Role of Nano-miRNAs in Diagnostics and Therapeutics
Abstract
:1. Introduction
MiRNA Detection and Biosensing
2. Outcome
2.1. SPR-Based Colorimetric Biosensors
2.2. Other SPR-Based Biosensors
2.3. Graphene-Based Biosensors
2.4. Carbon-Quantum-Dot-Based Biosensors
2.5. Chitosan-Molecular Beacon (CS-MB)
2.6. Electrochemical Biosensors
2.7. FRET for miRNA Detection
2.8. Nanoflares
2.9. Gold Nanoparticles (AuNPs)
2.10. Miscellaneous Section
3. Cancer Treatment
3.1. Extracellular Vesicles
3.2. Exosomes
3.3. Micelles
3.4. Niosomes
3.5. Nanoparticles
3.6. Nanoplatforms
4. Rationale behind Article Selection
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-QD@DNA NC | Triple-CdTe quantum-dot-labelled DNA nanocomposites |
| 3SPCE | Three-screen-printed carbon electrode |
| AgNCs | Silver nanoclusters |
| AIPC | Androgen-independent prostate cancer |
| Au-NPFe2O3NC | Gold-loaded nanoporous superparamagnetic iron oxide nano-cubes |
| AuNPs | Gold NPs |
| AuNPs/GQDs/GO | Gold nanoparticles/graphene quantum dots/graphene oxide |
| AuNPs-MoS2 | Gold-nanoparticle-decorated molybdenum sulfide |
| BCSCs | Breast cancer stem cells |
| BHQ1 | Black Hole Quencher 1 |
| BRCA1 | Breast cancer 1 gene mutation |
| CA 15-3 | Cancer antigen 15-3 |
| C-dots | Carbon dots |
| CESA | Cyclic enzymatic signal boost |
| CHA | Catalytic hairpin assembly |
| CQDs | Carbon quantum dots |
| CS | Nanoparticles of chitosan |
| CS-MB | Chitosan-molecular beacon |
| d PG-NH2 | Polyglycerol dendritic nanocarrier |
| DMHs | DNA mini hexahedrons |
| DPSCs | Dental pulp MSCs |
| DPV | Differential pulse voltammetry |
| DSA | Double signal amplification |
| DSN | Double-strand specific nuclease |
| DTPs | DNA tetrahedron probes |
| DTPs-Au | DNA tetrahedron probes onto gold films |
| EATR | Enzyme-assisted target recycling |
| EGFR | Epidermal growth-factor receptor |
| EVs | Extracellular vesicles |
| FC60 | Fullerene nanoparticles |
| FRET | Förster or fluorescent resonance energy transfer |
| FUS | Focused ultrasound |
| GBM | Glioblastoma |
| GCE | Glassy carbon electrode |
| GNPs | Plasmonic gold nanoparticles |
| GNRs | Gold nanorods |
| GO | Graphene oxide |
| GO–AuNPs | Graphene oxide–gold nanoparticles |
| GP | Nanocomposite of graphene |
| GQDs | Graphene quantum dots |
| HA | Hyaluronic acid |
| HCC | Hepatocellular carcinoma |
| HP-AuNPs | Hairpin-modified gold nanoparticles |
| HPs | Hairpin probes |
| LOD | Limit of detection |
| LRET | Luminescence resonance energy transfer |
| LSPR | Surface plasmon resonance |
| MB | Molecular beacon |
| MBs | Microbubbles |
| MCF-7 | Breast cancer cells |
| miRNA | microRNA |
| MPNPs | Magneto-plasmonic nanoparticles |
| MSCs-Exo | Exosomes from bone-marrow-derived mesenchymal stem cells |
| MSNs | Mesoporous silica nanoparticles |
| nano-miRs | Nanoparticles containing miRNA |
| NGS | Next-generation sequencing |
| NMOFs | Metal–organic framework nanoparticles |
| NPs | Nanoparticles |
| PC | Photonic crystal |
| PEG2k-PEI | Polyethylenimine (PEI) modifications including PEGylation |
| PEI | Polyethylenimine |
| PEI | Polyetherimide |
| PolyGIONs | Polyfunctional gold–iron oxide nanoparticles |
| PPY | Polypyrrole |
| PRAM | Photonic resonator absorption microscopy |
| QDs | Quantum dots |
| qRT-PCR | Real-time polymerase chain reaction |
| RCA | Rolling-circle amplification |
| RNAP | RNA probes |
| RSV | Resveratrol |
| SA-aptamer | Streptavidin aptamer |
| SERS | Surface-enhanced Raman scattering |
| SiO2NPs | Silica dioxide nanoparticles |
| SM | Sphingomyelin |
| SPCE | Screen-printed carbon electrode |
| SPIONs, SP | Superparamagnetic iron oxide nanoparticles |
| SPR | Surface plasmon resonance |
| ssDNA | Single-stranded DNA |
| SWV | Square-wave voltammetry |
| TEVs | Tumour-cell-derived extracellular vesicles |
| TF | Transferrin |
| TF-TQ-Np | Thymoquinone nanoparticles using transferrin |
| TMSD | Toehold-mediated strand displacement |
| TNBC | Triple-negative breast cancer |
| TQ-Np | Thymoquinone nanoparticles |
| UCL | Upconversion luminescence |
| UPNPs | Gold nanorods with upconverting nanoparticles |
References
- Revythis, A.; Shah, S.; Kutka, M.; Moschetta, M.; Ozturk, M.A.; Pappas-Gogos, G.; Ioannidou, E.; Sheriff, M.; Rassy, E.; Boussios, S. Unraveling the Wide Spectrum of Melanoma Biomarkers. Diagnostics 2021, 11, 1341. [Google Scholar] [CrossRef] [PubMed]
- De Planell-Saguer, M.; Rodicio, M.C. Analytical aspects of microRNA in diagnostics: A review. Anal. Chim. Acta 2011, 699, 134–152. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kowdley, K.V. MicroRNAs in common human diseases. Genom. Proteom. Bioinform. 2012, 10, 246–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wark, A.W.; Lee, H.J.; Corn, R.M. Multiplexed detection methods for profiling microRNA expression in biological samples. Angew. Chem. Int. Ed. Engl. 2008, 47, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Hunt, E.A.; Broyles, D.; Head, T.; Deo, S.K. MicroRNA Detection: Current Technology and Research Strategies. Annu. Rev. Anal. Chem. 2015, 8, 217–237. [Google Scholar] [CrossRef]
- Fiammengo, R. Can nanotechnology improve cancer diagnosis through miRNA detection? Biomark. Med. 2017, 11, 69–86. [Google Scholar] [CrossRef] [Green Version]
