Maternal Fluoride Exposure Exerts Different Toxicity Patterns in Parotid and Submandibular Glands of Offspring Rats
Abstract
:1. Introduction
2. Results
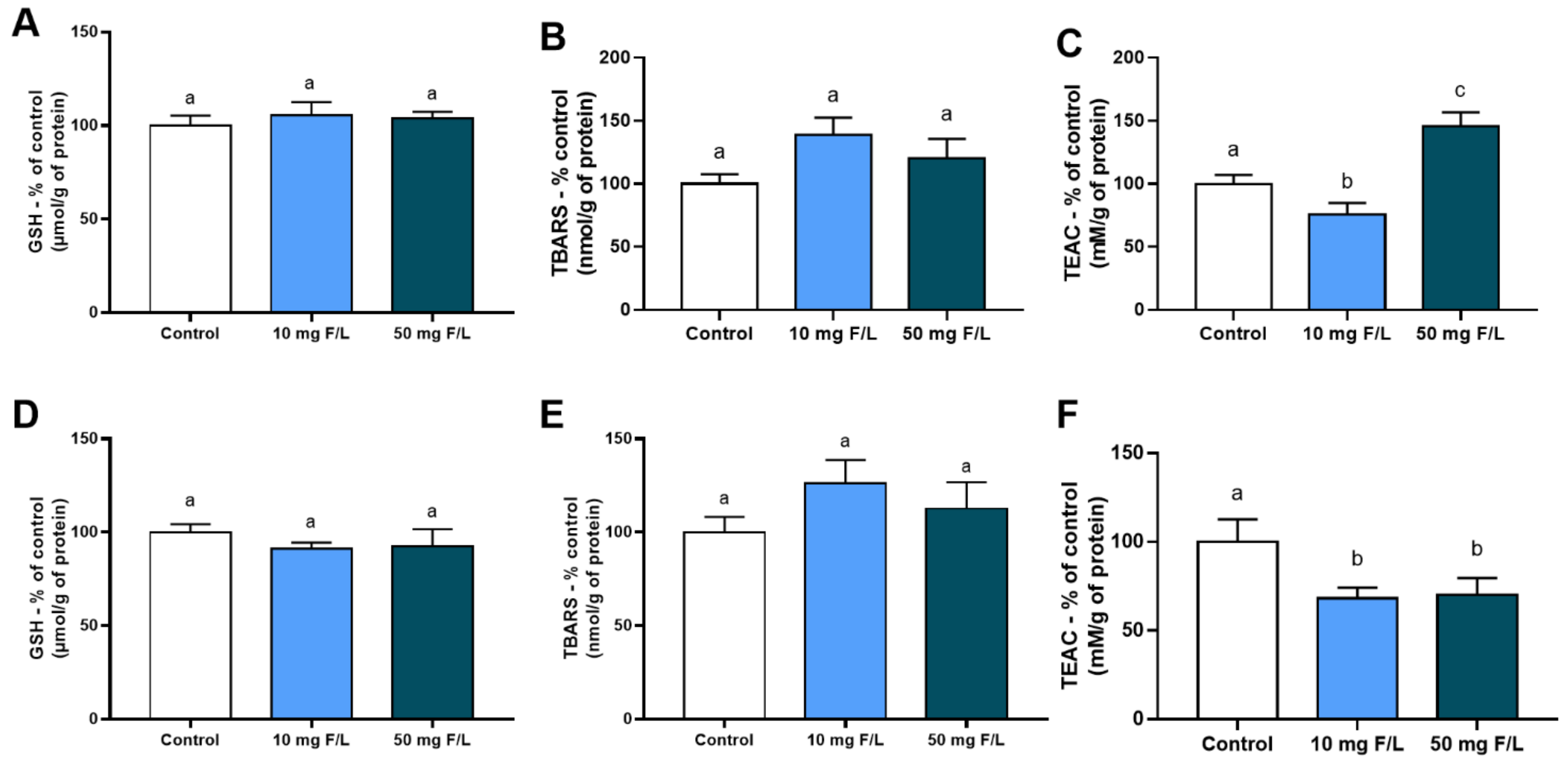
2.1. Indirect Exposure to F during the Intrauterine and Neonatal Periods Modulated the Antioxidant Competence of Offspring Rats’ Salivary Glands
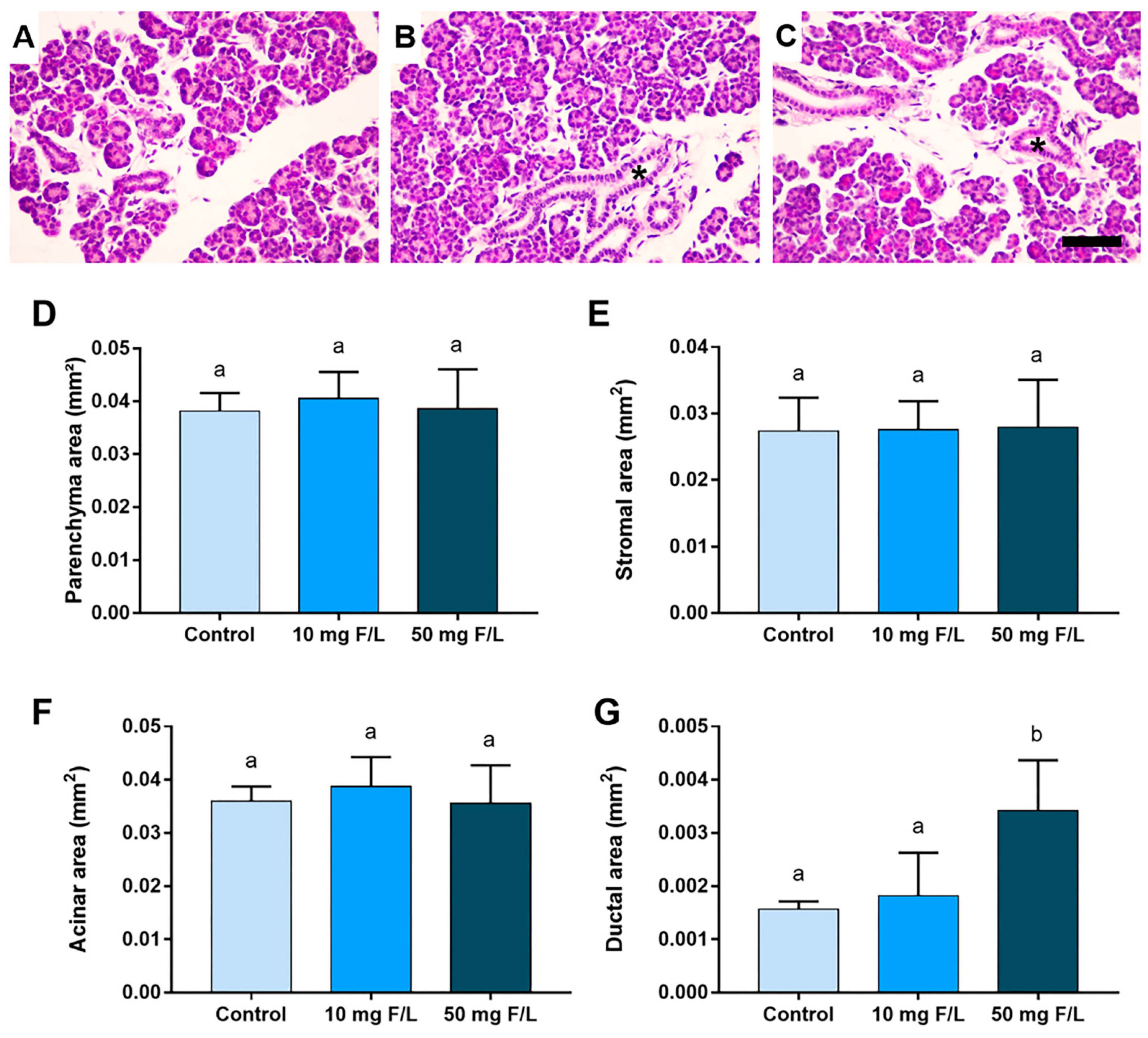
2.2. Indirect Exposure to F Was Able to Alter the Duct Area of the Parotid and Submandibular Glands of the Offspring at Higher Levels of F, but without Changes in the Other Morphometric Parameters
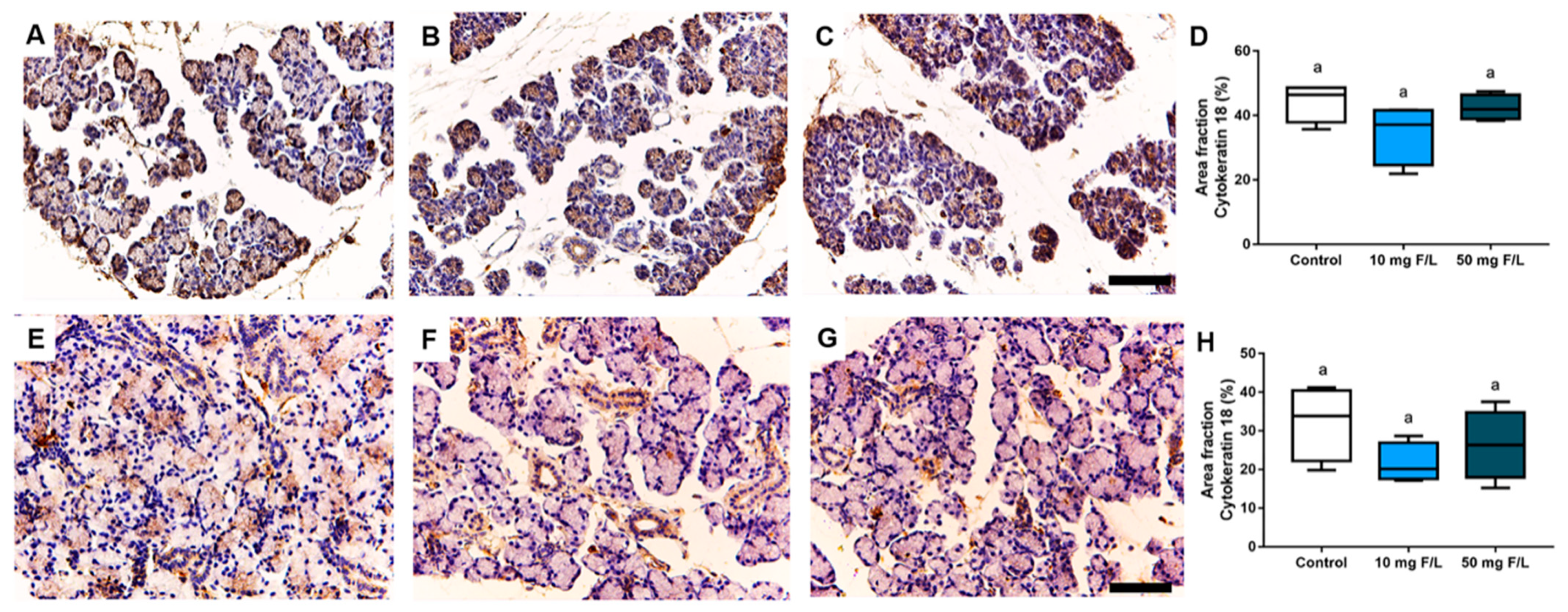
2.3. Indirect Exposure to F during Pregnancy and Lactation Did Not Trigger Changes in Cytokeratin-18 (CK-18) Filaments in the Salivary Glands of the Offspring
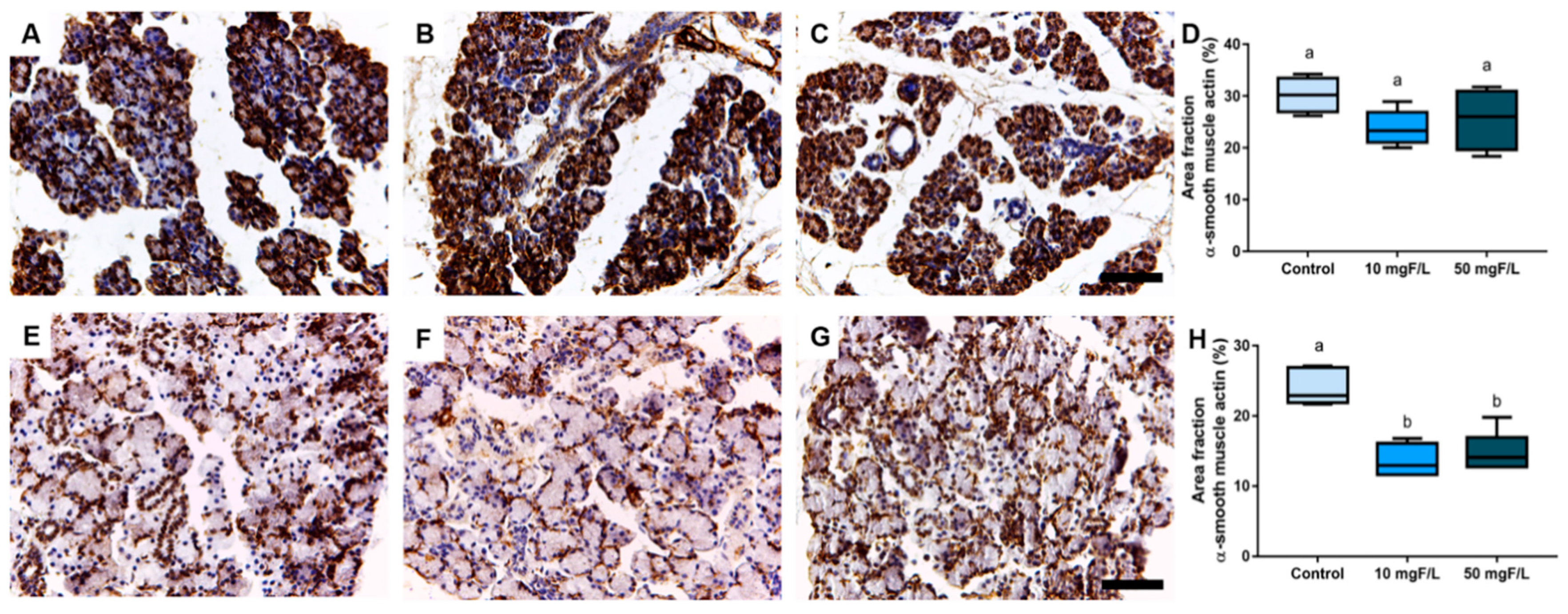
2.4. Indirect Exposure to F during Gestational and Lactation Periods Triggered Changes in the Immunostained Area Fraction of Myoepithelial Cells in the Submandibular Glands of Offspring Rats
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Fluoride Exposure Protocol
4.3. Sample Preparation and Collection Procedures
4.4. Biochemical Analyses
4.4.1. Oxidative Stress Assessment Assays
4.4.2. Determination of GSH Levels
4.4.3. Determination of TBARS Levels
4.4.4. Determination of the TEAC Levels
4.5. Histological Evaluation
4.5.1. Morphometric Analyses
4.5.2. Immunohistochemical Analyses
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, Z.N.; Sabino, I.T.; de Souza Melo, C.G.; Martini, T.; da Silva Pereira, H.A.B.; Buzalaf, M.A.R. Liver proteome of mice with distinct genetic susceptibilities to fluorosis treated with different concentrations of F in the drinking water. Biol. Trace Elem. Res. 2019, 187, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Buzalaf, M.A.R.; Levy, S.M. Fluoride intake of children: Considerations for dental caries and dental fluorosis. Fluoride Oral Environ. 2011, 22, 1–19. [Google Scholar]
