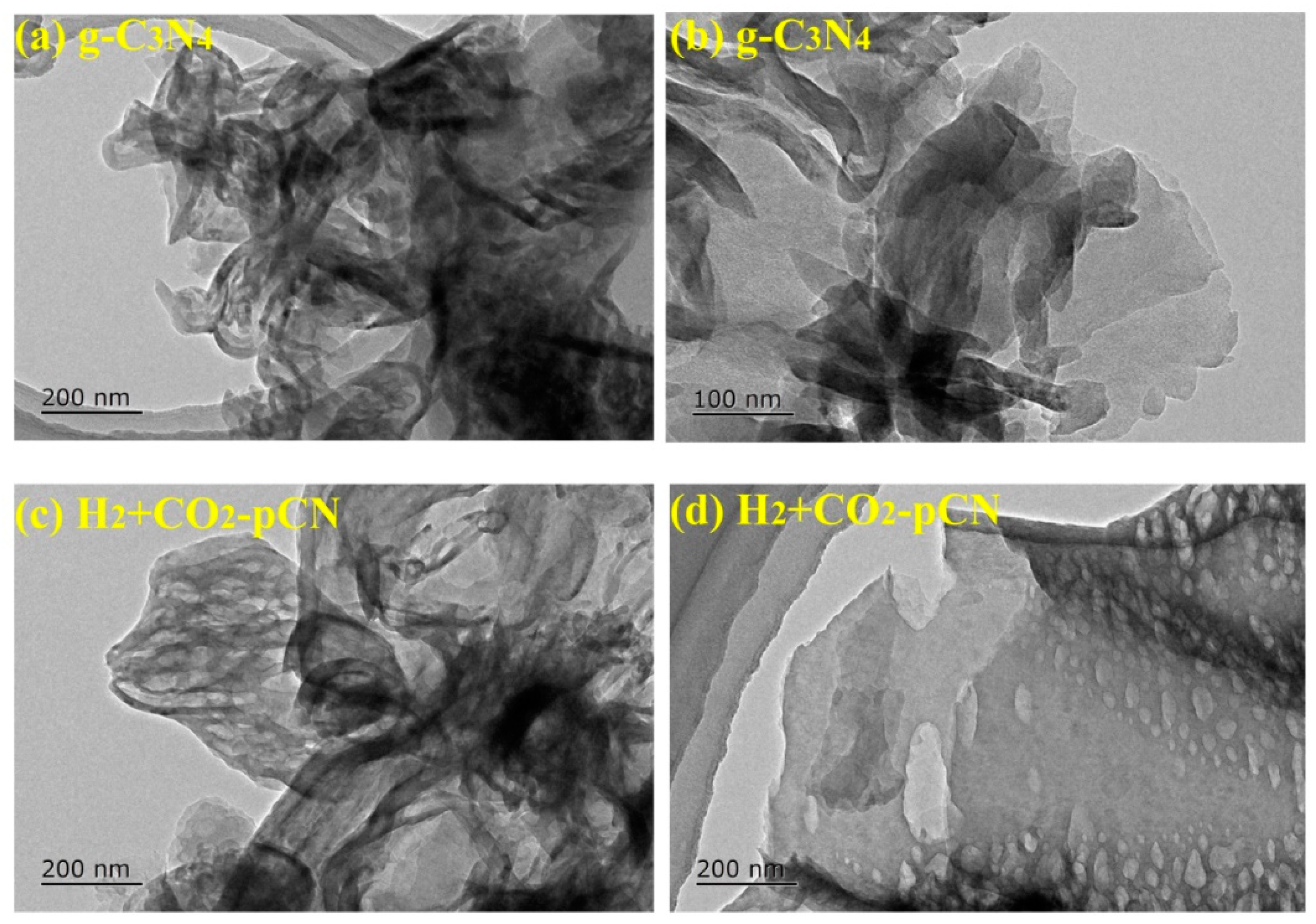
2.2. Morphology
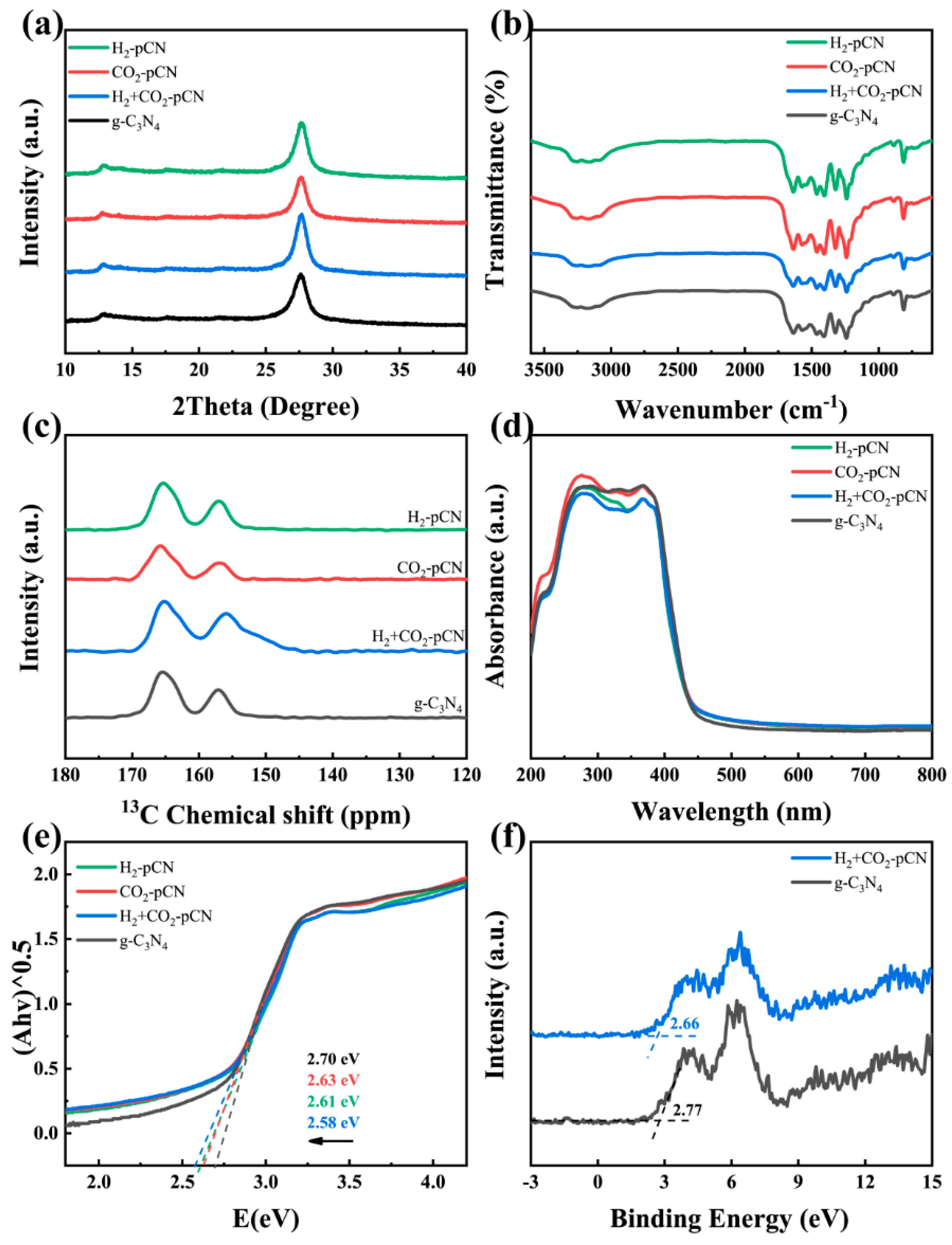
2.3. Physicochemical Characterization
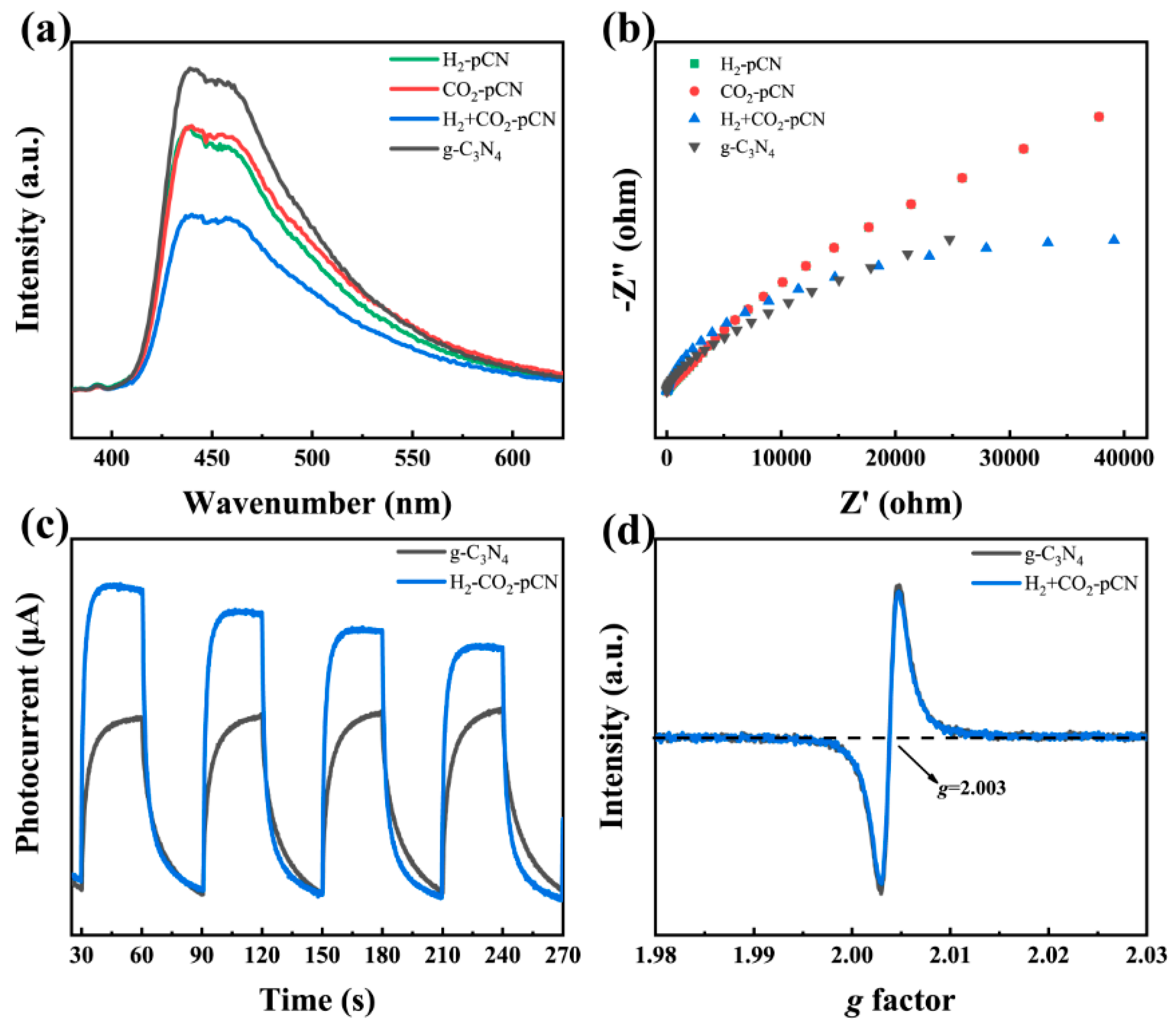
2.4. Electronic and Electrochemical Properties
2.5. Visible-Light-Driven H2 Evolution Performances and Optimization Mechanism
3. Materials and Methods
3.1. Materials
3.2. Preparation of Raw g-C3N4 and Carboxyl-Defective g-C3N4
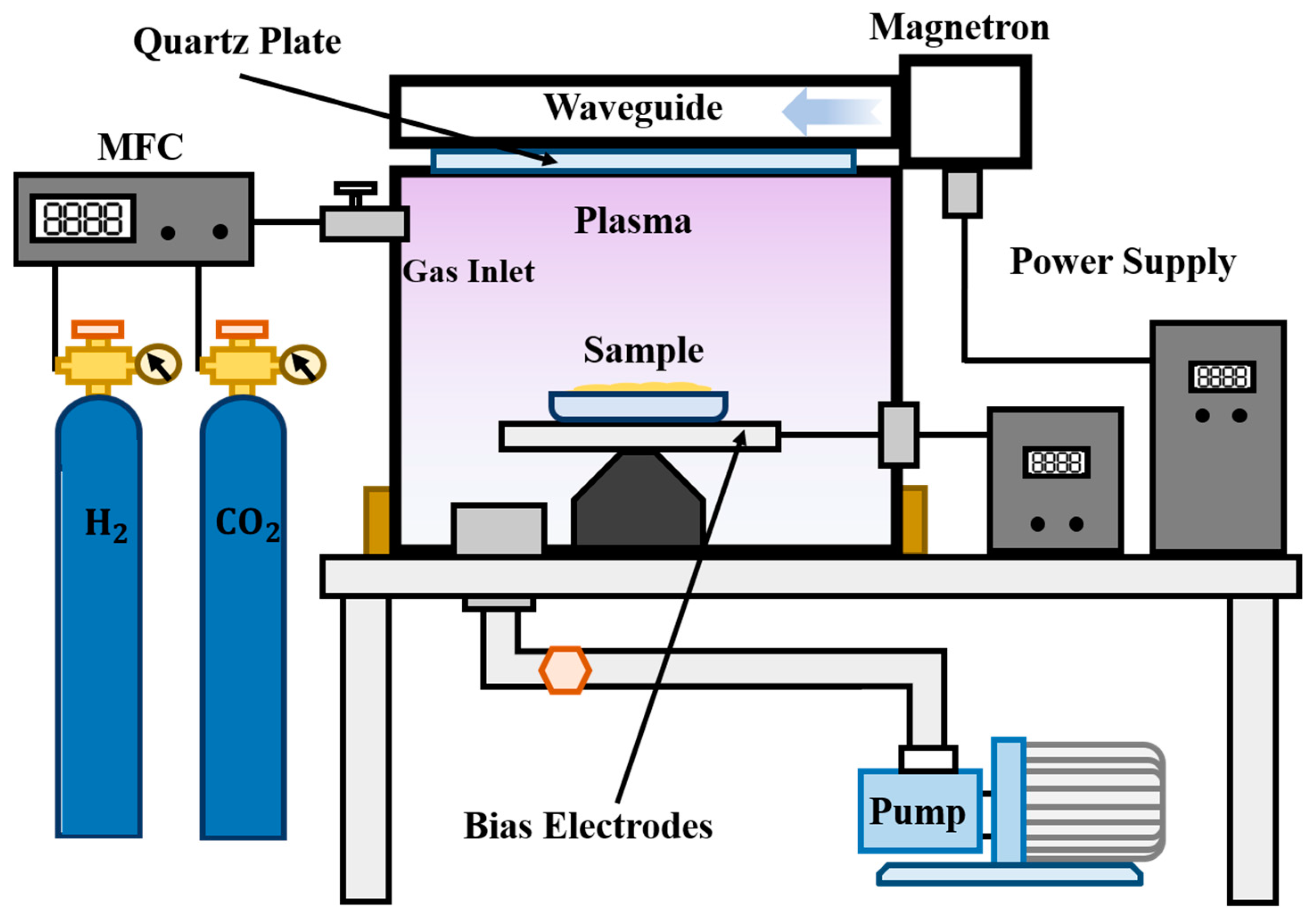
3.3. Plasma Equipment and Process
3.4. Characterization of Carboxyl-Defective g-C3N4
3.5. DFT Calculation
3.6. Photocatalytic Degradation Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takata, T.; Jiang, J.; Sakata, Y.; Nakabayashi, M.; Shibata, N.; Nandal, V.; Seki, K.; Hisatomi, T.; Domen, K. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 2020, 581, 411–414. [Google Scholar] [CrossRef]
- Tao, X.P.; Zhao, Y.; Wang, S.Y.; Li, C.; Li, R.G. Recent advances and perspectives for solar-driven water splitting using particulate photocatalysts. Chem. Soc. Rev. 2022, 51, 3561–3608. [Google Scholar] [CrossRef] [PubMed]
- Tay, Q.L.; Kanhere, P.; Ng, C.F.; Chen, S.; Chakraborty, S.; Huan, A.C.H.; Sum, T.C.; Ahuja, R.; Chen, Z. Defect engineered g-C3N4 for efficient visible light photocatalytic hydrogen production. Chem. Mater. 2015, 27, 4930–4933. [Google Scholar] [CrossRef]
- Dong, J.Q.; Zhang, Y.; Hussain, M.I.; Zhou, W.J.; Chen, Y.Z.; Wang, L.N. g-C3N4: Properties, pore modifications, and photocatalytic applications. Nanomaterials 2021, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Nor, N.U.M.; Mazalan, E.; Amin, N.A.S. Insights into enhancing photocatalytic reduction of CO2: Substitutional defect strategy of modified g-C3N4 by experimental and theoretical calculation approaches. J. Alloys Compd. 