Advances in Membrane-Bound Catechol-O-Methyltransferase Stability Achieved Using a New Ionic Liquid-Based Storage Formulation
Abstract
:1. Introduction
2. Results and Discussion
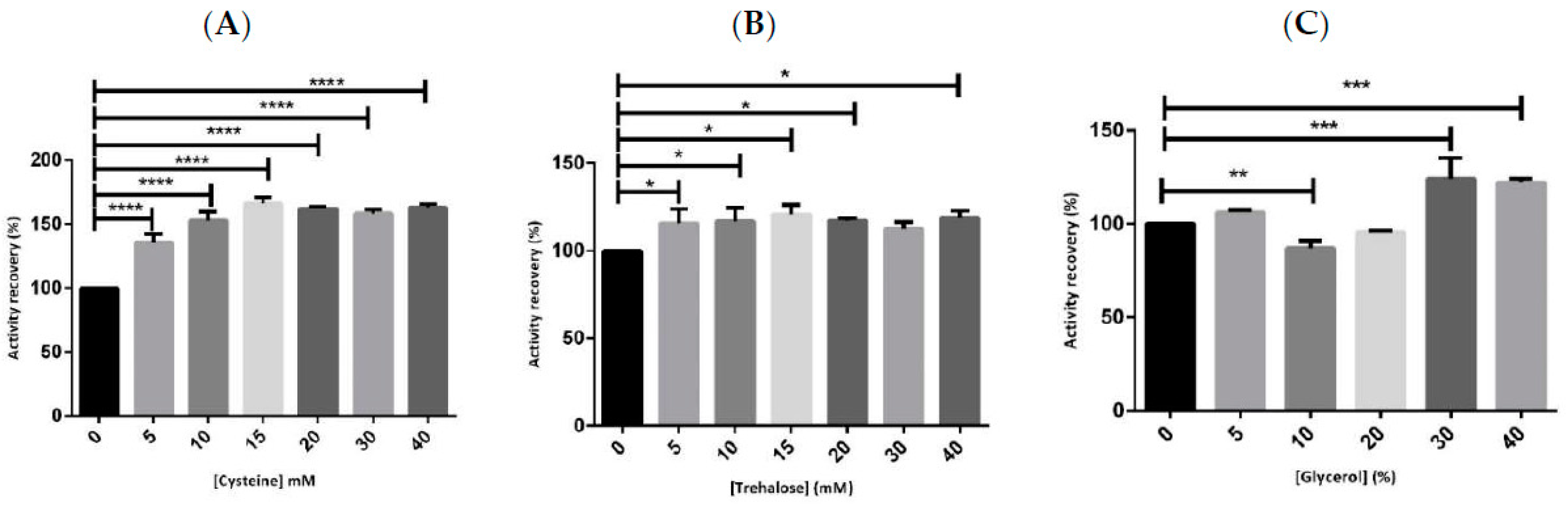
2.1. Preliminary Studies for hMBCOMT Stabilization
2.2. Enzymatic Stability with Multicomponent Buffer
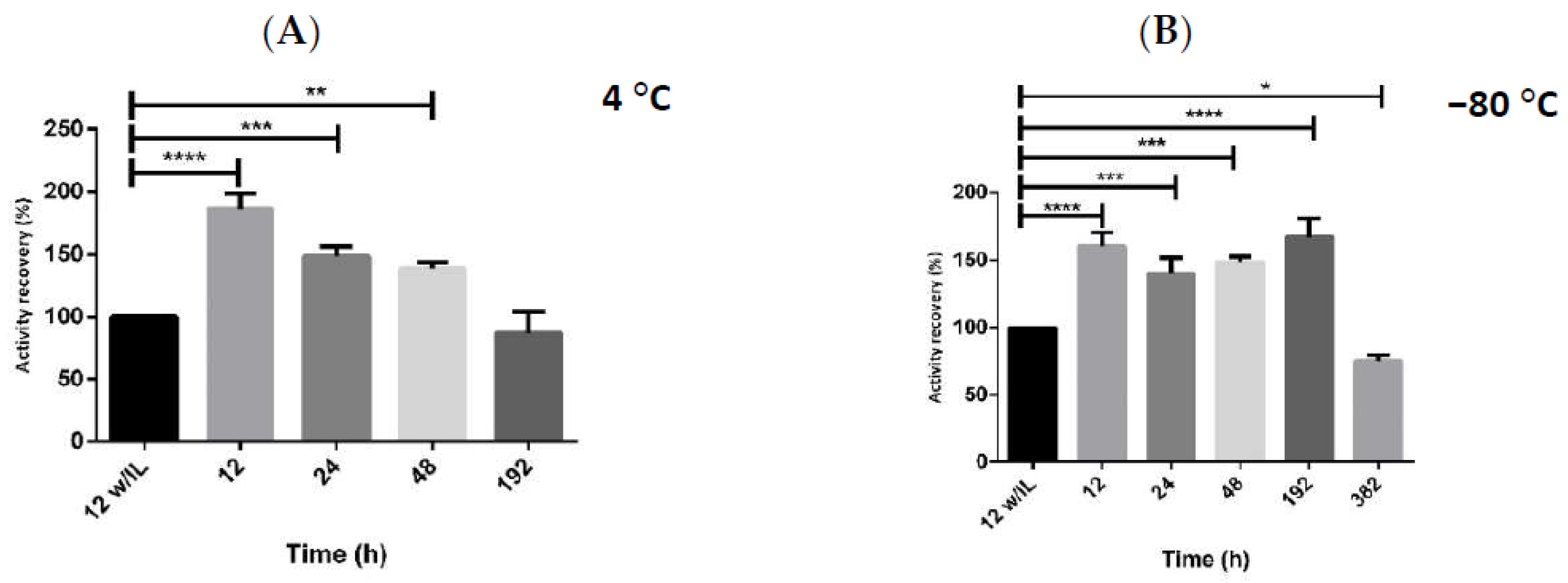
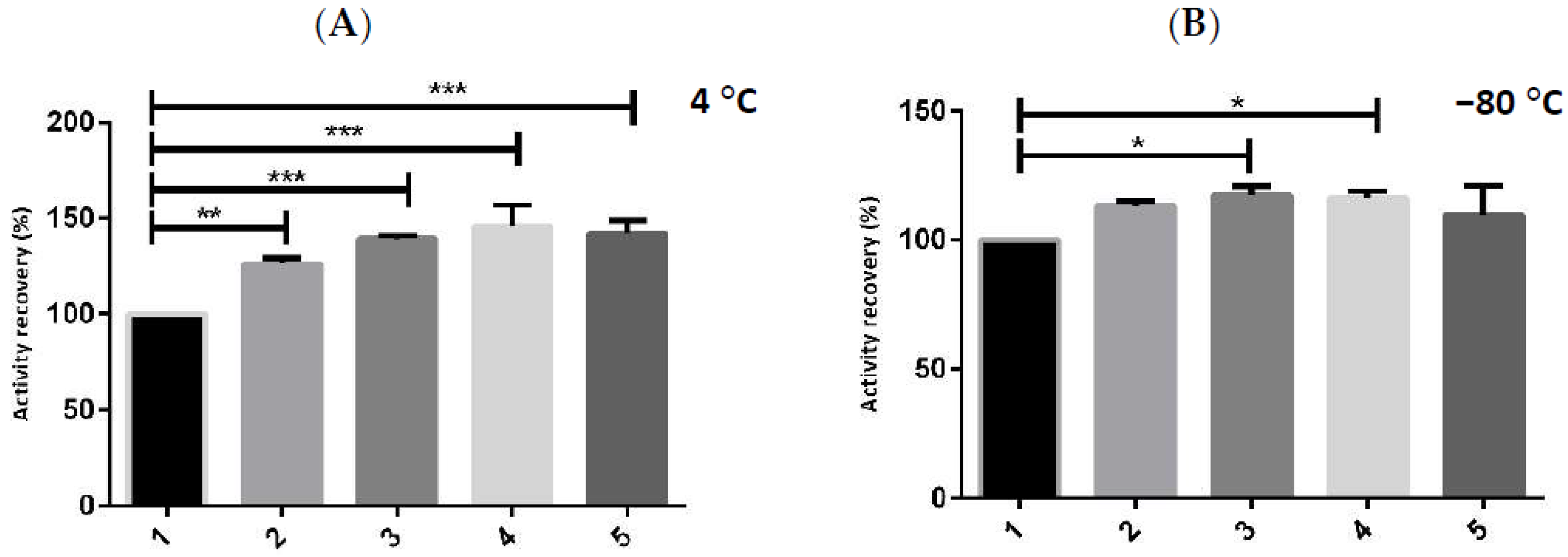
2.3. Storage Stability of hMBCOMT with DoE
2.4. Model Generation and Statistical Analysis
2.5. Model Generation and Statistical Analysis
2.6. Output Optimization and Model Validation
3. Materials and Methods
3.1. Instruments, Softwares, Materials and Reagents
3.2. Recombinant hMBCOMT Biosynthesis and Recuperation
3.3. hMBCOMT Stabilization Studies at Different Time and Temperatures
3.4. Design of Experiments (DoE)
3.5. Output Determination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Antonini, A.; Abbruzzese, G.; Barone, P.; Bonuccelli, U.; Lopiano, L.; Onofrj, M.; Zappia, M.; Quattrone, A. COMT inhibition with tolcapone in the treatment algorithm of patients with Parkinson’s disease (PD): Relevance for motor and non-motor features. Neuropsychiatr. Dis. Treat. 2008, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Boivin, S.; Kozak, S.; Meijers, R. Optimization of protein purification and characterization using Thermofluor screens. Protein Expr. Purif. 2013, 91, 192–206. [Google Scholar] [CrossRef]
- Apud, J.A.; Weinberger, D.R. Treatment of cognitive deficits associated with schizophrenia: Potential role of catechol-O-methyltransferase inhibitors. CNS Drugs 2007, 21, 535–557. [Google Scholar] [CrossRef]
- Müller, T. Catechol-O-methyltransferase inhibitors in Parkinson’s disease. Drugs 2015, 75, 157–174. [Google Scholar] [CrossRef]
- Pfeiffer, E.; Wefers, D.; Hildebrand, A.A.; Fleck, S.C.; Metzler, M. Catechol metabolites of the mycotoxin zearalenone are poor substrates but potent inhibitors of catechol-O-methyltransferase. Mycotoxin Res. 2013, 29, 177–183. [Google Scholar] [CrossRef]
- Yager, J.D. Catechol-O-methyltransferase: Characteristics, polymorphisms and role in breast cancer. Drug Discov. Today. Dis. Mech. 2012, 9, e41–e46. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Olson, J.; Zhang, J.; Hildebrandt, M.; Wang, L.; Ingle, J.; Fredericksen, Z.; Sellers, T.; Miller, W.; Dixon, J.M.; et al. Breast cancer risk reduction and membrane-bound catechol O-methyltransferase genetic polymorphisms. Cancer Res. 2008, 68, 5997–6005. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.T. On the mechanism of homocysteine pathophysiology and pathogenesis: A unifying hypothesis. Histol. Histopathol. 2002, 17, 1283–1291. [Google Scholar] [CrossRef]
