Genome-Wide Analysis and Expression Profiling of the Phenylalanine Ammonia-Lyase Gene Family in Solanum tuberosum
Abstract
:1. Introduction
2. Results
2.1. Identification of Members of PAL Gene Family in Potato
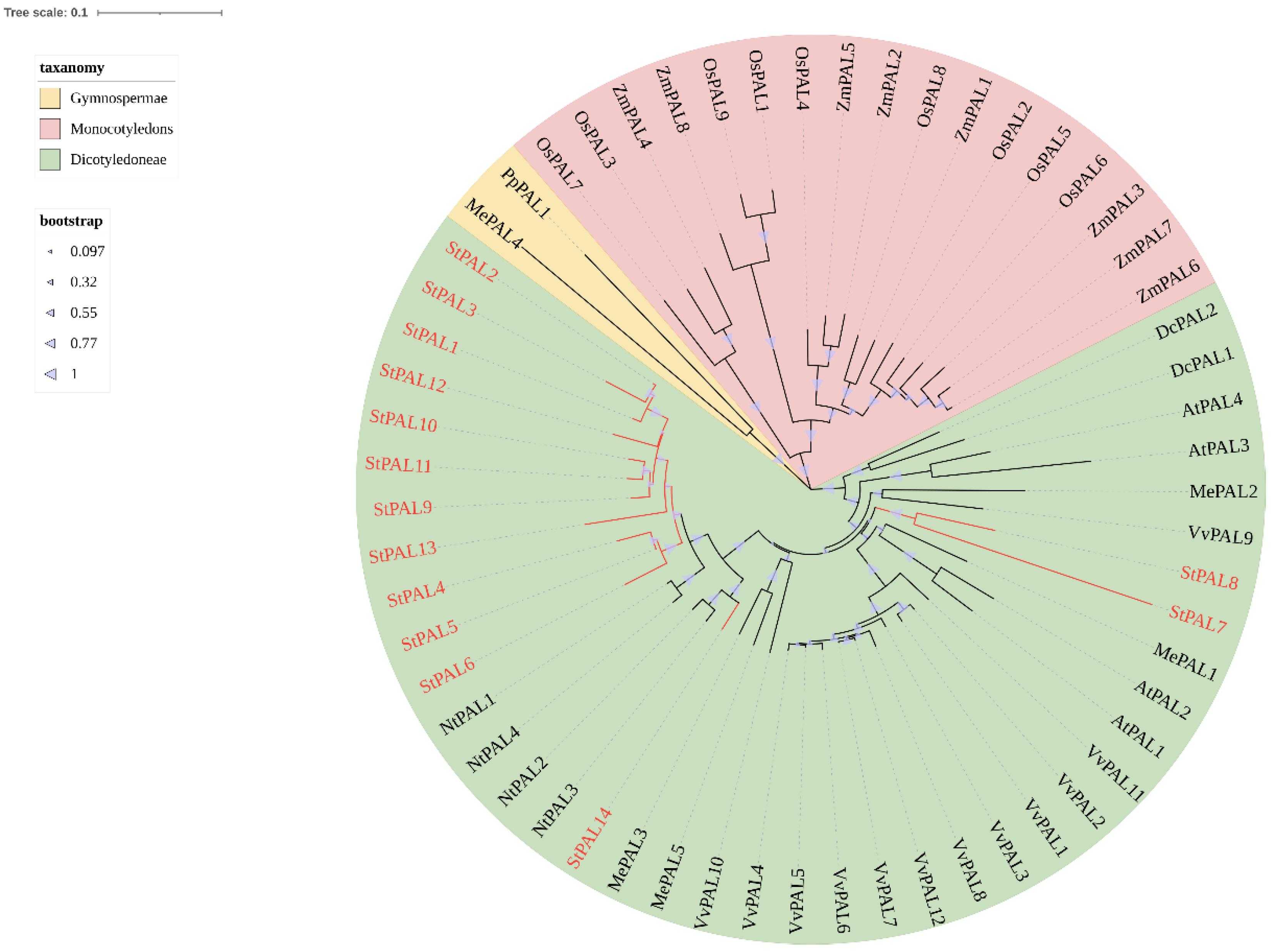
2.2. Phylogenetic Analysis of PAL Gene Family in Potato
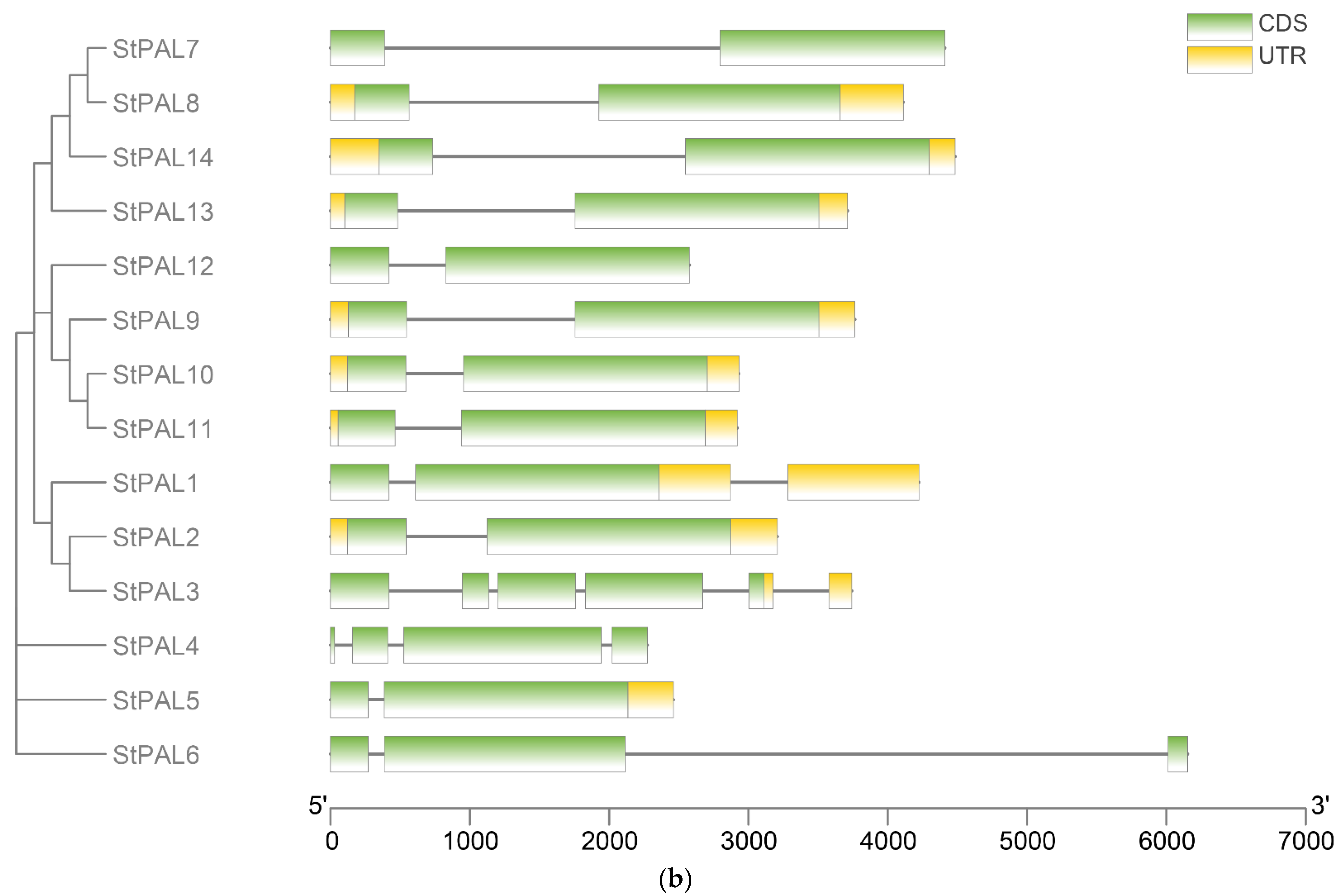
2.3. Chromosomal Location and Tandem Duplication Genes of Potato PAL Gene Family
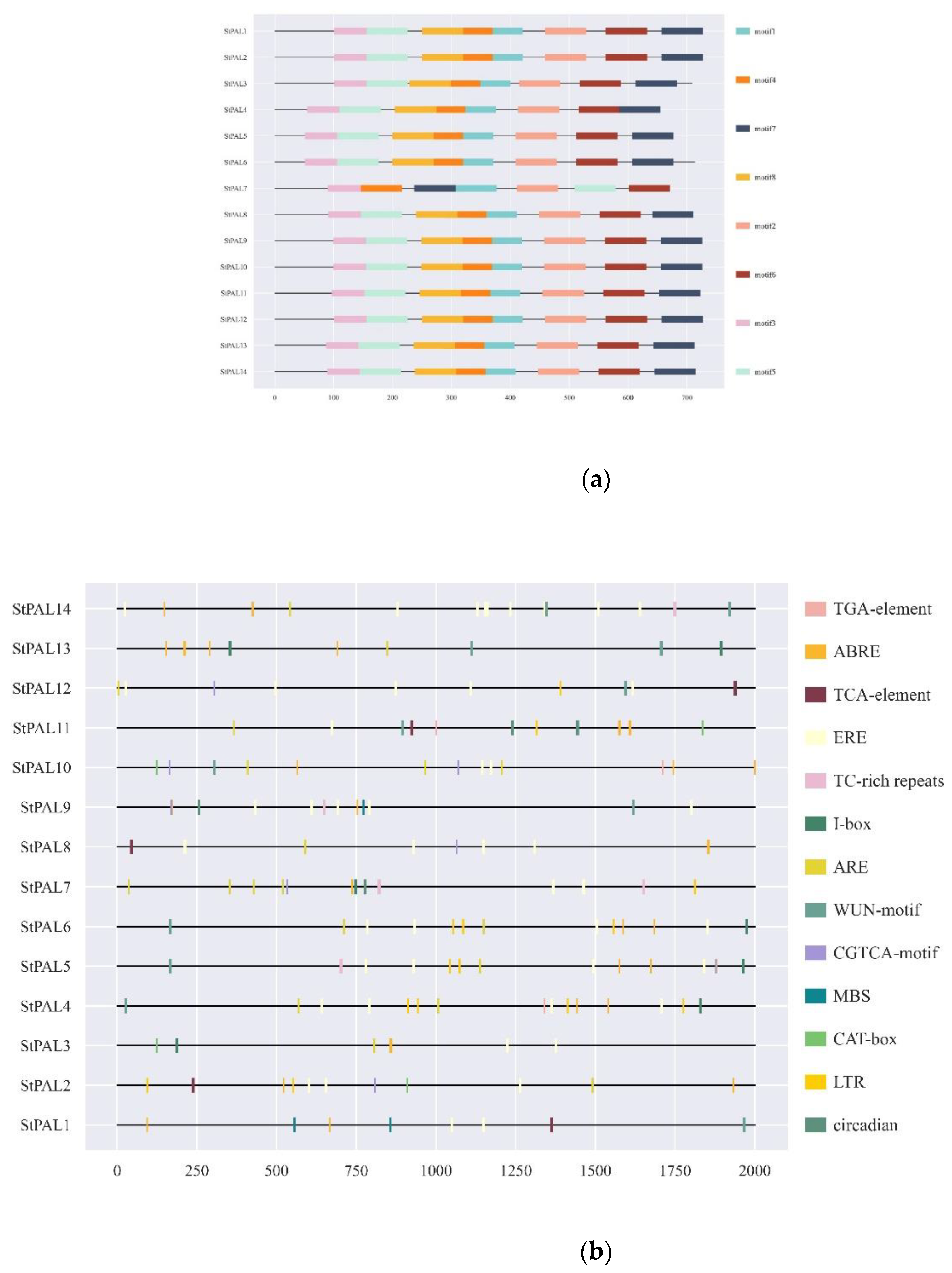
2.4. Cis-Acting Elements Analysis and Conserved Motif Identification
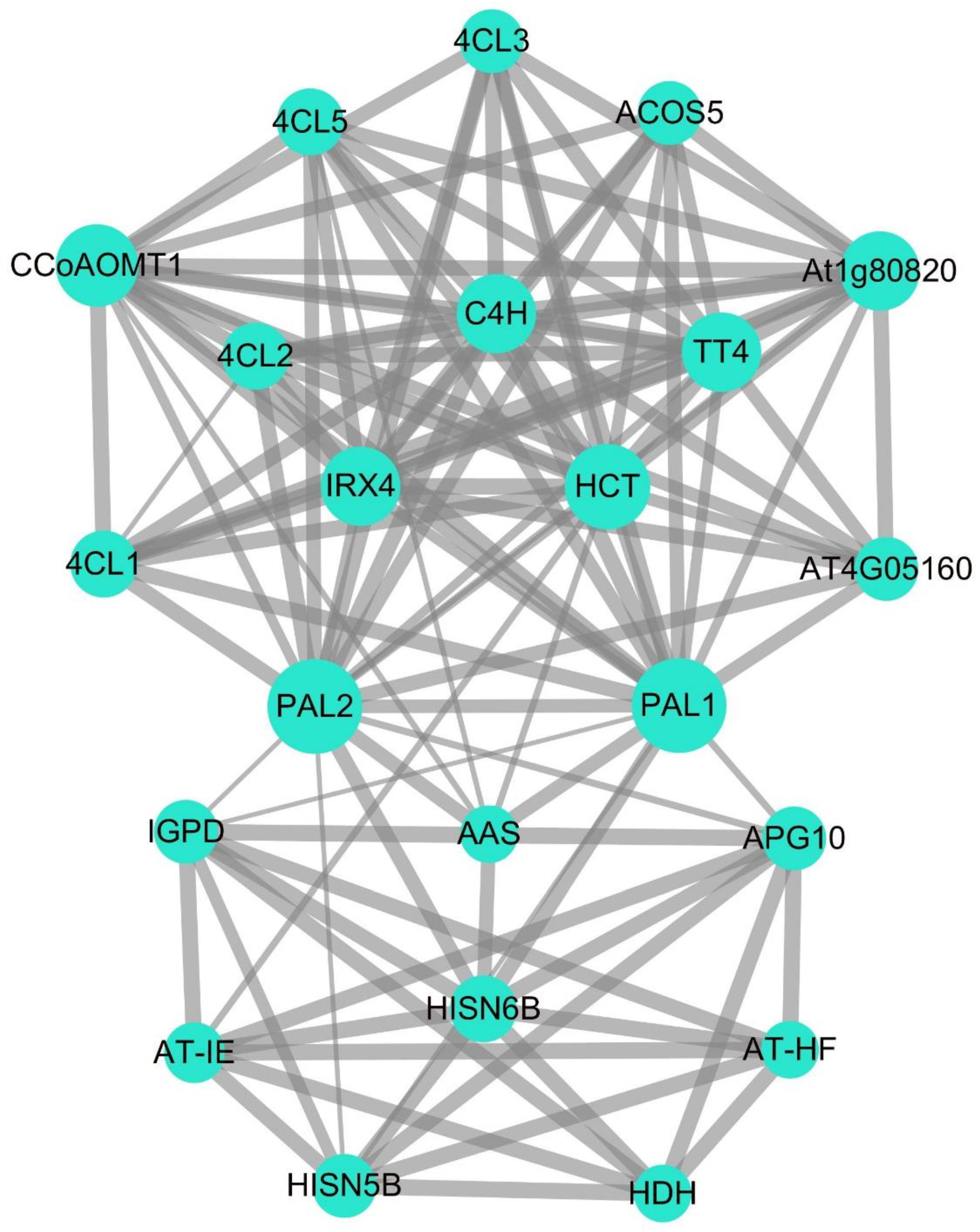
2.5. Potato PAL Protein Interaction Analysis
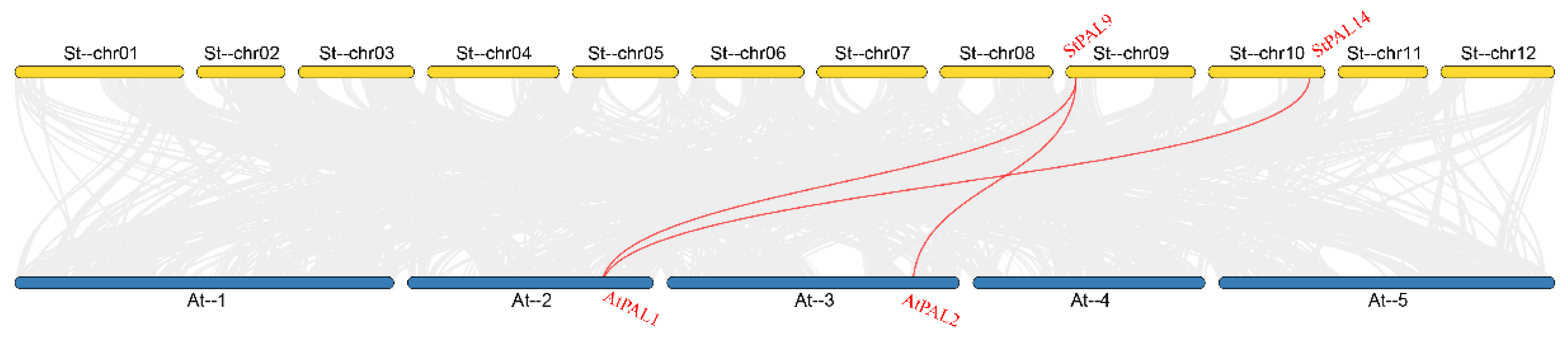
2.6. Collinear Analysis of PAL Genes in Potato and Arabidopsis
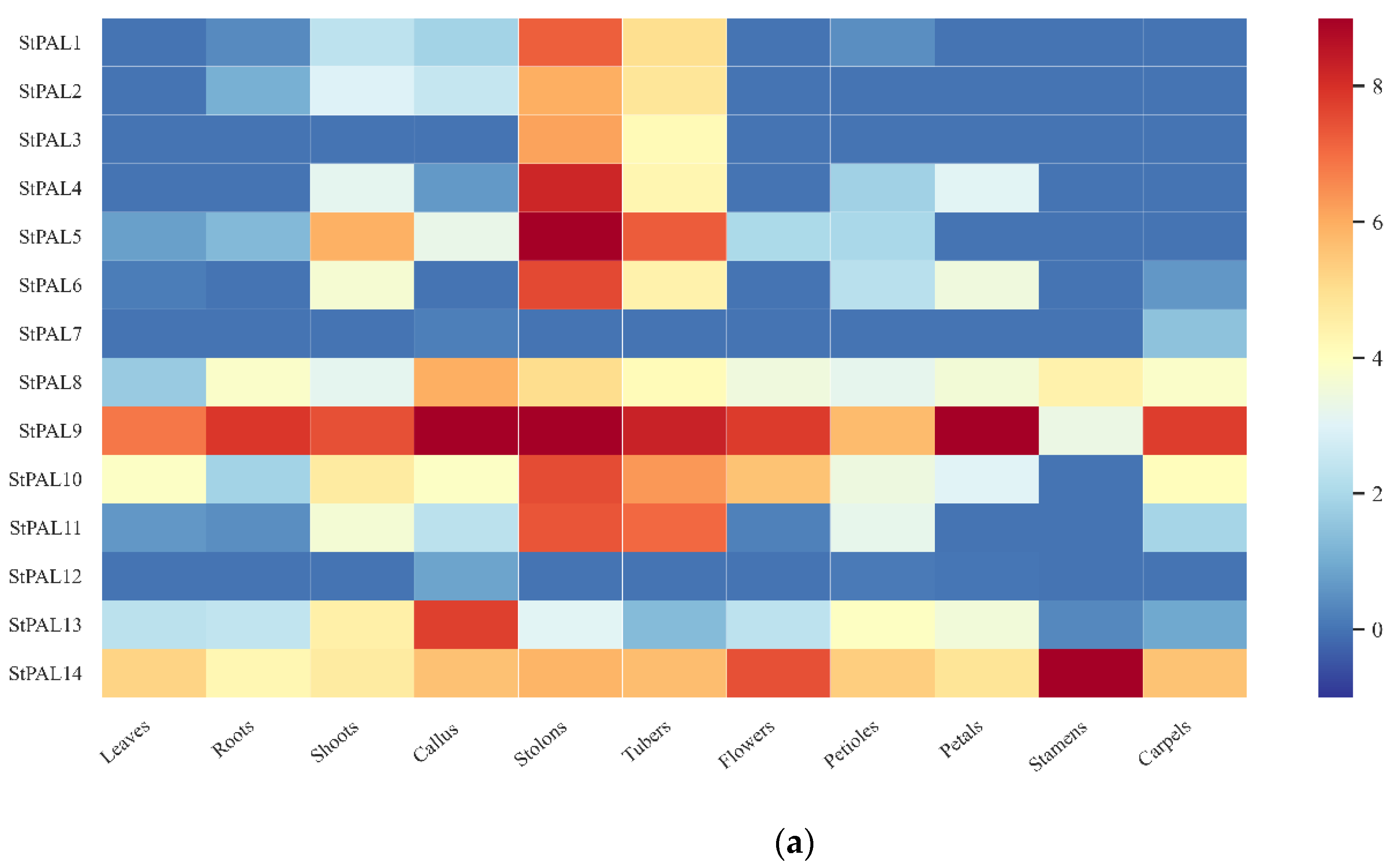
2.7. Tissue Expression of Potato PAL Gene and Expression Analysis of Stress Treatment
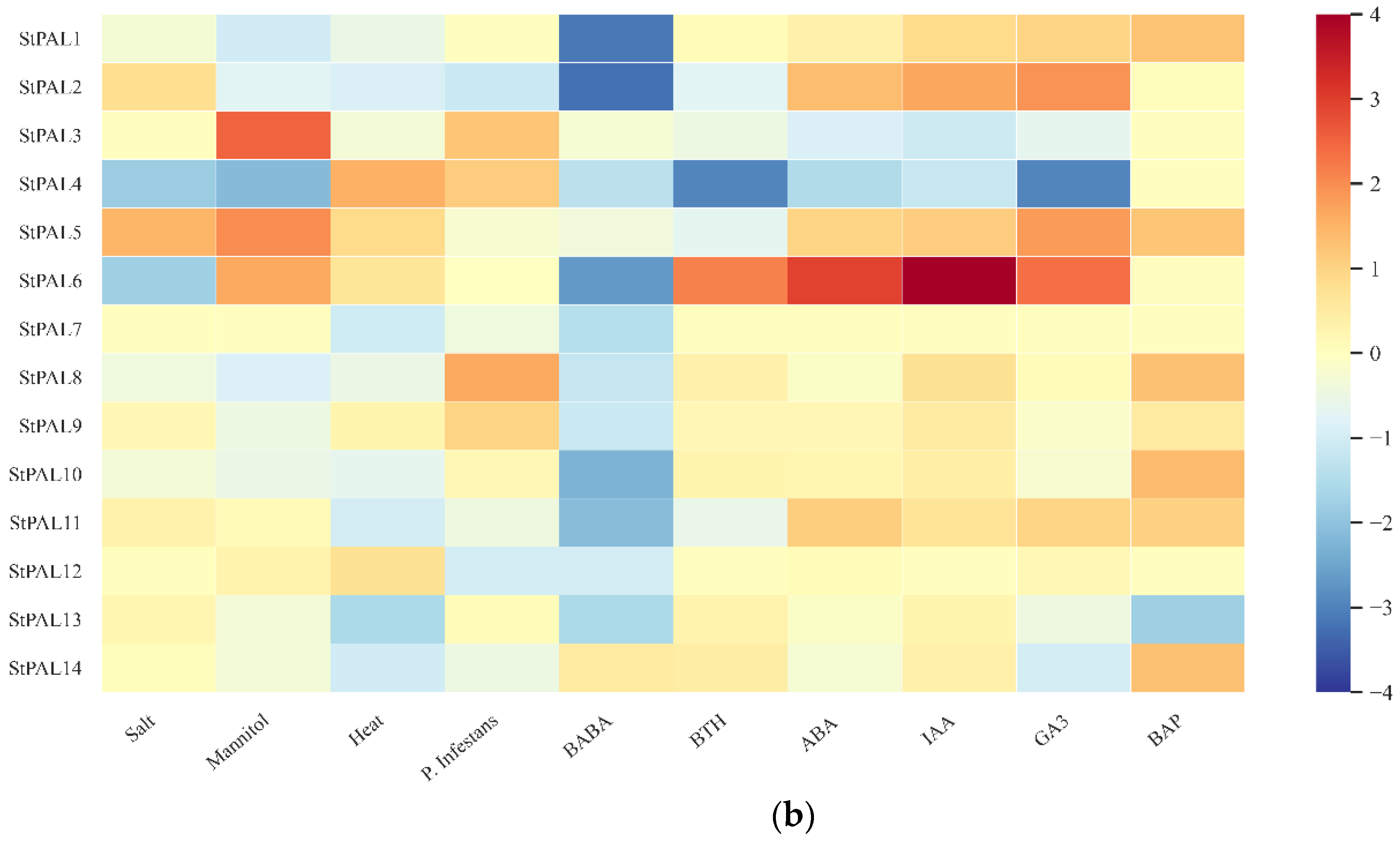
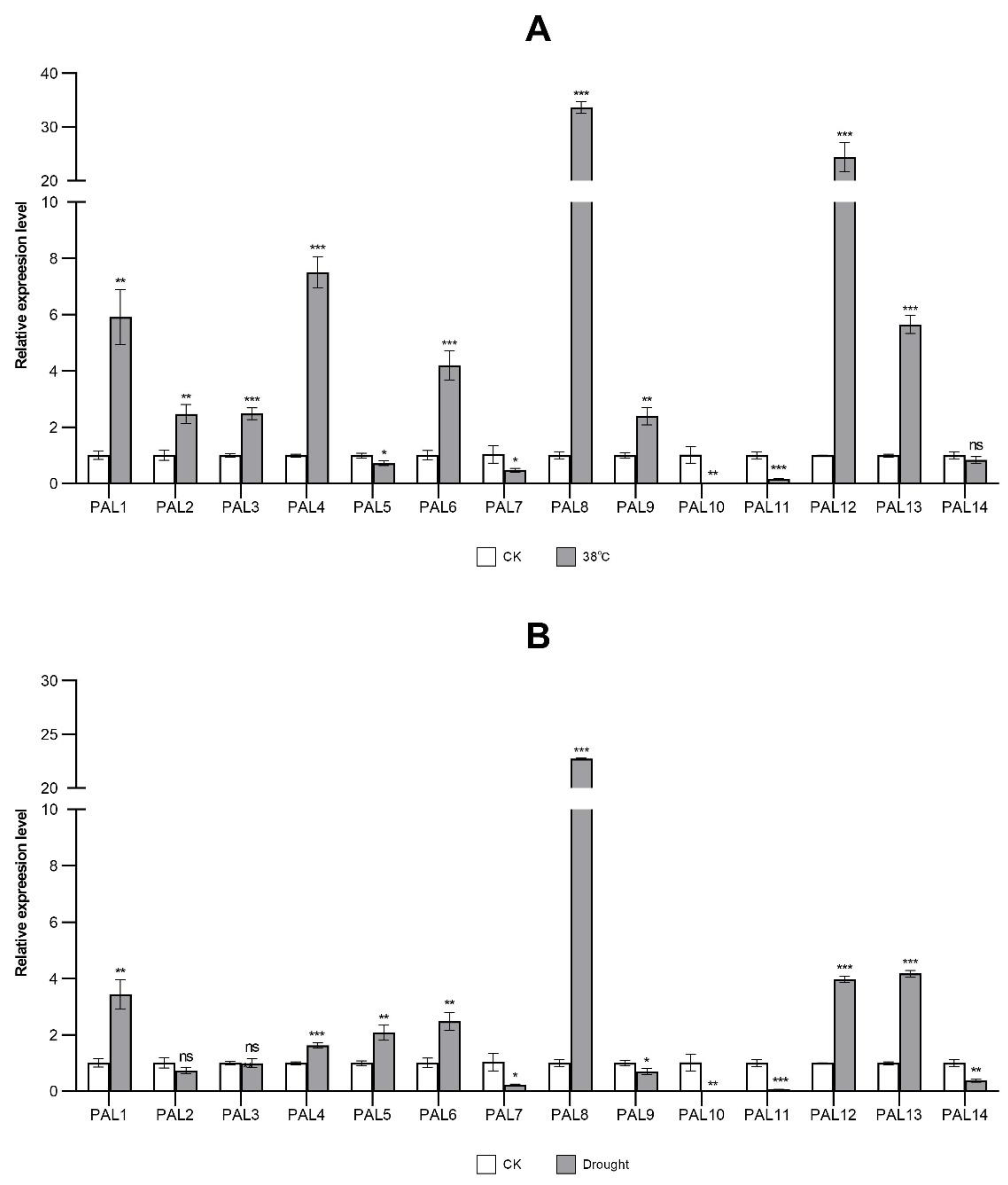
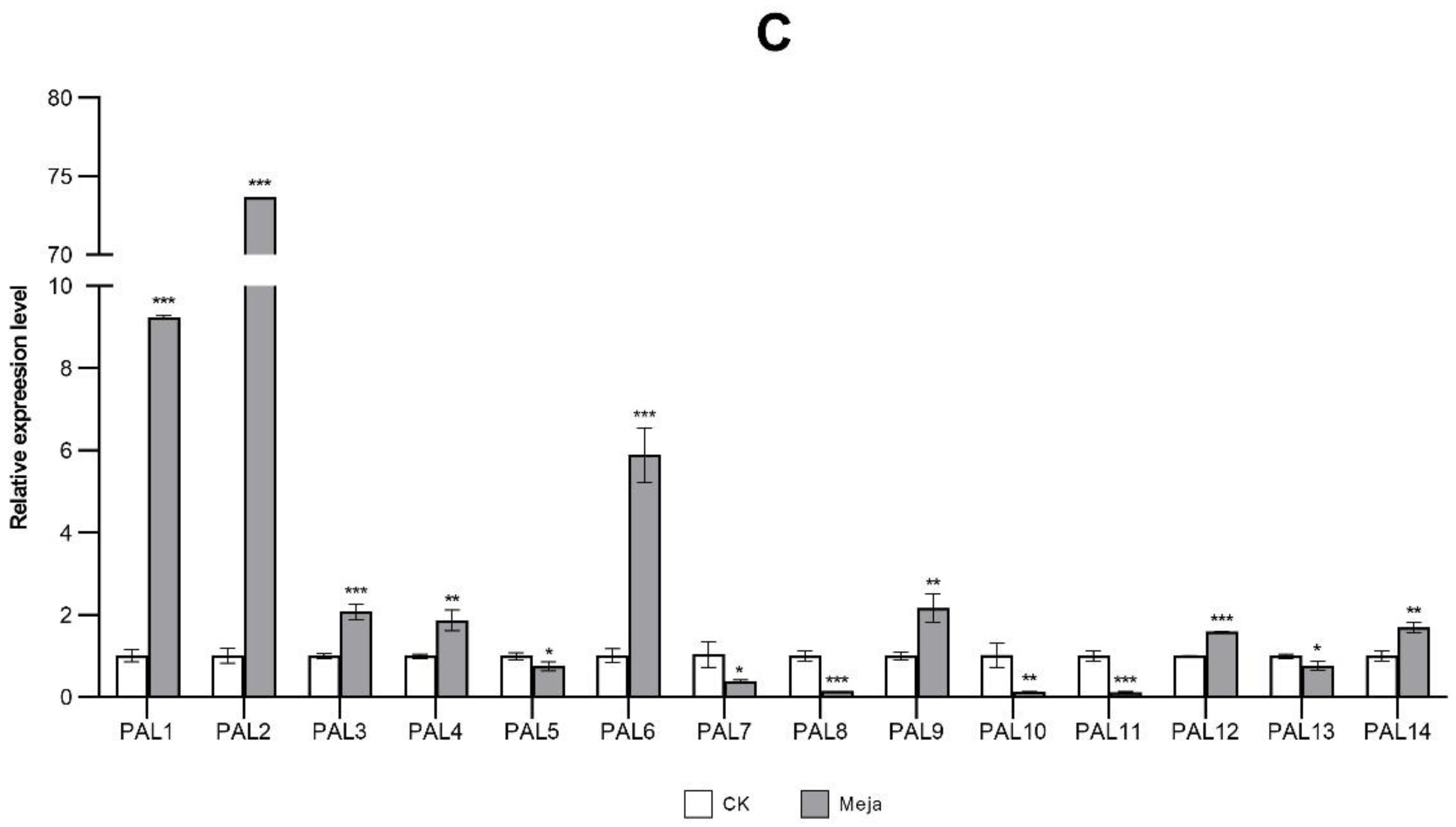
2.8. Expression Analysis of StPAL Genes in Different Treatments
3. Discussion
4. Materials and Methods
4.1. Plant Materials Preparation
4.2. Identification of PAL Gene Family in Potato
4.3. Multiple Sequence Alignment and Phylogenetic Tree Construction
4.4. Chromosomal Location Analysis and Tandem Replicated Genes and Gene Structure
4.5. Conserved Motif Identification and Cis-Acting Elements Analysis
4.6. Analysis of PAL Protein Interaction in Potato
4.7. Interspecific Collinearity Analysis of PAL Gene in Potato
4.8. Tissue Expression and Stress Treatment Expression Analysis of the Potato PAL Genes
4.9. RNAIsolation and qRT-PCR Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Gene Name | Locus ID |
|---|---|---|
| Arabidopsis thaliana | AtPAL1 | AT2G37040 |
| AtPAL2 | AT3G53260 | |
| AtPAL3 | AT5G04230 | |
| AtPAL4 | AT3G10340 | |
| Dioscorea cayenensis | DcPAL1 | XP_039137778 |
| DcPAL2 | XP_039132878 | |
| Manihot esculenta Crantz | MePAL1 | XP_021620397 |
| MePAL2 | XP_021611089 | |
| MePAL3 | AAK62030 | |
| MePAL4 | XP_021597003 | |
| MePAL5 | XP_021618700 | |
| Nicotiana tabacum | NtPAL1 | M84466 |
| NtPAL2 | D17467 | |
| NtPAL3 | X78269 | |
| NtPAL4 | EU883669/70 | |
| Oryza sativa L. | OsPAL1 | XP_015620761 |
| OsPAL2 | XP_015640196 | |
| OsPAL3 | NP_001388835 | |
| OsPAL4 | XP_015625125 | |
| OsPAL5 | XP_015625126 | |
| OsPAL6 | XP_015626729 | |
| OsPAL7 | BAG94561.1 | |
| OsPAL8 | XP_015633749 | |
| OsPAL9 | XP_015615944 | |
| Pinus pinaster | PpPAL1 | AAT66434 |
| Solanum tuberosum | StPAL1 | Soltu.DM.03G004870 |
| StPAL2 | Soltu.DM.03G004900 | |
| StPAL3 | Soltu.DM.03G004920 | |
| StPAL4 | Soltu.DM.03G011440 | |
| StPAL5 | Soltu.DM.03G011480 | |
| StPAL6 | Soltu.DM.03G011490 | |
| StPAL7 | Soltu.DM.05G017030 | |
| StPAL8 | Soltu.DM.05G026870 | |
| StPAL9 | Soltu.DM.09G005690 | |
| StPAL10 | Soltu.DM.09G005700 | |
| StPAL11 | Soltu.DM.09G005710 | |
| StPAL12 | Soltu.DM.09G005720 | |
| StPAL13 | Soltu.DM.10G005900 | |
| StPAL14 | Soltu.DM.10G020990 | |
| Vitis vinifera L. | VvPAL1 | XP_002267953 |
| VvPAL2 | XP_003633985 | |
| VvPAL3 | XP_002268181 | |
| VvPAL4 | NP_001384847 | |
| VvPAL5 | XP_003633986 | |
| VvPAL6 | XP_002268732 | |
| VvPAL7 | RVW78684 | |
| VvPAL8 | XP_010662075 | |
