A Novel Mouse Model of TGFβ2-Induced Ocular Hypertension Using Lentiviral Gene Delivery
Abstract
1. Introduction
2. Results
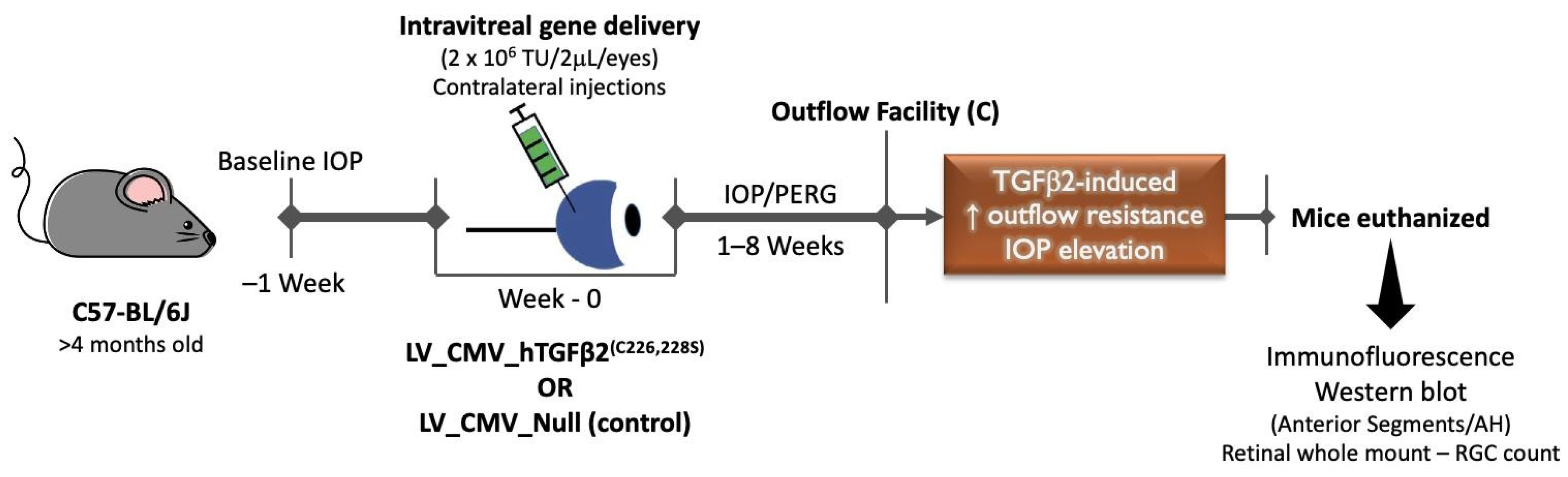
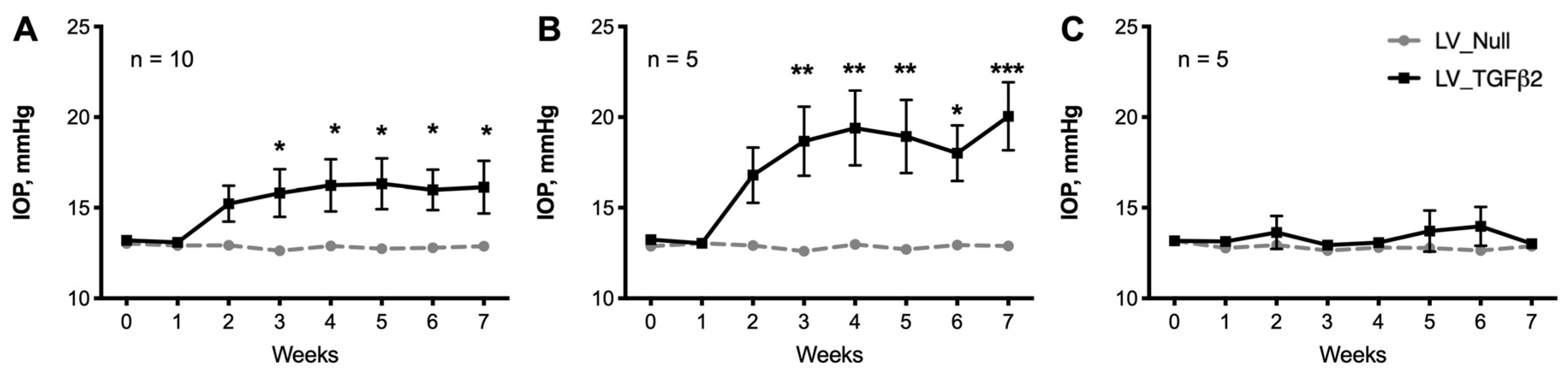
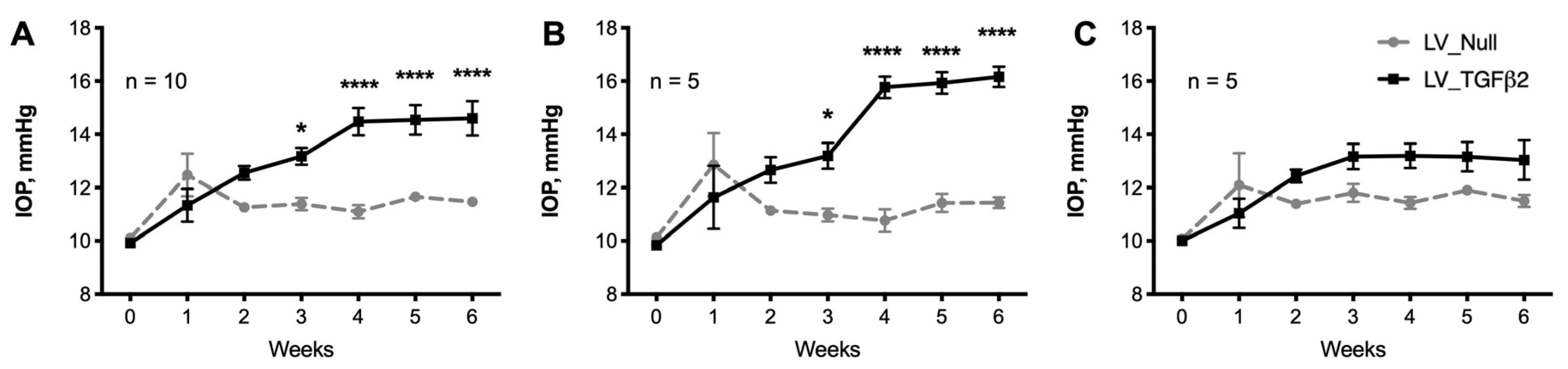
2.1. Intravitreal Injection of Lentiviral Vector Expressing hTGFβ2C226/228S Leads to Sustained IOP Elevation in Mice
2.2. Decrease of Aqueous Humor Outflow Facility in LV_TGFβ2 Expressing Eyes
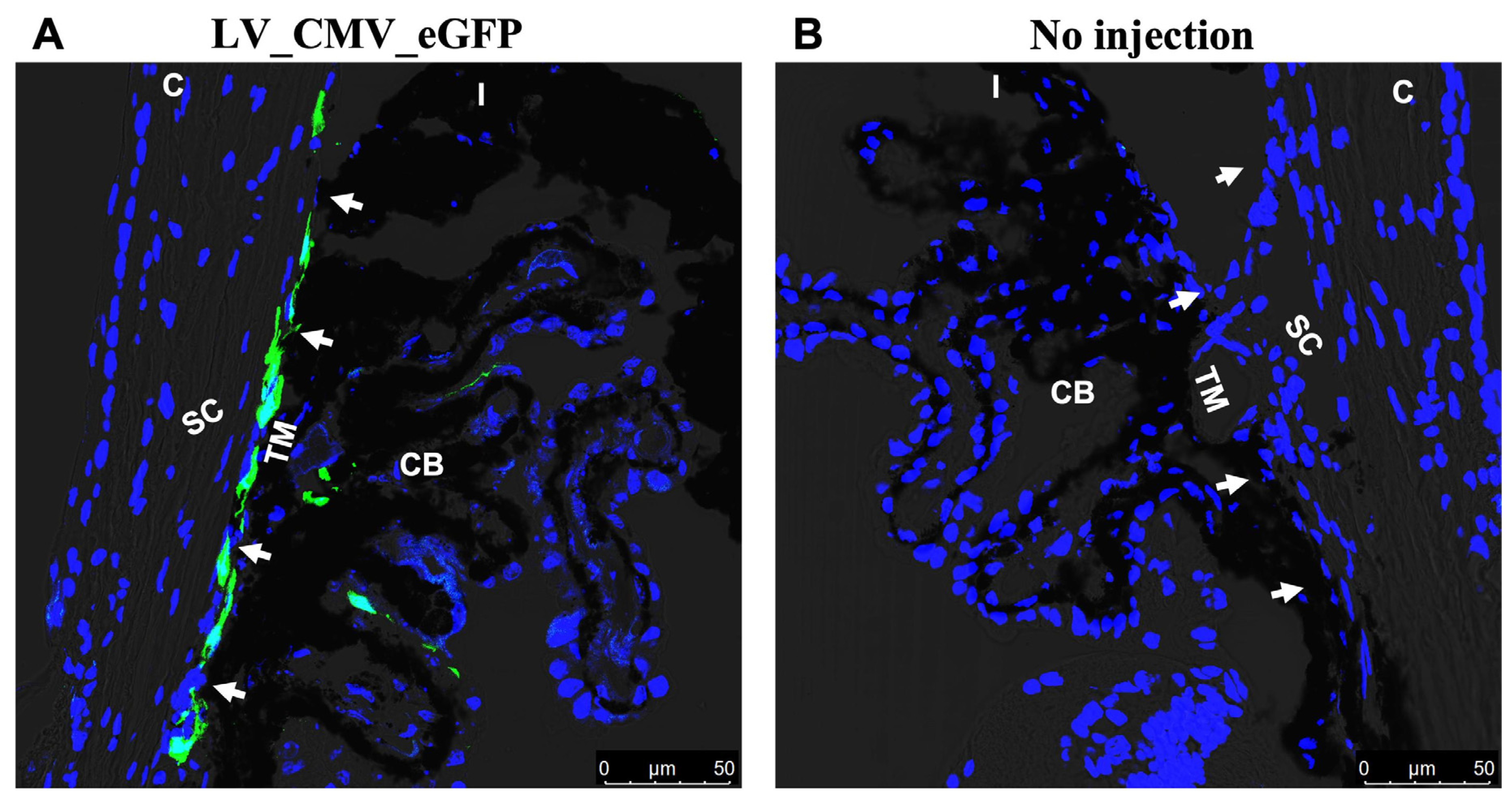
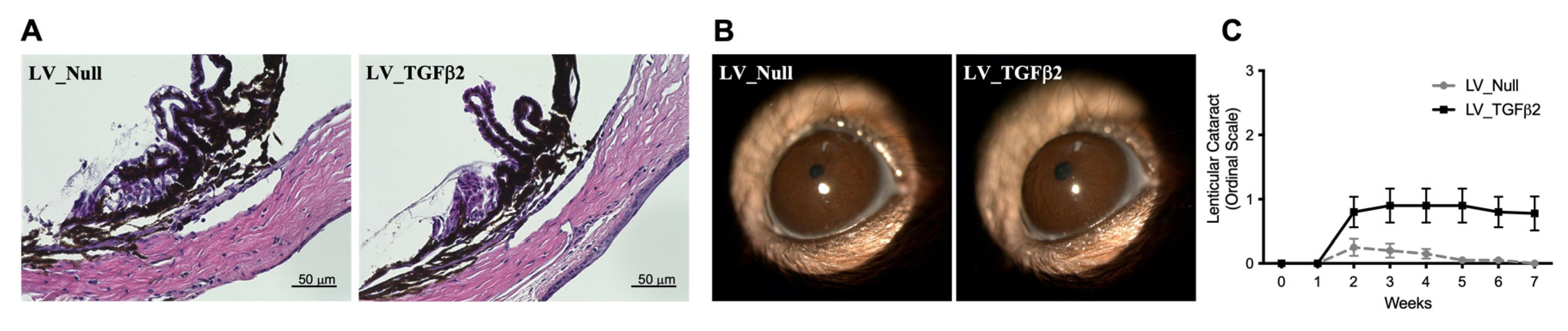
2.3. Gross Morphological Assessment of Ocular Tissues in LV-Injected Mice
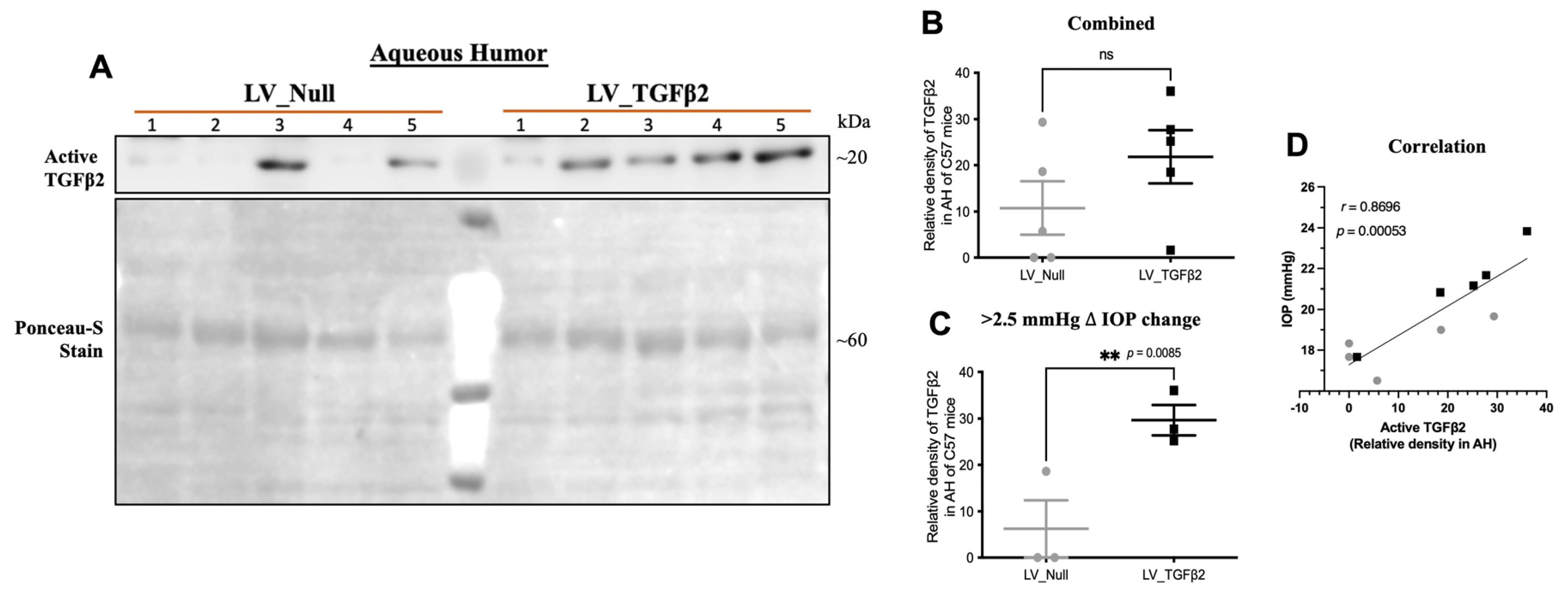
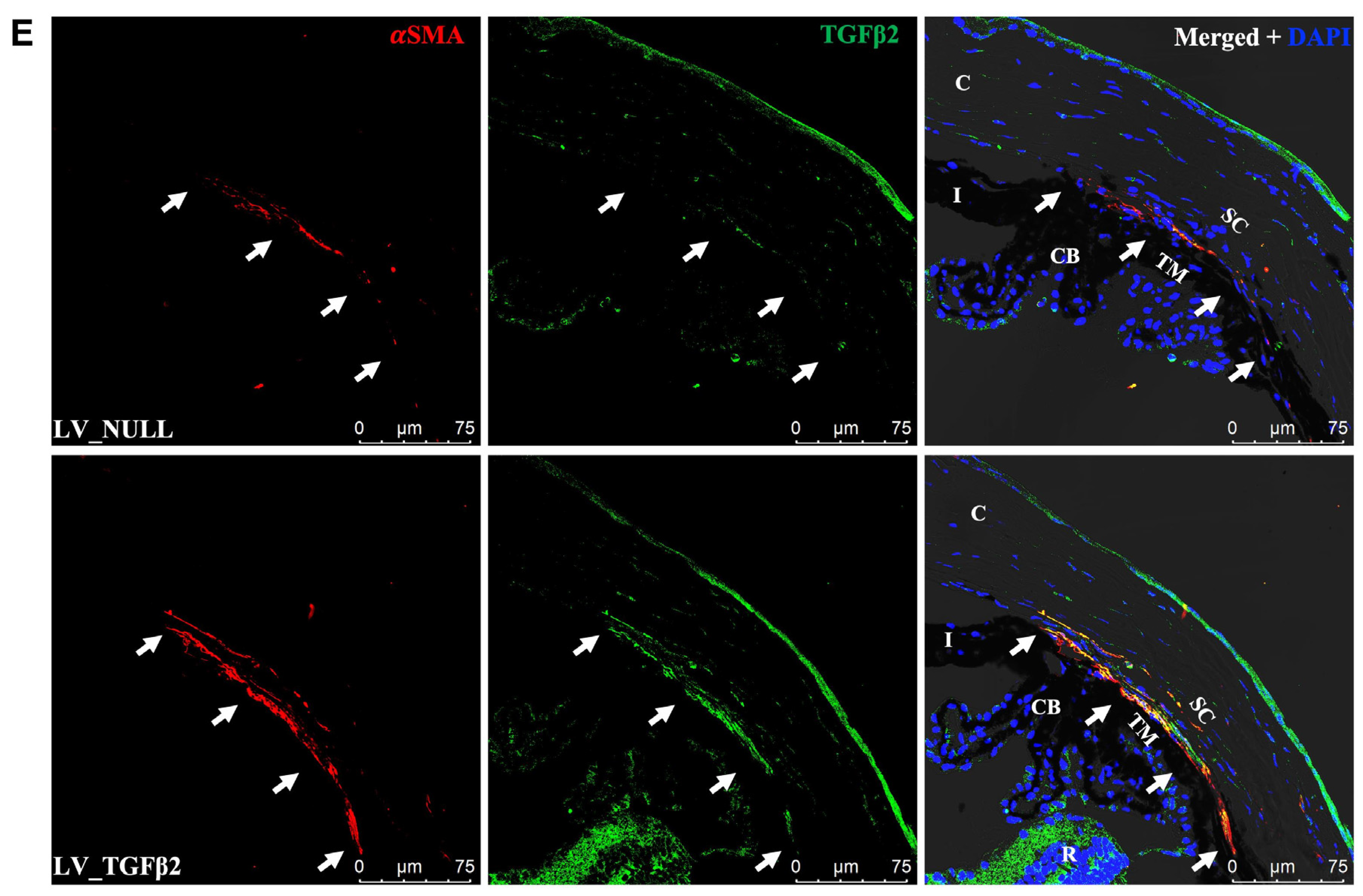
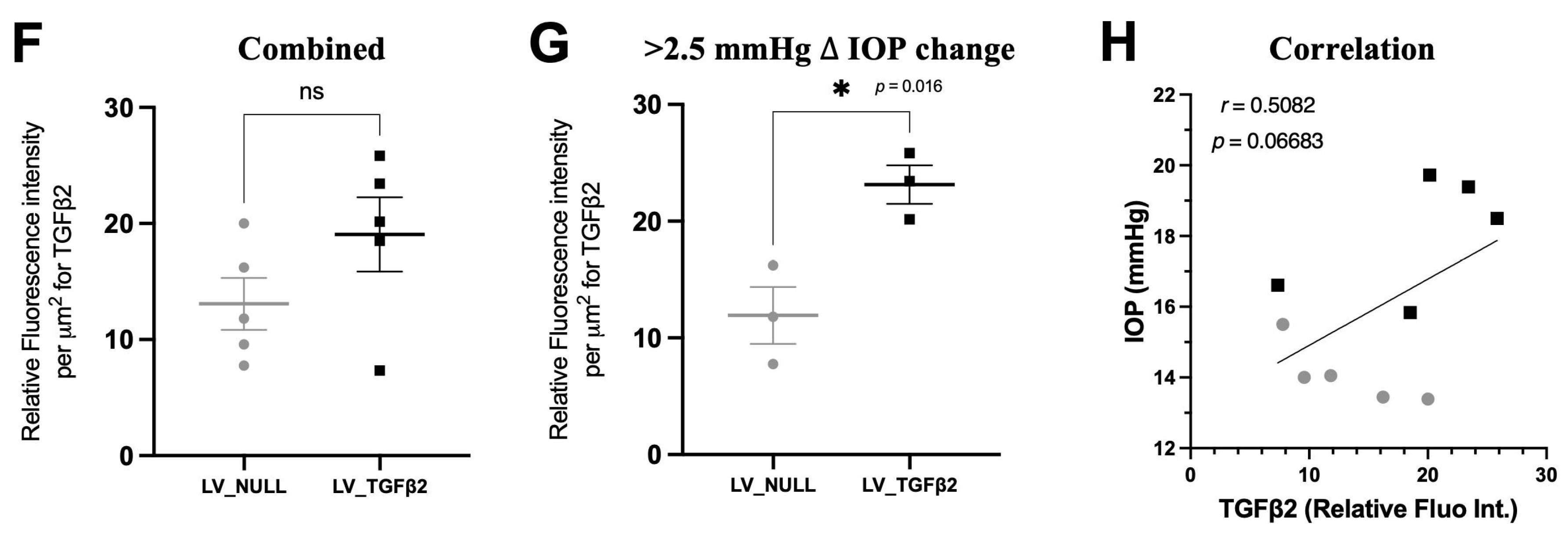
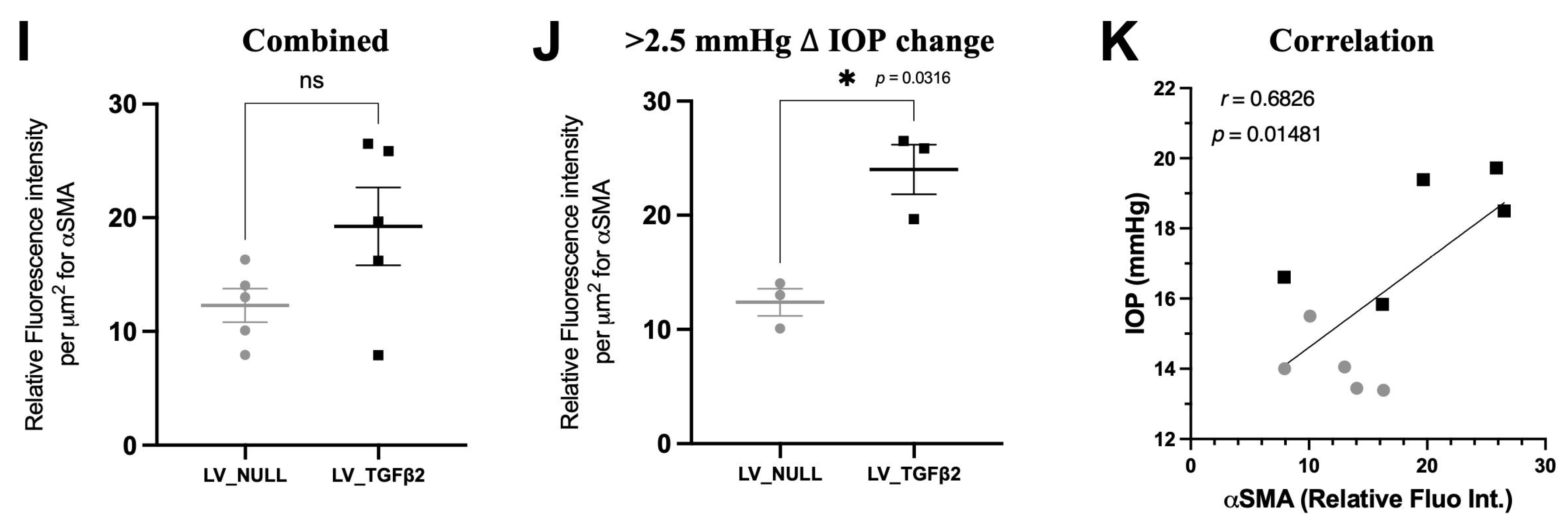
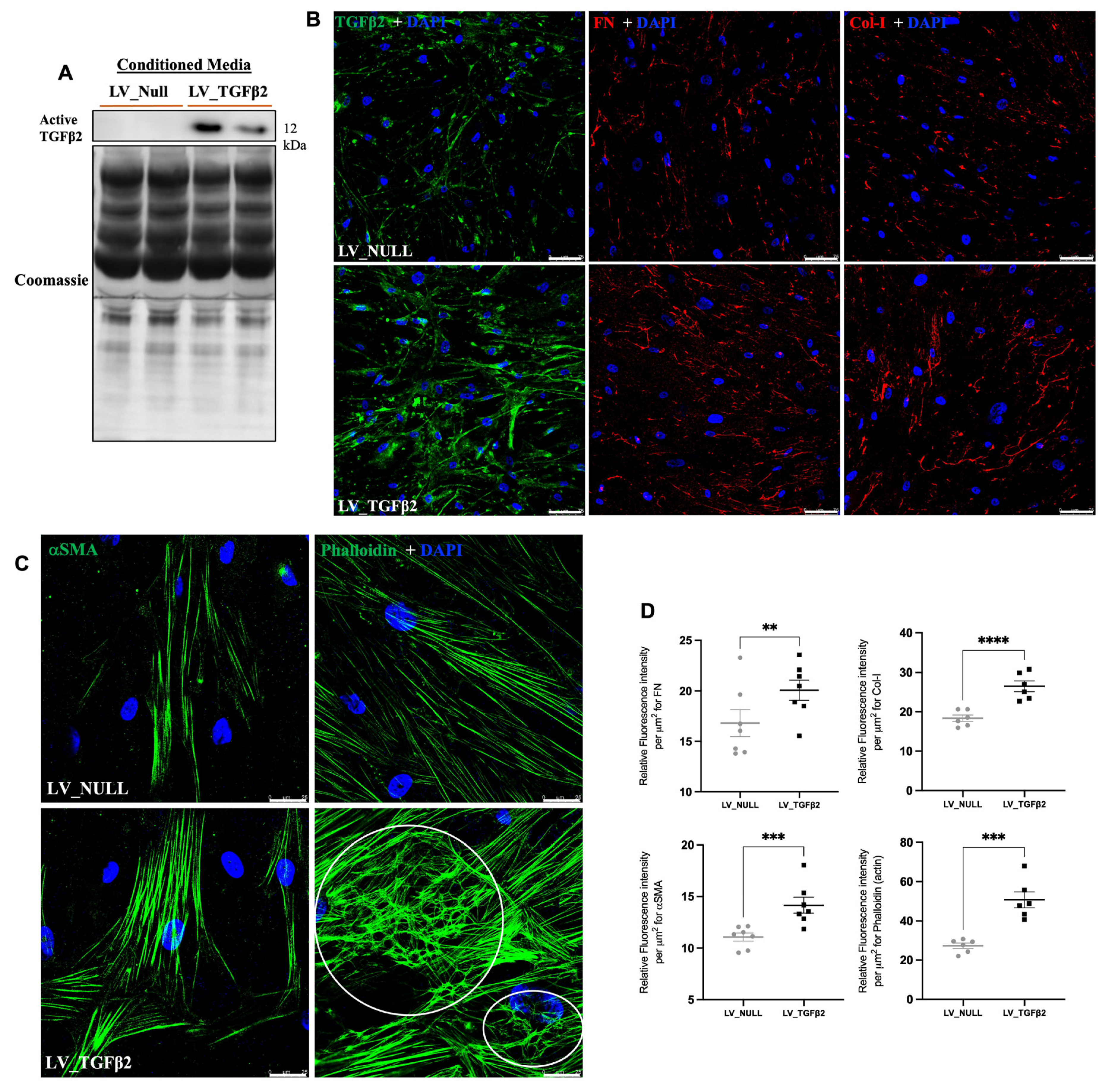
2.4. TGFβ2 Expression in AH and Anterior Segment Tissue of LV_TGFβ2 Injected Eyes
2.5. LV_TGFβ2 Stimulates ECM Deposition and Actin Reorganization in Primary TM Cells
3. Discussion
4. Methods and Materials
4.1. Mice
4.2. Viral Vector Injections
4.3. IOP Measurements
4.4. Outflow Facility Measurements
4.5. Ophthalmoscopy and Slit-Lamp Examination
4.6. Ocular Sample Collection
4.7. Lentiviral Transduction of Primary Human Trabecular Meshwork Cells
4.8. H&E Staining
4.9. Immunostaining
4.10. Western Blotting
4.11. Pattern Electroretinography
4.12. RGC Counts from Retinal Whole Mounts
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.S.; Wolfs, R.C.; O’Colmain, B.J.; Klein, B.E.; Taylor, H.R.; West, S.; Leske, M.C.; Mitchell, P.; Congdon, N.; Kempen, J.; et al. Prevalence of open-angle glaucoma among adults in the United States. Arch. Ophthalmol. 2004, 122, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Leung, C.K.; Crowston, J.G.; Medeiros, F.A.; Friedman, D.S.; Wiggs, J.L.; Martin, K.R. Primary open-angle glaucoma. Nat. Rev. Dis. Primers 2016, 2, 16067. [Google Scholar] [CrossRef]
- Rosenthal, J.; Leske, M.C. Open-angle glaucoma risk factors applied to clinical area. J. Am. Optom. Assoc. 1980, 51, 1017–1024. [Google Scholar]
- Friedman, D.S.; Wilson, M.R.; Liebmann, J.M.; Fechtner, R.D.; Weinreb, R.N. An evidence-based assessment of risk factors for the progression of ocular hypertension and glaucoma. Am. J. Ophthalmol. 2004, 138, S19–S31. [Google Scholar] [CrossRef]
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous humor dynamics: A review. Open Ophthalmol. J. 2010, 4, 52–59. [Google Scholar] [CrossRef]
- Rohen, J.W. Why is intraocular pressure elevated in chronic simple glaucoma? Anatomical considerations. Ophthalmology 1983, 90, 758–765. [Google Scholar] [CrossRef]
- Gabelt, B.T.; Kaufman, P.L. Changes in aqueous humor dynamics with age and glaucoma. Prog. Retin. Eye Res. 2005, 24, 612–637. [Google Scholar] [CrossRef]