- O’Mullane, D.M.; Baez, R.J.; Jones, S.; Lennon, M.A.; Petersen, P.E.; Rugg-Gunn, A.J.; Whelton, H.; Whitford, G.M. Fluoride and oral health. Community Dent. Health 2016, 33, 69–99. [Google Scholar] [PubMed]
- World Health Organization (WHO); World Health Organisation Staff. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2004; Volume 1, pp. 370–373. [Google Scholar]
- Cury, J.A.; Ricomini-Filho, A.P.; Berti, F.L.P.; Tabchoury, C.P. Systemic effects (risks) of water fluoridation. Braz. Dent. J. 2019, 30, 421–428. [Google Scholar] [CrossRef]
- Zuo, H.; Chen, L.; Kong, M.; Qiu, L.; Lü, P.; Wu, P.; Yanhua, Y.; Chen, K. Toxic effects of fluoride on organisms. Life Sci. 2018, 198, 18–24. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, L.D.; Liu, H.; Li, H.H.; Ren, C.; Zhang, P.; Guo, K.T.; Zhang, H.X.; Zhang, C.Y. Fluoride induces neuroinflammation and alters Wnt signaling pathway in BV2 microglial cells. Inflammation 2017, 40, 1123–1130. [Google Scholar] [CrossRef]
- Wei, M.; Ye, Y.; Ali, M.M.; Chamba, Y.; Tang, J.; Shang, P. Effect of Fluoride on Cytotoxicity Involved in Mitochondrial Dysfunction: A Review of Mechanism. Front. Vet. Sci. 2022, 9, 850771. [Google Scholar] [CrossRef]
- Dec, K.; Łukomska, A.; Maciejewska, D.; Jakubczyk, K.; Baranowska-Bosiacka, I.; Chlubek, D.; Wąsik, A.; Gutowska, I. The Influence of Fluorine on the Disturbances of Homeostasis in the Central Nervous System. Biol. Trace Elem. Res. 2017, 177, 224–234. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, A.A.; Yousefi, M.; Yaseri, M.; Jalilzadeh, M.; Mahvi, A.H. Skeletal fluorosis in relation to drinking water in rural areas of West Azerbaijan, Iran. Sci. Rep. 2017, 7, 17300. [Google Scholar] [CrossRef] [Green Version]
- Iano, F.G.; Ferreira, M.C.; Quaggio, G.B.; Fernandes, M.S.; Oliveira, R.C.; Ximenes, V.F.; Buzalaf, M.A.R. Effects of chronic fluoride intake on the antioxidant systems of the liver and kidney in rats. J. Fluor. Chem. 2014, 68, 212–217. [Google Scholar] [CrossRef]
- Fujibayashi, S.Y.; Archetti, F.B.; Pizzatto, S.; Losso, E.M.; Pizzatto, E. Severidade de fluorose dental em um grupo de escolares. Rev. Sul-Bras. Odontol. 2011, 8, 168–173. [Google Scholar]