- Bellassai, N.; Spoto, G. Biosensors for liquid biopsy: Circulating nucleic acids to diagnose and treat cancer. Anal. Bioanal. Chem. 2016, 408, 7255–7264. [Google Scholar] [CrossRef]
- Mariani, S.; Minunni, M. Surface plasmon resonance applications in clinical analysis. Anal. Bioanal. Chem. 2014, 406, 2303–2323. [Google Scholar] [CrossRef]
- Chaudhary, V.; Jangra, S.; Yadav, N.R. Nanotechnology based approaches for detection and delivery of microRNA in healthcare and crop protection. J. Nanobiotechnol. 2018, 16, 40. [Google Scholar] [CrossRef] [Green Version]
- Degliangeli, F.; Pompa, P.P.; Fiammengo, R. Nanotechnology-based strategies for the detection and quantification of microRNA. Chemistry 2014, 20, 9476–9492. [Google Scholar] [CrossRef]
- Ghose, A.; Gullapalli, S.V.N.; Chohan, N.; Bolina, A.; Moschetta, M.; Rassy, E.; Boussios, S. Applications of Proteomics in Ovarian Cancer: Dawn of a New Era. Proteomes 2022, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Mollasalehi, H.; Shajari, E. A colorimetric nano-biosensor for simultaneous detection of prevalent cancers using unamplified cell-free ribonucleic acid biomarkers. Bioorg. Chem. 2021, 107, 104605. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.; Moschetta, M.; Pappas-Gogos, G.; Sheriff, M.; Boussios, S. Genetic Aberrations of DNA Repair Pathways in Prostate Cancer: Translation to the Clinic. Int. J. Mol. Sci. 2021, 22, 9783. [Google Scholar] [CrossRef] [PubMed]
- Coradduzza, D.; Cruciani, S.; Arru, C.; Garroni, G.; Pashchenko, A.; Jedea, M.; Zappavigna, S.; Caraglia, M.; Amler, E.; Carru, C.; et al. Role of miRNA-145, 148, and 185 and Stem Cells in Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 1626. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Lee, C.Y.; Kang, S.; Kim, H.; Park, K.S.; Park, H.G. Universal, colorimetric microRNA detection strategy based on target-catalyzed toehold-mediated strand displacement reaction. Nanotechnology 2018, 29, 085501. [Google Scholar] [CrossRef]
- Cheung, A.; Shah, S.; Parker, J.; Soor, P.; Limbu, A.; Sheriff, M.; Boussios, S. Non-Epithelial Ovarian Cancers: How Much Do We Really Know? Int. J. Environ. Res. Public Health 2022, 19, 1106. [Google Scholar] [CrossRef]
- Ye, C.; Wang, M.Q.; Luo, H.Q.; Li, N.B. Label-Free Photoelectrochemical “Off-On” Platform Coupled with G-Wire-Enhanced Strategy for Highly Sensitive MicroRNA Sensing in Cancer Cells. Anal. Chem. 2017, 89, 11697–11702. [Google Scholar] [CrossRef]
- Cai, J.; Ding, L.; Gong, P.; Huang, J. A colorimetric detection of microRNA-148a in gastric cancer by gold nanoparticle–RNA conjugates. Nanotechnology 2019, 31, 095501. [Google Scholar] [CrossRef]
- Hearty, S.; Leonard, P.; Ma, H.; O’Kennedy, R. Measuring antibody-antigen binding kinetics using surface plasmon resonance. In Antibody Engineering; Springer: Berlin/Heidelberg, Germany, 2018; pp. 421–455. [Google Scholar]
- Carroll, J.; Raum, M.; Forsten-Williams, K.; Tauber, U.C. Ligand-receptor binding kinetics in surface plasmon resonance cells: A Monte Carlo analysis. Phys. Biol. 2016, 13, 066010. [Google Scholar] [CrossRef]
- Teran, M.; Nugent, M.A. Characterization of receptor binding kinetics for vascular endothelial growth factor-A using SPR. Anal. Biochem. 2019, 564, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, D.; Chen, F.C.; Hamal, S.; Bridgman, R.C. Kinetic Analysis and Epitope Mapping of Monoclonal Antibodies to Salmonella Typhimurium Flagellin Using a Surface Plasmon Resonance Biosensor. Antibodies 2019, 8, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Li, Q.; Yang, X.; Wang, K.; Du, S.; Zhang, H.; Nie, Y. Graphene oxide-gold nanoparticles hybrids-based surface plasmon resonance for sensitive detection of microRNA. Biosens. Bioelectron. 2016, 77, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Q.; Yang, X.; Wang, K.; Zhang, H.; Nie, W. High sensitivity surface plasmon resonance biosensor for detection of microRNA and small molecule based on graphene oxide-gold nanoparticles composites. Talanta 2017, 174, 521–526. [Google Scholar] [CrossRef]
- Li, J.; Lei, P.; Ding, S.; Zhang, Y.; Yang, J.; Cheng, Q.; Yan, Y. An enzyme-free surface plasmon resonance biosensor for real-time detecting microRNA based on allosteric effect of mismatched catalytic hairpin assembly. Biosens. Bioelectron. 2016, 77, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.J.; Wang, Q.; Li, Q.; Yang, X.H.; Wang, K.M.; Nie, W.Y. Surface plasmon resonance biosensor for sensitive detection of microRNA and cancer cell using multiple signal amplification strategy. Biosens. Bioelectron. 2017, 87, 433–438. [Google Scholar] [CrossRef]
- Nie, W.; Wang, Q.; Yang, X.; Zhang, H.; Li, Z.; Gao, L.; Zheng, Y.; Liu, X.; Wang, K. High sensitivity surface plasmon resonance biosensor for detection of microRNA based on gold nanoparticles-decorated molybdenum sulfide. Anal. Chim. Acta 2017, 993, 55–62. [Google Scholar] [CrossRef]
- Nie, W.; Wang, Q.; Zou, L.; Zheng, Y.; Liu, X.; Yang, X.; Wang, K. Low-Fouling Surface Plasmon Resonance Sensor for Highly Sensitive Detection of MicroRNA in a Complex Matrix Based on the DNA Tetrahedron. Anal. Chem. 2018, 90, 12584–12591. [Google Scholar] [CrossRef]
- Portela, A.; Calvo-Lozano, O.; Estevez, M.C.; Escuela, A.M.; Lechuga, L.M. Optical nanogap antennas as plasmonic biosensors for the detection of miRNA biomarkers. J. Mater. Chem. B 2020, 8, 4310–4317. [Google Scholar] [CrossRef]