- Stamer, W.D.; Acott, T.S. Current understanding of conventional outflow dysfunction in glaucoma. Curr. Opin. Ophthalmol. 2012, 23, 135–143. [Google Scholar] [CrossRef]
- Dismuke, W.M.; Overby, D.R.; Civan, M.M.; Stamer, W.D. The Value of Mouse Models for Glaucoma Drug Discovery. J. Ocul. Pharmacol. Ther. 2016, 32, 486–487. [Google Scholar] [CrossRef] [PubMed]
- Pang, I.H.; Clark, A.F. Inducible rodent models of glaucoma. Prog. Retin. Eye Res. 2020, 75, 100799. [Google Scholar] [CrossRef] [PubMed]
- Bouhenni, R.A.; Dunmire, J.; Sewell, A.; Edward, D.P. Animal models of glaucoma. J. Biomed. Biotechnol. 2012, 2012, 692609. [Google Scholar] [CrossRef]
- Pang, I.H.; Millar, J.C.; Clark, A.F. Elevation of intraocular pressure in rodents using viral vectors targeting the trabecular meshwork. Exp. Eye Res. 2015, 141, 33–41. [Google Scholar] [CrossRef]
- Vranka, J.A.; Kelley, M.J.; Acott, T.S.; Keller, K.E. Extracellular matrix in the trabecular meshwork: Intraocular pressure regulation and dysregulation in glaucoma. Exp. Eye Res. 2015, 133, 112–125. [Google Scholar] [CrossRef]
- Fuchshofer, R.; Tamm, E.R. Modulation of extracellular matrix turnover in the trabecular meshwork. Exp. Eye Res. 2009, 88, 683–688. [Google Scholar] [CrossRef]
- Keller, K.E.; Aga, M.; Bradley, J.M.; Kelley, M.J.; Acott, T.S. Extracellular matrix turnover and outflow resistance. Exp. Eye Res. 2009, 88, 676–682. [Google Scholar] [CrossRef]
- Yang, Y.F.; Sun, Y.Y.; Acott, T.S.; Keller, K.E. Effects of induction and inhibition of matrix cross-linking on remodeling of the aqueous outflow resistance by ocular trabecular meshwork cells. Sci. Rep. 2016, 6, 30505. [Google Scholar] [CrossRef]
- Wang, K.; Li, G.; Read, A.T.; Navarro, I.; Mitra, A.K.; Stamer, W.D.; Sulchek, T.; Ethier, C.R. The relationship between outflow resistance and trabecular meshwork stiffness in mice. Sci. Rep. 2018, 8, 5848. [Google Scholar] [CrossRef]
- Hoare, M.J.; Grierson, I.; Brotchie, D.; Pollock, N.; Cracknell, K.; Clark, A.F. Cross-linked actin networks (CLANs) in the trabecular meshwork of the normal and glaucomatous human eye in situ. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1255–1263. [Google Scholar] [CrossRef]
- Bermudez, J.Y.; Montecchi-Palmer, M.; Mao, W.; Clark, A.F. Cross-linked actin networks (CLANs) in glaucoma. Exp. Eye Res. 2017, 159, 16–22. [Google Scholar] [CrossRef]
- Read, A.T.; Chan, D.W.; Ethier, C.R. Actin structure in the outflow tract of normal and glaucomatous eyes. Exp. Eye Res. 2007, 84, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Rayana, N.P.; Dai, J.; Sugali, C.K.; Baidouri, H.; Suresh, A.; Raghunathan, V.K.; Mao, W. Cross-linked actin networks (CLANs) affect stiffness and/or actin dynamics in transgenic transformed and primary human trabecular meshwork cells. Exp. Eye Res. 2022, 220, 109097. [Google Scholar] [CrossRef] [PubMed]