- Grandjean, P. Developmental fluoride neurotoxicity: An updated review. Environ. Health 2019, 18, 110. [Google Scholar] [CrossRef] [Green Version]
- Duan, Q.; Jiao, J.; Chen, X.; Wang, X. Association between water fluoride and the level of children’s intelligence: A dose–response meta-analysis. Public Health 2018, 154, 87–97. [Google Scholar] [CrossRef]
- Dong, H.; Yang, X.; Zhang, S.; Wang, X.; Guo, C.; Zhang, X.; Ma, J.; Niu, P.; Chen, T. Associations of low level of fluoride exposure with dental fluorosis among US children and adolescents, NHANES 2015–2016. Ecotoxicol. Environ. Saf. 2021, 221, 112439. [Google Scholar] [CrossRef]
- Dhar, V.; Bhatnagar, M. Physiology and toxicity of fluoride. Indian J. Dent. Res. 2009, 20, 350. [Google Scholar] [CrossRef]
- Lima, L.A.O.; Miranda, G.H.N.; Aragão, W.A.B.; Bittencourt, L.O.; Dos Santos, S.M.; de Souza, M.P.C.; Nogueira, L.S.; de Oliveira, E.H.C.; Monteiro, M.C.; Dionizio, A.; et al. Effects of Fluoride on Submandibular Glands of Mice: Changes in Oxidative Biochemistry, Proteomic Profile, and Genotoxicity. Front. Pharmacol. 2021, 12, 715394. [Google Scholar] [CrossRef]
- Miranda, G.H.N.; Alencar de Oliveira Lima, L.; Bittencourt, L.O.; Dos Santos, S.M.; Platini Caldas de Souza, M.; Nogueira, L.S.; de Oliveira, E.H.C.; Monteiro, M.C.; Dionizio, A.; Leite, A.L.; et al. Effects of long-term fluoride exposure are associated with oxidative biochemistry impairment and global proteomic modulation, but not genotoxicity, in parotid glands of mice. PLoS ONE 2022, 17, e0261252. [Google Scholar] [CrossRef]
- Ekström, J.; Khosravani, N. Regulatory mechanisms and salivary gland functions. In Salivary Gland Disorders and Diseases: Diagnosis and Management; Georg Thieme Verlag: New York, NY, USA, 2011; pp. 10–18. [Google Scholar]
- Ekstrand, J.; Oliveby, A. Fluoride in the oral environment. Acta Odontol. Scand. 1999, 57, 330–333. [Google Scholar] [CrossRef]
- Pedersen, A.M.L.; Sørensen, C.E.; Proctor, G.B.; Carpenter, G.H.; Ekström, J. Salivary secretion in health and disease. J. Oral Rehabil. 2018, 45, 730–746. [Google Scholar] [CrossRef]