- Li, J.; Koo, K.M.; Wang, Y.; Trau, M. Native MicroRNA Targets Trigger Self-Assembly of Nanozyme-Patterned Hollowed Nanocuboids with Optimal Interparticle Gaps for Plasmonic-Activated Cancer Detection. Small 2019, 15, e1904689. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, T.; Zhang, X. Graphene-Based Biosensors for Detection of Biomarkers. Micromachines 2020, 11, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treerattrakoon, K.; Jiemsakul, T.; Tansarawiput, C.; Pinpradup, P.; Iempridee, T.; Luksirikul, P.; Khoothiam, K.; Dharakul, T.; Japrung, D. Rolling circle amplification and graphene-based sensor-on-a-chip for sensitive detection of serum circulating miRNAs. Anal. Biochem. 2019, 577, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, S.; Cho, Y.S.; Kim, Y.; Tae, J.H.; No, T.I.; Shim, J.S.; Jeong, Y.; Kang, S.H.; Lee, K.H. Electrical Cartridge Sensor Enables Reliable and Direct Identification of MicroRNAs in Urine of Patients. ACS Sens. 2021, 6, 833–841. [Google Scholar] [CrossRef]
- Semeniuk, M.; Yi, Z.; Poursorkhabi, V.; Tjong, J.; Jaffer, S.; Lu, Z.H.; Sain, M. Future Perspectives and Review on Organic Carbon Dots in Electronic Applications. ACS Nano 2019, 13, 6224–6255. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.M.; Lee, K. Nanomaterials for theranostics: Recent advances and future challenges. Chem. Rev. 2015, 115, 327–394. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Salimi, A. Fluorometric determination of microRNA-155 in cancer cells based on carbon dots and MnO2 nanosheets as a donor-acceptor pair. Microchim. Acta 2018, 185, 372. [Google Scholar] [CrossRef]
- Mahani, M.; Mousapour, Z.; Divsar, F.; Nomani, A.; Ju, H. A carbon dot and molecular beacon based fluorometric sensor for the cancer marker microRNA-21. Mikrochim. Acta 2019, 186, 132. [Google Scholar] [CrossRef]
- Mohammadi, S.; Mohammadi, S.; Salimi, A. A 3D hydrogel based on chitosan and carbon dots for sensitive fluorescence detection of microRNA-21 in breast cancer cells. Talanta 2021, 224, 121895. [Google Scholar] [CrossRef]
- Kuntip, N.; Japrung, D.; Pongprayoon, P. What Happens When a Complementary DNA Meets miR-29a Cancer Biomarker in Complex with a Graphene Quantum Dot. ACS Appl. Bio Mater. 2021, 4, 8368–8376. [Google Scholar] [CrossRef]
- Wang, Z.; Zong, S.; Liu, Y.; Qian, Z.; Zhu, K.; Yang, Z.; Wang, Z.; Cui, Y. Simultaneous detection of multiple exosomal microRNAs for exosome screening based on rolling circle amplification. Nanotechnology 2020, 32, 085504. [Google Scholar] [CrossRef]
- Kim, J.; Ahn, S.Y.; Um, S.H. Bead-Immobilized Multimodal Molecular Beacon-Equipped DNA Machinery for Specific RNA Target Detection: A Prototypical Molecular Nanobiosensor. Nanomaterials 2021, 11, 1617. [Google Scholar] [CrossRef] [PubMed]
- Azimzadeh, M.; Rahaie, M.; Nasirizadeh, N.; Ashtari, K.; Naderi-Manesh, H. An electrochemical nanobiosensor for plasma miRNA-155, based on graphene oxide and gold nanorod, for early detection of breast cancer. Biosens. Bioelectron. 2016, 77, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Nikokar, I.; Zokaei, M.; Bozorgzadeh, E. Design, development and evaluation of microRNA-199a-5p detecting electrochemical nanobiosensor with diagnostic application in Triple Negative Breast Cancer. Talanta 2018, 189, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, S.; Fang, X.; Kong, J. Double signal amplification strategy for ultrasensitive electrochemical biosensor based on nuclease and quantum dot-DNA nanocomposites in the detection of breast cancer 1 gene mutation. Biosens. Bioelectron. 2019, 142, 111544. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, T.; Bai, Y.; Li, Y.; Qiu, J.; Yu, W.; Zhang, S. Dual-amplified strategy for ultrasensitive electrochemical biosensor based on click chemistry-mediated enzyme-assisted target recycling and functionalized fullerene nanoparticles in the detection of microRNA-141. Biosens. Bioelectron. 2020, 150, 111964. [Google Scholar] [CrossRef]
- Hakimian, F.; Ghourchian, H. Ultrasensitive electrochemical biosensor for detection of microRNA-155 as a breast cancer risk factor. Anal. Chim. Acta 2020, 1136, 1–8. [Google Scholar] [CrossRef]
- Yazdanparast, S.; Benvidi, A.; Azimzadeh, M.; Tezerjani, M.D.; Ghaani, M.R. Experimental and theoretical study for miR-155 detection through resveratrol interaction with nucleic acids using magnetic core-shell nanoparticles. Microchim. Acta 2020, 187, 479. [Google Scholar] [CrossRef]
- Pothipor, C.; Aroonyadet, N.; Bamrungsap, S.; Jakmunee, J.; Ounnunkad, K. A highly sensitive electrochemical microRNA-21 biosensor based on intercalating methylene blue signal amplification and a highly dispersed gold nanoparticles/graphene/polypyrrole composite. Analyst 2021, 146, 2679–2688. [Google Scholar] [CrossRef]
- Pothipor, C.; Jakmunee, J.; Bamrungsap, S.; Ounnunkad, K. An electrochemical biosensor for simultaneous detection of breast cancer clinically related microRNAs based on a gold nanoparticles/graphene quantum dots/graphene oxide film. Analyst 2021, 146, 4000–4009. [Google Scholar] [CrossRef]