2021, 871, 159464. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.E.; Liu, Y.L.; Cen, W.L.; Luo, X.B. Functional group effects on the HOMO–LUMO gap of g-C3N4. Nanomaterials 2018, 8, 589. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chen, J.W.; Wan, Y.F.; Liu, H.W.; Chen, W.; Wang, G.; Wang, R.L. Defect engineering in atomic-layered graphitic carbon nitride for greatly extended visible-light photocatalytic hydrogen evolution. ACS Appl. Mater. Interfaces 2020, 12, 13805–13812. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, P.J.; Wang, C.; Gan, L.-Y.; Chen, X.J.; Zhang, P.; Wang, Y.; Li, H.; Wang, L.H.; Zhou, X.Y.; et al. Unraveling the dual defect sites in graphite carbon nitride for Ultra-high photocatalytic H2O2 evolution. Energy Environ. Sci. 2022, 15, 830–842. [Google Scholar] [CrossRef]
- Zhang, F.Z.; Liao, J.B.; Lu, J.J.; Niu, J.F. Extensive incorporation of carboxyl groups into g-C3N4 by integrated oxygen doping and HNO3 oxidation for enhanced catalytic ozonation of para-chlorobenzoic acid and atrazine. Sep. Purif. Technol. 2021, 256, 117806. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Wang, J.J.; Dong, L.; Zhang, W.T.; Lu, Z.H.; Liang, J.J.; Pan, D.Q.; Fan, Q.H. Carboxyl groups on g-C3N4 for boosting the photocatalytic U (VI) reduction in the presence of carbonates. Chem. Eng. J. 2021, 414, 128810. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.F.; Tan, J.; Meng, Y.; Lu, Y.; Zhang, T.T. First-principles study of S-doped point defects with different charge states in monolayer g-C3N4. Appl. Surf. Sci. 2021, 554, 149601. [Google Scholar] [CrossRef]