- Dawes, C.; Pedersen, A.L.; Villa, A.; Ekström, J.; Proctor, G.B.; Vissink, A.; Aframian, D.; McGowan, R.; Aliko, A.; Narayana, N.; et al. The functions of human saliva: A review sponsored by the World Workshop on Oral Medicine VI. Arch. Oral Biol. 2015, 60, 863–874. [Google Scholar] [CrossRef]
- De Paula, F.; Teshima, T.H.N.; Hsieh, R.; Souza, M.M.; Nico, M.M.S.; Lourenco, S.V. Overview of human salivary glands: Highlights of morphology and developing processes. Anat. Rec. 2017, 300, 1180–1188. [Google Scholar] [CrossRef] [Green Version]
- Kwong, W.Y.; Wild, A.E.; Roberts, P.; Willis, A.C.; Fleming, T.P. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development 2000, 127, 4195–4202. [Google Scholar] [CrossRef]
- Ferreira, M.K.M.; Aragão, W.A.B.; Bittencourt, L.O.; Puty, B.; Dionizio, A.; Souza, M.P.C.; Buzalaf, M.A.R.; de Oliveira, E.H.; Crespo-Lopez, M.E.; Lima, R.R. Fluoride exposure during pregnancy and lactation triggers oxidative stress and molecular changes in hippocampus of offspring rats. Ecotoxicol. Environ. Saf. 2021, 208, 111437. [Google Scholar] [CrossRef]
- Miranda, G.H.N.; Gomes, B.A.Q.; Bittencourt, L.O.; Aragão, W.A.B.; Nogueira, L.S.; Dionizio, A.S.; Dionizio, A.S.; Buzalaf, M.A.R.; Monteiro, M.C.; Lima, R.R. Chronic exposure to sodium fluoride triggers oxidative biochemistry misbalance in mice: Effects on peripheral blood circulation. Oxid. Med. Cell. Longev. 2018, 2018, 8379123. [Google Scholar] [CrossRef]
- Angwa, L.M.; Jiang, Y.; Pei, J.; Sun, D. Antioxidant phytochemicals for the prevention of fluoride-induced oxidative stress and apoptosis: A review. Biol. Trace Elem. Res. 2022, 200, 1418–1441. [Google Scholar] [CrossRef]
- Casati, L.; Pagani, F.; Limonta, P.; Vanetti, C.; Stancari, G.; Sibilia, V. Beneficial effects of δ-tocotrienol against oxidative stress in osteoblastic cells: Studies on the mechanisms of action. Eur. J. Nutr. 2020, 59, 1975–1987. [Google Scholar] [CrossRef] [Green Version]