| VvPAL9 | XP_002285277 | |
| VvPAL10 | RVW84295 | |
| VvPAL11 | XP_002281799 | |
| VvPAL12 | RVW78687 | |
| Zea mays L. | ZmPAL1 | NP_001147433 |
| ZmPAL2 | NP_001151482 | |
| ZmPAL3 | NP_001147922 | |
| ZmPAL4 | NP_001105334 | |
| ZmPAL5 | NP_001141469 | |
| ZmPAL6 | XP_008645952 | |
| ZmPAL7 | NP_001168086 | |
| ZmPAL8 | XP_020402425 |
| Gene | Leaves | Roots | Shoots | Callus | Stolons | Tubers | Flowers | Petioles | Petals | Stamens | Carpels |
|---|---|---|---|---|---|---|---|---|---|---|---|
| StPAL1 | 0.00 | 0.41 | 2.34 | 1.88 | 7.21 | 4.99 | 0.00 | 0.48 | 0.00 | 0.00 | 0.00 |
| StPAL2 | 0.00 | 1.07 | 2.96 | 2.51 | 5.96 | 4.78 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| StPAL3 | 0.00 | 0.00 | 0.00 | 0.00 | 6.17 | 4.18 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| StPAL4 | 0.00 | 0.00 | 3.15 | 0.67 | 8.19 | 4.28 | 0.00 | 1.85 | 3.10 | 0.00 | 0.00 |
| StPAL5 | 0.79 | 1.29 | 5.94 | 3.30 | 9.11 | 7.25 | 2.04 | 1.99 | 0.00 | 0.00 | 0.00 |
| StPAL6 | 0.17 | 0.00 | 3.67 | 0.00 | 7.58 | 4.40 | 0.00 | 2.27 | 3.47 | 0.00 | 0.62 |
| StPAL7 | 0.00 | 0.00 | 0.00 | 0.20 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.49 |
| StPAL8 | 1.67 | 3.81 | 3.17 | 5.98 | 5.04 | 4.12 | 3.46 | 3.22 | 3.60 | 4.39 | 3.84 |
| StPAL9 | 6.84 | 7.89 | 7.47 | 9.48 | 9.79 | 8.29 | 7.82 | 5.74 | 9.33 | 3.34 | 7.77 |
| StPAL10 | 3.88 | 1.85 | 4.63 | 3.91 | 7.52 | 6.33 | 5.57 | 3.42 | 3.04 | 0.00 | 4.07 |
| StPAL11 | 0.64 | 0.45 | 3.63 | 2.30 | 7.40 | 7.07 | 0.23 | 3.23 | 0.00 | 0.00 | 1.90 |
| StPAL12 | 0.00 | 0.00 | 0.00 | 0.87 | 0.00 | 0.00 | 0.00 | 0.11 | 0.05 | 0.00 | 0.00 |
| StPAL13 | 2.31 | 2.40 | 4.48 | 7.71 | 3.10 | 1.32 | 2.36 | 3.93 | 3.56 | 0.36 | 0.92 |
| StPAL14 | 5.25 | 4.24 | 4.66 | 5.63 | 5.86 | 5.71 | 7.45 | 5.34 | 4.83 | 9.12 | 5.59 |
| Gene | Salt | Mannitol | Heat | P. infestans | BABA | BTH | ABA | IAA | GA3 | BAP |
|---|---|---|---|---|---|---|---|---|---|---|
| StPAL1 | −0.31 | −1.01 | −0.55 | 0.03 | −3.13 | 0.07 | 0.35 | 0.85 | 0.99 | 1.27 |
| StPAL2 | 0.79 | −0.73 | −0.89 | −1.12 | −3.23 | −0.72 | 1.37 | 1.68 | 1.91 | 0.05 |
| StPAL3 | 0.00 | 2.53 | −0.34 | 1.24 | −0.27 | −0.49 | −0.88 | −1.09 | −0.64 | 0.00 |
| StPAL4 | −1.83 | −2.13 | 1.55 | 1.12 | −1.37 | −2.94 | −1.52 | −1.17 | −2.94 | 0.00 |
| StPAL5 | 1.49 | 2.01 | 0.87 | −0.23 | −0.38 | −0.66 | 0.98 | 1.11 | 1.83 | 1.23 |
| StPAL6 | −1.76 | 1.63 | 0.65 | −0.02 | −2.69 | 2.14 | 2.94 | 4.09 | 2.35 | 0.00 |
| StPAL7 | 0.00 | 0.00 | −1.04 | −0.42 | −1.41 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| StPAL8 | −0.42 | −0.86 | −0.50 | 1.65 | −1.22 | 0.35 | −0.12 | 0.74 | 0.10 | 1.29 |
| StPAL9 | 0.20 | −0.48 | 0.25 | 0.97 | −1.12 | 0.21 | 0.20 | 0.52 | −0.13 | 0.52 |
| StPAL10 | −0.32 | −0.54 | −0.63 | 0.16 | −2.27 | 0.27 | 0.23 | 0.38 | −0.25 | 1.40 |
| StPAL11 | 0.32 | 0.15 | −0.98 | −0.45 | −2.12 | −0.57 | 1.08 | 0.70 | 0.99 | 1.02 |
| StPAL12 | 0.00 | 0.29 | 0.74 | −0.98 | −0.98 | 0.00 | 0.11 | 0.00 | 0.18 | 0.00 |
| StPAL13 | 0.24 | −0.34 | −1.57 | 0.12 | −1.57 | 0.30 | −0.10 | 0.26 | −0.45 | −1.76 |
| StPAL14 | 0.04 | −0.33 | −1.01 | −0.47 | 0.51 | 0.46 | −0.26 | 0.37 | −0.98 | 1.28 |
| Species | Sequence (in 5′ → 3′ Order) | |
|---|---|---|
| Forward | Reverse | |
| StPAL1 | TCTAATCTGACAGCAGGAAGGA | CCGAGCAGTAAGAAGCCATC |