- Radovic, L.R.; Mora-Vilches, C.V.; Salgado-Casanova, A.J.; Buljan, A. Graphene functionalization: Mechanism of carboxyl group formation. Carbon 2018, 130, 340–349. [Google Scholar] [CrossRef]
- Şahin, Ö.; Yardim, Y.; Baytar, O.; Saka, C. Enhanced electrochemical double-layer capacitive performance with CO2 plasma treatment on activated carbon prepared from pyrolysis of pistachio shells. Int. J. Hydrogen Energy 2020, 45, 8843–8852. [Google Scholar] [CrossRef]
- Yoo, S.; Seok, D.; Jung, Y.; Lee, K. Hydrophilic surface treatment of carbon powder using CO2 plasma activated gas. Coatings 2021, 11, 925. [Google Scholar] [CrossRef]
- Qu, X.Y.; Hu, S.Z.; Li, P.; Li, Z.; Wang, H.; Ma, H.F.; Li, W. The effect of embedding N vacancies into g-C3N4 on the photocatalytic H2O2 production ability via H2 plasma treatment. Diam. Relat. Mater. 2018, 86, 159–166. [Google Scholar] [CrossRef]
- Martinez, H.; Reyes, P.; Vergara-Sanchez, J.; Contreras, V.; Cisneros, C.; Yousif, F. Langmuir probe, optical, and mass characterization of a DC CO2–H2 plasma. Phys. Plasmas 2020, 27, 083501. [Google Scholar] [CrossRef]
- Xiang, Q.J.; Li, F.; Zhang, D.N.; Liao, Y.L.; Zhou, H.P. Plasma-based surface modification of g-C3N4 nanosheets for highly efficient photocatalytic hydrogen evolution. Appl. Surf. Sci. 2019, 495, 143520. [Google Scholar] [CrossRef]
- Ruan, D.; Kim, S.; Fujitsuka, M.; Majima, T. Defects rich g-C3N4 with mesoporous structure for efficient photocatalytic H2 production under visible light irradiation. Appl. Catal. B Environ. 2018, 238, 638–646. [Google Scholar] [CrossRef]
- Lv, H.L.; Zhou, X.D.; Wu, G.L.; Kara, U.I.; Wang, X.G. Engineering defects in 2D g-C3N4 for wideband, efficient electromagnetic absorption at elevated temperature. J. Mater. Chem. A 2021, 9, 19710–19718. [Google Scholar] [CrossRef]