- Ghani, M.A.; Barril, C.; Bedgood, D.R., Jr.; Prenzler, P.D. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem. 2017, 230, 195–207. [Google Scholar] [CrossRef]
- Nicolau, J.; Sassaki, K.T. Metabolism of carbohydrate in the major salivary glands of rats. Arch. Oral Biol. 1976, 21, 659–661. [Google Scholar] [CrossRef]
- Nagler, R.M.; Klein, I.; Zarzhevsky, N.; Drigues, N.; Reznick, A.Z. Characterization of the differentiated antioxidant profile of human saliva. Free Radic. Biol. Med. 2002, 32, 268–277. [Google Scholar] [CrossRef]
- Yamaguti, P.M.; Simões, A.; Ganzerla, E.; Souza, D.N.; Nogueira, F.N.; Nicolau, J. Effects of single exposure of sodium fluoride on lipid peroxidation and antioxidant enzymes in salivary glands of rats. Oxid. Med. Cell Longev. 2013, 2013, 674593. [Google Scholar] [CrossRef] [Green Version]
- Ullah, R.; Zafar, M.S.; Shahani, N. Potential fluoride toxicity from oral medicaments: A review. Iran. J. Basic Med. Sci. 2017, 20, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Araujo, T.T.; Barbosa Silva Pereira, H.A.; Dionizio, A.; Sanchez, C.; de Souza Carvalho, T.; da Silva Fernandes, M.; Rabelo Buzalaf, M.A. Changes in energy metabolism induced by fluoride: Insights from inside the mitochondria. Chemosphere 2019, 236, 124357. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.M.L.; Sørensen, C.E.; Dynesen, A.W.; Jensen, S.B. Salivary gland structure and functions and regulation of saliva secretion in health and disease. In Salivary Glands: Anatomy, Functions in Digestion and Role in Disease, 1st ed.; Nova Science Publishers: New York, NY, USA, 2013; pp. 1–44. [Google Scholar]
- Carpenter, G.H.; Cotroneo, E. Salivary gland regeneration. Front. Oral Biol. 2010, 14, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.I.; Gobe, G.C. Cell death and cell proliferation during atrophy of the rat parotid gland induced by duct obstruction. J. Pathol. 1987, 153, 333–344. [Google Scholar] [CrossRef]
- Millsop, J.W.; Wang, E.A.; Fazel, N. Etiology, evaluation, and management of xerostomia. Clin. Dermatol. 2017, 35, 468–476. [Google Scholar] [CrossRef]