| StPAL2 | CCTCGGGTGATCTTGTACCTT | TAACACCAGCCACACGGAAA |
| StPAL3 | GAATGGCACAGCAGTTGGT | TTCCGTTCATCACTTCAGCAA |
| StPAL4 | TGCACAAAATGGACATAAAGCCAA | AGAAAGTTCCACTTTGACCCCAC |
| StPAL5 | ATTACCCCGTGTTTGCCCCT | ACCATTGGGTCCAACAGCCT |
| StPAL6 | GCCATCTAATCTCACAGCAGGAAGG | AAGTTCCGAGCAGTAAGAAGCCATC |
| StPAL7 | GCCGGTGATCCGACTAGGTG | CCGACAGCTCCACCTTCACA |
| StPAL8 | TGCAGCCCTACAGAAAGAGC | CCTCACTAGCATAGCTGCCC |
| StPAL9 | TGCTGATGATCCCTGCAGTTCA | GGGTTGCCACTTTCAAGCATAG |
| StPAL10 | TCTTGAAAGTGGCAACCCTGT | CTCCTCACCGGGAGATCGAA |
| StPAL11 | GCTATGCTTGAGAGTGGAAACCC | GTCAATCTCCTCACCGGGCG |
| StPAL12 | CCCGTTGTCATACATTGTTGGG | GCTGTGCCATTCACAAGTGC |
| StPAL13 | TGCTGAAGAAGCGTTCCGTGTT | ATGCCATACCAGAGCCAACTGC |
| StPAL14 | GCCAGAGTCGCGTTGGAAAG | CAATTCCGTCCCGAGCTCCT |
| ef1α | GGAAAAGCTTGCCTATGTGG | CTGCTCCTGGCAGTTTCAA |
References
- Jones, D.H. Phenylalanine ammonia-lyase: Regulation of its induction, and its role in plant development. Phytochemistry 1984, 23, 1349–1359. [Google Scholar] [CrossRef]
- Weisshaar, B.; Jenkins, G.I. Phenylpropanoid Biosynthesis and Its Regulation. Curr. Opin. Plant Biol. 1998, 1, 251–257. [Google Scholar] [CrossRef]
- Whetten, R.; Sederoff, R. Lignin Biosynthesis. Plant Cell 1995, 7, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, M.H. Characterization of the phenylalanine ammonia-lyase gene (SlPAL5) from tomato (Solanum lycoper sicum L.). Mol. Biol. Rep. 2009, 36, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.J.; Reddy, M.S.; Wang, L. The phenylpropanoid pathway and plant defense—A genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef]
- Khakdan, F.; Alizadeh, H.; Ranjbar, M. Molecular cloning, functional characterization and expression of a drought inducible phenylalanine ammonia-lyase gene (ObPAL) from Ocimum basilicum L. Plant Physiol. Biochem. 2018, 130, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Wen, P.F.; Kong, W.F.; Pan, Q.H.; Zhan, J.C.; Li, J.M.; Wan, S.B.; Huang, W.D. Effect of salicylic acid on phenylpropanoids and phenylalanine ammonia-lyase in harvested grape berries. Postharvest Biol. Technol. 2006, 40, 64–72. [Google Scholar] [CrossRef]
- Jiang, Y.M.; Joyce, D.C. ABA effects on ethylene production, PAL activity, anthocyanin and phenolic contents of strawberry fruit. Plant Growth Regul. 2003, 39, 171–174. [Google Scholar] [CrossRef]
- Ritter, H.; Schulz, G.E. Structural basis for the entrance into the phenylpropanoid metabolism catalyzed by phenylalanine ammonia-lyase. Plant Cell 2004, 16, 3426–3436. [Google Scholar] [CrossRef]
- Koukol, J.; Conn, E.E. The metabolism of aromatic compounds in higher plants. IV. Purification and properties of the phenylalanine deaminase of Hordeum vulgare. J. Biol. Chem. 1961, 236, 2692–2698. [Google Scholar] [CrossRef]
- MacDonald, M.J.; D’Cunha, G.B. A modern view of phenylalanine ammonia lyase. Biochem. Cell Biol. 2007, 85, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, S.; Sadeghi, M.; Pöppel, A. Differential inductions of phenylalanine ammonia-lyase and Chalcone synthase during wounding, salicylic acid treatment, and salinity stress in safflower, Carthamus tinctorius. Biosci. Rep. 2014, 34, e00114. [Google Scholar] [CrossRef]