- Bai, H.W.; Shim, J.Y.; Yu, J.; Zhu, B.T. Biochemical and molecular modeling studies of the O-methylation of various endogenous and exogenous catechol substrates catalyzed by recombinant human soluble and membrane-bound catechol-O-methyltransferases. Chem. Res. Toxicol. 2007, 20, 1409–1425. [Google Scholar] [CrossRef]
- Cotton, N.J.; Stoddard, B.; Parson, W.W. Oxidative inhibition of human soluble catechol-O-methyltransferase. J. Biol. Chem. 2004, 279, 23710–23718. [Google Scholar] [CrossRef] [Green Version]
- Santos, F.M.; Pedro, A.Q.; Soares, R.F.; Martins, R.; Bonifácio, M.J.; Queiroz, J.A.; Passarinha, L.A. Performance of hydrophobic interaction ligands for human membrane-bound catechol-O-methyltransferase purification. J. Sep. Sci. 2013, 36, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Vagenende, V.; Yap, M.G.; Trout, B.L. Mechanisms of protein stabilization and prevention of protein aggregation by glycerol. Biochemistry 2009, 48, 11084–11096. [Google Scholar] [CrossRef]
- Pedro, A.Q.; Pereira, P.; Bonifácio, M.J.; Queiroz, J.A.; Passarinha, L.A. Purification of Membrane-Bound Catechol-O-Methyltransferase by Arginine-Affinity Chromatography. Chromatographia 2015, 78, 1339–1348. [Google Scholar] [CrossRef]
- Pedro, A.Q.; Gonçalves, A.M.; Queiroz, J.A.; Passarinha, L.A. Purification of Histidine-Tagged Membrane-Bound Catechol-O-Methyltransferase from Detergent-Solubilized Pichia pastoris Membranes. Chromatographia 2018, 81, 425–434. [Google Scholar] [CrossRef]
- Correia, F.F.; Santos, F.M.; Pedro, A.Q.; Bonifácio, M.J.; Queiroz, J.A.; Passarinha, L.A. Recovery of biological active catechol-O-methyltransferase isoforms from Q-sepharose. J. Sep. Sci. 2014, 37, 20–29. [Google Scholar] [CrossRef]
- Galamba, N. Mapping structural perturbations of water in ionic solutions. J. Phys. Chem. B 2012, 116, 5242–5250. [Google Scholar] [CrossRef]
- Schröder, C. Proteins in Ionic Liquids: Current Status of Experiments and Simulations. Top. Curr. Chem. 2017, 375, 25. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Cremer, P.S. Chemistry of Hofmeister anions and osmolytes. Annu. Rev. Phys. Chem. 2010, 61, 63–83. [Google Scholar] [CrossRef] [Green Version]
