Therapeutic Potential of Allicin and Aged Garlic Extract in Alzheimer’s Disease
Abstract
:1. Introduction
2. Garlic Bioactive Compounds and Chemistry
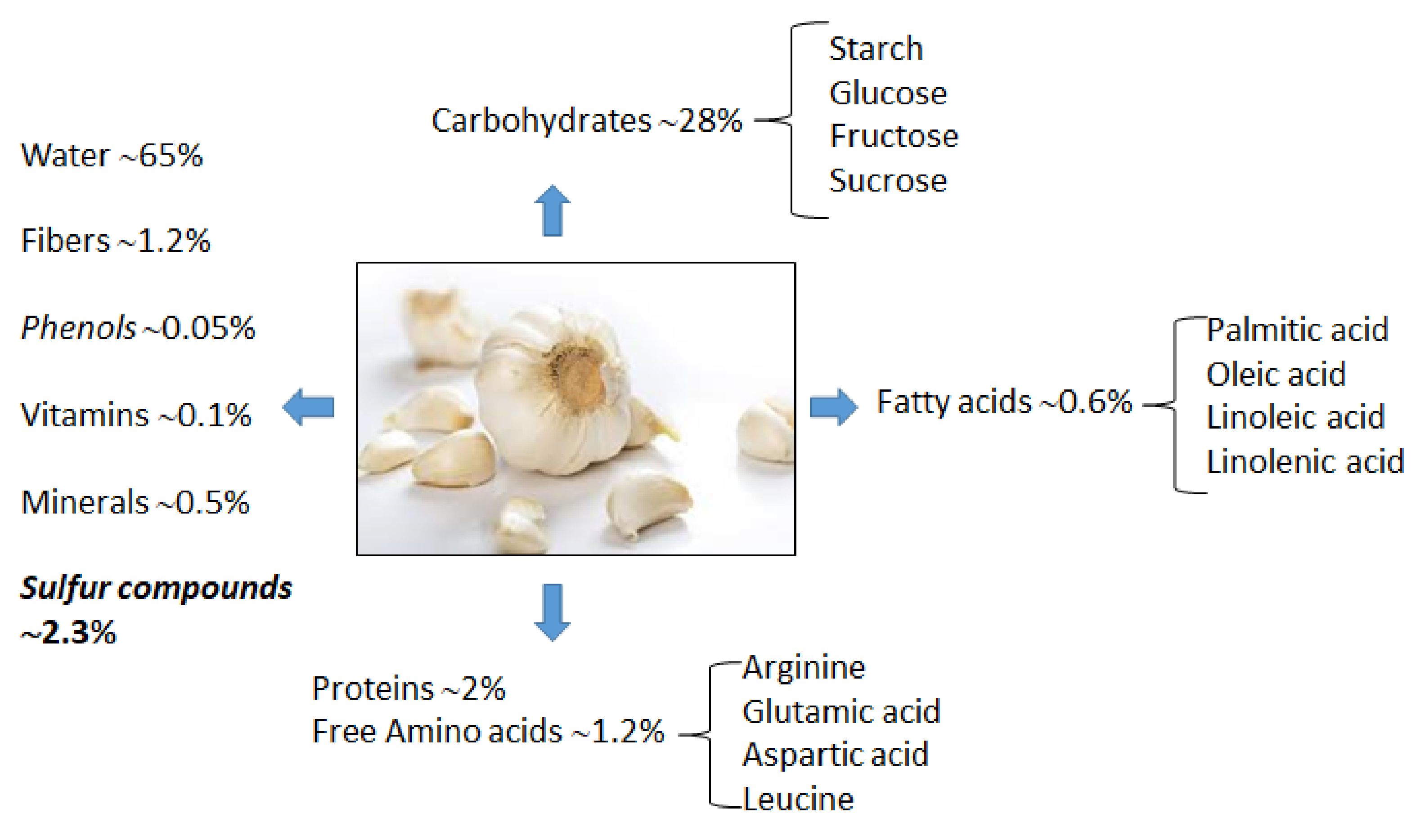
2.1. Garlic Proximate Composition
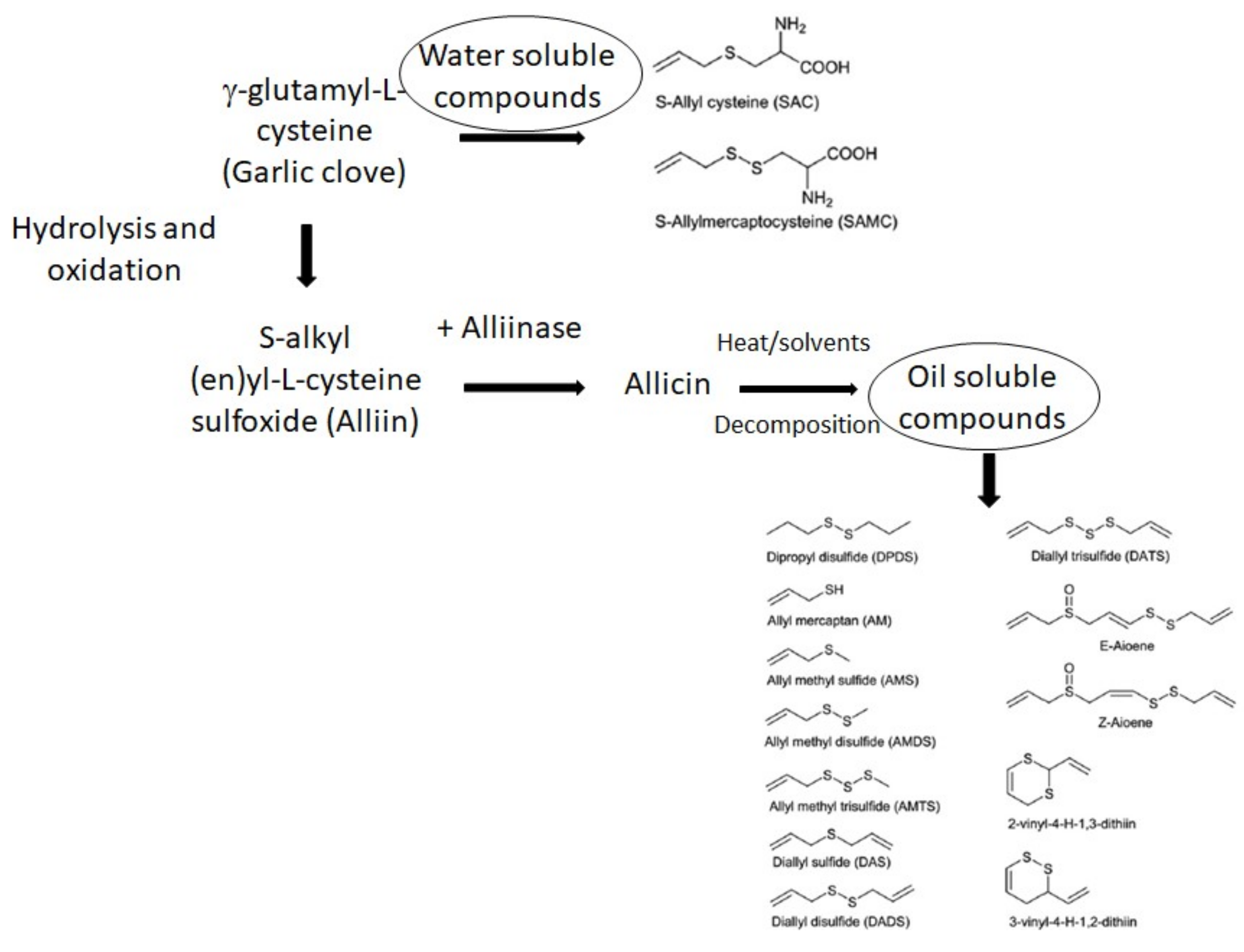
2.2. Allicin and Organo-Sulfur-Containing Compounds (OSCs)
2.3. Aged Garlic Extract
3. Metabolism and Bioavailability
4. AD
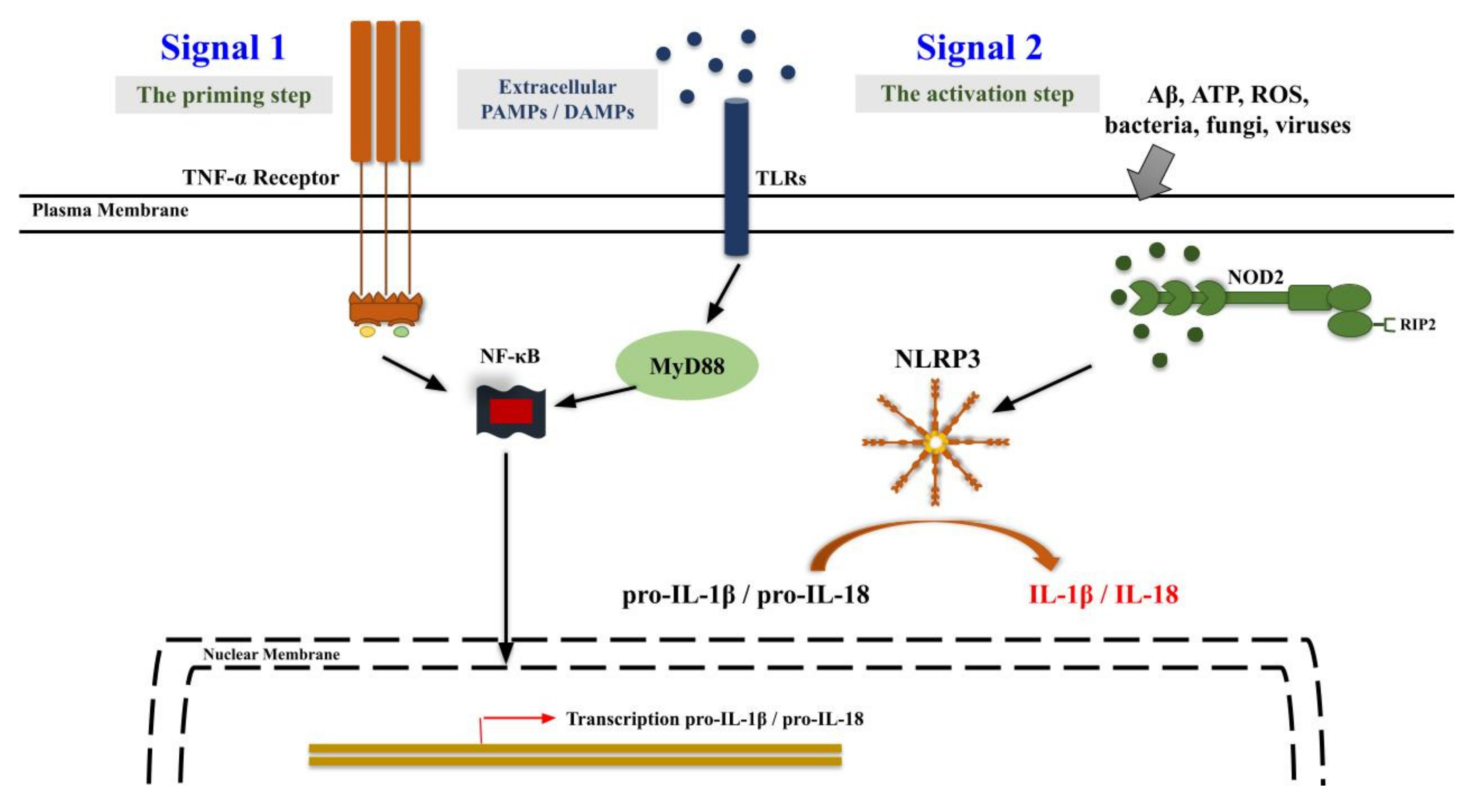
5. Neuroinflammation
6. Garlic Bioactive Compounds as Neuroprotective Agents against AD
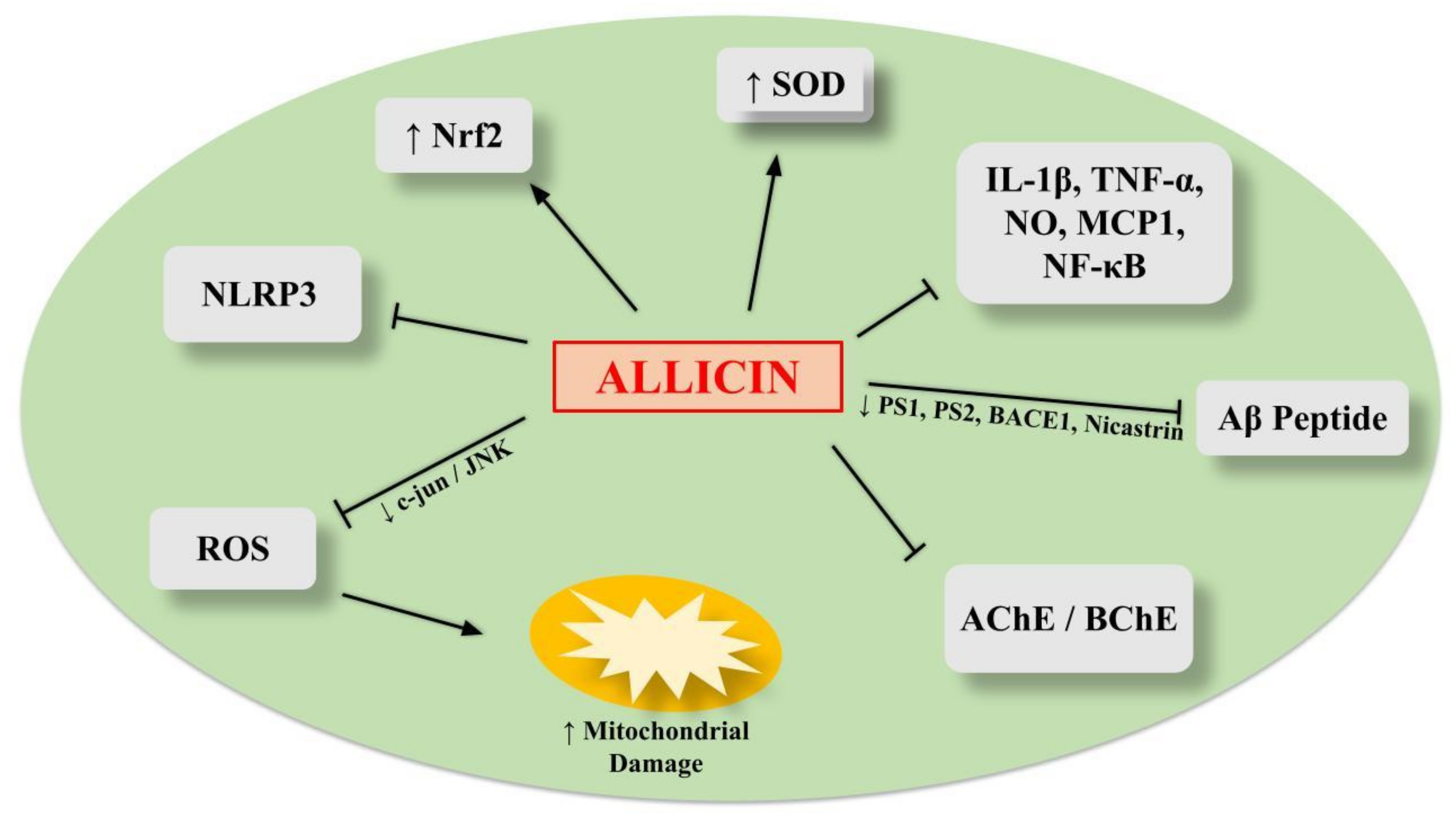
6.1. Allicin
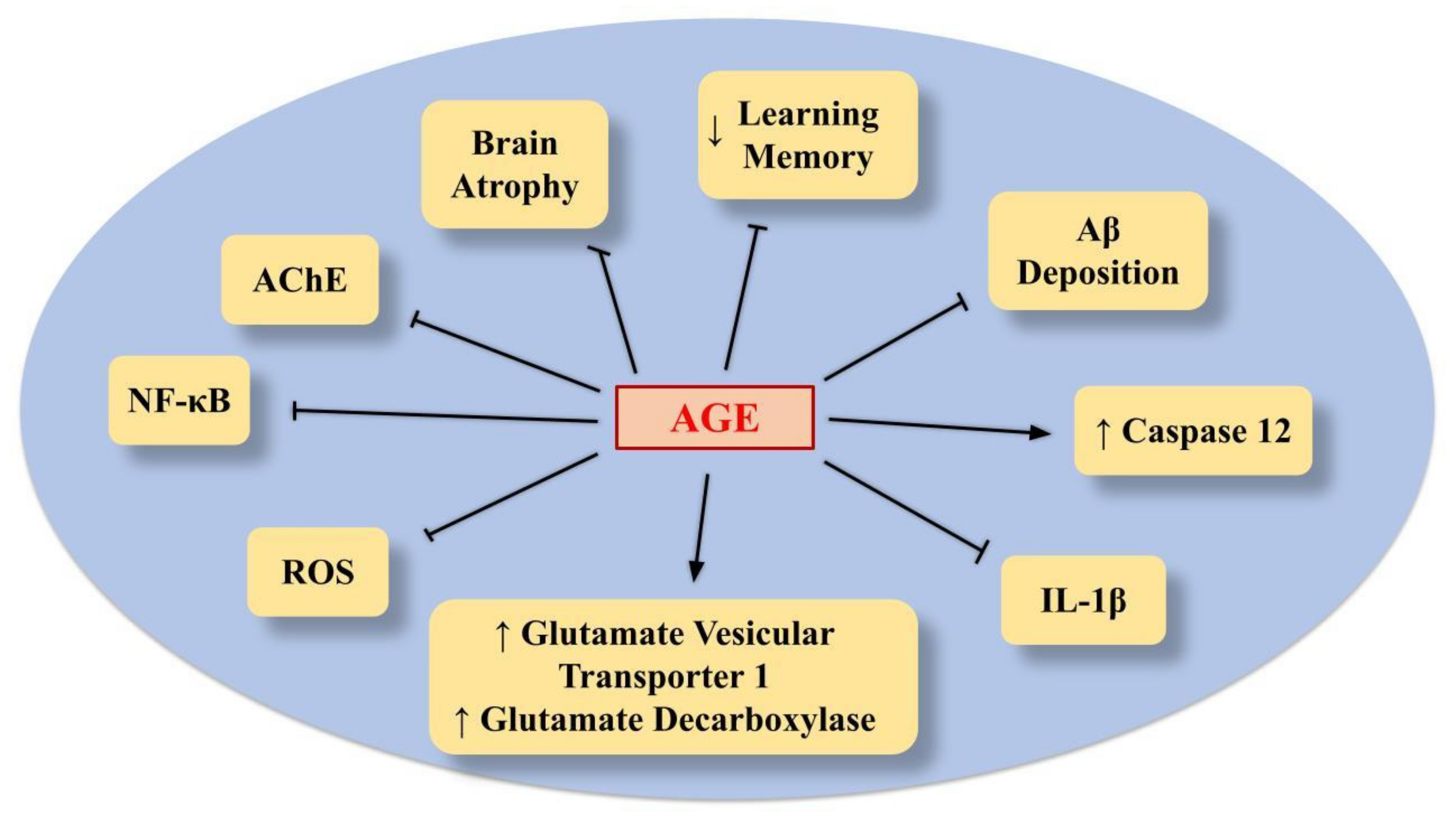
6.2. AGE
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mazza, G.; Oomah, B.D. Garlic constituents and disease prevention. In Herbs, Botanicals & Teas, Functional Foods & Nutraceuticals Series, 1st ed.; Lancaster Publishing Co., Inc.: Lancaster, UK, 2000; Volume 1, pp. 1–16. [Google Scholar]
- Brandolini, V.; Tedeschi, P.; Cereti, E.; Maietti, A.; Barile, D.; Coisson, J.D.; Mazzotta, D.; Arlorio, M.; Martelli, A. Chemical and genomic combined approach applied to the characterization and identification of Italian Allium sativum L. J. Agric. Food Chem. 2005, 53, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Amagase, H.; Petesch, B.L.; Matsuura, H.; Kasuga, S.; Itakura, Y. Intake of Garlic and Its Bioactive Components. J. Nutr. 2001, 131, 955S–962S. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.A.; Federici, M.F.; Altamirano, J.C.; Camargo, A.B.; Luco, J.M. Permeability Data of Organosulfur Garlic Compounds Estimated by Immobilized Artificial Membrane Chromatography: Correlation Across Several Biological Barriers. Front. Chem. 2021, 9, 690707. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, M.S.; Nandagopal, M.S.; Nordin, S.A.; Karuppiah Thilakavathy, K.; Joseph, N. Prevailing Knowledge on the Bioavailability and Biological Activities of Sulphur Compounds from Alliums: A Potential Drug Candidate. Molecules 2020, 25, 4111. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.J.; Holub, B.J. Effect of Garlic and Fish-Oil Supplementation on Serum Lipid and Lipoprotein Concentrations in Hypercholesterolemic Men. Am. J. Clin. Nutr. 1997, 65, 445–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ried, K.; Toben, C.; Fakler, P. Effect of garlic on serum lipids: An updated meta-analysis. Nutr. Rev. 2013, 71, 282–299. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xue, Z.; Ma, Q.; Xing, L.; Santhanam, R.K.; Zhang, M.; Chen, H. Antioxidant and antihypertensive effects of garlic proteine and its hydrolysates and the related mechanism. J. Food Biochem. 2020, 44, 13126. [Google Scholar] [CrossRef]
- Liu, W.; Chen, S.; Yu, C.; Li, Y.; Zhang, J.Y. Antihypertensive effects of allicin on spontaneously hypertensive rats via vasorelaxation and hydrogen sulfide mechanisms. Biomed. Pharmacother. 2020, 128, 110240. [Google Scholar]
- Toygar, I.; Tureyen, A.; Demir, D.; Cetinkalp, S. Effect of allicin on wound healing: An experimental diabetes model. J. Wound Care 2020, 29, 388–392. [Google Scholar] [CrossRef]
- Nadeem, M.S.; Kazmi, I.; Ullah, I.; Muhammad, K.; Anwar, F. Allicin, an Antioxidant and Neuroprotective Agent, Ameliorates Cognitive Impairment. Antioxidants 2022, 11, 87. [Google Scholar] [CrossRef]
- Li, W.Q.; Zhang, J.Y.; Ma, J.L.; Li, Z.X.; Zhang, L.; Zhang, Y.; Guo, Y.; Zhou, T.; Li, J.Y.; Shen, L.; et al. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: Follow-up of a randomized intervention trial. BMJ 2019, 366, l5016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Liu, X.; Ruan, J.; Zhuang, X.; Zhang, X.; Li, Z. Phytochemicals of garlic: Promising candidates for cancer therapy. Biomed. Pharmacother. 2020, 123, 109730. [Google Scholar] [CrossRef] [PubMed]