- Raes, J.; Rohde, A.; Christensen, J.H.; Van de Peer, Y.; Boerjan, W. Genome-wide characterization of the lignification toolbox in Arabidopsis. Plant Physiol. 2003, 133, 1051–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichert, A.I.; He, X.Z.; Dixon, R.A. Phenylalanine ammonia-lyase (PAL) from tobacco (Nicotiana tabacum): Characterization of the four tobacco PAL genes and active heterotetrameric enzymes. Biochem. J. 2009, 424, 233–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamberger, B.; Ellis, M.; Friedmann, M.; de Azevedo Sousa, C.; Barbazuk, B.; Douglas, C. Genome-wide analyses of phenylpropanoid-related genes in Populus trichocarpa, Arabidopsis thaliana, and Oryza sativa: The Populus lignin toolbox and conservation and diversification of angiosperm gene families. Botany 2007, 14, 1182–1201. [Google Scholar]
- Feng, Y.T.; Huang, Q.L.; Zhang, R.; Li, J.Y.; Luo, K.; Chen, Y.H. Molecular characterisation of PAL gene family reveals their role in abiotic stress response in lucerne (Medicago sativa). Crop Pasture Sci. 2022, 73, 300–311. [Google Scholar] [CrossRef]
- Shang, Q.M.; Li, L.; Dong, C.J. Multiple tandem duplication of the phenyalanine ammonia-lyase genes in Cucumis sativus L. Planta 2012, 236, 1093–1105. [Google Scholar] [CrossRef]
- Dong, C.-J.; Cao, N.; Zhang, Z.-G.; Shang, Q.-M. Phenylalanine ammonia-lyase gene families in cucurbit species: Structure, evolution, and expression. J. Integr. Agric. 2016, 15, 1239–1255. [Google Scholar] [CrossRef] [Green Version]
- Hyun, M.W.; Yun, Y.H.; Kim, J.Y.; Kim, S.H. Fungal and plant phenylalanine ammonia-lyase. Mycobiology 2011, 39, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Hahlbrock, K.; Scheel, D. Physiology and Molecular Biology of Phenylpropanoid Metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 341–369. [Google Scholar] [CrossRef]
- Rohde, A.; Morreel, K.; Ralph, J.; Goeminne, G.; Hostyn, V.; De Rycke, R.; Kushnir, S.; Van Doorsselaere, J.; Joseleau, J.P.; Vuylsteke, M.; et al. Molecular phenotyping of the pal1 and pal2 mutants of Arabidopsis thaliana reveals far-reaching consequences on phenylpropanoid, amino acid, and carbohydrate metabolism. Plant Cell 2004, 16, 2749–2771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizutani, M.; Ohta, D.; Sato, R. Isolation of a cDNA and a genomic clone encoding cinnamate 4-hydroxylase from Arabidopsis and its expression manner in planta. Plant Physiol. 1997, 113, 755–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, K.M.; Lea, U.S.; Slimestad, R.; Verheul, M.; Lillo, C. Differential expression of four Arabidopsis PAL genes; PAL1 and PAL2 have functional specialization in abiotic environmental triggered flavonoid synthesis. J. Plant Physiol. 2008, 165, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Hijmans, R.J. Global distribution of the potato crop. Am. J. Pot Res. 2001, 78, 403–412. [Google Scholar] [CrossRef]
- Joos, H.-J.; Hahlbrock, K. Phenylalanine ammonia-lyase in potato (Solanum tuberosum L.). Eur. J. Biochem. 1992, 204, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Borchert, R. Time course and spatial distribution of phenylalanine ammonia-lyase and peroxidase activity in wounded potato tuber tissue. Plant Physiol. 1978, 62, 789–793. [Google Scholar] [CrossRef] [Green Version]