- Leibly, D.J. Stabilizing Additives Added during Cell Lysis Aid in the Solubilization of Recombinant Proteins. PLoS ONE 2012, 7, e52482. [Google Scholar] [CrossRef]
- Kiritsi, M.N.; Fragoulis, E.G.; Sideris, D.C. Essential cysteine residues for human RNase kappa catalytic activity. FEBS J. 2012, 279, 1318–1326. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Luo, X.; Li, J.; Reed, S.A.; Xiao, H.; Young, T.S.; Schultz, P.G. Enhancing protein stability with extended disulfide bonds. Proc. Natl. Acad. Sci. USA 2016, 113, 5910–5915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, G.R. Properties of soluble alpha-chymotrypsin in neat glycerol and water. Enzym. Microb. Technol. 2000, 27, 143–150. [Google Scholar] [CrossRef]
- Naushad, M.; Alothman, Z.A.; Khan, A.B.; Ali, M. Effect of ionic liquid on activity, stability, and structure of enzymes: A review. Int. J. Biol. Macromol. 2012, 51, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.D.; Seddon, K.R. Ionic Liquids—Solvents of the Future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef]
- Han, Q.; Brown, S.J.; Drummond, C.J.; Greaves, T.L. Protein aggregation and crystallization with ionic liquids: Insights into the influence of solvent properties. J. Colloid Interface Sci. 2022, 608, 1173–1190. [Google Scholar] [CrossRef]
- Ventura, S.P.; e Silva, F.A.; Gonçalves, A.M.; Pereira, J.L.; Gonçalves, F.; Coutinho, J.A. Ecotoxicity analysis of cholinium-based ionic liquids to Vibrio fischeri marine bacteria. Ecotoxicol. Environ. Saf. 2014, 102, 48–54. [Google Scholar] [CrossRef]
- Fujita, K.; Forsyth, M.; MacFarlane, D.R.; Reid, R.W.; Elliott, G.D. Unexpected improvement in stability and utility of cytochrome c by solution in biocompatible ionic liquids. Biotechnol. Bioeng. 2006, 94, 1209–1213. [Google Scholar] [CrossRef]
- Fujita, K.; MacFarlane, D.R.; Forsyth, M.; Yoshizawa-Fujita, M.; Murata, K.; Nakamura, N.; Ohno, H. Solubility and stability of cytochrome c in hydrated ionic liquids: Effect of oxo acid residues and kosmotropicity. Biomacromolecules 2007, 8, 2080–2086. [Google Scholar] [CrossRef]
- Vrikkis, R.M.; Fraser, K.J.; Fujita, K.; Macfarlane, D.R.; Elliott, G.D. Biocompatible ionic liquids: A new approach for stabilizing proteins in liquid formulation. J. Biomech. Eng. 2009, 131, 074514. [Google Scholar] [CrossRef]