- Fuchshofer, R.; Tamm, E.R. The role of TGF-beta in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 2012, 347, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Sethi, A.; Mao, W.; Wordinger, R.J.; Clark, A.F. Transforming growth factor-beta induces extracellular matrix protein cross-linking lysyl oxidase (LOX) genes in human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5240–5250. [Google Scholar] [CrossRef] [PubMed]
- Lutjen-Drecoll, E.; Futa, R.; Rohen, J.W. Ultrahistochemical studies on tangential sections of the trabecular meshwork in normal and glaucomatous eyes. Investig. Ophthalmol. Vis. Sci. 1981, 21, 563–573. [Google Scholar]
- Fleenor, D.L.; Shepard, A.R.; Hellberg, P.E.; Jacobson, N.; Pang, I.H.; Clark, A.F. TGFbeta2-induced changes in human trabecular meshwork: Implications for intraocular pressure. Investig. Ophthalmol. Vis. Sci. 2006, 47, 226–234. [Google Scholar] [CrossRef]
- Montecchi-Palmer, M.; Bermudez, J.Y.; Webber, H.C.; Patel, G.C.; Clark, A.F.; Mao, W. TGFbeta2 Induces the Formation of Cross-Linked Actin Networks (CLANs) in Human Trabecular Meshwork Cells Through the Smad and Non-Smad Dependent Pathways. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1288–1295. [Google Scholar] [CrossRef]
- McDowell, C.M.; Tebow, H.E.; Wordinger, R.J.; Clark, A.F. Smad3 is necessary for transforming growth factor-beta2 induced ocular hypertension in mice. Exp. Eye Res. 2013, 116, 419–423. [Google Scholar] [CrossRef]
- Swaminathan, S.S.; Oh, D.J.; Kang, M.H.; Shepard, A.R.; Pang, I.H.; Rhee, D.J. TGF-beta2-mediated ocular hypertension is attenuated in SPARC-null mice. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4084–4097. [Google Scholar] [CrossRef]
- Hernandez, H.; Medina-Ortiz, W.E.; Luan, T.; Clark, A.F.; McDowell, C.M. Crosstalk Between Transforming Growth Factor Beta-2 and Toll-Like Receptor 4 in the Trabecular Meshwork. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Gleizes, P.E.; Munger, J.S.; Nunes, I.; Harpel, J.G.; Mazzieri, R.; Noguera, I.; Rifkin, D.B. TGF-beta latency: Biological significance and mechanisms of activation. Stem Cells 1997, 15, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.C.; Li, J.; Chan, W.F.; Tripathi, B.J. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp. Eye Res. 1994, 59, 723–727. [Google Scholar] [CrossRef]
- Min, S.H.; Lee, T.I.; Chung, Y.S.; Kim, H.K. Transforming growth factor-beta levels in human aqueous humor of glaucomatous, diabetic and uveitic eyes. Korean J. Ophthalmol. 2006, 20, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Daher, A.M.; Agarwal, R. Aqueous humor TGF-beta2 levels in patients with open-angle glaucoma: A meta-analysis. Mol. Vis. 2015, 21, 612–620. [Google Scholar]