- Valcarcel, J.; Reilly, K.; Gaffney, M.; O’Brien, N.M. Levels of potential bioactive compounds including carotenoids, vitamin C and phenolic compounds, and expression of their cognate biosynthetic genes vary significantly in different varieties of potato (Solanum tuberosum L.) grown under uniform cultural conditions. J. Sci. Food Agric. 2016, 96, 1018–1026. [Google Scholar]
- Gerasimova, N.G.; Pridvorova, S.M.; Ozeretskovskaya, O.L. Role of L-phenylalanine ammonia lyase in the induced resistance and susceptibility of potato plants. Appl. Biochem. Microbiol. 2005, 41, 103–105. [Google Scholar]
- Cramer, C.L.; Edwards, K.; Dron, M.; Liang, X.; Dildine, S.L.; Bolwell, G.P.; Dixon, R.A.; Lamb, C.J.; Schuch, W. Phenylalanine ammonia lyase gene organization and structure. Plant Mol. Biol. 1989, 12, 67–383. [Google Scholar] [CrossRef]
- Kumar, A.; Ellis, B.E. The Phenylalanine ammonia-lyase gene family in raspberry: Structure, expression, and evolution. Plant Physiol. 2001, 127, 230–239. [Google Scholar] [CrossRef] [Green Version]
- Du, D.; Cheng, T.; Pan, H. Genome-wide identification, molecular evolution and expression analyses of the phospholipase D gene family in three Rosaceae species. Sci. Hortic. 2013, 153, 13–21. [Google Scholar]
- Winkel-Shirley, B. Flavonoid biosynthesis. a colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.L.; Dai, W.; Sun, B.M.; Zhao, Y.; Ma, Q. Genome-wide identification and comparative analysis of the TUBBY-like protein gene family in maize. Genes Genom. 2016, 38, 25–36. [Google Scholar]
- Li, G.; Wang, H.; Cheng, X.; Su, X.; Zhao, Y.; Jiang, T.; Jin, Q.; Lin, Y.; Cai, Y.P. Comparative genomic analysis of the PAL genes in five Rosaceae species and functional identification of Chinese white pear. PeerJ 2019, 7, e8064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Deng, G.; Cheng, S.; Zhang, W.; Huang, X.; Li, L.; Cheng, H.; Rong, X.; Li, J. Molecular Cloning, Characterization and Expression of the Phenylalanine Ammonia-Lyase Gene from Juglans regia. Molecules 2012, 17, 7810–7823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayaz, A.; Saqib, S.; Huang, H.; Zaman, W.; Lü, S.; Zhao, H. Genome-wide comparative analysis of long-chain acyl-CoA synthetases (LACSs) gene family: A focus on identification, evolution and expression profiling related to lipid synthesis. Plant Physiol. Biochem. 2021, 161, 1–11. [Google Scholar] [CrossRef]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef] [Green Version]
- Chang, A.; Lim, M.-H.; Lee, S.-W.; Robb, E.J.; Nazar, R.N. Tomato phenylalanine ammonia-lyase gene family, highly redundant but strongly underutilized. J. Biol. Chem. 2008, 283, 33591–33601. [Google Scholar] [CrossRef] [Green Version]
- Ren, W.; Wang, Y.; Xu, A.; Zhao, Y.G. Genome-wide identification and characterization of the phenylalanine ammonia-lyase (PAL) gene family in Medicago truncatula. Legume Rese Structural basis for the entrance into the phenylpropanoid metabolism catalyzed by phenylalanine ammonia-lyase. Legume Res. 2019, 42, 461–466. [Google Scholar]
- Dong, C.J.; Shang, Q.M. Genome-Wide characterization of phenylalanine ammonia-lyase gene family in watermelon (Citrullus lanatus). Planta 2013, 238, 35–49. [Google Scholar] [CrossRef]