- Brugnoli, F.; Tedeschi, P.; Grassilli, S.; Maietti, A.; Brandolini, V.; Bertagnolo, V. Ethanol-based garlic extract prevents malignant evolution of non-invasive breast tumor cells induced by moderate hypoxia. Biomed. Pharm. 2021, 142, 112052. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, B.; Xiang, T.; Peng, W.; Qiu, Z.; Wan, J.; Zhang, L.; Li, H.; Li, H.; Ren, G. Diallyl disulfide inhibits growth and metastatic potential of human triple-negative breast cancer cells through inactivation of the beta-catenin signaling pathway. Mol. Nutr. Food Res. 2015, 59, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Desai, G.; Schelske-Santos, M.; Nazario, C.M.; Rosario-Rosado, R.V.; Mansilla-Rivera, I.; Ramírez-Marrero, F.; Nie, J.; Myneni, A.A.; Zhang, Z.F.; Freudenheim, J.L.; et al. Onion and Garlic Intake and Breast Cancer, a Case-Control Study in Puerto Rico. Nutr. Cancer 2020, 72, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Patiño-Morales, C.C.; Jaime-Cruz, R.; Sánchez-Gómez, C.; Corona, J.C.; Hernández-Cruz, E.Y.; Kalinova-Jelezova, I.; Pedraza-Chaverri, J.; Maldonado, P.D.; Silva-Islas, C.A.; Salazar-García, M. Antitumor Effects of Natural Compounds Derived from Allium sativum on Neuroblastoma: An Overview. Antioxidants 2021, 11, 48. [Google Scholar] [CrossRef]
- Juan-García, A.; Agahi, F.; Drakonaki, M.; Tedeschi, P.; Font, G.; Juan, C. Cytoprotection assessment against mycotoxins on HepG2 cells by extracts from Allium sativum L. Food Chem. Toxicol. 2021, 151, 112129. [Google Scholar] [CrossRef]
- Agahi, F.; Penalva-Olcina, R.; Font, G.; Juan-García, A.; Juan, C. Effects of Voghiera garlic extracts in neuronal human cell line against zearalenone’s derivates and beauvericin. Food Chem. Toxicol. 2022, 162, 112905. [Google Scholar] [CrossRef]
- Tedeschi, P.; Leis, M.; Pezzi, M.; Civolani, S.; Maietti, A.; Brandolini, V. Insecticidal activity and fungitoxicity of plant extracts and components of horseradish (Armoracia rusticana) and garlic (Allium sativum). J. Environ. Sci. Health B 2011, 46, 486–490. [Google Scholar]
- Mylona, K.; Garcia-Cela, E.; Sulyok, M.; Medina, A.; Magan, N. Influence of two garlic-derived compounds, propyl propane thiosulfonate (PTS) and propyl propane thiosulfinate (PTSO), on growth and mycotoxin production by Fusarium species in vitro and in stored cereals. Toxins 2019, 11, 495. [Google Scholar] [CrossRef] [Green Version]
- Alorainy, M.S. Evaluation of antimicrobial activity of garlic (Allium sativum) against E. coli O 157:H 7. J. Agric. Vet. Sci. 2011, 4, 149–157. [Google Scholar]
- Chakraborty, D.; Majumder, A. Garlic (Lahsun)–an immunity booster against SARS-CoV-2. Biotica Res. Today 2020, 2, 755–757. [Google Scholar]
- Khubber, S.; Hashemifesharaki, R.; Mohammadi, M.; Gharibzahedi, S.M.T. Garlic (Allium sativum L.): A potential unique therapeutic food rich in organosulfur and flavonoid compounds to fight with COVID-19. Nutr. J. 2020, 19, 124. [Google Scholar] [CrossRef] [PubMed]
- Sripanidkulchai, B. Benefits of aged garlic extract on Alzheimer’s disease: Possible mechanisms of action. Exp. Ther. Med. 2020, 19, 1560–1564. [Google Scholar] [CrossRef] [Green Version]
- Seki, K.; Ishikawa, J.; Okada, Y. Contribution of 2-Propenesulfenic acid to the Antioxidant activity of allicin. J. Food Sci. 2018, 83, 1265–1270. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Rybczynska-Tkaczyk, K.; Gawel-Beben, K.; Swieca, M.; Karas, M.; Jakubczyk, A.; Matysiak, M.; Binduga, U.E.; Gminski, J. Characterization of active compounds of different garlic (Allium sativum L.) cultivars. Pol. J. Food Nutr. Sci. 2018, 68, 73–81. [Google Scholar] [CrossRef]
- Cruz-Villalon, G. Synthesis of allicin and purification by solid-phase extraction. Anal. Biochem. 2001, 290, 376–378. [Google Scholar] [CrossRef]
- Mansingh, D.P.; Dalpati, N.; Sali, V.K.; Vasanthi, A.H.R. Alliin the precursor of allicin in garlic extract mitigates proliferation of gastric adenocarcinoma cells by modulating apoptosis. Pharmacogn. Mag. 2018, 14, 84–91. [Google Scholar]
- Shang, A.; Cao, S.; Xu, X.; Gan, R.; Tang, G.; Corke, H. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef] [Green Version]
- Lawson, L.D.; Hunsaker, S.M. Allicin Bioavailability and Bioequivalence from Garlic Supplements and Garlic Foods. Nutrients 2018, 10, 812. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, F.; Leontiev, R.; Jacob, C.; Slusarenko, A.J. An optimized facile procedure to synthesize and purify allicin. Molecules 2017, 22, 770. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, H.; Suma, K.; Origuchi, K.; Seki, T.; Ariga, T. Thermostability of allicin determined by chemical and biological assays. Biosci. Biotechno. Biochem. 2008, 72, 2877–2883. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Zucca, P.; Orhan, I.E.; Azzini, E.; Adetunji, C.O.; Mohammed, S.A.; Ahmad, Z. Allicin and health: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 502–516. [Google Scholar] [CrossRef]