- Chen, X.; Ji, Y.; Wang, J. Improvement on the crystallization of lysozyme in the presence of hydrophilic ionic liquid. Analyst 2010, 135, 2241–2248. [Google Scholar] [CrossRef]
- Pusey, M.L.; Paley, M.S.; Turner, M.B.; Rogers, R.D. Protein Crystallization Using Room Temperature Ionic Liquids. Cryst. Growth Des. 2007, 7, 787–793. [Google Scholar] [CrossRef]
- Otrelo-Cardoso, A.R.; Schwuchow, V.; Rodrigues, D.; Cabrita, E.J.; Leimkühler, S.; Romão, M.J.; Santos-Silva, T. Biochemical, stabilization and crystallization studies on a molecular chaperone (PaoD) involved in the maturation of molybdoenzymes. PLoS ONE 2014, 9, e87295. [Google Scholar] [CrossRef] [PubMed]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of Cellose with Ionic Liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Lange, C.; Patil, G.; Rudolph, R. Ionic liquids as refolding additives: N′-alkyl and N′-(omega-hydroxyalkyl) N-methylimidazolium chlorides. Protein Sci. 2005, 14, 2693–2701. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, R.; Tischer, A.; Patil, G.; Rudolph, R.; Lange, C. Ionic liquids as refolding additives: Variation of the anion. J. Biotechnol. 2010, 150, 64–72. [Google Scholar] [CrossRef]
- Golovanov, A.P.; Hautbergue, G.M.; Wilson, S.A.; Lian, L.Y. A simple method for improving protein solubility and long-term stability. J. Am. Chem. Soc. 2004, 126, 8933–8939. [Google Scholar] [CrossRef]
- Barreca, D.; Lagana, G.; Magazu, S.; Migliardo, F.; Gattuso, G.; Bellocco, E. FTIR, ESI-MS, VT-NMR and SANS study of trehalose thermal stabilization of lysozyme. Int. J. Biol. Macromol. 2014, 63, 225–232. [Google Scholar] [CrossRef]
- Tilgmann, C.; Ulmanen, I. Purification methods of mammalian catechol-O-methyltransferases. J. Chromatogr. B Biomed. Appl. 1996, 684, 147–161. [Google Scholar] [CrossRef]
- Pedro, A.Q.; Oppolzer, D.; Bonifácio, M.J.; Maia, C.J.; Queiroz, J.A.; Passarinha, L.A. Evaluation of Mut(S) and Mut⁺ Pichia pastoris strains for membrane-bound catechol-O-methyltransferase biosynthesis. Appl. Biochem. Biotechnol. 2015, 175, 3840–3855. [Google Scholar] [CrossRef]
- Costa, S.R.; Bonifacio, M.J.; Queiroz, J.A.; Passarinha, L.A. Analysis of hSCOMT adsorption in bioaffinity chromatography with immobilized amino acids: The influence of pH and ionic strength. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 1704–1706. [Google Scholar] [CrossRef]