- Faraji, S.; Filiz, E.; Kazemitabar, S.K.; Vannozzi, A.; Palumbo, F.; Barcaccia, G.; Heidari, P. The AP2/ERF Gene Family in Triticum durum: Genome-Wide Identification and Expression Analysis under Drought and Salinity Stresses. Genes 2020, 11, 1464. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, A.; Huang, H.; Zheng, M.; Zaman, W.; Li, D.; Saqib, S.; Zhao, H.; Lü, S. Molecular Cloning and Functional Analysis of GmLACS2-3 Reveals Its Involvement in Cutin and Suberin Biosynthesis along with Abiotic Stress Tolerance. Int. J. Mol. Sci. 2021, 22, 9175. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.M.; Chapple, C. The phenylpropanoid pathway in Arabidopsis. Arab. Book 2011, 9, e0152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elkind, Y.; Edwards, R.; Mavandad, M.; Hedrick, S.A.; Ribak, O.; Dixon, R.A.; Lamb, C.J. Abnormal plant development and downregulation of phenylpropanoid biosynthesis in transgenic tobacco containing a heterologous phenylalanine ammonia-lyase gene. Proc. Natl. Acad. Sci. USA 1990, 87, 9057–9061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reams, A.B.; Neidle, E.L. Selection for gene clustering by tandem duplication. Annu. Rev. Microbiol. 2004, 58, 119–142. [Google Scholar] [CrossRef]
- Lu, B.B.; Du, Z.; Ding, R.X.; Zhang, L.; Yu, X.J.; Liu, C.H.; Chen, W.S. Cloning and characterization of a differentially expressed phenylalanine ammonialase gene (IiPAL) after genome duplication from Tetraploid Isatis indigotica Fort. J. Integr. Plant Biol. 2006, 48, 1439–1449. [Google Scholar] [CrossRef]
- Manghwar, H.; Hussain, A.; Ali, Q.; Liu, F. Brassinosteroids (BRs) Role in Plant Development and Coping with Different Stresses. Int. J. Mol. Sci. 2022, 23, 1012. [Google Scholar] [CrossRef]
- Sanchez-Ballesta, M.T.; Lafuente, M.T.; Zacarias, L.; Granell, A. Involvement of phenylalanine ammonia-lyase in the response of Fortune mandarin fruits to cold temperature. Physiol. Plant 2000, 108, 382–389. [Google Scholar] [CrossRef]
- Rasool, F.; Uzair, M.; Naeem, M.K.; Rehman, N.; Afroz, A.; Shah, H.; Khan, M.R. Phenylalanine Ammonia-Lyase (PAL) Genes Family in Wheat (Triticum aestivum L.): Genome-Wide Characterization and Expression Profiling. Agronomy 2021, 11, 2511. [Google Scholar] [CrossRef]
- Nguyen, T.-N.; Son, S.; Jordan, M.C.; David, B.; Belay, T. Lignin biosynthesis in wheat (Triticum aestivum L.): Its response to waterlogging and association with hormonal levels. BMC Plant Biol. 2016, 16, 28. [Google Scholar] [CrossRef] [Green Version]
- Dixon, R.A.; Maxwell, C.A.; Ni, W.; Oommen, A.; Paiva, N.L. Genetic manipulation of lignin and phenylpropanoid compounds involved in interactions with microorganisms. In Genetic Engineering of Plant Secondary Metabolism; Springer: Berlin/Heidelberg, Germany, 1994; pp. 153–178. [Google Scholar]
- Thulke, O.; Conrath, U. Salicylic acid has a dual role in activation of defence-related genes in parsley. Plant J. 1998, 14, 35–42. [Google Scholar] [CrossRef]