- Inatani, M.; Tanihara, H.; Katsuta, H.; Honjo, M.; Kido, N.; Honda, Y. Transforming growth factor-beta 2 levels in aqueous humor of glaucomatous eyes. Graefes Arch. Clin. Exp. Ophthalmol. 2001, 239, 109–113. [Google Scholar] [CrossRef]
- Jampel, H.D.; Roche, N.; Stark, W.J.; Roberts, A.B. Transforming growth factor-beta in human aqueous humor. Curr. Eye Res. 1990, 9, 963–969. [Google Scholar] [CrossRef]
- Yamamoto, N.; Itonaga, K.; Marunouchi, T.; Majima, K. Concentration of transforming growth factor beta2 in aqueous humor. Ophthalmic Res. 2005, 37, 29–33. [Google Scholar] [CrossRef]
- Ochiai, Y.; Ochiai, H. Higher concentration of transforming growth factor-beta in aqueous humor of glaucomatous eyes and diabetic eyes. Jpn. J. Ophthalmol. 2002, 46, 249–253. [Google Scholar] [CrossRef]
- Picht, G.; Welge-Luessen, U.; Grehn, F.; Lutjen-Drecoll, E. Transforming growth factor beta 2 levels in the aqueous humor in different types of glaucoma and the relation to filtering bleb development. Graefes Arch. Clin. Exp. Ophthalmol. 2001, 239, 199–207. [Google Scholar] [CrossRef]
- Shepard, A.R.; Millar, J.C.; Pang, I.H.; Jacobson, N.; Wang, W.H.; Clark, A.F. Adenoviral gene transfer of active human transforming growth factor-{beta}2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2067–2076. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Stamenkovic, I. Transforming growth factor-beta facilitates breast carcinoma metastasis by promoting tumor cell survival. Clin. Exp. Metastasis 2004, 21, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Loewen, R.; Parikh, H.A.; Roy, P.; Loewen, N.A. Gene transfer to the outflow tract. Exp. Eye Res. 2017, 158, 73–84. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, X.; Brandt, C.R.; Gabelt, B.T.; Bryar, P.J.; Smith, M.E.; Kaufman, P.L. Herpes simplex virus mediated gene transfer to primate ocular tissues. Exp. Eye Res. 1999, 69, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Spencer, B.; Agarwala, S.; Miskulin, M.; Smith, M.; Brandt, C.R. Herpes simplex virus-mediated gene delivery to the rodent visual system. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1392–1401. [Google Scholar]
- Borras, T.; Gabelt, B.T.; Klintworth, G.K.; Peterson, J.C.; Kaufman, P.L. Non-invasive observation of repeated adenoviral GFP gene delivery to the anterior segment of the monkey eye in vivo. J. Gene Med. 2001, 3, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J. Immune response following intraocular delivery of recombinant viral vectors. Gene Ther. 2003, 10, 977–982. [Google Scholar] [CrossRef]