- Talha, B.; Swarnkar, S.A. Xerostomia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Nascimento, P.C.; Ferreira, M.K.M.; Balbinot, K.M.; Alves, S.M., Jr.; de Jesus Pinherio, J.; Silveira, F.M.; Martins, M.D.; Crespo-Lopex, M.E.; Lima, R.R. Methylmercury-Induced Toxicopathologic Findings in Salivary Glands of Offspring Rats After Gestational and Lactational Exposure. Biol. Trace Elem. Res. 2021, 199, 2983–2991. [Google Scholar] [CrossRef]
- Kusama, K.; Jiang, Y.; Ohno, J.; Shikata, H.; Ishikawa, F.; Taguchi, K.; Kikuchi, K.; Mori, K.; Sakashita, H.; Sakagami, H.; et al. Immunohistochemical detection of cytokeratin 18 and its neo-epitope in human salivary glands and pleomorphic adenomas. Anticancer Res. 2000, 20, 2485–2487. [Google Scholar]
- Rojas, M.; Martínez-García, F.; Cobo, P.; Palacios, J.; Nistal, M.; Regadera, J. Keratinas: Biologia celular y significado funcional normal y patologico. Rev. Chil. Anat. 1998, 16, 15–31. [Google Scholar] [CrossRef]
- Moll, R.; Divo, M.; Langbein, L. The human keratins: Biology and pathology. Histochem. Cell Biol. 2008, 129, 705–733. [Google Scholar] [CrossRef] [Green Version]
- Oriolo, A.S.; Wald, F.A.; Ramsauer, V.P.; Salas, P.J. Intermediate filaments: A role in epithelial polarity. Exp. Cell Res. 2007, 313, 2255–2264. [Google Scholar] [CrossRef] [Green Version]
- Garrett, J.R.; Emmelin, N. Activities of salivary myoepithelial cells: A review. Med. Biol. 1979, 57, 1–28. [Google Scholar]
- Gervais, E.M.; Sequeira, S.J.; Wang, W.; Abraham, S.; Kim, J.H.; Leonard, D.; DeSantis, K.A.; Larsen, M. Par-1b is required for morphogenesis and differentiation of myoepithelial cells during salivary gland development. Organogenesis 2016, 12, 194–216. [Google Scholar] [CrossRef] [Green Version]
- Porcheri, C.; Mitsiadis, T.A. Physiology, Pathology and Regeneration of Salivary Glands. Cells 2019, 8, 976. [Google Scholar] [CrossRef] [Green Version]
- Sisto, M.; Lisi, S.; Ribatti, D. The role of the epithelial-to-mesenchymal transition (EMT) in diseases of the salivary glands. Histochem. Cell Biol. 2018, 150, 133–147. [Google Scholar] [CrossRef]