- Fraissinet-Tachet, L.; Baltz, R.; Chong, J.; Kauffmann, S.; Friting, B.; Saindrenan, P. Two tobacco genes induced by infection, elicitor and salicylic acid encode glucosyltransferases acting on phenylpropanoids and benzoic acid derivatives, including salicylic acid. FEBS Lett. 1998, 437, 319–323. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Zhu, X.F.; Tian, W.G.; Cheng, W.H.; Sun, J.; Jin, S.X.; Zhu, H.G. Genome-wide identification and expression analysis of polyamine oxidase genes in upland cotton (Gossypium hirsutum L.). Plant Cell Tiss. Organ Cult. 2017, 129, 237–249. [Google Scholar] [CrossRef]
- Manghwar, H.; Hussain, A. Mechanism of tobacco osmotin gene in plant responses to biotic and abiotic stress tolerance: A brief history. Biocell 2022, 46, 623–632. [Google Scholar] [CrossRef]
- Yang, C.; Wang, D.; Zhang, C.; Ye, M.; Chen, Q. Comprehensive Analysis and Expression Profiling of PIN, AUX/LAX, and ABCB Auxin Transporter Gene Families in Solanum tuberosum under Phytohormone Stimuli and Abiotic Stresses. Biology 2020, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, Y.; Yuan, D.; Duan, M.; Liu, Y.; Shen, Z.; Yang, C.; Qiu, Z.; Liu, D.; Wen, P.; et al. An R2R3 MYB transcription factor confers brown planthopper resistance by regulating the phenylalanine ammonia-lyase pathway in rice. Proc. Natl. Acad. Sci. USA 2020, 117, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, R.M.; Yao, W.; Wang, Y.J.; Zhang, C.H.; Li, Y. Genome-wide identification and characterisation of phenylalanine ammonia-lyase gene family in grapevine. J. Hortic. Sci. Biotechnol. 2021, 96, 456–468. [Google Scholar] [CrossRef]
- Yuan, J.; Amend, A.; Borkowski, J.; Demarco, R.; Bailey, W.; Liu, Y.; Xie, G.; Blevins, R. MULTICLUSTAL: A systematic method for surveying Clustal W alignment parameters. Bioinformatics 1999, 15, 862–863. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Guo, K.; Yu, L.; Tu, Y.; Peng, L. Expression profiling and integrative analysis of the CESA/CSL superfamily in rice. BMC Plant Biol. 2010, 10, 282. [Google Scholar] [CrossRef] [Green Version]
- Timothy, B.; Mikael, B.; Buske, F.A.; Martin, F.; Charles, E.G.; Luca, C.; Ren, J.Y.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. Plant Care, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cao, M.; Chen, X.; Ye, M.; Zhao, P.; Nan, Y.; Li, W.; Zhang, C.; Kong, L.; Kong, N.; et al. Genome-Wide Analysis of the Lateral Organ Boundaries Domain (LBD) Gene Family in Solanum tuberosum. Int. J. Mol. Sci. 2019, 20, 5360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Zhang, G.; Murphy, A.; Koeyer, D.D.; Tai, H.; Bizimungu, B.; Si, H.; Li, X.Q. Differences between the Bud End and Stem End of Potatoes in Dry Matter Content, Starch Granule Size, and Carbohydrate Metabolic Gene Expression at the Growing and Sprouting Stages. J. Agric. Food Chem. 2016, 64, 1176–1184. [Google Scholar] [CrossRef] [PubMed]


| Gene 1 | Gene ID 1 | Chromosome Location 1 (bp) 1 | ORF Length (bp) 1 | No. of Exons 1 | Protein 2 | Subcellular Localization | ||
|---|---|---|---|---|---|---|---|---|
| Length (aa) | MW (Da) | pI | ||||||
| StPAL1 | Soltu.DM.03G004870 | Chr03:5254948—5259172 (+) | 2171 | 2 | 723 | 78,585.86 | 6.11 | Cytoplasm |
| StPAL2 | Soltu.DM.03G004900 | Chr03:5293236—5296444 (+) | 2172 | 2 | 723 | 78,749.96 | 6.00 | Cytoplasm |
| StPAL3 | Soltu.DM.03G004920 | Chr03: 5304263—5308005 (+) | 2172 | 5 | 708 | 77,814.62 | 6.23 | Plasma membrane |