- Yang, H.H.; Qian, X.R.; Zhang, N.; Zhang, L.; Zhou, M.J. KNO3-Assisted incorporation of K dopants and N defects into g-C3N4 with enhanced visible light driven photocatalytic H2O2 production. New J. Chem. 2021, 45, 22591–22601. [Google Scholar] [CrossRef]
- Liao, J.Z.; Cui, W.; Li, J.Y.; Sheng, J.P.; Wang, H.; Dong, X.; Chen, P.; Jiang, G.M.; Wang, Z.M.; Dong, F. Nitrogen defect structure and NO+ intermediate promoted photocatalytic NO removal on H2 treated g-C3N4. Chem. Eng. J. 2020, 379, 122282. [Google Scholar] [CrossRef]
- Sun, S.Q.; Wu, Y.C.; Zhu, J.F.; Lu, C.J.; Sun, Y.; Wang, Z.; Chen, J. Stabilizing plasma-induced highly nitrogen-deficient g-C3N4 by heteroatom-refilling for excellent lithium-ion battery anodes. Chem. Eng. J. 2022, 427, 131032. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Sun, Y.; Chang, X.; Zhang, P. Template-free synthesis of one-dimensional g-C3N4 chain nanostructures for efficient photocatalytic hydrogen evolution. Front. Chem. 2021, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, D.; Kędzierski, T.; Aleksandrzak, M.; Mijowska, E.; Zielińska, B. Influence of hydrogenation on morphology, chemical structure and photocatalytic efficiency of graphitic carbon nitride. Int. J. Mol. Sci. 2021, 22, 13096. [Google Scholar] [CrossRef]
- Wu, C.B.; Han, Q.; Qu, L.T. Functional group defect design in polymeric carbon nitride for photocatalytic application. APL Mater. 2020, 8, 120703. [Google Scholar] [CrossRef]