- Taga, R.; Sesso, A. Ultrastructural Studies on Developing Parotid Gland of the Rat at Early Postnatal Periods. Arch. Histol. Jpn. 1979, 42, 427–444. [Google Scholar] [CrossRef] [Green Version]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLOS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Dunipace, A.; Brizendine, E.; Zhang, W.; Wilson, M.; Miller, L.; Katz, B.; Warrick, J.; Stookey, G. Effect of Aging on Animal Response to Chronic Fluoride Exposure. J. Dent. Res. 1995, 74, 358–368. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Kohn, H.I.; Liversedge, M. On a new aerobic metabolite whose production by brain is inhibited by apomorphine, emetine, ergotamine, epinephrine, and menadione. J. Pharmacol. Exp. Ther. 1944, 82, 292–300. [Google Scholar]
- Percario, S.; Vital, A.C.C.; Jablonka, F. Dosagem do malondialdeido. Newslab 1994, 2, 46–50. [Google Scholar]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A Novel Method for Measuring Antioxidant Capacity and its Application to Monitoring the Antioxidant Status in Premature Neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ferreira, R.O.; Aragão, W.A.; Bittencourt, L.O.; Fernandes, L.P.; Balbinot, K.M.; Alves-Junior, S.M.; Pinheiro, J.D.J.V.; Maia, C.D.S.F.; Crespo-Lopez, M.E.; Lima, R.R. Ethanol binge drinking during pregnancy and its effects on salivary glands of offspring rats: Oxidative stress, morphometric changes and salivary function impairments. Biomed. Pharmacother. 2021, 133, 110979. [Google Scholar] [CrossRef]
- Pereira, F.L.M.; Bruno, T.F.; Melo, A.-J.S.; Viana, P.J.D.J.; Ferraz, M.C.S.; Rodrigues, L.R. Immunohistochemical changes and atrophy after chronic ethanol intoxication in rat salivary glands. Histol. Histopathol. 2015, 30, 1069–1078. [Google Scholar] [CrossRef]
- Ogawa, Y. Immunocytochemistry of myoepithelial cells in the salivary glands. Prog. Histochem. Cytochem. 2003, 38, 343–426. [Google Scholar] [CrossRef]