- Pothipor, C.; Bamrungsap, S.; Jakmunee, J.; Ounnunkad, K. A gold nanoparticle-dye/poly (3-aminobenzylamine)/two dimensional MoSe2/graphene oxide electrode towards label-free electrochemical biosensor for simultaneous dual-mode detection of cancer antigen 15-3 and microRNA-21. Colloids Surf. B Biointerfaces 2022, 210, 112260. [Google Scholar] [CrossRef]
- Hussain, S.A.; Dey, D.; Chakraborty, S.; Saha, J.; Roy, A.D.; Chakraborty, S.; Debnath, P.; Bhattacharjee, D. Fluorescence resonance energy transfer (FRET) sensor. arXiv 2014, arXiv:1408.6559. [Google Scholar]
- Li, J.; Wang, A.; Yang, X.; Wang, K.; Huang, J. Orderly Assembled, Self-Powered FRET Flares for MicroRNA Imaging in Live Cells. Anal. Chem. 2021, 93, 6270–6277. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Howes, P.D.; Kim, E.; Spicer, C.D.; Thomas, M.R.; Lin, Y.; Crowder, S.W.; Pence, I.J.; Stevens, M.M. Duplex-specific nuclease-amplified detection of MicroRNA using compact quantum dot–DNA conjugates. ACS Appl. Mater. Interfaces 2018, 10, 28290–28300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Huang, J.; Yang, X.; Yang, Y.; Quan, K.; Xie, N.; Wu, Y.; Ma, C.; Wang, K. Two-Color-Based Nanoflares for Multiplexed MicroRNAs Imaging in Live Cells. Nanotheranostics 2018, 2, 96–105. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, C.; Li, Y.; Ma, Y.; Deng, J.; Li, L.; Sun, J. Thermophoretic Detection of Exosomal microRNAs by Nanoflares. J. Am. Chem. Soc. 2020, 142, 4996–5001. [Google Scholar] [CrossRef]
- Qing, Z.; Luo, G.; Xing, S.; Zou, Z.; Lei, Y.; Liu, J.; Yang, R. Pt–S Bond-Mediated Nanoflares for High-Fidelity Intracellular Applications by Avoiding Thiol Cleavage. Angew. Chem. Int. Ed. 2020, 59, 14044–14048. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [Green Version]
- Abdulbari, H.A.; Basheer, E.A.M. Electrochemical Biosensors: Electrode Development, Materials, Design, and Fabrication. Chembioeng. Rev. 2017, 4, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Zeng, S.; Baillargeat, D.; Ho, H.P.; Yong, K.T. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem. Soc. Rev. 2014, 43, 3426–3452. [Google Scholar] [CrossRef]
- Liz-Marzán, L.M. Tailoring surface plasmons through the morphology and assembly of metal nanoparticles. Langmuir 2006, 22, 32–41. [Google Scholar] [CrossRef]
- Kawasaki, E.S.; Player, A. Nanotechnology, nanomedicine, and the development of new, effective therapies for cancer. Nanomedicine 2005, 1, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili-Bandboni, A.; Amini, S.M.; Faridi-Majidi, R.; Bagheri, J.; Mohammadnejad, J.; Sadroddiny, E. Cross-linking gold nanoparticles aggregation method based on localised surface plasmon resonance for quantitative detection of miR-155. IET Nanobiotechnol. 2018, 12, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, Y.; Jiang, L.P.; Bi, S.; Zhu, J.J. Cascade Amplification-Mediated In Situ Hot-Spot Assembly for MicroRNA Detection and Molecular Logic Gate Operations. Anal. Chem. 2018, 90, 4544–4551. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Shangguan, J.; Guo, Q.; Ma, W.; Wang, H.; Jia, R.; Ye, Z.; He, X.; Wang, K. Colorimetric and fluorescent dual-mode detection of microRNA based on duplex-specific nuclease assisted gold nanoparticle amplification. Analyst 2019, 144, 4917–4924. [Google Scholar] [CrossRef] [PubMed]
- Hwu, S.; Blickenstorfer, Y.; Tiefenauer, R.F.; Gonnelli, C.; Schmidheini, L.; Luchtefeld, I.; Hoogenberg, B.J.; Gisiger, A.B.; Voros, J. Dark-Field Microwells toward High-Throughput Direct miRNA Sensing with Gold Nanoparticles. ACS Sens. 2019, 4, 1950–1956. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Nie, A.X.; Lu, Z.C.; Li, J.J.; Shu, M.B.; Han, H. Catalytic hairpin assembly-assisted lateral flow assay for visual determination of microRNA-21 using gold nanoparticles. Microchim. Acta 2019, 186, 661. [Google Scholar] [CrossRef] [PubMed]
- Qu, A.; Sun, M.; Xu, L.; Hao, C.; Wu, X.; Xu, C.; Kotov, N.A.; Kuang, H. Quantitative zeptomolar imaging of miRNA cancer markers with nanoparticle assemblies. Proc. Natl. Acad. Sci. USA 2019, 116, 3391–3400. [Google Scholar] [CrossRef] [Green Version]
- Canady, T.D.; Li, N.; Smith, L.D.; Lu, Y.; Kohli, M.; Smith, A.M.; Cunningham, B.T. Digital-resolution detection of microRNA with single-base selectivity by photonic resonator absorption microscopy. Proc. Natl. Acad. Sci. USA 2019, 116, 19362–19367. [Google Scholar] [CrossRef] [Green Version]
- Che, C.Y.; Xue, R.Y.; Li, N.T.; Gupta, P.; Wang, X.J.; Zhao, B.; Singamaneni, S.; Nie, S.M.; Cunningham, B.T. Accelerated Digital Biodetection Using Magneto-plasmonic Nanoparticle-Coupled Photonic Resonator Absorption Microscopy. ACS Nano 2022, 16, 2345–2354. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, W.; Xu, Y.; Yuan, H.; Li, F. Sensitive multiplex detection of MicroRNAs based on liquid suspension nano-chip. Anal. Chim. Acta 2020, 1112, 24–33. [Google Scholar] [CrossRef]