- Liu, P.; Weng, R.; Sheng, X.; Wang, X.; Zhang, W.; Qian, Y.; Qiu, J. Profiling of organosulfur compounds and amino acids in garlic from different regions of China. Food Chem. 2020, 305, 125499. [Google Scholar] [CrossRef] [PubMed]
- Iciek, M.; Kwiecien, I.; Włodek, L. Biological Properties of Garlic and Garlic-Derived Organosulfur Compounds. Environ. Mol. Mutagen. 2009, 50, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, S.; Takada, N.; Wanibuchi, H.; Hori, T.; Min, W.; Ogawa, M. Suppression of chemical carcinogenesis by water-soluble organosulfur compounds. J. Nutr. 2001, 131, 1049S–1053S. [Google Scholar] [CrossRef]
- Farhat, Z.; Hershberger, P.A.; Freudenheim, J.L.; Mammen, M.J.; Hageman Blair, R.; Aga, D.S.; Mu, L. Types of garlic and their anticancer and antioxidant activity: A review of the epidemiologic and experimental evidence. Eur. J. Nutr. 2021, 60, 3585–3609. [Google Scholar] [CrossRef]
- Lanzotti, V.; Scala, F.; Bonanomi, G. Compounds from Allium species with cytotoxic and antimicrobial activity. Phytochem. Rev. 2014, 13, 769–791. [Google Scholar] [CrossRef]
- Gourmaud, S.; Paquet, C.; Dumurgier, J.; Pace, C.; Bouras, C.; Gray, F.; Hugon, J. Increased levels of cerebrospinal fluid JNK3 associated with amyloid pathology: Links to cognitive decline. J. Psychiatry Neurosci. 2015, 40, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Putnik, P.; Gabrić, D.; Roohinej, S.; Barba, F.J.; Granato, D.; Mallikarjunan, K.; Lorenzo, J.M.; Bursać Kovačević, D. An overview of organosulfur compounds from Allium spp.: From processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties. Food Chem. 2019, 276, 680–691. [Google Scholar] [CrossRef]
- Barba, F.J.; Orlien, V. Processing, bioaccessibility and bioavailability of bioactive sulfur compounds: Facts and gaps. J. Food Compos. Anal. 2017, 61, 1–3. [Google Scholar] [CrossRef]
- Barba, F.J.; Mariutti, L.R.B.; Bragagnolo, N.; Mercadante, A.Z.; Barbosa-Cánovas, G.V.; Orlien, V. Bioaccessibility of bioactive compounds from fruits and vegetables after thermal and nonthermal processing. Trends Food Sci. Technol. 2017, 67, 195–206. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Sanches-Silva, A.; Daglia, M.; Nabavi, S.F.; Jafari, N.J.; Nabavi, S.M. Antifungal and antibacterial activities of allicin: A review. Trends Food Sci. Technol. 2016, 52, 49–56. [Google Scholar] [CrossRef]
- Rahman, M.S. Allicin and other functional active components in garlic: Health benefits and bioavailability. Int. J. Food Prop. 2007, 10, 245–268. [Google Scholar] [CrossRef]
- Lawson, L.D.; Gardner, C.D. Composition, stability, and bioavailability of garlic products used in a clinical trial. J. Agric. Food Chem. 2005, 53, 6254–6261. [Google Scholar] [CrossRef] [Green Version]
- Lahiri, D.K.; Chen, D.; Ge, Y.W.; Bondy, S.C.; Sharman, E.H. Dietary supplementation with melatonin reduces levels of amyloid beta-peptides in the murine cerebral cortex. J. Pineal Res. 2004, 36, 224–231. [Google Scholar] [CrossRef]
- Lahiri, D.K.; Maloney, B.; Zawia, N.H. The LEARn model: An epigenetic explanation for idiopathic neurobiological diseases. Mol. Psychiatry 2009, 14, 992–1003. [Google Scholar] [CrossRef]
- Lahiri, D.K.; Maloney, B. The ‘LEARn’ (Latent Early-life Associated Regulation) model integrates environmental risk factors and the developmental basis of Alzheimer’s disease, and proposes remedial steps. Exp. Gerontol. 2010, 45, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Price, D.L.; Sisodia, S.S.; Borchelt, D.R. Genetic neurodegenerative diseases: The human illness and transgenic models. Science 1998, 282, 1079–1083. [Google Scholar] [CrossRef]
- Varadarajan, S.; Yatin, S.; Aksenova, M.; Butterfield, D.A. Review: Alzheimer’s amyloid beta-peptide-associated free radical oxidative stress and neurotoxicity. J. Struct. Biol. 2000, 130, 184–208. [Google Scholar] [CrossRef]