- Borras, T.; Xue, W.; Choi, V.W.; Bartlett, J.S.; Li, G.; Samulski, R.J.; Chisolm, S.S. Mechanisms of AAV transduction in glaucoma-associated human trabecular meshwork cells. J. Gene Med. 2006, 8, 589–602. [Google Scholar] [CrossRef]
- Buie, L.K.; Rasmussen, C.A.; Porterfield, E.C.; Ramgolam, V.S.; Choi, V.W.; Markovic-Plese, S.; Samulski, R.J.; Kaufman, P.L.; Borras, T. Self-complementary AAV virus (scAAV) safe and long-term gene transfer in the trabecular meshwork of living rats and monkeys. Investig. Ophthalmol. Vis. Sci. 2010, 51, 236–248. [Google Scholar] [CrossRef]
- Yi, Y.; Noh, M.J.; Lee, K.H. Current advances in retroviral gene therapy. Curr. Gene Ther. 2011, 11, 218–228. [Google Scholar] [CrossRef]
- Challa, P.; Luna, C.; Liton, P.B.; Chamblin, B.; Wakefield, J.; Ramabhadran, R.; Epstein, D.L.; Gonzalez, P. Lentiviral mediated gene delivery to the anterior chamber of rodent eyes. Mol. Vis. 2005, 11, 425–430. [Google Scholar] [PubMed]
- Barraza, R.A.; Rasmussen, C.A.; Loewen, N.; Cameron, J.D.; Gabelt, B.T.; Teo, W.L.; Kaufman, P.L.; Poeschla, E.M. Prolonged transgene expression with lentiviral vectors in the aqueous humor outflow pathway of nonhuman primates. Hum. Gene Ther. 2009, 20, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Loewen, N.; Fautsch, M.P.; Peretz, M.; Bahler, C.K.; Cameron, J.D.; Johnson, D.H.; Poeschla, E.M. Genetic modification of human trabecular meshwork with lentiviral vectors. Hum. Gene Ther. 2001, 12, 2109–2119. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, B.; Wang, J.M.; Li, G.G.; Zhang, H.; Manyande, A.; Tian, X.B. Gene transfer to human trabecular meshwork cells in vitro and ex vivo using HIV-based lentivirus. Int. J. Ophthalmol. 2014, 7, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Millar, J.C.; Pang, I.H.; Wang, W.H.; Wang, Y.; Clark, A.F. Effect of immunomodulation with anti-CD40L antibody on adenoviral-mediated transgene expression in mouse anterior segment. Mol. Vis. 2008, 14, 10–19. [Google Scholar] [PubMed]
- McDowell, C.M.; Luan, T.; Zhang, Z.; Putliwala, T.; Wordinger, R.J.; Millar, J.C.; John, S.W.; Pang, I.H.; Clark, A.F. Mutant human myocilin induces strain specific differences in ocular hypertension and optic nerve damage in mice. Exp. Eye Res. 2012, 100, 65–72. [Google Scholar] [CrossRef]
- Robertson, J.V.; Nathu, Z.; Najjar, A.; Dwivedi, D.; Gauldie, J.; West-Mays, J.A. Adenoviral gene transfer of bioactive TGFbeta1 to the rodent eye as a novel model for anterior subcapsular cataract. Mol. Vis. 2007, 13, 457–469. [Google Scholar]
- Liu, Y.; Allingham, R.R. Major review: Molecular genetics of primary open-angle glaucoma. Exp. Eye Res. 2017, 160, 62–84. [Google Scholar] [CrossRef]