- Boussios, S.; Ozturk, M.A.; Moschetta, M.; Karathanasi, A.; Zakynthinakis-Kyriakou, N.; Katsanos, K.H.; Christodoulou, D.K.; Pavlidis, N. The Developing Story of Predictive Biomarkers in Colorectal Cancer. J. Pers. Med. 2019, 9, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.N.; Masud, M.K.; Nguyen, N.T.; Gopalan, V.; Alamri, H.R.; Alothman, Z.A.; Al Hossain, M.S.; Yamauchi, Y.; Lam, A.K.; Shiddiky, M.J.A. Gold-loaded nanoporous ferric oxide nanocubes for electrocatalytic detection of microRNA at attomolar level. Biosens. Bioelectron. 2018, 101, 275–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, J.; Dong, H.; Dai, W.; Meng, X.; Zhang, K.; Cao, Y.; Yang, F.; Zhang, X. Functional DNA hexahedron for real-time detection of multiple microRNAs in living cells. Anal. Chim. Acta 2019, 1078, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.F.; Cheng, L.X.; Li, M.; Zuo, D.; Zhang, N.; Zhuang, H.J.; Xie, D.; Zeng, Q.D.; Hutchison, J.A.; Zhao, Y.L. Frequency Shift Raman-Based Sensing of Serum MicroRNAs for Early Diagnosis and Discrimination of Primary Liver Cancers. Anal. Chem. 2018, 90, 10144–10151. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.J.C.; Uday Kumar, S.; Zeng, Y.; Afjei, R.; Robinson, E.; Lau, K.; Bermudez, A.; Habte, F.; Pitteri, S.J.; Sinclair, R. Tumor cell-derived extracellular vesicle-coated nanocarriers: An efficient theranostic platform for the cancer-specific delivery of anti-miR-21 and imaging agents. ACS Nano 2018, 12, 10817–10832. [Google Scholar] [CrossRef]
- Fernandez-Piñeiro, I.; Badiola, I.; Sanchez, A. Nanocarriers for microRNA delivery in cancer medicine. Biotechnol. Adv. 2017, 35, 350–360. [Google Scholar] [CrossRef]
- Talluri, S.V.; Kuppusamy, G.; Karri, V.V.S.R.; Tummala, S.; Madhunapantula, S.V. Lipid-based nanocarriers for breast cancer treatment—Comprehensive review. Drug Deliv. 2016, 23, 1291–1305. [Google Scholar] [CrossRef]
- Gallego, L.; Cena, V. Nanoparticle-mediated therapeutic compounds delivery to glioblastoma. Expert Opin. Drug Deliv. 2020, 17, 1541–1554. [Google Scholar] [CrossRef]
- Pottoo, F.H.; Barkat, M.A.; Ansari, M.A.; Javed, M.N.; Jamal, Q.M.S.; Kamal, M.A. Nanotechnological based miRNA intervention in the therapeutic management of neuroblastoma. Semin. Cancer Biol. 2021, 69, 100–108. [Google Scholar] [CrossRef]
- Tiram, G.; Segal, E.; Krivitsky, A.; Shreberk-Hassidim, R.; Ferber, S.; Ofek, P.; Udagawa, T.; Edry, L.; Shomron, N.; Roniger, M.; et al. Identification of Dormancy-Associated MicroRNAs for the Design of Osteosarcoma-Targeted Dendritic Polyglycerol Nanopolyplexes. ACS Nano 2016, 10, 2028–2045. [Google Scholar] [CrossRef]
- Sun, S.; Wang, Y.; Zhou, R.; Deng, Z.; Han, Y.; Han, X.; Tao, W.; Yang, Z.; Shi, C.; Hong, D. Targeting and regulating of an oncogene via nanovector delivery of MicroRNA using patient-derived xenografts. Theranostics 2017, 7, 677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, D.; Liu, H.; Zhao, P.; Ye, L.; Zou, H.; Zhao, X.; Dai, H.; Kong, X.; Liu, P. Programing Assembling/Releasing Multifunctional miRNA Nanomedicine to Treat Prostate Cancer. ACS Appl. Mater. Interfaces 2020, 12, 9032–9040. [Google Scholar] [CrossRef] [PubMed]
- Javanmardi, S.; Tamaddon, A.M.; Aghamaali, M.R.; Ghahramani, L.; Abolmaali, S.S. Redox-sensitive, PEG-shielded carboxymethyl PEI nanogels silencing MicroRNA-21, sensitizes resistant ovarian cancer cells to cisplatin. Asian J. Pharm. Sci. 2020, 15, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Nagachinta, S.; Bouzo, B.L.; Vazquez-Rios, A.J.; Lopez, R.; Fuente, M. Sphingomyelin-Based Nanosystems (SNs) for the Development of Anticancer miRNA Therapeutics. Pharmaceutics 2020, 12, 189. [Google Scholar] [CrossRef] [Green Version]
- Elfiky, A.M.; Mohamed, R.H.; Abd El-Hakam, F.E.; Yassin, M.A.; El Hefnawi, M. Targeted delivery of miR-218 via decorated hyperbranched polyamidoamine for liver cancer regression. Int. J. Pharm. 2021, 610, 121256. [Google Scholar] [CrossRef]
- Nakase, I.; Noguchi, K.; Aoki, A.; Takatani-Nakase, T.; Fujii, I.; Futaki, S. Arginine-rich cell-penetrating peptide-modified extracellular vesicles for active macropinocytosis induction and efficient intracellular delivery. Sci. Rep. 2017, 7, 1991. [Google Scholar] [CrossRef]
- Boca, S.; Gulei, D.; Zimta, A.A.; Onaciu, A.; Magdo, L.; Tigu, A.B.; Ionescu, C.; Irimie, A.; Buiga, R.; Berindan-Neagoe, I. Nanoscale delivery systems for microRNAs in cancer therapy. Cell Mol. Life Sci. 2020, 77, 1059–1086. [Google Scholar] [CrossRef]
- Naseri, Z.; Oskuee, R.K.; Jaafari, M.R.; Forouzandeh Moghadam, M. Exosome-mediated delivery of functionally active miRNA-142-3p inhibitor reduces tumorigenicity of breast cancer in vitro and in vivo. Int. J. Nanomed. 2018, 13, 7727–7747. [Google Scholar] [CrossRef] [Green Version]
- Vakhshiteh, F.; Rahmani, S.; Ostad, S.N.; Madjd, Z.; Dinarvand, R.; Atyabi, F. Exosomes derived from miR-34a-overexpressing mesenchymal stem cells inhibit in vitro tumor growth: A new approach for drug delivery. Life Sci. 2021, 266, 118871. [Google Scholar] [CrossRef]
- Moradi-Chaleshtori, M.; Shojaei, S.; Mohammadi-Yeganeh, S.; Hashemi, S.M. Transfer of miRNA in tumor-derived exosomes suppresses breast tumor cell invasion and migration by inducing M1 polarization in macrophages. Life Sci. 2021, 282, 119800. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, J.; Xie, J.; Wang, Y.; Sun, J.; Liu, T.; Zhao, Y.; Zhao, X.; Wang, X.; Ma, Y.; et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat. Biomed. Eng. 2020, 4, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wang, H.; Li, Z.; Shu, D.; Guo, P. RNA micelles for the systemic delivery of anti-miRNA for cancer targeting and inhibition without ligand. ACS Nano 2018, 13, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Chen, Y.; Chen, Z.; Feng, Y.; Wang, J.; Li, T.; Li, S.; Qin, X.; Wu, C.; Zheng, C.; et al. Polymeric Hybrid Nanomicelles for Cancer Theranostics: An Efficient and Precise Anticancer Strategy for the Codelivery of Doxorubicin/miR-34a and Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2019, 11, 43865–43878. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dai, Y.; Zhang, X.J.; Chen, J.H. Three-layered polyplex as a microRNA targeted delivery system for breast cancer gene therapy. Nanotechnology 2017, 28, 285101. [Google Scholar] [CrossRef]