- Vassar, R.; Kovacs, D.M.; Yan, R.; Wong, P.C. The beta-secretase enzyme BACE in health and Alzheimer’s disease: Regulation, cell biology, function, and therapeutic potential. J. Neurosci. 2009, 29, 12787–12794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Khoury, J.; Hickman, S.E.; Thomas, C.A.; Cao, L.; Silverstein, S.C.; Loike, J.D. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature 1996, 382, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Coraci, I.S.; Husemann, J.; Berman, J.W.; Hulette, C.; Dufour, J.H.; Campanella, G.K.; Luster, A.D.; Silverstein, S.C.; El-Khoury, J.B. CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer’s disease brains and can mediate production of reactive oxygen species in response to beta-amyloid fibrils. Am. J. Pathol. 2002, 160, 101–112. [Google Scholar] [CrossRef]
- Yamada, K.; Nabeshima, T. Animal models of Alzheimer’s disease and evaluation of anti-dementia drugs. Pharmacol. Ther. 2000, 88, 93–113. [Google Scholar] [CrossRef]
- Nakai, T.; Yamada, K.; Mizoguchi, H. Alzheimer’s Disease Animal Models: Elucidation of Biomarkers and Therapeutic Approaches for Cognitive Impairment. Int. J. Mol. Sci. 2021, 22, 5549. [Google Scholar] [CrossRef]
- Yamada, K.; Ren, X.; Nabeshima, T. Perspectives of pharmaco-therapy in Alzheimer’s disease. Jpn. J. Pharmacol. 1999, 80, 9–14. [Google Scholar]
- Park, K.; Kim, E.; Han, H.; Shim, Y.; Kwon, J.; Ku, B.; Park, K.H.; Yi, H.A.; Kim, K.K.; Yang, D.W. Efficacy and Tolerability of Rivastigmine Patch Therapy in Patients with Mild-To-Moderate Alzheimer’s Dementia Associated with Minimal and Moderate Ischemic white Matter Hyperintensities: A Multicenter Prospective Open-Label Clinical Trial. PLoS ONE 2017, 12, e0182123. [Google Scholar] [CrossRef] [Green Version]
- Stoiljkovic, M.; Horvath, T.L.; Hajós, M. Therapy for Alzheimer’s disease: Missing targets and functional markers? Ageing Res. Rev. 2021, 68, 101318. [Google Scholar] [CrossRef]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- Cummings, J. New Approaches to Symptomatic Treatments for Alzheimer’s Disease. Mol. Neurodegener. 2021, 16, 2. [Google Scholar] [CrossRef]
- Whitehouse, P.J.; Saini, V. Making the Case for the Accelerated Withdrawal of Aducanumab. J. Alzheimers Dis. 2022, 87, 1003–1007, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K. Waste Clearance in the Brain and Neuroinflammation: A Novel Perspective on Biomarker and Drug Target Discovery in Alzheimer’s Disease. Cells 2022, 11, 919. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.G. Defining activation states of microglia in human brain tissue: An unresolved issue for Alzheimer’s disease. Neuroimmunol. Neuroinfam. 2020, 7, 194–214. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinfammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef] [PubMed]
- McFarland, K.N.; Chakrabarty, P. Microglia in Alzheimer’s Disease: A Key Player in the Transition Between Homeostasis and Pathogenesis. Neurotherapeutics 2022, 19, 186–208. [Google Scholar] [CrossRef]
- Neniskyte, U.; Vilalta, A.; Brown, G.C. Tumour necrosis factor alpha-induced neuronal loss is mediated by microglial phagocytosis. FEBS Lett. 2014, 588, 2952–2956. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Du, L.; Ju, Q.; Chen, Z.; Ma, Y.; Bai, T.; Ji, G.; Wu, Y.; Liu, Z.; Shao, Y.; et al. Silencing TLR4/MyD88/NF-κB signaling pathway alleviated inflammation of corneal epithelial cells infected by ISE. Inflammation 2021, 44, 633–644. [Google Scholar] [CrossRef]
- Lindhout, I.A.; Murray, T.E.; Richards, C.M.; Klegeris, A. Potential neurotoxic activity of diverse molecules released by microglia. Neurochem. Int. 2021, 148, 105117. [Google Scholar] [CrossRef]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation pathways: A general review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef]
- Hanamsagar, R.; Hanke, M.L.; Kielian, T. Toll-like receptor (TLR) and inflammasome actions in the central nervous system. Trends Immunol. 2012, 33, 333–342. [Google Scholar] [CrossRef] [Green Version]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Petrilli, V.; Papin, S.; Dostert, C.; Mayor, A.; Martinon, F.; Tschopp, J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007, 14, 1583–1589. [Google Scholar] [CrossRef]