- Pedro, A.; Soares, R.; Oppolzer, D.; Santos, F.; Rocha, L.; Gonçalves, A.; Bonifacio, M.; Queiroz, J.; Gallardo, E.; Passarinha, L. An improved HPLC method for quantification of metanephrine with coulometric detection. J. Chromatogr. Sep. Tech. 2014, 5, 17–24. [Google Scholar]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K. Ionic Liquids as Stabilization and Refolding Additives and Solvents for Proteins. Adv. Biochem. Engin./Biotechnol. 2019, 168, 215–226. [Google Scholar] [CrossRef]
- Zhao, H. Protein Stabilization and Enzyme Activation in Ionic Liquids: Specific Ion Effects. J. Chem. Technol. Biotechnol. 2016, 91, 25–50. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Bisht, M.; Venkatesu, P. Biocompatibility of ionic liquids towards protein stability: A comprehensive overview on the current understanding and their implications. Int. J. Biol. Macromol. 2017, 96, 611–651. [Google Scholar] [CrossRef]
- Fujita, K.; Kajiyama, M.; Liu, Y.; Nakamura, N.; Ohno, H. Hydrated ionic liquids as a liquid chaperon for refolding of aggregated recombinant protein expressed in Escherichia coli. Chem. Comm. 2016, 52, 13491–13494. [Google Scholar] [CrossRef]
- Reslan, M.; Ranganathan, V.; Macfarlane, D.R.; Kayser, V. Choline ionic liquid enhances the stability of Herceptin® (trastuzumab). Chem. Comm. 2018, 54, 10622–10625. [Google Scholar] [CrossRef] [PubMed]
- Tarver, C.L.; Yuan, Q.; Pusey, M.L. Ionic Liquids as Protein Crystallization Additives. Crystals 2021, 11, 1166. [Google Scholar] [CrossRef]
- Yamamoto, E.; Yamaguchi, S.; Nagamune, T. Protein Refolding by N-Alkylpyridinium and N-Alkyl-N-methylpyrrolidinium Ionic Liquids. Appl. Biochem. Biotechnol. 2011, 164, 957–967. [Google Scholar] [CrossRef]
- Zhao, H. Methods for stabilizing and activating enzymes in ionic liquids—A review. J. Chem. Technol. Biotechnol. 2010, 85, 891–907. [Google Scholar] [CrossRef]
- Park, S.; Kazlauskas, R.J. Biocatalysis in ionic liquids—Advantages beyond green technology. Curr. Opin. Biotechnol. 2003, 14, 432–437. [Google Scholar] [CrossRef]
- Fukaya, Y.; Iizuka, Y.; Sekikawa, K.; Ohno, H. Bio ionic liquids: Room temperature ionic liquids composed wholly of biomaterials. Green Chem. 2007, 9, 1155–1157. [Google Scholar] [CrossRef]