- Lv, S.W.; Liu, J.M.; Li, C.Y.; Zhao, N.; Wang, Z.H.; Wang, S. In situ growth of benzothiadiazole functionalized UiO-66-NH2 on carboxyl modified g-C3N4 for enhanced photocatalytic degradation of sulfamethoxazole under visible light. Catal. Sci. Technol. 2020, 10, 4703–4711. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Cui, L.F.; Zhang, Y.; Klausen, L.H.; Chen, M.Y.; Sun, D.; Xu, S.Y.; Kang, S.F.; Shi, J.Y. Regulation of carboxyl groups and structural defects of graphitic carbon nitride via environmental-friendly glucose oxidase ring-opening modulation. Appl. Catal. B Environ. 2021, 297, 120441. [Google Scholar] [CrossRef]
- Hernández-Uresti, D.B.; Vázquez, A.; Sanchez-Martinez, D.; Obregón, S. Performance of the polymeric g-C3N4 photocatalyst through the degradation of pharmaceutical pollutants under UV–vis irradiation. J. Photochem. Photobiol. A Chem. 2016, 324, 47–52. [Google Scholar] [CrossRef]
- Yu, F.Y.; Chen, L.Y.; Shen, X.S.; Li, X.Z.; Duan, C.Y. NH2-UiO-66/g-C3N4/CdTe composites for photocatalytic CO2 reduction under visible light. APL Mater. 2019, 7, 101101. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yang, P. The edge-epitaxial growth of yellow g-C3N4 on red g-C3N4 nanosheets with superior photocatalytic activities. Chem. Commun. 2021, 57, 3119–3122. [Google Scholar] [CrossRef]
- Pan, J.Q.; Jiang, Z.Y.; Feng, S.X.; Zhao, C.; Dong, Z.J.; Wang, B.B.; Wang, J.J.; Song, C.S.; Zheng, Y.Y.; Li, C.R. The enhanced photocatalytic hydrogen production of the fusiform g-C3N4 modification CaTiO3 nano-heterojunction. Int. J. Hydrogen Energy 2018, 43, 19019–19028. [Google Scholar] [CrossRef]
- Teye, G.K.; Huang, J.Y.; Li, Y.; Li, K.; Chen, L.; Darkwah, W.K. Photocatalytic degradation of sulfamethoxazole, nitenpyram and tetracycline by composites of core shell g-C3N4@ ZnO, and ZnO defects in aqueous phase. Nanomaterials 2021, 11, 2609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Zhang, J.X.; Xia, Y.Y.; Xun, M.H.; Chen, H.; Liu, X.H.; Yin, X. Metal-free