- Dostert, C.; Pétrilli, V.; Van Bruggen, R.; Steele, C.; Mossman, B.T.; Tschopp, J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 2008, 320, 674–677. [Google Scholar] [CrossRef] [Green Version]
- Craven, R.R.; Gao, X.; Allen, I.; Gris, D.; Wardenburg, J.B.; McElvania-TeKippe, E.; Ting, J.P.; Duncan, J.A. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS ONE 2009, 4, e7446. [Google Scholar] [CrossRef]
- Schroder, K.; Zhou, R.; Tschopp, J. The NLRP3 inflammasome: A sensor for metabolic danger? Science 2010, 327, 296–300. [Google Scholar] [CrossRef]
- Hornung, V.; Bauernfeind, F.; Halle, A.; O Samstad, E.; Kono, H.; Rock, K.L.; Fitzgerald, K.; Latz, E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008, 9, 847–856. [Google Scholar] [CrossRef]
- Han, L.P.; Li, C.J.; Sun, B.; Xie, Y.; Guan, Y.; Ma, Z.J.; Chenm, L.M. Protective effects of celastrol on diabetic liver injury via TLR4/MyD88/NF-κB signaling pathway in type 2 diabetic rats. J. Diabetes Res. 2016, 2016, 2641248. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Wu, S.; Yang, Y.; Li, G.Q.; Meng, L.; Zheng, Q.Y.; Li, Y.; Xu, G.L.; Zhang, K.Q.; Peng, K.F. LIGHT aggravates sepsis-associated acute kidney injury via TLR4-MyD88-NF-κB pathway. J. Cell. Mol. Med. 2020, 24, 11936–11948. [Google Scholar] [CrossRef]
- El-Sahar, A.E.; Shiha, N.A.; El Sayed, N.S.; Ahmed, L.A. Alogliptin attenuates lipopolysaccharide-induced neuroinflammation in mice through modulation of TLR4/MYD88/NF-κB and miRNA-155/SOCS-1 signaling pathways. Int. J. Neuropsychopharmacol. 2021, 24, 158–169. [Google Scholar] [CrossRef]
- Aıd, S.; Bosetti, F. Targeting cyclooxygenases-1 and -2 in neuroinflammation: Therapeutic implications. Biochimie 2011, 93, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Ghazanfari, N.; van Waarde, A.; Dierckx, R.A.; Doorduin, J.; de Vries, E.F. Is cyclooxygenase-1 involved in neuroinflammation? J. Neurosci. Res. 2021, 99, 2976–2998. [Google Scholar] [CrossRef]
- Xian, M.; Cai, J.; Zheng, K.; Liu, Q.; Liu, Y.; Lin, H.; Liang, S.; Wang, S. Aloe-emodin prevents nerve injury and neuroinflammation caused by ischemic stroke via the PI3K/AKT/mTOR and NF-κB pathway. Food Funct. 2021, 12, 8056–8067. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, A.; Xu, L.; Zhang, X. The role of TLR4-mediated PTEN/PI3K/AKT/NF-κB signaling pathway in neuroinflammation in hippocampal neurons. Neuroscience 2014, 269, 93–101. [Google Scholar] [CrossRef]
- Wang, L.; Kou, M.C.; Weng, C.Y.; Hu, L.W.; Wang, Y.J.; Wu, M.J. Arsenic modulates heme oxygenase-1, interleukin-6, and vascular endothelial growth factor expression in endothelial cells: Roles of ROS, NF-κB, and MAPK pathways. Arch. Toxicol. 2012, 86, 879–896. [Google Scholar] [CrossRef]
- Srivastava, I.N.; Shperdheja, J.; Baybis, M.; Ferguson, T.; Crino, P.B. mTOR pathway inhibition prevents neuroinflammation and neuronal death in a mouse model of cerebral palsy. Neurobiol. Dis. 2016, 85, 144–154. [Google Scholar] [CrossRef]
- Merighi, S.; Bencivenni, S.; Vincenzi, F.; Varani, K.; Borea, P.A.; Gessi, S. A(2B) adenosine receptors stimulate IL-6 production in primary murine microglia through p38 MAPK kinase pathway. Pharmacol. Res. 2017, 117, 9–19. [Google Scholar] [CrossRef]
- Mocayar Marón, F.J.; Camargo, A.B.; Manucha, W. Allicin pharmacology: Common molecular mechanisms against neuroinflammation and cardiovascular diseases. Life Sci. 2020, 249, 117513. [Google Scholar] [CrossRef]
- Ho, S.; Su, M. Evaluating the anti-neuroinflammatory capacity of raw and steamed garlic as well as five organosulfur compounds. Molecules 2014, 19, 17697–17714. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wang, P.; Xue, Y.; Liu, L.; Li, Z.; Liu, Y. Allicin ameliorates cognitive impairment in APP/PS1 mice via suppressing oxidative stress by blocking JNK signaling pathways. Tissue Cell 2018, 50, 89–95. [Google Scholar] [CrossRef]
- Li, X.H.; Li, C.Y.; Lu, J.M.; Tian, R.B.; Wei, J. Allicin ameliorates cognitive deficits ageing-induced learning and memory deficits through enhancing of Nrf2 antioxidant signaling pathways. Neurosci. Lett. 2012, 514, 46–50. [Google Scholar] [CrossRef]