- Shepard, A.R.; Jacobson, N.; Millar, J.C.; Pang, I.H.; Steely, H.T.; Searby, C.C.; Sheffield, V.C.; Stone, E.M.; Clark, A.F. Glaucoma-causing myocilin mutants require the Peroxisomal targeting signal-1 receptor (PTS1R) to elevate intraocular pressure. Hum. Mol. Genet. 2007, 16, 609–617. [Google Scholar] [CrossRef][Green Version]
- Tan, J.; Fan, N.; Wang, N.; Feng, B.; Yang, M.; Liu, G.; Wang, Y.; Zhu, X.; Kaufman, P.L.; Pang, I.H.; et al. Effects of Lentivirus-Mediated C3 Expression on Trabecular Meshwork Cells and Intraocular Pressure. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4937–4944. [Google Scholar] [CrossRef]
- Kasetti, R.B.; Patel, P.D.; Maddineni, P.; Patil, S.; Kiehlbauch, C.; Millar, J.C.; Searby, C.C.; Raghunathan, V.; Sheffield, V.C.; Zode, G.S. ATF4 leads to glaucoma by promoting protein synthesis and ER client protein load. Nat. Commun. 2020, 11, 5594. [Google Scholar] [CrossRef] [PubMed]
- Prendes, M.A.; Harris, A.; Wirostko, B.M.; Gerber, A.L.; Siesky, B. The role of transforming growth factor beta in glaucoma and the therapeutic implications. Br. J. Ophthalmol. 2013, 97, 680–686. [Google Scholar] [CrossRef]
- Braunger, B.M.; Fuchshofer, R.; Tamm, E.R. The aqueous humor outflow pathways in glaucoma: A unifying concept of disease mechanisms and causative treatment. Eur. J. Pharm. Biopharm. 2015, 95, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Sappington, R.M.; Carlson, B.J.; Crish, S.D.; Calkins, D.J. The microbead occlusion model: A paradigm for induced ocular hypertension in rats and mice. Investig. Ophthalmol. Vis. Sci. 2010, 51, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Nicou, C.M.; Pillai, A.; Passaglia, C.L. Effects of acute stress, general anesthetics, tonometry, and temperature on intraocular pressure in rats. Exp. Eye Res. 2021, 210, 108727. [Google Scholar] [CrossRef]
- Carreon, T.A.; Edwards, G.; Wang, H.; Bhattacharya, S.K. Segmental outflow of aqueous humor in mouse and human. Exp. Eye Res. 2017, 158, 59–66. [Google Scholar] [CrossRef]
- Li, G.; Gonzalez, P.; Camras, L.J.; Navarro, I.; Qiu, J.; Challa, P.; Stamer, W.D. Optimizing gene transfer to conventional outflow cells in living mouse eyes. Exp. Eye Res. 2013, 109, 8–16. [Google Scholar] [CrossRef][Green Version]
- Loewen, N.; Fautsch, M.P.; Teo, W.L.; Bahler, C.K.; Johnson, D.H.; Poeschla, E.M. Long-term, targeted genetic modification of the aqueous humor outflow tract coupled with noninvasive imaging of gene expression in vivo. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3091–3098. [Google Scholar] [CrossRef][Green Version]
- Barraza, R.A.; McLaren, J.W.; Poeschla, E.M. Prostaglandin pathway gene therapy for sustained reduction of intraocular pressure. Mol. Ther. 2010, 18, 491–501. [Google Scholar] [CrossRef]