carbon quantum dots implant graphitic carbon nitride: Enhanced photocatalytic dye wastewater purification with simultaneous hydrogen production. Int. J. Mol. Sci. 2020, 21, 1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, C.Y.; Zhou, Y.Z.; Zhang, L.L.; Yu, X.H.; Gao, S.; Sun, X.J.; Cheng, C.; Liu, Q.Q.; Yang, J. Enhanced n→ π* electron transition of porous P-doped g-C3N4 nanosheets for improved photocatalytic H2 evolution performance. Ceram. Int. 2020, 46, 8444–8451. [Google Scholar] [CrossRef]
- Wu, X.H.; Wang, X.F.; Wang, F.Z.; Yu, H.G. Soluble g-C3N4 nanosheets: Facile synthesis and application in photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2019, 247, 70–77. [Google Scholar] [CrossRef]
- Long, D.; Chen, W.L.; Zheng, S.H.; Rao, X.; Zhang, Y.P. Barium-and phosphorus-codoped g-C3N4 microtubes with efficient photocatalytic H2 evolution under visible light irradiation. Ind. Eng. Chem. Res. 2020, 59, 4549–4556. [Google Scholar] [CrossRef]
- Yan, J.T.; Liu, J.H.; Sun, Y.; Ding, D.; Wang, C.L.; Sun, L.-B.; Li, X.F. Exfoliation-induced O-doped g-C3N4 nanosheets with improved photoreactivity towards RhB degradation and H2 evolution. Inorg. Chem. Front. 2022, 9, 1423–1433. [Google Scholar] [CrossRef]
- Yu, T.; Xie, T.; Zhou, W.; Zhang, Y.Z.; Chen, Y.L.; Shao, B.Y.; Guo, W.-Q.; Tan, X. Fumaric acid assistant band structure tunable nitrogen defective g-C3N4 fabrication for enhanced photocatalytic hydrogen evolution. ACS Sustain. Chem. Eng. 2021, 9, 7529–7540. [Google Scholar] [CrossRef]
- Berthelot, A.; Bogaerts, A. Modeling of CO2 plasma: Effect of uncertainties in the plasma chemistry. Plasma Sources Sci. Technol. 2017, 26, 115002. [Google Scholar] [CrossRef]