- Ghaffari, M.; Kalantar, S.M.; Hemati, M.; Firoozabadi, A.D.; Asri, A.; Shams, A.; Ghalekohneh, S.J.; Haghiralsadat, F. Co-delivery of miRNA-15a and miRNA-16-1 using cationic PEGylated niosomes downregulates Bcl-2 and induces apoptosis in prostate cancer cells. Biotechnol. Lett. 2021, 43, 981–994. [Google Scholar] [CrossRef]
- Binzel, D.W.; Shu, Y.; Li, H.; Sun, M.; Zhang, Q.; Shu, D.; Guo, B.; Guo, P. Specific Delivery of MiRNA for High Efficient Inhibition of Prostate Cancer by RNA Nanotechnology. Mol. Ther. 2016, 24, 1267–1277. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chen, Y.; Li, J.; Zhang, Z.; Huang, C.; Lian, G.; Yang, K.; Chen, S.; Lin, Y.; Wang, L. Co-delivery of micro RNA-21 antisense oligonucleotides and gemcitabine using nanomedicine for pancreatic cancer therapy. Cancer Sci. 2017, 108, 1493–1503. [Google Scholar] [CrossRef]
- Yoo, B.; Kavishwar, A.; Wang, P.; Ross, A.; Pantazopoulos, P.; Dudley, M.; Moore, A.; Medarova, Z. Therapy targeted to the metastatic niche is effective in a model of stage IV breast cancer. Sci. Rep. 2017, 7, 45060. [Google Scholar] [CrossRef] [Green Version]
- Tekie, F.S.M.; Soleimani, M.; Zakerian, A.; Dinarvand, M.; Amini, M.; Dinarvand, R.; Arefian, E.; Atyabi, F. Glutathione responsive chitosan-thiolated dextran conjugated miR-145 nanoparticles targeted with AS1411 aptamer for cancer treatment. Carbohydr. Polym. 2018, 201, 131–140. [Google Scholar] [CrossRef]
- Vandghanooni, S.; Eskandani, M.; Barar, J.; Omidi, Y. AS1411 aptamer-decorated cisplatin-loaded poly(lactic-co-glycolic acid) nanoparticles for targeted therapy of miR-21-inhibited ovarian cancer cells. Nanomedicine 2018, 13, 2729–2758. [Google Scholar] [CrossRef]
- Lopez-Bertoni, H.; Kozielski, K.L.; Rui, Y.; Lal, B.; Vaughan, H.; Wilson, D.R.; Mihelson, N.; Eberhart, C.G.; Laterra, J.; Green, J.J. Bioreducible Polymeric Nanoparticles Containing Multiplexed Cancer Stem Cell Regulating miRNAs Inhibit Glioblastoma Growth and Prolong Survival. Nano Lett. 2018, 18, 4086–4094. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, F.; Climent, M.; Malvindi, M.A.; Pompa, P.P.; Bonetti, P.; Nicassio, F. Delivery of biologically active miR-34a in normal and cancer mammary epithelial cells by synthetic nanoparticles. Nanomedicine 2019, 19, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, U.K.; Bose, R.J.C.; Malhotra, M.; Babikir, H.A.; Afjei, R.; Robinson, E.; Zeng, Y.T.; Chang, E.; Habte, F.; Sinclair, R.; et al. Intranasal delivery of targeted polyfunctional gold-iron oxide nanoparticles loaded with therapeutic microRNAs for combined theranostic multimodality imaging and presensitization of glioblastoma to temozolomide. Biomaterials 2019, 218, 119342. [Google Scholar] [CrossRef]
- Chiang, C.L.; Goswami, S.; Frissora, F.W.; Xie, Z.; Yan, P.S.; Bundschuh, R.; Walker, L.A.; Huang, X.; Mani, R.; Mo, X.M.; et al. ROR1-targeted delivery of miR-29b induces cell cycle arrest and therapeutic benefit in vivo in a CLL mouse model. Blood 2019, 134, 432–444. [Google Scholar] [CrossRef]
- Perepelyuk, M.; Sacko, K.; Thangavel, K.; Shoyele, S.A. Evaluation of MUC1-Aptamer Functionalized Hybrid Nanoparticles for Targeted Delivery of miRNA-29b to Nonsmall Cell Lung Cancer. Mol. Pharm. 2018, 15, 985–993. [Google Scholar] [CrossRef]
- Qian, R.C.; Lv, J.; Long, Y.T. Controllable Aggregation-Induced Exocytosis Inhibition (CAIEI) of Plasmonic Nanoparticles in Cancer Cells Regulated by MicroRNA. Mol. Pharm. 2018, 15, 4031–4037. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Luo, G.F.; Sohn, Y.S.; Nechushtai, R.; Willner, I. miRNA-Specific Unlocking of Drug-Loaded Metal–Organic Framework Nanoparticles: Targeted Cytotoxicity toward Cancer Cells. Small 2019, 15, 1900935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ouyang, Y.; Sohn, Y.S.; Nechushtai, R.; Pikarsky, E.; Fan, C.H.; Willner, I. pH- and miRNA-Responsive DNA-Tetrahedra/Metal-Organic Framework Conjugates: Functional Sense-and-Treat Carriers. ACS Nano 2021, 15, 6645–6657. [Google Scholar] [CrossRef]
- Yin, H.R.; Xiong, G.F.; Guo, S.J.; Xu, C.C.; Xu, R.; Guo, P.X.; Shu, D. Delivery of Anti-miRNA for Triple-Negative Breast Cancer Therapy Using RNA Nanoparticles Targeting Stem Cell Marker CD133. Mol. Ther. 2019, 27, 1252–1261. [Google Scholar] [CrossRef] [Green Version]
- Ahir, M.; Upadhyay, P.; Ghosh, A.; Sarker, S.; Bhattacharya, S.; Gupta, P.; Ghosh, S.; Chattopadhyay, S.; Adhikary, A. Delivery of dual miRNA through CD44-targeted mesoporous silica nanoparticles for enhanced and effective triple-negative breast cancer therapy. Biomater. Sci. 2020, 8, 2939–2954. [Google Scholar] [CrossRef]