- Lee, E.S.; Gabelt, B.T.; Faralli, J.A.; Peters, D.M.; Brandt, C.R.; Kaufman, P.L.; Bhattacharya, S.K. COCH transgene expression in cultured human trabecular meshwork cells and its effect on outflow facility in monkey organ cultured anterior segments. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2060–2066. [Google Scholar] [CrossRef]
- Cronin, J.; Zhang, X.Y.; Reiser, J. Altering the tropism of lentiviral vectors through pseudotyping. Curr. Gene Ther. 2005, 5, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Everett, R.S.; Evans, H.K.; Hodges, B.L.; Ding, E.Y.; Serra, D.M.; Amalfitano, A. Strain-specific rate of shutdown of CMV enhancer activity in murine liver confirmed by use of persistent [E1(-), E2b(-)] adenoviral vectors. Virology 2004, 325, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Medina-Ortiz, W.E.; Belmares, R.; Neubauer, S.; Wordinger, R.J.; Clark, A.F. Cellular fibronectin expression in human trabecular meshwork and induction by transforming growth factor-beta2. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6779–6788. [Google Scholar] [CrossRef] [PubMed]
- Pattabiraman, P.P.; Rao, P.V. Mechanistic basis of Rho GTPase-induced extracellular matrix synthesis in trabecular meshwork cells. Am. J. Physiol. Cell Physiol. 2010, 298, C749–C763. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Millar, J.C.; Pang, I.H.; Wax, M.B.; Clark, A.F. Noninvasive measurement of rodent intraocular pressure with a rebound tonometer. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4617–4621. [Google Scholar] [CrossRef]
- Millar, J.C.; Clark, A.F.; Pang, I.H. Assessment of aqueous humor dynamics in the mouse by a novel method of constant-flow infusion. Investig. Ophthalmol. Vis. Sci. 2011, 52, 685–694. [Google Scholar] [CrossRef]
- Millar, J.C.; Phan, T.N.; Pang, I.H.; Clark, A.F. Strain and Age Effects on Aqueous Humor Dynamics in the Mouse. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5764–5776. [Google Scholar] [CrossRef]
- Zode, G.S.; Kuehn, M.H.; Nishimura, D.Y.; Searby, C.C.; Mohan, K.; Grozdanic, S.D.; Bugge, K.; Anderson, M.G.; Clark, A.F.; Stone, E.M.; et al. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J. Clin. Investig. 2011, 121, 3542–3553. [Google Scholar] [CrossRef]
- Keller, K.E.; Bhattacharya, S.K.; Borras, T.; Brunner, T.M.; Chansangpetch, S.; Clark, A.F.; Dismuke, W.M.; Du, Y.; Elliott, M.H.; Ethier, C.R.; et al. Consensus recommendations for trabecular meshwork cell isolation, characterization and culture. Exp. Eye Res. 2018, 171, 164–173. [Google Scholar] [CrossRef]
- Kasetti, R.B.; Maddineni, P.; Kiehlbauch, C.; Patil, S.; Searby, C.C.; Levine, B.; Sheffield, V.C.; Zode, G.S. Autophagy stimulation reduces ocular hypertension in a murine glaucoma model via autophagic degradation of mutant myocilin. JCI Insight 2021, 6, e143359. [Google Scholar] [CrossRef]