- Gao, W.; Wang, W.; Liu, G.; Zhang, J.; Yang, J.; Deng, Z. Allicin attenuated chronic social defeat stress induced depressive-like behaviors through suppression of NLRP3 inflammasome. Metab. Brain Dis. 2019, 34, 319–329. [Google Scholar] [CrossRef]
- Lv, R.; Mao, N.; Wu, J.; Lu, C.; Ding, M.; Gu, X.; Wu, Y.; Shi, Z. Neuroprotective effect of allicin in a rat model of acute spinal cord injury. Life Sci. 2015, 143, 114–123. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Li, X.H.; Yuan, Z.P.; Li, C.Y.; Tian, R.B.; Jia, W.; Xiao, Z.P. Allicin improves endoplasmic reticulum stress-related cognitive deficits via PERK/Nrf2 antioxidative signaling pathway. Eur. J. Pharmacol. 2015, 762, 239–246. [Google Scholar] [CrossRef]
- Li, X.H.; Li, C.Y.; Xiang, Z.G.; Zhong, F.; Chen, Z.Y.; Lu, J.M. Allicin can reduce neuronal death and ameliorate the spatial memory impairment in Alzheimer’s disease models. Neurosciences 2010, 15, 237–243. [Google Scholar]
- Kaur, S.; Raj, K.; Gupta, Y.K.; Singh, S. Allicin ameliorates aluminium- and copper-induced cognitive dysfunction in Wistar rats: Relevance to neuro-inflammation, neurotransmitters and Abeta((1–42)) analysis. J. Biol. Inorg. Chem. 2021, 26, 495–510. [Google Scholar] [CrossRef]
- Killick, R.; Ribe, E.M.; Al-Shawi, R.; Malik, B.; Hooper, C.; Fernandes, C.; Lovestone, S. Clusterin regulates beta-amyloid toxicity via Dickkopf-1-driven induction of the wnt-PCP-JNK pathway. Mol. Psychiatry 2014, 19, 88–98. [Google Scholar] [CrossRef]
- Ahn, J.H.; So, S.P.; Kim, N.Y.; Kim, H.J.; Yoon, S.Y.; Kim, D.H. c-Jun N-terminal Kinase (JNK) induces phosphorylation of amyloid precursor protein (APP) at Thr668, in okadaic acid-induced neurodegeneration. BMB Rep. 2016, 49, 376–381. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S. Dual inhibition of acetylcholinesterase and butyrylcholinesterase enzymes by allicin. Indian J. Pharmacol. 2015, 47, 444–446. [Google Scholar] [CrossRef]
- Ray, B.; Chauhan, N.B.; Lahiri, D.K. The "aged garlic extract:" (AGE) and one of its active ingredients S-allyl-L-cysteine (SAC) as potential preventive and therapeutic agents for Alzheimer’s disease (AD). Curr. Med. Chem. 2011, 18, 3306–3313. [Google Scholar] [CrossRef]
- Qu, Z.; Mossine, V.V.; Cui, J.; Sun, G.Y.; Gu, Z. Protective Effects of AGE and Its Components on Neuroinflammation and Neurodegeneration. Neuromolecular Med. 2016, 18, 474–482. [Google Scholar] [CrossRef]
- Ahangar-Sirous, R.; Poudineh, M.; Ansari, A.; Nili, A.; Dana, S.M.M.A.; Nasiri, Z.; Hosseini, Z.; Karami, D.; Mokhtari, M.; Deravi, N. Pharmacotherapeutic Potential of Garlic in Age-Related Neurological Disorders. CNS Neurol. Disord. Drug Targets 2022, 21, 377–398. [Google Scholar] [CrossRef]
- Takeda, T.; Hosokawa, M.; Higuchi, K. Senescence-accelerated mouse (SAM): A novel murine model of accelerated senescence. J. Am. Geriatr. Soc. 1991, 39, 911–919. [Google Scholar] [CrossRef]
- Nishiyama, N.; Moriguchi, T.; Saito, H. Beneficial effects of aged garlic extract on learning and memory impairment in the senescence-accelerated mouse. Exp. Gerontol. 1997, 32, 149–160. [Google Scholar] [CrossRef]
- Griffin, B.; Selassie, M.; Gwebu, E.T. Effect of Aged Garlic Extract on the Cytotoxicity of Alzheimer beta-Amyloid Peptide in Neuronal PC12 Cells. Nutr. Neurosci. 2000, 3, 139–142. [Google Scholar] [CrossRef]
- Peng, Q.; Buz’Zard, A.R.; Lau, B.H. Neuroprotective effect of garlic compounds in amyloid-beta peptide-induced apoptosis in vitro. Med. Sci. Monit. 2002, 8, 328–337. [Google Scholar]
- Ito, Y.; Ito, M.; Takagi, N.; Saito, H.; Ishige, K. Neurotoxicity induced by amyloid beta-peptide and ibotenic acid in organotypic hippocampal cultures: Protection by S-allyl-L-cysteine, a garlic compound. Brain Res. 2003, 985, 98–107. [Google Scholar] [CrossRef]
- Ray, B.; Chauhan, N.B.; Lahiri, D.K. Oxidative insults to neurons and synapse are prevented by aged garlic extract and S-allyl-L-cysteine treatment in the neuronal culture and APP-Tg mouse model. J. Neurochem. 2011, 117, 388–402. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.H.; Jeong, H.R.; Jo, Y.N.; Kim, H.J.; Shin, J.H.; Heo, H.J. Ameliorating effects of aged garlic extracts against Abeta-induced neurotoxicity and cognitive impairment. BMC Complement. Altern. Med. 2013, 13, 268. [Google Scholar] [CrossRef] [Green Version]