- Jerome, L.M.; Well, A.D.; Lorch, R.F., Jr. Research Design and Statistical Analysis, 3rd ed.; Routtedge: Abingdon, UK, 2010. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 10th ed.; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
- Carlson, R. Design of Experiments, Principles and Applications, L. Eriksson, E. Johansson, N. Kettaneh- Wold, C. Wikström and S. Wold, Umetrics AB, Umeå Learnways AB, Stockholm, 2000, ISBN 91-973730-0-1, xii + 329 pp. J. Chemom. 2001, 15, 495–496. [Google Scholar] [CrossRef]
| % of hMBCOMT Activity Recovery | |||||
|---|---|---|---|---|---|
| [IL] (mM) | [Ch][Glu] | [Ch][DHP] | [Ch]Cl | [C12mim]Cl | [C4mim]Cl |
| 5 | 127% ± 4.15 | 126% ± 1.83 | 131% ± 2.77 | - | 128% ± 1.38 |
| 10 | 132% ± 6.23 | 199% ± 10.97 | 132% ± 8.88 | - | 130% ± 10.96 |
| 50 | 135% ± 6.79 | 199% ± 2.96 | 91% ± 1.37 | - | 101% ± 31.72 |
| 125 | 114% ± 2.3 | 101.37% ± 0.75 | 105% ± 0.49 | - | 90% ± 5.79 |
| 250 | 142% ± 2.9 | - | 106% ± 0.35 | - | 83% ± 2.76 |
| 500 | Not tested | - | 110% ± 5.37 | - | - |
| Assay Number | Ionic Liquid Concentration (mM) | Time (h) | Temperature (°C) | % of hMBCOMT Activity |
|---|---|---|---|---|
| 1 | 7.5 | 24 | −80 | 148.2% ± 1.9 |
| 2 | 7.5 | 24 | 4 | 106.66% ± 3.6 |
| 3 | 10 | 24 | −80 | 142% ± 0.44 |
| 4 | 10 | 24 | 4 | 98.7% ± 0.8 |
| 5 | 12.5 | 24 | −80 | 122.9% ± 0.7 |
| 6 | 12.5 | 24 | 4 | 93.09% ± 1.8 |
| 7 | 7.5 | 48 | −80 | 135% ± 1.9 |
| 8 | 7.5 | 48 | 4 | 91.27% ± 1.5 |
| 9 | 10 | 48 | −80 | 125.9% ± 5.3 |
| 10 | 10 | 48 | −80 | 127.6% ± 3.1 |
| 11 | 10 | 48 | −80 | 128.5% ± 3.4 |
| 12 | 10 | 48 | 4 | 88.14% ± 4.2 |
| 13 | 10 | 48 | 4 | 81.4% ± 2.9 |
| 14 | 10 | 48 | 4 | 79.2% ± 0.14 |
| 15 | 12.5 | 48 | −80 | 114.7% ± 1.0 |
| 16 | 12.5 | 48 | 4 | 77.9% ± 1.8 |
| 17 | 7.5 | 72 | −80 | 127.8% ± 0.6 |
| 18 | 7.5 | 72 | 4 | 52.9% ± 1.8 |
| 19 | 10 | 72 | −80 | 108% ± 1.5 |
| 20 | 10 | 72 | 4 | 54.4% ± 4.8 |
| 21 | 12.5 | 72 | −80 | 117.3% ± 3.9 |
| 22 | 12.5 | 72 | 4 | 42.1% ± 0.9 |
| Output | R2 | Adjust R2 | Predicted R2 | Adequate Precision |
|---|---|---|---|---|
| % Activity recovery | 0.9770 | 0.9629 | 0.9074 | 27.056 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value |
|---|---|---|---|---|---|
| Model | 18,053.09 | 8 | 2256.64 | 69.15 | 0.0001 |
| Concentration of [Ch][DHP] (A) | 733.83 | 1 | 733.83 | 22.49 | 0.0004 |
| Time (B) | 3641.83 | 1 | 3641.83 | 111.59 | <0.0001 |