- Chu, P.G.; Weiss, L.M. Keratin expression in human tissues and neoplasms. Histopathology 2002, 40, 403–439. [Google Scholar] [CrossRef]
- Lima, L.A.O.; Bittencourt, L.O.; Puty, B.; Fernandes, R.M.; Nascimento, P.C.; Silva, M.C.F.; de Melo Alves Júnior, S.; Pinheiro, J.J.V.; Lima, R.R. Methylmercury intoxication promotes metallothionein response and cell damage in salivary glands of rats. Biol. Trace Elem. Res. 2018, 185, 135–142. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, V.R.N.; Ferreira, M.K.M.; Bittencourt, L.O.; Mendes, P.F.S.; Souza-Monteiro, D.; Balbinot, K.M.; Pinheiro, J.d.J.V.; Charone, S.; Pessan, J.P.; Lima, R.R. Maternal Fluoride Exposure Exerts Different Toxicity Patterns in Parotid and Submandibular Glands of Offspring Rats. Int. J. Mol. Sci. 2022, 23, 7217. https://doi.org/10.3390/ijms23137217
dos Santos VRN, Ferreira MKM, Bittencourt LO, Mendes PFS, Souza-Monteiro D, Balbinot KM, Pinheiro JdJV, Charone S, Pessan JP, Lima RR. Maternal Fluoride Exposure Exerts Different Toxicity Patterns in Parotid and Submandibular Glands of Offspring Rats. International Journal of Molecular Sciences. 2022; 23(13):7217. https://doi.org/10.3390/ijms23137217
Chicago/Turabian Styledos Santos, Vinicius Ruan Neves, Maria Karolina Martins Ferreira, Leonardo Oliveira Bittencourt, Paulo Fernando Santos Mendes, Deiweson Souza-Monteiro, Karolyny Martins Balbinot, João de Jesus Viana Pinheiro, Senda Charone, Juliano Pelim Pessan, and Rafael Rodrigues Lima. 2022. "Maternal Fluoride Exposure Exerts Different Toxicity Patterns in Parotid and Submandibular Glands of Offspring Rats" International Journal of Molecular Sciences 23, no. 13: 7217. https://doi.org/10.3390/ijms23137217
APA Styledos Santos, V. R. N., Ferreira, M. K. M., Bittencourt, L. O., Mendes, P. F. S., Souza-Monteiro, D., Balbinot, K. M., Pinheiro, J. d. J. V., Charone, S., Pessan, J. P., & Lima, R. R. (2022). Maternal Fluoride Exposure Exerts Different Toxicity Patterns in Parotid and Submandibular Glands of Offspring Rats. International Journal of Molecular Sciences, 23(13), 7217. https://doi.org/10.3390/ijms23137217