- Unal, O.; Akkoc, Y.; Kocak, M.; Nalbat, E.; Dogan-Ekici, A.I.; Yagci Acar, H.; Gozuacik, D. Treatment of breast cancer with autophagy inhibitory microRNAs carried by AGO2-conjugated nanoparticles. J. Nanobiotechnol. 2020, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, P.; Sarker, S.; Ghosh, A.; Gupta, P.; Das, S.; Ahir, M.; Bhattacharya, S.; Chattopadhyay, S.; Ghosh, S.; Adhikary, A. Transferrin-decorated thymoquinone-loaded PEG-PLGA nanoparticles exhibit anticarcinogenic effect in non-small cell lung carcinoma via the modulation of miR-34a and miR-16. Biomater. Sci. 2019, 7, 4325–4344. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Liang, G.F.; Li, Y.; Li, J.H.; Jing, A.H.; Feng, W.P.; Li, G.D.; Du, J.X.; Feng, S.Y. Preparation and Evaluation of Upconversion Nanoparticles Based miRNA Delivery Carrier in Colon Cancer Mice Model. J. Biomed. Nanotechnol. 2019, 15, 2240–2250. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Wang, R.; Sun, Y.; Yue, Z.; Sun, H.; Wang, X.; Wang, P.; Sun, G.; Hu, J.; Sun, H.; et al. Delivery of MicroRNA-let-7c-5p by Biodegradable Silica Nanoparticles Suppresses Human Cervical Carcinoma Cell Proliferation and Migration. J. Biomed. Nanotechnol. 2020, 16, 1600–1611. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, M.; Jiao, M.; Yan, C.; Xu, S.; Du, Q.; Morsch, M.; Yin, J.; Shi, B. Polymeric nanoparticle mediated inhibition of miR-21 with enhanced miR-124 expression for combinatorial glioblastoma therapy. Biomaterials 2021, 276, 121036. [Google Scholar] [CrossRef]
- Maghsoudnia, N.; Baradaran Eftekhari, R.; Naderi Sohi, A.; Norouzi, P.; Akbari, H.; Ghahremani, M.H.; Soleimani, M.; Amini, M.; Samadi, H.; Dorkoosh, F.A. Mitochondrial delivery of microRNA mimic let-7b to NSCLC cells by PAMAM-based nanoparticles. J. Drug Target 2020, 28, 818–830. [Google Scholar] [CrossRef]
- Wang, H.; Ellipilli, S.; Lee, W.J.; Li, X.; Vieweger, M.; Ho, Y.S.; Guo, P. Multivalent rubber-like RNA nanoparticles for targeted co-delivery of paclitaxel and MiRNA to silence the drug efflux transporter and liver cancer drug resistance. J. Control. Release 2021, 330, 173–184. [Google Scholar] [CrossRef]
- Zhou, X.; You, M.; Wang, F.; Wang, Z.; Gao, X.; Jing, C.; Liu, J.; Guo, M.; Li, J.; Luo, A. Multifunctional graphdiyne–cerium oxide nanozymes facilitate microRNA delivery and attenuate tumor hypoxia for highly efficient radiotherapy of esophageal cancer. Adv. Mater. 2021, 33, 2100556. [Google Scholar] [CrossRef]
- Hwang, D.W.; Kim, H.Y.; Li, F.Y.; Park, J.Y.; Kim, D.; Park, J.H.; Han, H.S.; Byun, J.W.; Lee, Y.S.; Jeong, J.M.; et al. In vivo visualization of endogenous miR-21 using hyaluronic acid-coated graphene oxide for targeted cancer therapy. Biomaterials 2017, 121, 144–154. [Google Scholar] [CrossRef]
- Assali, A.; Akhavan, O.; Mottaghitalab, F.; Adeli, M.; Dinarvand, R.; Razzazan, S.; Arefian, E.; Soleimani, M.; Atyabi, F. Cationic graphene oxide nanoplatform mediates miR-101 delivery to promote apoptosis by regulating autophagy and stress. Int. J. Nanomed. 2018, 13, 5865–5886. [Google Scholar] [CrossRef] [Green Version]
- Assali, A.; Akhavan, O.; Adeli, M.; Razzazan, S.; Dinarvand, R.; Zanganeh, S.; Soleimani, M.; Dinarvand, M.; Atyabi, F. Multifunctional core-shell nanoplatforms (gold@ graphene oxide) with mediated NIR thermal therapy to promote miRNA delivery. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1891–1903. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Niu, G.; Yi, H.; Li, Q.; Wu, Z.; Wang, J.; Yang, J.; Li, B.; Peng, Y.; Liang, Y.; et al. Nanomedicine promotes ferroptosis to inhibit tumour proliferation in vivo. Redox Biol. 2021, 42, 101908. [Google Scholar] [CrossRef] [PubMed]
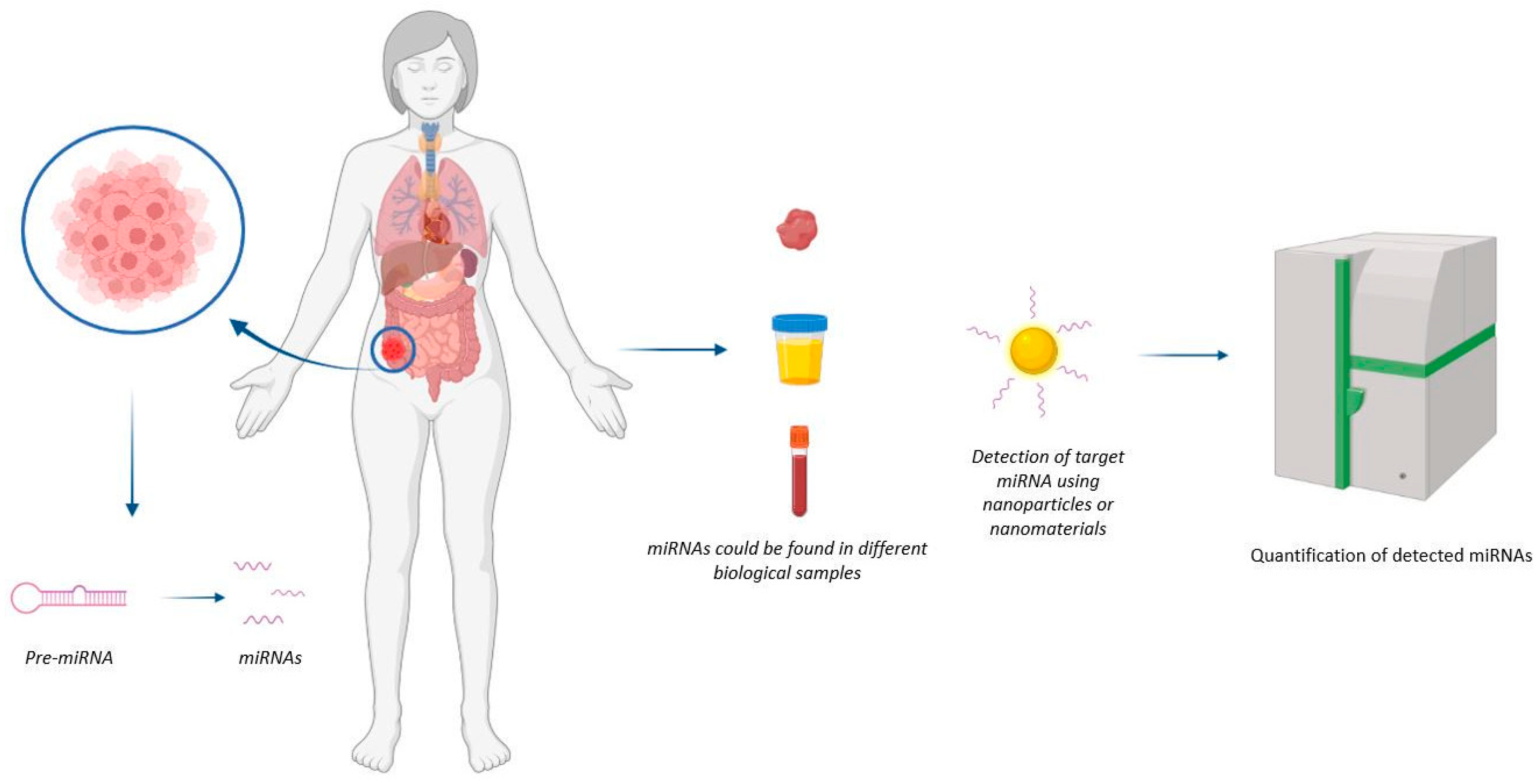
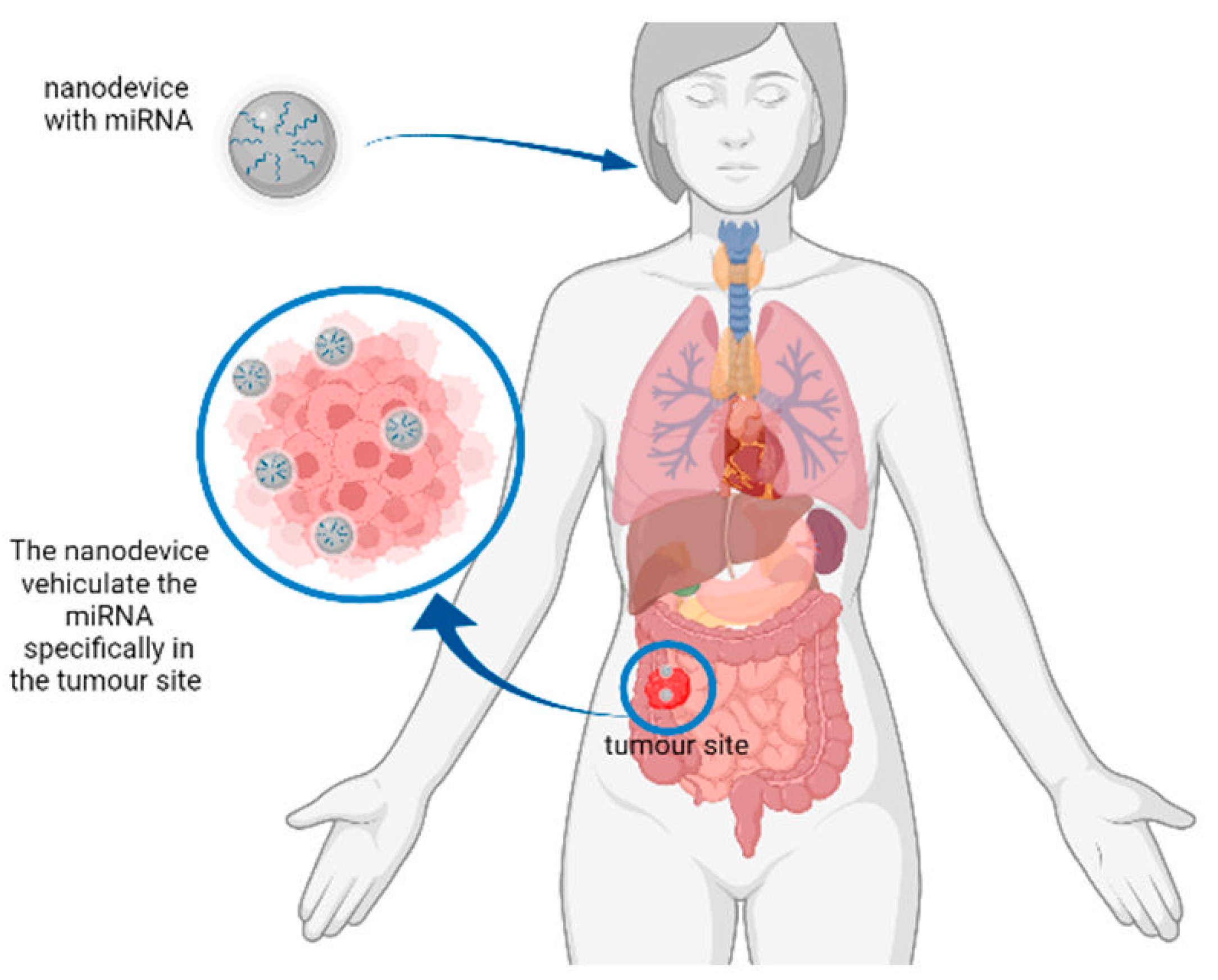
| Type of Biosensor/Sensing System | Detected miRNAs/miRNA Precursors | Detection Limit | Types of Samples | References |
|---|---|---|---|---|
| Colorimetric Biosensor | let-7d | ca. 1.06 nM | -- | [16]—Park Y et al. (2018) |
| miR-148a | ~1.9 nM | Cancer cells | [18]—Ye C et al. (2017) | |
| ~1.9 nM | Cancer cells | [19]—Cai J et al. (2019) | ||
| miR-21, miR-155 | <1 ng/µL | Cancer cell lines | [12]—Mollasalehi H et al. (2021) | |
| Surface plasmon resonance (SPR) | miR-141 | 1 fM | Cell extractions | [24]—Wang et al. (2016) |
| 0.5 fM | -- | [28]—Nie et al. (2017) | ||
| 0.6 fM | -- | [27]—Liu et al. (2017) | ||
| miR-21 | 1 pM | Human total RNA | [26]—Li et al. (2016) | |
| let-7a | 0.8 fM | Human serum and cell lysate | [29]—Wenyan Nie et al. (2018) | |
| miR-148a | ~1.9 nM | -- | [19]—Jun Cai et al. (2020) | |
| miR-210 | 0.78 nM | -- | [30]—Portela et al. (2020) | |
| Graphene | miR-29a, miR-144 | 0.05 pmol | Human serum and HeLa cells | [34]—Treerattrakoon et al. (2019) |
| miR-21, miR-1246, miR-Let7b | 10 fM | Urine | [34]—Kim et al. (2020) | |
| Carbon dots | miR-155 | 0.1 aM | MCF-7 and human serum | [37]—Mohammadi et al. (2018) |
| miR-21 | 0.3 nM | -- | [38]—Mahani M et al. (2019) | |
| 0.03 fM | MCF-7 | [39]—Mohammadi S et al. (2021) | ||
| Molecular beacon | miR-21, miR-122, miR-155 | -- | Cell lines | [41]—Wang et al. (2021) |
| miR-155-5p | -- | -- | [42]—Kim et al. (2021) | |
| Electrochemical biosensor | miR-155 | 0.6 fM | -- | [43]—Azimzadeh et al. (2016) |
| miR199a-5p | 4.5 fM | -- | [44]—Ebrahimi et al. (2018) | |
| microRNA | 1.2 aM | -- | [67]—Cheng et al. (2019) | |