- Kosuge, Y.; Koen, Y.; Ishige, K.; Minami, K.; Urasawa, H.; Saito, H.; Ito, Y. S-allyl-L-cysteine selectively protects cultured rat hippocampal neurons from amyloid beta-protein- and tunic-amycin-induced neuronal death. Neuroscience 2003, 122, 885–895. [Google Scholar] [CrossRef]
- Imai, T.; Kosuge, Y.; Ishige, K.; Ito, Y. Amyloid beta-protein potentiates tunicamycin-induced neuronal death in organotypic hippocampal slice cultures. Neuroscience 2007, 147, 639–651. [Google Scholar] [CrossRef]
- Chauhan, N.B. Anti-amyloidogenic effect of Allium sativum in Alzheimer’s transgenic model Tg2576. J. Herb. Pharmacother. 2003, 3, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.B. Effect of aged garlic extract on APP processing and tau phosphorylation in Alzheimer’s transgenic model Tg2576. J. Ethnopharmacol. 2006, 108, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.B.; Sandoval, J. Amelioration of early cognitive deficits by aged garlic extract in Alzheimer’s transgenic mice. Phytother. Res. 2007, 21, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.J.; Chiu, C.P.; Yang, H.T.; Yin, M.C. s-Allyl cysteine, s-ethyl cysteine, and spropyl cysteine alleviate beta-amyloid, glycative, and oxidative injury in brain of mice treated by D-galactose. J. Agric. Food Chem. 2011, 59, 6319–6326. [Google Scholar] [CrossRef]
- Javed, H.; Khan, M.M.; Khan, A.; Vaibhav, K.; Ahmad, A.; Khuwaja, G.; Ahmed, M.E.; Raza, S.S.; Ashafaq, M.; Tabassum, R.; et al. S-allyl cysteine attenuates oxidative stress associated cognitive impairment and neurodegeneration in mouse model of streptozotocin-induced experimental dementia of Alzheimer’s type. Brain Res. 2011, 1389, 133–142. [Google Scholar] [CrossRef]
- Zarezadeh, M.; Baluchnejadmojarad, T.; Kiasalari, Z.; Afshin-Majd, S.; Roghani, M. Garlic active constituent s-allyl cysteine protects against lipopolysaccharide-induced cognitive deficits in the rat: Possible involved mechanisms. Eur. J. Pharmacol. 2017, 795, 13–21. [Google Scholar] [CrossRef]
- Thorajak, P.; Pannangrong, W.; Welbat, J.U.; Chaijaroonkhanarak, W.; Sripanidkulchai, K.; Sripanidkulchai, B. Effects of Aged Garlic Extract on Cholinergic, Glutamatergic and GABAergic Systems with Regard to Cognitive Impairment in Aβ-Induced Rats. Nutrients 2017, 9, 686. [Google Scholar] [CrossRef] [Green Version]
- Nillert, N.; Pannangrong, W.; Welbat, J.U.; Chaijaroonkhanarak, W.; Sripanidkulchai, K.; Sripanidkulchai, B. Neuroprotective Effects of Aged Garlic Extract on Cognitive Dysfunction and Neuroinflammation Induced by β-Amyloid in Rats. Nutrients 2017, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Kim, M.R. Effect of Aged Garlic Ethyl Acetate Extract on Oxidative Stress and Cholinergic Function of Scopolamine-Induced Cognitive Impairment in Mice. Prev. Nutr. Food Sci. 2019, 24, 165–170. [Google Scholar] [CrossRef]
- Borek, C. Antioxidant health effects of aged garlic extract. J. Nutr. 2001, 131, 1010S–1015S. [Google Scholar] [CrossRef] [Green Version]
- Borek, C. Garlic reduces dementia and heart-disease risk. J. Nutr. 2006, 136, 810S–812S. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.; McNeil, B.; Taylor, C.; Holl, G.; Ruff, D.; Gwebu, E.T. Effect of aged garlic extract on caspase-3 activity, in vitro. Nutr. Neurosci. 2002, 5, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.F.; Dong, Y.; Chen, J.Y.; Lu, J.H. The effect and underlying mechanisms of garlic extract against cognitive impairment and Alzheimer’s disease: A systematic review and meta-analysis of experimental animal studies. J. Ethnopharmacol. 2021, 280, 114423. [Google Scholar] [CrossRef] [PubMed]
- Merighi, S.; Nigro, M.; Travagli, A.; Pasquini, S.; Borea, P.A.; Varani, K.; Vincenzi, F.; Gessi, S. A2A Adenosine Receptor: A Possible Therapeutic Target for Alzheimer’s Disease by Regulating NLRP3 Inflammasome Activity? Int. J. Mol. Sci. 2022, 23, 5056. [Google Scholar] [CrossRef]
| References | Experimental Model | Chemicals Concentration | Results |
|---|---|---|---|
| [90] | APP/PS1 double transgenic mice | 10 mg/kg/day allicin via intragastric administration on alternate days for 3 months |