- Kelly, S.; Sullivan, J.A. CO2 decomposition in CO2 and CO2/H2 spark-like plasma discharges at atmospheric pressure. ChemSusChem 2019, 12, 3785–3791. [Google Scholar] [CrossRef]
- Zhao, J.S.; Sun, Z.; Ren, Y.X.; Song, L.; Wang, S.Z.; Liu, W.; Yu, Z.; Wei, Y.H. Experimental characteristics of 2.45 GHz microwave reconfigurable plasma antennas. J. Phys. D Appl. Phys. 2019, 52, 295202. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Zhang, Z.; Xu, S.; Guo, Y.; Kang, S.; Chang, X. H2+CO2 Synergistic Plasma Positioning Carboxyl Defects in g-C3N4 with Engineered Electronic Structure and Active Sites for Efficient Photocatalytic H2 Evolution. Int. J. Mol. Sci. 2022, 23, 7381. https://doi.org/10.3390/ijms23137381
Wang D, Zhang Z, Xu S, Guo Y, Kang S, Chang X. H2+CO2 Synergistic Plasma Positioning Carboxyl Defects in g-C3N4 with Engineered Electronic Structure and Active Sites for Efficient Photocatalytic H2 Evolution. International Journal of Molecular Sciences. 2022; 23(13):7381. https://doi.org/10.3390/ijms23137381
Chicago/Turabian StyleWang, Daqian, Zhihao Zhang, Shuchuan Xu, Ying Guo, Shifei Kang, and Xijiang Chang. 2022. "H2+CO2 Synergistic Plasma Positioning Carboxyl Defects in g-C3N4 with Engineered Electronic Structure and Active Sites for Efficient Photocatalytic H2 Evolution" International Journal of Molecular Sciences 23, no. 13: 7381. https://doi.org/10.3390/ijms23137381
APA StyleWang, D., Zhang, Z., Xu, S., Guo, Y., Kang, S., & Chang, X. (2022). H2+CO2 Synergistic Plasma Positioning Carboxyl Defects in g-C3N4 with Engineered Electronic Structure and Active Sites for Efficient Photocatalytic H2 Evolution. International Journal of Molecular Sciences, 23(13), 7381. https://doi.org/10.3390/ijms23137381