| miR-141 | 7.78 fM | -- | [46]—Zhou et al. (2020) | |
| miR-155 | 20 zmol | -- | [47]—Hakimian et al. (2020) | |
| 0.15 fM | Human serum | [48]—Yazdanparast et al. (2020) | ||
| miR-21 | 0.020 fM | -- | [49]—Pothipor et al. (2021) | |
| miR-21, miR-155, and miR-210 | -- | -- | [50]—Pothipor C et al. (2021) | |
| miR-21 | 0.14 U/mL | -- | [51]—Pothipor C et.al (2022) | |
| Förster resonance energy transfer (FRET) | miR-21 | -- | Cancer cells | [53]—Li et al. (2021) |
| miR-148 | 42 fM | Human cancer- and normal-cell lines | [54]—Wang et al. (2018) | |
| Magneto-plasmonic Nanoparticle | miR-375 | 61.9 aM | Human serum | [70]—Che C et al. (2022) |
| Nanoflares | miR-21, miR-141 | -- | Cancer cells | [55]—Li J et al. (2018) |
| miR-375 | 0.36 fM | Human serum | [56]—Zhao et al. (2020) | |
| miR-21 | -- | Living cells | [57]—Qing Z et al. (2020) | |
| Polystyrene nanoparticles | miRNAs, miR-106a, miR-15a, and miR-21 | -- | -- | [71]—Wang et al. (2020) |
| Iron oxide nanocubes | miR-107 | 100 aM | -- | [73]—Nazmul et al. (2018) |
| DNA mini hexahedron (DMH) and DNA-based probe | miR-21, miR-1246 | -- | Healthy and cancerous cells | [74]—Dong et al. (2019) |
| Gold nanoparticles (AuNPs) | miR-155 | 10 nM | -- | [63]—Esmaeili-bandboni (2018) |
| miR-141 | 25.1 aM | -- | [64]—Yu et al. (2018) | |
| miR-21 | 50 pM | -- | [65]—Huang et al. (2019) | |
| microRNA | -- | Cell lysate | [66]—Hwu et al. (2019) | |
| miR-21 | 0.89 pM | Cell extracts and serum samples | [67]—Wang et al. (2019) | |
| miR-21, miR-200b | Zeptomolar range | -- | [68]—Qu et al. (2019) | |
| Silver nanoparticle (AgNFs) | miR-26a-5p, miR-223 and miR-27a-3p | -- | -- | [75]—Zhu et al. (2018) |
| Type of Nanocarrier | Target miRNAs | Target | References |
|---|---|---|---|
| Nanoparticles | MiR-17, miR-21 | Prostate cancer cell | [97]—Binzel D et al. (2016) |
| miR-21 | Pancreatic cancer cell | [98]—Li Y et al. (2017) | |
| MiR-10b-a | Tumour | [99]—Yoo B et al. (2017) | |
| miR-145 | Cancer | [100]—Tekie F et al. (2018) | |
| miR-21 | Ovarian cancer cells | [101]—Vandghanooni S et al. (2018) | |
| miR-148a, miR-296-5p | Glioblastoma cells | [102]—Lopez-Bertoni H et al. (2018) | |
| miR-34a | Breast cancer cells | [103]—Panebianco F et al. (2019) | |
| miR-100, miR-21 | Glioblastoma | [104]—Sukumar U et al. (2019) | |
| miR-29b | Leukaemia | [105]—Chiang C et al. (2019) | |
| miR-29b | Lung cancer | [106]—Perepelyuk M et al. (2018) | |
| miR-21 | Breast cancer | [107]—Qian R et al. (2018) | |
| miR-21, miR-221 | Breast cancer | [108]—Chen W et al. (2019) | |
| miR-21, miR-155 | Cancer | [109]—Zhang P et al. (2021) | |
| MiR-21 | Triple-negative breast cancer | [110]—Yin H et al. (2019) | |
| miR-34a, miR-10b | Triple-negative breast cancer | [111]—Ahir M et al. (2020) | |
| miR-376b | HER2-positive breast cancer | [112]—Unal O et al. (2020) | |
| miR-34a, miR-16 | Non-small-cell lung cells | [113]—Upadhyay P et al. (2019) | |
| miRNA-145 | Colon cancer cells | [114]—Shi H et al. (2019) | |
| miR-let-7c-5p | HeLa cells | [115]—Shao L et al. (2020) | |
| miRNA-124, miRNA-21 | Glioblastoma cells | [116]—Liu Y et al. (2021) | |
| miR-let-7b | Lung cancer cells | [117]—Maghsoudnia N et al. (2020) | |
| miR-122 | Tumour cells | [118]—Wang H et al. (2021) | |
| miR-181 | Oesophageal cancer | [119]—Zhou X et al. (2021) | |
| Nanoplatforms/nanosystems | miR-21 | Breast cancer cells | [120]—Kim H et al. (2017) |
| miR-101 | MCF7, MDA- MB-231 | [121]—Assali A et al. (2018) | |
| MiR-101 | Breast cancer cells | [122]—Assali A et al. (2018) | |
| Breast cancer cells | [123]—Luo Y et al. (2021) | ||
| Extracellular vesicles | miR-21 | SKBR3 cells | [76]—JC Bose R et al. (2018) |
| Exosomes | miR-142-3p, miR-150 | Breast cancer cells | [89]—Nseri Z et al. (2018) |
| miR-34a | Breast cancer cells | [90]—Vakhshiteh F et al. (2021) | |
| miR-130 | Breast cancer cells | [91]—Moradi-Chaleshtori M et al. (2021) | |
| Micelles | miR-21 | Cancer cells | [93]—Yin H et al. (2018) |
| miR-210 | Breast cancer cells | [95]—Li Y et al. (2017) | |
| Niosomes | miR-15a, miR-16-1 | Prostate cancer cells | [96]—Ghaffari M et al. (2021) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coradduzza, D.; Bellu, E.; Congiargiu, A.; Pashchenko, A.; Amler, E.; Necas, A.; Carru, C.; Medici, S.; Maioli, M. Role of Nano-miRNAs in Diagnostics and Therapeutics. Int. J. Mol. Sci. 2022, 23, 6836. https://doi.org/10.3390/ijms23126836
Coradduzza D, Bellu E, Congiargiu A, Pashchenko A, Amler E, Necas A, Carru C, Medici S, Maioli M. Role of Nano-miRNAs in Diagnostics and Therapeutics. International Journal of Molecular Sciences. 2022; 23(12):6836. https://doi.org/10.3390/ijms23126836
Chicago/Turabian StyleCoradduzza, Donatella, Emanuela Bellu, Antonella Congiargiu, Aleksei Pashchenko, Evzen Amler, Alois Necas, Ciriaco Carru, Serenella Medici, and Margherita Maioli. 2022. "Role of Nano-miRNAs in Diagnostics and Therapeutics" International Journal of Molecular Sciences 23, no. 12: 6836. https://doi.org/10.3390/ijms23126836
APA StyleCoradduzza, D., Bellu, E., Congiargiu, A., Pashchenko, A., Amler, E., Necas, A., Carru, C., Medici, S., & Maioli, M. (2022). Role of Nano-miRNAs in Diagnostics and Therapeutics. International Journal of Molecular Sciences, 23(12), 6836. https://doi.org/10.3390/ijms23126836