| StPAL4 | Soltu.DM.03G011440 | Chr03: 31804241—31801966 (−) | 1956 | 4 | 651 | 70,751.77 | 6.75 | Cytoplasm |
| StPAL5 | Soltu.DM.03G011480 | Chr03: 31877150—31874688 (−) | 2022 | 2 | 673 | 73,124.54 | 6.21 | Cytoplasm |
| StPAL6 | Soltu.DM.03G011490 | Chr03: 31902002—31895849 (−) | 2142 | 3 | 713 | 77,929.94 | 7.97 | Plasma membrane |
| StPAL7 | Soltu.DM.05G017030 | Chr03: 40539165—40534755 (−) | 2004 | 2 | 667 | 73,689.12 | 5.41 | Cytoplasm |
| StPAL8 | Soltu.DM.05G026870 | Chr05: 54865269—54869381 (+) | 2124 | 2 | 707 | 77,510.48 | 6.07 | Cytoplasm |
| StPAL9 | Soltu.DM.09G005690 | Chr09: 5255916—5252152 (−) | 2169 | 2 | 722 | 78,590.86 | 6.32 | Cytoplasm |
| StPAL10 | Soltu.DM.09G005700 | Chr09: 5262994—5260062 (−) | 2169 | 2 | 722 | 78,488.71 | 6.04 | Cytoplasm |
| StPAL11 | Soltu.DM.09G005710 | Chr09: 5280698—5283618 (+) | 2160 | 2 | 719 | 78,330.48 | 6.15 | Cytoplasm |
| StPAL12 | Soltu.DM.09G005720 | Chr09: 5287714—5290290 (+) | 2172 | 2 | 723 | 78,819.07 | 6.00 | Cytoplasm |
| StPAL13 | Soltu.DM.10G005900 | Chr10: 5983790—5987501 (+) | 2130 | 2 | 709 | 77,707.78 | 6.16 | Cytoplasm |
| StPAL14 | Soltu.DM.10G020990 | Chr10: 52868738—52864254 (−) | 2136 | 2 | 711 | 77,543.40 | 5.86 | Cytoplasm |
| Motif | Length | Amino Acid Sequence |
|---|---|---|
| Motif1 | 50 | QKPKQDRYALRTSPQWLGPQIEVIRAATKMIEREINSVNDNPLIDVSRNK |
| Motif2 | 50 | DYGFKGAEIAMASYCSELQFLANPVTNHVQSAEQHNQDVNSLGLISARKT |
| Motif3 | 50 | SDWVMDSMSKGTDSYGVTTGFGATSHRRTKNGGALQKELIRFLNAGVFGN |
| Motif4 | 50 | VMNGKPEFTDYLTHKLKHHPGQIEAAAIMEHILDGSSYVKAAQKLHEMDP |
| Motif5 | 50 | HTLPHSATRAAMLVRINTLLQGYSGIRFEILEAITKLINSNITPCLPLRG |
| Motif6 | 50 | ELHPARFCEKELLRVVDREYLFAYADDPCSSNYPLMQKLRQVLVDHAMKN |
| Motif7 | 50 | NRITECRSYPLYRLVRZELGTELLTGEKVRSPGEEIDKVFTAMCNGQIID |
| Motif8 | 50 | VSGGFFELQPKEGLALVNGTAVGSGMASIVLFESNILAVMSEVLSAIFAE |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, F.; Li, L.; Zhang, C.; Yang, C.; Chen, G.; Niu, Y.; Si, J.; Liu, T.; Sun, X.; Wang, S.; et al. Genome-Wide Analysis and Expression Profiling of the Phenylalanine Ammonia-Lyase Gene Family in Solanum tuberosum. Int. J. Mol. Sci. 2022, 23, 6833. https://doi.org/10.3390/ijms23126833
Mo F, Li L, Zhang C, Yang C, Chen G, Niu Y, Si J, Liu T, Sun X, Wang S, et al. Genome-Wide Analysis and Expression Profiling of the Phenylalanine Ammonia-Lyase Gene Family in Solanum tuberosum. International Journal of Molecular Sciences. 2022; 23(12):6833. https://doi.org/10.3390/ijms23126833
Chicago/Turabian StyleMo, Fangyu, Long Li, Chao Zhang, Chenghui Yang, Gong Chen, Yang Niu, Jiaxin Si, Tong Liu, Xinxin Sun, Shenglan Wang, and et al. 2022. "Genome-Wide Analysis and Expression Profiling of the Phenylalanine Ammonia-Lyase Gene Family in Solanum tuberosum" International Journal of Molecular Sciences 23, no. 12: 6833. https://doi.org/10.3390/ijms23126833
APA StyleMo, F., Li, L., Zhang, C., Yang, C., Chen, G., Niu, Y., Si, J., Liu, T., Sun, X., Wang, S., Wang, D., Chen, Q., & Chen, Y. (2022). Genome-Wide Analysis and Expression Profiling of the Phenylalanine Ammonia-Lyase Gene Family in Solanum tuberosum. International Journal of Molecular Sciences, 23(12), 6833. https://doi.org/10.3390/ijms23126833