| Temperature (C) | 12,871.50 | 1 | 12,871.50 | 394.41 | <0.0001 |
| AB | 38.59 | 1 | 38.59 | 1.18 | 0.2966 |
| AC | 28.09 | 1 | 28.09 | 0.86 | 0.3704 |
| BC | 660.83 | 1 | 660.83 | 20.25 | 0.006 |
| A2 | 0.073 | 1 | 0.072 | 2.245 × 10−3 | 0.9629 |
| B2 | 74.02 | 1 | 74.02 | 2.27 | 0.1560 |
| Residual | 424.25 | 13 | 32.63 | - | - |
| Lack of Fit | 377.37 | 4 | 41.93 | 3.58 | 0.1160 |
| Output | Predicted Mean | SE Mean | 95% CI Low | 95% CI High | SE Predicted | 95% PI Low | 95% PI High |
|---|---|---|---|---|---|---|---|
| % Activity recovery | 144.884 | 3.71267 | 136.787 | 152.961 | 6.83244 | 130.123 | 159.645 |
| Stabilizer | Feature | Concentration Range |
| Cysteine | Stabilization of disulfide bonds | 5 to 40 mM |
| Trehalose | Thermal stabilizers/promote protein folding and refolding | 5 to 40 mM |
| Glycerol | Cryo-protector | 5 to 40% |
| [Ch][DHP] | Thermal stabilizer/aggregation behavior | 5–125 mM |
| [Ch]Cl | 5–500 mM | |
| [C12mim]Cl | 5–500 mM | |
| [C4mim]Cl | 5–500 mM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, A.M.; Sousa, Â.; Pedro, A.Q.; Romão, M.J.; Queiroz, J.A.; Gallardo, E.; Passarinha, L.A. Advances in Membrane-Bound Catechol-O-Methyltransferase Stability Achieved Using a New Ionic Liquid-Based Storage Formulation. Int. J. Mol. Sci. 2022, 23, 7264. https://doi.org/10.3390/ijms23137264
Gonçalves AM, Sousa Â, Pedro AQ, Romão MJ, Queiroz JA, Gallardo E, Passarinha LA. Advances in Membrane-Bound Catechol-O-Methyltransferase Stability Achieved Using a New Ionic Liquid-Based Storage Formulation. International Journal of Molecular Sciences. 2022; 23(13):7264. https://doi.org/10.3390/ijms23137264
Chicago/Turabian StyleGonçalves, Ana M., Ângela Sousa, Augusto Q. Pedro, Maria J. Romão, João A. Queiroz, Eugénia Gallardo, and Luís A. Passarinha. 2022. "Advances in Membrane-Bound Catechol-O-Methyltransferase Stability Achieved Using a New Ionic Liquid-Based Storage Formulation" International Journal of Molecular Sciences 23, no. 13: 7264. https://doi.org/10.3390/ijms23137264
APA StyleGonçalves, A. M., Sousa, Â., Pedro, A. Q., Romão, M. J., Queiroz, J. A., Gallardo, E., & Passarinha, L. A. (2022). Advances in Membrane-Bound Catechol-O-Methyltransferase Stability Achieved Using a New Ionic Liquid-Based Storage Formulation. International Journal of Molecular Sciences, 23(13), 7264. https://doi.org/10.3390/ijms23137264