- Zode, G.S.; Sharma, A.B.; Lin, X.; Searby, C.C.; Bugge, K.; Kim, G.H.; Clark, A.F.; Sheffield, V.C. Ocular-specific ER stress reduction rescues glaucoma in murine glucocorticoid-induced glaucoma. J. Clin. Investig. 2014, 124, 1956–1965. [Google Scholar] [CrossRef] [PubMed]
- Zode, G.S.; Bugge, K.E.; Mohan, K.; Grozdanic, S.D.; Peters, J.C.; Koehn, D.R.; Anderson, M.G.; Kardon, R.H.; Stone, E.M.; Sheffield, V.C. Topical ocular sodium 4-phenylbutyrate rescues glaucoma in a myocilin mouse model of primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.H.; Bohorquez, J.; Toft-Nielsen, J.; Ozdamar, O.; Porciatti, V. Robust mouse pattern electroretinograms derived simultaneously from each eye using a common snout electrode. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2469–2475. [Google Scholar] [CrossRef]
- Maddineni, P.; Kasetti, R.B.; Patel, P.D.; Millar, J.C.; Kiehlbauch, C.; Clark, A.F.; Zode, G.S. CNS axonal degeneration and transport deficits at the optic nerve head precede structural and functional loss of retinal ganglion cells in a mouse model of glaucoma. Mol. Neurodegener 2020, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]

| Antibodies | Catalog # | Company | Dilutions |
|---|---|---|---|
| Transforming Growth Factor-β2 | Ab36495 | Abcam, Cambridge, MA, USA | 1:100 |
| Fibronectin (FN) | Ab2413 | Abcam | 1:400 |
| Collagen-1 (Col-1) | NB600-408 | Novus Biologicals, Littleton, CO, USA | 1:100 |
| α-Smooth Muscle Actin (αSMA) | Ab7817 | Abcam | 1:100 |
| Phalloidin-Alexa Conjugate | 8878S | Cell Signaling Technology, Danvers, MA, USA | 1:20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, S.V.; Kasetti, R.B.; Millar, J.C.; Zode, G.S. A Novel Mouse Model of TGFβ2-Induced Ocular Hypertension Using Lentiviral Gene Delivery. Int. J. Mol. Sci. 2022, 23, 6883. https://doi.org/10.3390/ijms23136883
Patil SV, Kasetti RB, Millar JC, Zode GS. A Novel Mouse Model of TGFβ2-Induced Ocular Hypertension Using Lentiviral Gene Delivery. International Journal of Molecular Sciences. 2022; 23(13):6883. https://doi.org/10.3390/ijms23136883
Chicago/Turabian StylePatil, Shruti V., Ramesh B. Kasetti, J. Cameron Millar, and Gulab S. Zode. 2022. "A Novel Mouse Model of TGFβ2-Induced Ocular Hypertension Using Lentiviral Gene Delivery" International Journal of Molecular Sciences 23, no. 13: 6883. https://doi.org/10.3390/ijms23136883
APA StylePatil, S. V., Kasetti, R. B., Millar, J. C., & Zode, G. S. (2022). A Novel Mouse Model of TGFβ2-Induced Ocular Hypertension Using Lentiviral Gene Delivery. International Journal of Molecular Sciences, 23(13), 6883. https://doi.org/10.3390/ijms23136883