|
| [91] | 3- and 20-month-old C57BL/6 mice | Diet supplemented with 180 mg/kg/day of allicin for 8 consecutive weeks |
|
| [92] | 8-week-old male C57BL/6 J mice (depressive-like model) | 2, 10, 50 mg/kg allicin once day via intraperitoneal injection |
|
| [93] | Rats with acute traumatic spinal cord injury (TSCI) | 2, 10, 50 mg/kg intraperitoneal injection of allicin for 21 days |
|
| [94] | Adult male rats 72 h of lateral ventricular infusion of tunicamycin (TM), an endoplasmic reticulum stress stimulator (cognitive deficit induction) | Diet supplemented with 180 mg/kg/d of allicin for 16 weeks |
|
| [95] | AD mouse model via injection of Aß(1–42) (1 µL = 4 µg) into the bilateral hippocampi | Allicin via intraperitoneal injection for 14 days 180 mg/kg/day) |
|
| [96] | Male Wistar rats | Allicin via intraperitoneal injection (10 and 20 mg/kg) 7 days before metals (aluminum chloride, 200 mg/kg p.o; copper sulfate, 0.5 mg/kg p.o.) administration for 28 days |
|
| References | Experimental Model | Chemicals Concentration | Results |
|---|---|---|---|
| [104] | Senescence-Accelerated Mice (SAM) | Diet containing 2% (w/w) AGE |
|
| [105] | Neuronal PC12 cells treated with NGF for 4 days and injured with Aβ 95 nM | Cell growth medium containing 0.01% AGE |
|
| [106] | Undifferentiated PC12 cells injured with Aβ25-35 40 μM | AGE (1–8 mg/mL) SAC (1–4 mg/mL) |
|
| [107] | Hippocampal slice culture injured with Aβ25–35 25 μM | SAC (10–100 μM) |
|
| [108] | Neuronal PC12 cells treated with NGF for 12 days and APP-Tg mice | 0.3% or 1.0% AGE in PC12 cells diet 2% AGE in mice |
|
| [109] | NGF-treated neuronal PC12 cells injured with Aβ25-35 80 μM ICR mice administered Aβ25-35 via intracerebroventricular injection | AGE 25–200 µg/mL in PC12 cells Freeze-dried ethyl acetate fraction from AGE at concentrations of 5, 10 and 20 mg/kg in mice |
|
| [110] | rat hippocampal neurons injured with Aβ25–35 5 μM or TM (10 μg/mL) | SAC (1 µM) |
|
| [111] | rat hippocampal neurons injured with Aβ25–35 25 μM or TM (20–80 μg/mL) | SAC (100 µM) |
|
| [112] | Tg2576 mice | AGE (40 mg/kg/d/4 wks) |
|
| [113] | Tg2576 mice | AGE 2%, 20 mg SAC/kg and 20 mg DADS/kg |
|
| [114] | Tg2576 mice | Diet 2% AGE for 5 months |
|
| [115] | C57BL/6 mice treated with D-galactose (AD-like model) | SAC (1 g/L into drinking water for 7 weeks) |
|
| [116] | Intracerebroventricular infusion of streptozotocin (STZ) (model of memory impairment in mice) | SAC (30 mg/kg i.p. for 15 days) |
|
| [117] | LPS-treated rats (167 μg/kg for 7 days) | SAC (25, 50, 100 mg/kg/day p.o. for 7 days) |
|
| [118] | Rats injured with Aβ1–42 1 µg/µL intracerebroventricular infusion | AGE (125, 250 and 500 mg/kg p.o. for 65 days) |
|
| [119] | Rats injured with Aβ1–42 1 µg/µL intracerebroventricular infusion | AGE (125, 250 and 500 mg/kg body weight, p.o., daily for 56 days) |
|
| [120] | Scopolamine-treated mice (2 mg/kg) injected 30 min before the tests. | AGE (25 or 50 mg/kg p.o.) |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tedeschi, P.; Nigro, M.; Travagli, A.; Catani, M.; Cavazzini, A.; Merighi, S.; Gessi, S. Therapeutic Potential of Allicin and Aged Garlic Extract in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 6950. https://doi.org/10.3390/ijms23136950
Tedeschi P, Nigro M, Travagli A, Catani M, Cavazzini A, Merighi S, Gessi S. Therapeutic Potential of Allicin and Aged Garlic Extract in Alzheimer’s Disease. International Journal of Molecular Sciences. 2022; 23(13):6950. https://doi.org/10.3390/ijms23136950
Chicago/Turabian StyleTedeschi, Paola, Manuela Nigro, Alessia Travagli, Martina Catani, Alberto Cavazzini, Stefania Merighi, and Stefania Gessi. 2022. "Therapeutic Potential of Allicin and Aged Garlic Extract in Alzheimer’s Disease" International Journal of Molecular Sciences 23, no. 13: 6950. https://doi.org/10.3390/ijms23136950
APA StyleTedeschi, P., Nigro, M., Travagli, A., Catani, M., Cavazzini, A., Merighi, S., & Gessi, S. (2022). Therapeutic Potential of Allicin and Aged Garlic Extract in Alzheimer’s Disease. International Journal of Molecular Sciences, 23(13), 6950. https://doi.org/10.3390/ijms23136950









